Abstract
Background
Inadequate treatment of hypertension is a widespread problem, especially in South Asian countries where cardiovascular disease mortality rates are high. We aimed to explore the effect of a multicomponent intervention (MCI) on antihypertensive medication intensification among rural South Asians with hypertension.
Methods
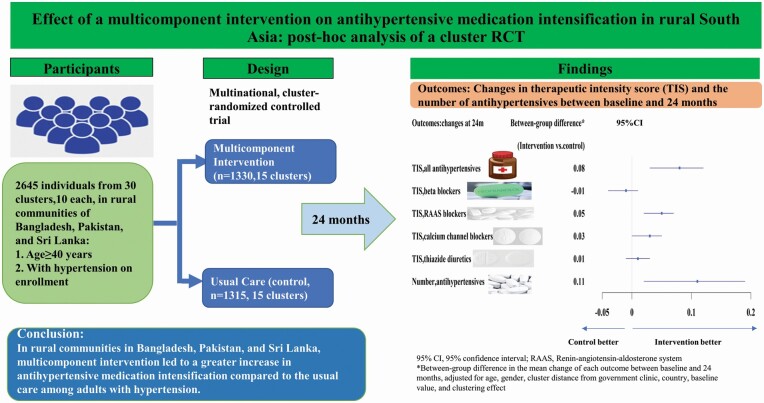
A post hoc analysis of a 2-year cluster-randomized controlled trial including 2,645 hypertensives aged ≥40 years from 30 rural communities, 10 each, in Bangladesh, Pakistan, and Sri Lanka. Independent assessors collected information on participants’ self-reports and physical inspection of medications. The main outcomes were the changes from baseline to 24 months in the following: (i) the therapeutic intensity score (TIS) for all (and class-specific) antihypertensive medications; (ii) the number of antihypertensive medications in all trial participants.
Results
At 24 months, the mean increase in the TIS score of all antihypertensive medications was 0.11 in the MCI group and 0.03 in the control group, with a between-group difference in the increase of 0.08 (95% confidence interval (CI, 0.03, 0.12); P = 0.002). In MCI compared with controls, a greater increase in the TIS of renin–angiotensin–aldosterone system blockers (0.05; 95% CI (0.02, 0.07); P < 0.001) and calcium channel blockers (0.03; 95% CI (0.00, 0.05); P = 0.031), and in the number of antihypertensive medications (0.11, 95% CI (0.02, 0.19); P = 0.016) was observed.
Conclusions
In rural communities in Bangladesh, Pakistan, and Sri Lanka, MCI led to a greater increase in antihypertensive medication intensification compared with the usual care among adults with hypertension.
Clinical trials registration
Trial Number NCT02657746.
Keywords: blood pressure, community health workers, hypertension, medications intensification, multicomponent intervention, physicians, South Asia
Graphical Abstract
Graphical Abstract.
Hypertension is the leading risk factor for cardiovascular and kidney disease and related deaths globally.1,2 Despite the established benefit of blood pressure (BP) lowering on vascular disease, and the publication of several international guidelines on hypertension management, BP control remains suboptimal, particularly in low- and middle-income countries (LMICs), where less than 10% have BP controlled to conventional targets of <140/90 mm Hg.3
Several determinants of uncontrolled hypertension have been identified at the patient, healthcare provider, and health systems levels.4 Of note, therapeutic inertia, or failure of healthcare providers to initiate or intensify antihypertensive drugs, i.e., addition of new antihypertensive medications or uptitration of existing ones by guidelines, has been shown to contribute substantially to uncontrolled hypertension in a variety of settings.4,5 Antihypertensive medication intensification has been shown to improve BP control.6 Meta-analyses of randomized trials showed that the addition of full or half dose of any major class of antihypertensive medication (thiazides, β blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, and calcium channel blockers (CCBs)) produced up to 9 and 5.5 mm Hg reduction in systolic BP (SBP) and diastolic BP (DBP), respectively.7 However, evidence remains scarce on the effect of a community-based health system intervention on antihypertensive intensification among individuals with hypertension from rural South Asia.
We recently reported findings from COBRA-BPS (Control of BP and Risk Attenuation—Bangladesh, Pakistan, and Sri Lanka)—a multicomponent intervention (MCI) including trained community health workers (CHWs) and physicians tailored to the rural setting in 3 countries, and showed a greater reduction in BP due to MCI relative to usual care.8 MCI also improved BP control, antihypertensive medication adherence, and some aspects of self-reported health.8 However, the effect of MCI on antihypertensive medication intensification and any variation in the latter due to patient characteristics has not been studied. Moreover, the potential contribution of antihypertensive medication intensification to the intervention effect on BP reduction has not been quantified.
