Abstract
Background:
Preoperative serum inflammatory markers have been correlated to outcome following resection of hepatocellular carcinoma (HCC), but studies have had conflicting results. We aimed to evaluate the association of 6 inflammatory markers with recurrence-free survival (RFS), overall survival (OS), and microvascular invasion (MVI), a well-known prognostic factor.
Methods:
In 370 patients who underwent resection of HCC from 1992 to 2016, we retrospectively evaluated their inflammatory indices and individual components, including neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), lymphocyte to monocyte ratio (LMR), prognostic nutritional index (PNI), aspartate aminotransferase to platelet ratio index (APRI), and aspartate aminotransferase to neutrophil ratio index (ANRI). Univariate and multivariate analyses were performed to evaluate these markers for RFS, OS, and MVI.
Results:
Median RFS was 23 months and median OS was 60 months. Factors independently associated with worse RFS were higher levels of PLR and alpha-fetoprotein, male gender, as well as presence of MVI and multiple nodules. Factors independently associated with worse OS were higher levels of PLR and international normalized ratio, male gender, older age, presence of MVI and multiple nodules, larger tumor, presence of cirrhosis, and absence of steatosis. MVI was identified in 47% of patients. Lower level of albumin, higher level of alpha-fetoprotein, and larger tumor on preoperative imaging were independently associated with MVI.
Conclusions:
In this largest western series to evaluate the utility of preoperative inflammatory markers in patients with HCC, we found that only PLR was associated with RFS and OS, and albumin was associated with MVI.
Keywords: Platelet to lymphocyte ratio, albumin, serum inflammatory indices, hepatocellular carcinoma, microvascular invasion, recurrence-free survival, overall survival
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide.1 Multiple staging systems and nomograms have shown to be prognostic but they are cumbersome to use and have low concordance index on external validation.2-5 Several serum inflammatory indices have also been proposed to improve survival prediction in patient following resection of HCC. They can be easily calculated in any patients with routine preoperative blood tests, including complete blood count and liver function tests. These serum inflammatory indices are reflective of the underlying immune health and systemic inflammation, which can affect cancer development and metastatic progression by promoting epigenetic changes, cell proliferation, angiogenesis, and cell invasion.6
Serum inflammatory indices that have been shown to be prognostic include neutrophil to lymphocyte ratio (NLR),7-9 platelet to lymphocyte ratio (PLR),10 lymphocyte to monocyte ratio (LMR),11 prognostic nutritional index (PNI),11,12 aspartate aminotransferase/platelet ratio index (APRI),13-15 and aspartate aminotransferase/neutrophil ratio index (ANRI).16 However, most of the prior studies only evaluated one or two serum inflammatory markers, most were limited to patients treated in Asia and Europe, and several had conflicting results.12,17-19 Thus, a comprehensive evaluation and validation of all of these serum inflammatory indices would be valuable in patients with HCC treated in the U.S.
In addition to evaluating these preoperative serum inflammatory indices for prognostic purposes, we also aimed to evaluate them for their association with MVI. MVI is a significant risk factor for worse RFS and OS in patients following resection of HCC.20-22 We hypothesized that preoperative serum inflammatory indices may also be utilized to predict MVI since both factors are prognostic. MVI is usually determined postoperatively on pathologic examination of the tumor since preoperative biopsy is not routinely performed.23 Ability to predict MVI preoperatively may help guide treatment recommendations for patients considered for liver transplantation or resection.
In this study, we aimed to comprehensively evaluate the utility of preoperative serum inflammatory indices and their individual components for their correlation to RFS and OS as well as MVI in patients following resection of HCC.
METHODS
Patients
With the approval of the Institutional Review Board at Memorial Sloan Kettering Cancer Center, we retrospectively reviewed 391 consecutive patients with complete macroscopic resection of HCC from 1992 to 2016. Of 391 patients, 21 (5%) who died or lost to follow up within 3 months of operation were excluded from the study in order to evaluate RFS and OS. We did not include patients with R2 resection, prior liver resection for HCC, or pathologically confirmed fibrolamellar HCC or combined HCC and cholangiocarcinoma. Patients were selected for resection given sufficient future liver remnant, absence of distant metastasis, and adequate general health. Patients were evaluated for resection and followed postoperatively for recurrence as previously described.24
Clinicopathologic variables
We reviewed demographic information and medical history, including age at resection, gender, Child Pugh classification, primary liver diseases, and history of metabolic syndrome. Metabolic syndrome was defined as meeting any 3 of the following risk factors: body mass index ≥25, type 2 diabetes, hypertension, and hyperlipidemia.25 We also obtained preoperative levels of AFP, serum inflammatory indices, and their individual components.
For the inflammatory markers, all test results were obtained within 1 month preoperatively and had concomitant white blood cell counts that were within the normal range of 4-11 K/uL. Inflammatory indices in patients with leukopenia and leukocytosis were excluded in the analysis given the likelihood of alteration from an acute infectious or inflammatory process instead of chronic cancer-related effects. NLR was calculated by the absolute count of neutrophils to lymphocytes, PLR was calculated by platelets to lymphocytes, and LMR was calculated by absolute lymphocytes to monocytes.9-11 PNI was measured by albumin (g/L) + 5 x absolute lymphocyte count.12 APRI was measured by [AST (units/L)/upper limit of normal range]/platelets (109/L) x 100 as previously described, and the upper limit of normal for AST was 37 units/L.15 ANRI was measured by AST/absolute neutrophils.16
In addition to evaluating preoperative laboratory tests, we also examined preoperative abdominal imaging, details of the operation, pathology from the resected tumor, as well as RFS and OS.