In this paper, we report findings from post hoc analysis of COBRA-BPS trial data to examine whether MCI compared with usual care led to greater treatment intensification of antihypertensive medications. Our secondary aim was to examine if the MCI effect on treatment intensification varied with patients’ demographics and clinical characteristics. Our working hypothesis was that patients receiving MCI would have a greater therapeutic intensification compared with those under usual care. In addition, we also conducted an exploratory mediation analysis9 to understand the potential contribution of antihypertensive medication intensification to the intervention on BP lowering at 24 months.
METHODS
Trial design
The design and methods of this trial were described previously.8,10 The trial was a multicountry, cluster-randomized, controlled trial in 30 rural villages from Bangladesh, Pakistan, and Sri Lanka. It was designed to compare MCI with usual care with regard to their effect on the levels of BP (ClinicalTrials.gov: NCT02657746). This paper presents the results of a secondary analysis. The study protocol was approved by the institutional review boards at all participating sites. Written informed consent was obtained from all participants.
Participants
Briefly, participants were eligible if they were ≥40 years, had hypertension defined as current use of antihypertensive medications or persistently elevated BP (SBP ≥140 mm Hg or DBP ≥90 mm Hg) based on each set of the last 2 of 3 measurements from 2 separate days. Pregnant women and persons with advanced illness, terminal illness, or an inability to travel to the clinic were excluded. A total of 2,914 participants were eligible, and 2,645 were enrolled.8
Randomization
The unit of randomization was a cluster of 250–300 households served by 1 or 2 CHWs and 1 government clinic.10,11 A total of 30 clusters were randomly selected from designated districts in the 3 countries (10 per country). Randomization was stratified according to country and distance from the government clinic, and clusters were assigned in a 1:1 ratio to either the MCI (intervention group) or usual care (control group) using a computer-generated program.
Intervention
The details of the intervention components are described in the trial protocol.10 The intervention included:
BP monitoring and use of a checklist to guide monitoring and referral to physicians. Government CHWs were trained in measuring BP and monitored participants’ BPs at 3 monthly home visits. Based on a checklist, participants with very poorly controlled BP (SBP ≥160 mm Hg or DBP ≥100 mm Hg) or at high cardiovascular disease risk were referred to a physician, at the government primary care facility.
Home health education by trained government CHWs was delivered to all participants and their family members at 3 monthly home visits.
Training physicians in BP monitoring, management of hypertension, and using a checklist. A treatment algorithm (Supplementary Section S1 online) based on Joint National Committee and 2013 European Society of Cardiology guidelines was followed.12 Use of low-cost generic antihypertensive agents was encouraged. The target BP was SBP <140 mm Hg and DBP <90 mm Hg.
Designated hypertension triage reception and hypertension care coordinator at the government clinics.
Compensation for additional health services and targeted subsidies. Compensation was paid to the CHWs at the discretion of the local District Health Office. The cost of medications was borne primarily by the patients in Bangladesh and Pakistan and by publicly funded clinics in Sri Lanka, in accordance with the local norms.
Usual care (Control) comprised of existing services in the community with routine home visits by CHWs for maternal and childcare only.
Baseline assessment
The trained research staff masked to randomization status visited all households and invited adults aged ≥40 years to participate. Data on the use of antihypertensives and other medications were collected through self-reports and physical inspection of pill bottles, boxes, packets, and other materials by trained data collectors for verification. All drug information including medication name, indication, dose, and frequency was recorded. Body mass index and waist circumference were measured. BP was measured with the individual in a sitting position using the standard protocol.13
Other baseline data included sociodemographics, economic status, smoking status, chronic conditions (self-reported heart disease and stroke, chronic kidney disease, and diabetes), and laboratory tests (serum creatinine, fasting blood glucose, lipid profile, urine albumin, and urine creatinine). Economic status was evaluated using the International Wealth Index.14 The baseline 10-year cardiovascular disease risk was estimated using the Pooled Cohort Equation.15
Outcomes
Participants were assessed every 6 months, and data collectors, masked to the group assignment, collected information on antihypertensive medications, as in baseline.
In this study, we evaluated the intensification of antihypertensive medications via the increase in the therapeutic intensity score (TIS) and the number of antihypertensive medications from baseline to 24 months at per participant level. TIS score is summative representation of medication dosing and has been reported to have good utility in both clinical and research settings.16 TIS for a single antihypertensive medication was calculated as the prescribed daily dose of the medication divided by the corresponding maximum Food and Drug Administration (FDA)-approved daily dose.17 The TIS for each current antihypertensive medication was then added to yield a single, summative TIS. For example, if an individual used 2 antihypertensive medications, 1 at 20% maximum FDA-approved dose and the other at 50% maximum dose, then the total TIS would be 0.70 (0.2 + 0.5).
The main outcomes for this analysis were the changes in (i) the TIS for all and each major class of antihypertensive medications between baseline and 24 months, and (ii) the number of antihypertensive medications between baseline and 24 months. The additional outcomes were (i) the percentage change in TIS of all and each major class of antihypertensive medications calculated as [(TIS at 24months – TIS at baseline)] × 100/TIS at baseline, and (ii) the time from baseline to the initiation of the increase in the TIS of all antihypertensive medications during the follow-up.
Statistical analysis
Baseline characteristics and addition of new classes of antihypertensive medications at 24 months were summarized using mean, SD, and percentages, where appropriate. The intention-to-treat approach was used as the primary analysis and was done for all participants (n = 2,645). However, addition of new class antihypertensive drugs was reported only among individuals whose TIS of all antihypertensive medications increased at 24 months.
Generalized linear mixed effect models with identity link and a random effect at the cluster level were used to test the effects of the intervention on the absolute and percentage changes in the TIS and the change in the number of antihypertensive medications at 24 months from baseline. A compound symmetry covariance matrix was selected for the random clustering effect. The fixed effects in the model included randomization group (MCI vs. usual care), age, gender, country, distance of cluster from the government clinic (far vs. near), and baseline measurement of the corresponding outcome. Subgroup analysis was conducted for the intervention effect on change in the TIS of all antihypertensive medications at 24 months. We chose the same subgroups as in the analysis of MCI effect on BP reduction.8
Intervention effect on the time to the initiation of the increase in the TIS of all antihypertensive medications was analyzed using Cox proportional-hazards regression models, with age, gender, country, and distance of cluster from the government clinic as confounders and clusters as random effects. The PROCESS macro for SAS was used to perform the mediation analysis to explore if antihypertensive medications intensification mediated BP reduction effect of MCI (Supplementary Section S2 online).9
SAS version 9.4 was used to obtain point estimates and standard errors and to test for differences between randomization groups. A 2-sided P value of <0.05 was considered statistically significant.
RESULTS
A total of 2,645 hypertensive individuals were analyzed in the study with a response rate of 90.8%. The 24-month follow-up ended with retention of 92.1% of the participants in the intervention group and 89.3% of those in the control group.8
Baseline characteristics
The mean age (SD) of the participants was 58.8 (11.5) years, and 64.3% (n = 1,701) were female. About 68.8% (n = 1,819) were on antihypertensive treatment: 35.0% (n = 926) were on renin–angiotensin–aldosterone system (RAAS) blockers; 27.9% (n = 737) on beta blockers; 23.4% (n = 619) on CCBs; 11.1% (n = 294) on thiazide-like diuretics, but 58.2% (n = 1,058) had not achieved target BP. The baseline characteristics were generally comparable between the intervention and control group (Table 1).
Table 1.
Baseline characteristics of individuals with hypertension from Bangladesh, Pakistan, and Sri Lanka (n = 2,645)
| Characteristics | Intervention (n = 1,330) | Control (n = 1,315) | All (n = 2,645) |
|---|---|---|---|
| Cluster, n | 15 | 15 | 30 |
| Far/near clusters | 6/9 | 6/9 | 12/18 |
| Age (years), mean (SD) | 58.5 (11.2) | 59.0 (11.8) | 58.8 (11.5) |
| Female, n (%) | 877 (65.9) | 824 (62.7) | 1,701 (64.3) |
| Formally educated, n (%) | 834 (62.7) | 725 (55.1) | 1,559 (58.9) |
| High economic statusa, n (%) | 745 (56.1) | 580 (44.2) | 1,325 (50.21) |
| Current smoking, n (%) | 138 (10.4) | 132 (10.0) | 270 (10.2) |
| Overweight or obeseb, n (%) | 814 (61.2) | 683 (51.9) | 1,497 (56.6) |
| Central obesityc, n (%) | 920 (69.4) | 811 (62.0) | 1,731 (65.4) |
| Self-reported heart disease, n (%) | 177 (13.3) | 167 (12.7) | 344 (13.0) |
| Self-reported stroke, n (%) | 165 (12.4) | 159 (12.1) | 324 (12.2) |
| Diabetesd (%) | 374 (28.1) | 308 (23.4) | 682 (25.8) |
| Chronic kidney diseasee, n (%) | 558 (42.0) | 549 (41.7) | 1,107 (41.9) |
| Cardiometabolic comorbiditiesf, n (%) | 359 (27.9) | 319 (25.1) | 678 (26.6) |
| Systolic BP (mm Hg), mean (SD) | 146.7 (22.4) | 144.7 (21.0) | 145.7 (21.8) |
| Diastolic BP (mm Hg), mean (SD) | 89.1 (14.7) | 87.8 (13.8) | 88.5 (14.3) |
| Uncontrolled BPg, n (%) | 934 (70.2) | 907 (69.0) | 1,841 (69.6) |
| Antihypertensive use, n (%) | 926 (69.6) | 893 (67.9) | 1,819 (68.8) |
| Class of antihypertensives, n (%) | |||
| Beta blockers | 342 (25.7) | 395 (30.0) | 737 (27.9) |
| Calcium channel blockers | 330 (24.8) | 289 (22.0) | 619 (23.4) |
| RAAS blockers | 480 (36.1) | 446 (33.9) | 926 (35.0) |
| Thiazide diuretics | 133 (10.0) | 161 (12.2) | 294 (11.1) |
| High 10-year CVD riskh | 630 (26.2) | 308 (25.2) | 322 (27.3) |
| Country, n (%) | |||
| Bangladesh | 447 (33.6) | 448 (34.1) | 895 (33.8) |
| Pakistan | 450 (33.8) | 444 (33.8) | 894 (33.8) |
| Sri Lanka | 433 (32.6) | 423 (32.2) | 856 (32.4) |
Abbreviations: BP, blood pressure; CVD, cardiovascular disease; RAAS, renin–angiotensin–aldosterone system.
aDefined as International Wealth Index score ≥country-specific median.
bDefined as body mass index ≥23.5 kg/m2.
cDefined as ≥90 cm for male and ≥80 cm for female.
dDefined as fasting plasma glucose ≥126 mg/dl or using antidiabetes medications or previously diagnosed.
eDefined as the Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate based on Pakistan data (CKD-EPIPK) <60 ml/min/1.73 m2 or urine albumin to creatinine ratio ≥30 mg/g.
fDefined as the presence of 2 or more chronic conditions including stroke, heart disease, chronic kidney disease, and diabetes.
gDefined as systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg.
hDefined as ≥20% of 10-year CVD risk based on Pooled Cohort Equation (PCE).
Intervention effect on TIS and the number of antihypertensive medications
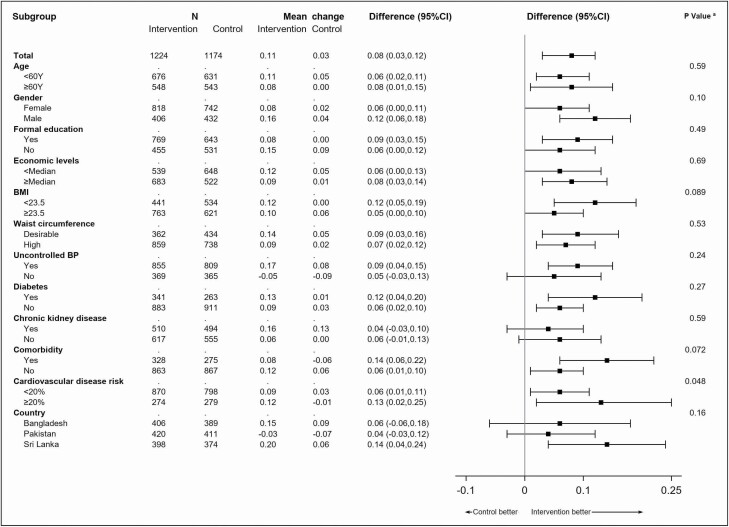
At 24 months, the adjusted mean TIS of all antihypertensive medications increased by 0.11 in the intervention group (n = 1,224) and by 0.03 in the control group (n = 1,174), with a difference of change of 0.08 (95% confidence interval (CI, 0.03, 0.12); P = 0.002; Table 2) at 24 months, which equated to a 8.5% between-group percentage increase in the TIS of all antihypertensive medications at 24 months (Supplementary Table S1 online). The net increase in the TIS of all antihypertensive medications was consistent by most baseline characteristics, except that a more pronounced increase was observed among the individuals with 10-year cardiovascular disease risk of ≥20% vs. <20% (P for interaction = 0.048) (Figure 1).
Table 2.
Adjusteda intervention effect on antihypertensive medication intensification at 24 months
| Mean (SD) at baseline | Adjusted mean change (95% CI) between baseline and 24 months | Adjusted mean between-group difference in change at 24 months since baseline (95% CI) | P value | |||
|---|---|---|---|---|---|---|
| Intervention (n = 1,330) | Control (n = 1,315) | Intervention (n = 1,224) | Control (n = 1,174) | |||
| TIS of all antihy pertensive medications | 0.53 (0.54) | 0.52 (0.54) | 0.11 (0.07, 0.14) | 0.03 (−0.00, 0.06) | 0.08 (0.03, 0.12) | 0.002 |
| TIS of beta blockers | 0.12 (0.24) | 0.14 (0.25) | −0.01 (−0.03, 0.01) | 0.00 (−0.01, 0.02) | −0.01 (−0.04, 0.01) | 0.33 |
| TIS of RAAS blockers | 0.20 (0.33) | 0.20 (0.33) | 0.05 (0.04, 0.07) | 0.01 (−0.01, 0.03) | 0.05 (0.02, 0.07) | <0.001 |
| TIS of calcium channel blockers | 0.14 (0.25) | 0.12 (0.23) | 0.04 (0.03, 0.06) | 0.02 (−0.00, 0.03) | 0.03 (0.00, 0.05) | 0.031 |
| TIS of thiazide diuretics | 0.06 (0.22) | 0.06 (0.20) | 0.02 (0.00, 0.03) | 0.00 (−0.01, 0.02) | 0.01 (−0.01, 0.03) | 0.16 |
| Number of antihypertensive medications | 0.99 (0.87) | 1.02 (0.93) | 0.15 (0.09, 0.21) | 0.04 (−0.02, 0.11) | 0.11 (0.02, 0.19) | 0.016 |
Abbreviations: 95% CI, 95% confidence interval; RAAS, renin–angiotensin–aldosterone system; TIS, therapeutic intensity score.
aAdjusted for age, gender, cluster distance from government clinic, country, baseline TIS, and clustering effect via generalized linear mixed effects modeling.
Figure 1.
Difference in the mean change of therapeutic intensity score (TIS) of all antihypertensive medications at 24 months from baseline between intervention and control groups in different subgroups. aP value was the test of interaction between treatment and each variable. Abbreviation: 95% CI, 95% confidence interval.
The intervention also resulted in a significantly higher rate of TIS increase in antihypertensive medication than the control (319 vs. 250 events per 1,000 person-years; hazard ratio, 1.29; 95% CI (1.13, 1.47)) (Supplementary Table S2 online).
The increase in the number of antihypertensive medications at 24 months was 0.11 (95% CI (0.02, 0.19)) more in the intervention than in the control group (P = 0.016; Table 2). Supplementary Tables S3–S8 online summarize the proportion of antihypertensive users in terms of classes and generic names of antihypertensive medications at baseline and 24 months.
Intervention effect on TIS of major class-specific antihypertensive medications
The between-group difference in the adjusted mean increase at 24 months in TIS was 0.05 (95% CI (0.02, 0.07); P < 0.001; Table 2) for RAAS blockers and 0.03 (95% CI (0.00, 0.05); P = 0.031; Table 2) for CCBs. Conversely, the between-group differences for beta blocker and thiazide diuretics were not significant (Table 2). The results were consistent when the percentage change in TIS (Supplementary Table S1 online) between baseline and 24 months was analyzed.
Intervention effect on addition of new class of antihypertensive medications
A total of 692 (28.8%) individuals out of total cohort in both groups (n = 2,398) had their TIS of all antihypertensive medications increased at 24 months (Supplementary Table S9 online).
Mediating effect of change in TIS and the number of antihypertensive medications on intervention effect of MCI on change in BP at 24 months
The change in the number of antihypertensive medications between baseline and 24 months was the only significant mediator (P = 0.007) (Table 3). The mediated effect (a × b) of the intervention was to reduce by about 0.8 mm Hg SBP through the increase in both the TIS and the number of antihypertensive medications, accounting for about 13.3% of the total intervention effect on SBP change (Table 3).
Table 3.
Mediated effects in path modelsa predicting change in systolic blood pressure from the intervention
| Mediator (change from baseline to 24 months) | Intervention effect on mediator, path a (SE) | Mediator effect on change in SBP in mm Hg from baseline to 24 months, path b (SE) | Mediated effect in mm Hg, a × b (95% CI) | Proportion of total effect due to mediated effect, (a × b)/c |
|---|---|---|---|---|
| Change in TIS of beta blockers | −0.00 (0.01) | 3.46 (2.22) | −0.00 (−0.09, 0.08) | 0.0% |
| Change in TIS of RAAS blockers | 0.04*** (0.01) | −3.09 (1.91) | −0.12 (−0.34, 0.05) | 1.90% |
| Change in TIS of calcium channel blockers | 0.02 (0.01) | −9.32*** (2.17) | −0.18 (−0.44, 0.01) | 2.85% |
| Change in TIS of thiazide diuretics | 0.01 (0.01) | −4.94* (2.26) | −0.07 (−0.22, 0.02) | 1.11% |
| Change in the number of antihypertensive medications | 0.11*** (0.03) | −4.24*** (0.91) | −0.47* (−0.88, −0.15) | 7.46% |
| All 5 mediators in the model | 13.32% |
Abbreviations: 95% CI, 95% confidence interval; RAAS, rennin–angiotensin–aldosterone system; SE, standard error; TIS, therapeutic intensity score.
aCovariates for multiple-mediator models were age, gender, distance to government clinic, and country.
*P < 0.05.
***P < 0.001.
DISCUSSION
In this pragmatic cluster-randomized trial of rural hypertensive individuals from Bangladesh, Pakistan, and Sri Lanka, we found that COBRA-BPS intervention led to a greater increase in the mean TIS of all antihypertensive medications at 24 months, specifically RAAS blockers and CCBs. The intervention also produced a greater increase in the number of antihypertensive medications at 24 months. Patients randomized to COBRA-BPS intervention had their TIS of antihypertensive medication increased significantly earlier than those in the control group. Furthermore, our results of the mediation analysis imply that an increase in the number of antihypertensive medications had a stronger influence on BP lowering than the dose increase. Thus, our findings indicate the remarkable improvement in therapeutic inertia due to COBRA-BPS intervention and underscore the importance of provider training in the updated pharmacologic management of hypertension as part of CHW-led MCI for the prevention of cardiovascular and kidney disease in rural LMICs. Such concerted efforts are likely to facilitate the Sustainable Development Goal target 3.4 for a 30% reduction in premature mortality from noncommunicable disease by 2030.18
Previous community-based interventions primarily conducted in urban LMICs also achieved greater antihypertensive intensification than the usual care.19,20 In a cluster-randomized clinical trial of 1,432 low-income patients with uncontrolled BP, the proportion of medication intensification defined as titration or addition of new antihypertensives was 13.7% higher in the physician–CHW collaboration group than the control group at 18 months.19 However, those studies did not quantify the magnitude of dose change, nor did they analyze class-specific information, or compare the timing of initiation of the increase in the dose of antihypertensive medications. Moreover, antihypertensive medications in most previous studies were provided free as part of the trial thus limiting generalizability and sustainability in a real-world setting.
We evaluated treatment intensification based on the TIS, permitting evaluation of expected BP effects with differential antihypertensive dosing across medication classes.16 We observed that increase in the TIS of antihypertensive medication in response to the intervention occurred primarily for RAAS blockers and CCBs. It is understandable because both RAAS blockers and CCBs were the first-line options recommended according to vascular risk profile in the antihypertensive treatment algorithm employed in the COBRA-BPS intervention. By contrast, the addition of thiazide diuretics was proposed only when the combination of RAAS blockers and CCBs was unable to control BP, and beta blockers were not indicated despite being a possible choice for antihypertensive treatment. We also observed that antihypertensive medications were intensified earlier in the intervention group than the control. Others have shown that early initiation of antihypertensive therapy is a key determinant of improved cardiovascular outcomes.21
Our exploratory mediation analysis underscores the importance of antihypertensive medication treatment intensification in a community-based hypertension control program. Furthermore, our findings of the increase in the number of antihypertensive medications being more effective in reducing BP than increasing the dose indicate that adding an antihypertensive agent before dose escalation is likely to be more effective. This is consistent with previous studies.22 Most of our study participants had their TIS increased via the addition of a new class of antihypertensive medication. We would also like to emphasize that our study was not designed or powered for mediation analysis and could underestimate antihypertensive medications’ relative contribution to BP lowering. Adoption of a healthy lifestyle advocated during home health education by CHW (i.e., weight loss strategies, physical activity, smoking cessation, avoiding excessive alcohol, low salt and saturated fat intake, and high fruit and vegetable intake) and other factors unaccounted for in our analysis could also mediate the benefit of MCI, perhaps even synergistically with antihypertensive medications. The relative contribution of antihypertensive medications (13.3%) to SBP decline observed in our mediation analysis is consistent with the previously reported relationship of change in TIS score with BP change.16 However, contribution of medication modification was very low in a community-based hypertension program in Argentina.23 Our findings highlight the importance of an increase in antihypertensive medications mediating the benefit of MCI, which probably reflects the success of training as one of the critical components of MCI.
The strength of the current study is its cluster-randomized design using a uniform protocol in 3 LMICs, the objective tool of TIS to evaluate treatment intensification, a long follow-up duration, a large sample size with a high retention rate (91%), minimization of contamination, and good generalizability of the findings to the community settings in other South Asian countries. There are several limitations in the study. First, the intervention effect could have been underestimated because participants in the control group may have modified their behavior in response to BP measurements performed by researchers to assess outcomes. Second, data on antihypertensive daily dose were self-reported. However, efforts were made by trained research staff to cross-validate the information from pillboxes and tablet leaflets. Moreover, our findings were consistent when the number of antihypertensive medications was analyzed. Thus, we believe our findings are robust. Third, our exploratory mediation analysis did not consider the within-cluster correlation of outcome variables (SBP change and the potential mediators). However, our findings are highly suggestive of an increase in the number of antihypertensive medications being a significant mediator of the effect of MCI on BP lowering.
In conclusion, our CHW-based MCI greatly improved the prescribing behaviors of trained physicians in that it not only significantly intensified antihypertensive treatment but also advanced treatment intensification among South Asians in rural communities. The treatment intensification was mainly attributed to the increase in the TIS of RAAS blockers and CCBs at 24 months, and partially explained the beneficial effects of the intervention on BP. Our recent study of the same sample has shown better BP control and higher adherence to antihypertensive medications in the MCI group than the control group.8 Our findings underscore the importance of physician–CHW collaborative care as an effective and workable approach to treatment inertia translating into better BP control and eventually reducing cardiovascular disease in rural South Asia.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the investigators, coordinators, and staff of the trial at the respective institutions, including at the International Center for Diarrheal Disease Research, Bangladesh (Dhaka); Aga Khan University (Karachi, Pakistan); the Faculty of Medicine, University of Kelaniya (Ragama, Sri Lanka); the London School of Hygiene and Tropical Medicine (London); and Duke-NUS Medical School (Singapore, Singapore). We also thank all the members of the trial steering committee and the data and safety monitoring committee and all the trial participants, without whose cooperation the trial would not have been possible.
FUNDING
The trial was supported by a grant (MR/N006178/1, PI THJ) from the Joint Global Health Trials scheme, which includes the Medical Research Council, the U.K. Department for International Development, the National Institute for Health Research, and the Wellcome Trust.
DISCLOSURE
The authors declared no conflict of interest.
DATA AVAILABILITY
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, Ferdinand KC, Ann Forciea M, Frishman WH, Jaigobin C, Kostis JB, Mancia G, Oparil S, Ortiz E, Reisin E, Rich MW, Schocken DD, Weber MA, Wesley DJ. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol 2011; 57:2037–2114. [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014; 129:399–410. [DOI] [PubMed] [Google Scholar]
- 3. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 2016; 134:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ogedegbe G. Barriers to optimal hypertension control. J Clin Hypertens (Greenwich) 2008; 10:644–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Phillips LS, Branch WT, Cook CB, Doyle JP, El-Kebbi IM, Gallina DL, Miller CD, Ziemer DC, Barnes CS. Clinical inertia. Ann Intern Med 2001; 135:825–834. [DOI] [PubMed] [Google Scholar]
- 6. Wang YR, Alexander GC, Stafford RS. Outpatient hypertension treatment, treatment intensification, and control in Western Europe and the United States. Arch Intern Med 2007; 167:141–147. [DOI] [PubMed] [Google Scholar]
- 7. Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ 2003; 326:1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jafar TH, Gandhi M, de Silva HA, Jehan I, Naheed A, Finkelstein EA, Turner EL, Morisky D, Kasturiratne A, Khan AH, Clemens JD, Ebrahim S, Assam PN, Feng L; COBRA-BPS Study Group . A community-based intervention for managing hypertension in rural South Asia. N Engl J Med 2020; 382:717–726. [DOI] [PubMed] [Google Scholar]
- 9. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. The Guilford Press: New York, 2013. [Google Scholar]
- 10. Jafar TH, Jehan I, de Silva HA, Naheed A, Gandhi M, Assam P, Finkelstein EA, Quigley HL, Bilger M, Khan AH, Clemens JD, Ebrahim S, Turner EL, Kasturiratne A; for COBRA-BPS Study Group . Multicomponent intervention versus usual care for management of hypertension in rural Bangladesh, Pakistan and Sri Lanka: study protocol for a cluster randomized controlled trial. Trials 2017; 18:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gandhi M, Assam PN, Turner EL, Morisky DE, Chan E, Jafar TH; COBRA-BPS Study Group . Statistical analysis plan for the control of blood pressure and risk attenuation-rural Bangladesh, Pakistan, Sri Lanka (COBRA-BPS) trial: a cluster randomized trial for a multicomponent intervention versus usual care in hypertensive patients. Trials 2018; 19:658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F; Task Force Members . 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 13. Grim CE, Grim CM. The Omron Elite 7300W home blood pressure monitor passes the European Society of Hypertension International Validation Protocol for women and men. Blood Press Monit 2009; 14:87–90. [DOI] [PubMed] [Google Scholar]
- 14. Smits J, Steendijk R. The international wealth index (IWI). Soc Indic Res 2015; 122:65–85. [Google Scholar]
- 15. Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 16. Levy PD, Willock RJ, Burla M, Brody A, Mahn J, Marinica A, Nasser SA, Flack JM. Total antihypertensive therapeutic intensity score and its relationship to blood pressure reduction. J Am Soc Hypertens 2016; 10:906–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National High Blood Pressure Education Program. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute: Bethesda, MD, 2004. Treatment. https://www.ncbi.nlm.nih.gov/books/NBK9626/ [Google Scholar]
- 18. Bennett JE, Stevens GA, Mathers CD, Bonita R, Rehm J, Kruk ME, Riley LM, Dain K, Kengne AP, Chalkidou K. NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet 2018; 392:1072–1088. [DOI] [PubMed] [Google Scholar]
- 19. He J, Irazola V, Mills KT, Poggio R, Beratarrechea A, Dolan J, Chen CS, Gibbons L, Krousel-Wood M, Bazzano LA, Nejamis A, Gulayin P, Santero M, Augustovski F, Chen J, Rubinstein A; HCPIA Investigators . Effect of a community health worker-led multicomponent intervention on blood pressure control in low-income patients in Argentina: a randomized clinical trial. JAMA 2017; 318:1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwalm JD, McCready T, Lopez-Jaramillo P, Yusoff K, Attaran A, Lamelas P, Camacho PA, Majid F, Bangdiwala SI, Thabane L, Islam S, McKee M, Yusuf S. A community-based comprehensive intervention to reduce cardiovascular risk in hypertension (HOPE 4): a cluster-randomised controlled trial. Lancet 2019; 394:1231–1242. [DOI] [PubMed] [Google Scholar]
- 21. Han X, McCombs J, Chu M, Dougherty JS, Fox DS. Impact of early initiation of antihypertensive medications for patients with hypertension or elevated blood pressure. J Hypertens 2019; 37:1276–1284. [DOI] [PubMed] [Google Scholar]
- 22. Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med 2009; 122:290–300. [DOI] [PubMed] [Google Scholar]
- 23. Mills KT, He H, Irazola V, Poggio R, Beratarrechea A, Chen C-S, Gibbons L, Chen J, Rubinstein A, He J. Factors contributing to the success of a multicomponent intervention for blood pressure control in Argentina: a mediation analysis [abstract]. Circulation 2019; 139:AP197. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.