Statistics
Categorical variables were expressed as frequency and percentage and were compared using Fisher’s exact test. Continuous variables were expressed as mean and standard deviation and were compared using the Wilcoxon rank sum test. Univariate analyses of categorical and continuous variables were performed using univariate logistic regression and Cox proportional hazard models, respectively. All significant preoperative and postoperative variables from univariate analysis were evaluated in the multivariate analysis of RFS and OS, whereas only significant preoperative variables from univariate analysis were evaluated in the multivariate analysis for prediction of MVI. Tumor size and number from histology were used in the multivariate model for RFS and OS, whereas tumor details from preoperative imaging were used in the multivariate analysis for prediction of MVI.
Multivariate analysis was performed using a two-step process. The first step selected the prognostic clinical factors using Cox proportional hazard models and separately selected serum inflammatory markers using Lasso penalized regression26. Lasso penalized regression was utilized in this study in order to examine all 6 serum inflammatory indices despite of moderate to high correlation of several serum inflammatory indices with each other. The second step combined the selected variables from the first step and refit using Cox proportional hazard model. Serum inflammatory markers were presented as a standard deviation from the mean, and their prognostic utility was evaluated by incremental increases in standard deviations. Kaplan-Meier curves were generated for RFS and OS using the MVI status or using the optimal cutoff for inflammatory marker as determined by the method of maximally selected rank statistics. P-values < 0.05 from 2-sided tests were considered significant. Statistical analyses were performed using R version 3.3.2 (cran.r-project.org) and SAS 9.4.
RESULTS
With a median follow-up of 56 months for all survivors, median RFS and OS were 23 and 60 months, respectively. Patients with high levels of AFP, platelets, NLR, and PLR, as well as low levels of albumin, LMR and PNI had worse RFS and OS on univariate analysis (Table 1). In addition, patients with worse prognosis included male gender, Child Pugh class B, high operative blood loss, local extrahepatic invasion, R1 margin, large tumors, multiple nodules, MVI, cirrhosis, and absence of steatosis.
Table 1.
Prognostic characteristics of the study patients.
| Characteristics | RFS p-value HR (95% CI) |
OS p-value HR (95% CI) |
|
|---|---|---|---|
| Demographic and history | |||
| Age* | 65 (12) | 0.144 1.1 (1.0-1.2) |
0.005 1.2 (1.1-1.4) |
| Male | 271 (73%) |
0.002 1.6 (1.2-2.1) |
< 0.001 1.8 (1.3-2.5) |
| Child Pugh class B | 7 (2%) |
0.028 2.3 (1.1-4.9) |
< 0.001 4.0 (1.9-8.5) |
| Primary liver disease | 0.846# 1.0 (0.8-1.2) |
0.168# 0.8 (0.6-1.1) |
|
| Hepatitis B | 93 (25%) | ||
| Hepatitis C | 68 (18%) | ||
| Hepatitis B + C | 6 (2%) | ||
| Alcoholic | 40 (11%) | ||
| Hemochromatosis | 6 (2%) | ||
| None | 157 (42%) | ||
| Metabolic syndrome | 101 (27%) | 0.741 1.0 (0.8-1.4) |
0.364 1.1 (0.9-1.5) |
| Preoperative tests | |||
| AFP, ng/mL* | 7799 (33988) |
<0.001 1.4 (1.2-1.6) |
0.045 1.2 (1.0-1.4) |
| Neutrophil, K/mcL* | 4.3 (1.4) | 0.229 1.1 (1.0-1.2) |
0.101 1.1 (1.0-1.3) |
| Lymphocyte, K/mcL* | 1.5 (0.6) | 0.110 0.9 (0.8-1.0) |
0.298 0.9 (0.8-1.1) |
| Monocyte, K/mcL* | 0.4(0.2) | 0.098 1.1 (1.0-1.3) |
0.037 1.1 (1.0-1.3) |
| Total bilirubin, mg/dL* | 0.7 (0.4) | 0.239 1.1 (1.0-1.2) |
0.163 1.1 (0.9-1.3) |
| INR* | 1.1 (0.2) | 0.208 1.1 (1.0-1.1) |
0.010 1.1 (1.0-1.2) |
| Creatinine, mg/dL* | 1.1 (0.4) | 0.340 1.1 (0.9-1.2) |
0.102 1.1 (1.0-1.3) |
| AST, units/L* | 59 (50) |
0.032 1.1 (1.0-1.2) |
0.117 1.1 (1.0-1.3) |
| Platelet, K/mcL* | 236 (106) |
0.016 1.2 (1.0-1.3) |
0.024 1.2 (1.0-1.3) |
| Albumin, g/dL* | 4.1 (0.4) |
0.007 0.8 (0.8-1.0) |
< 0.001 0.8 (0.7-0.9) |
| NLR* | 3.3 (2.1) |
0.007 1.2 (1.0-1.3) |
0.015 1.2 (1.0-1.4) |
| PLR* | 186 (135) |
< 0.001 1.3 (1.2-1.5) |
0.001 1.3 (1.1-1.4) |
| LMR* | 4.3 (2.1) |
0.019 0.8 (0.7-1.0) |
0.012 0.8 (0.7-1.0) |
| PNI* | 48.9 (5.9) |
0.002 0.8 (0.7-0.9) |
< 0.001 0.8 (0.7-0.9) |
| APRI* | 0.8 (0.8) | 0.087 1.1 (1.0-1.2) |
0.483 1.1 (0.9-1.2) |
| ANRI* | 14.9 (13.6) | 0.268 1.1 (1.0-1.2) |
0.233 1.1 (0.9-1.2) |
| Operative data | |||
| Major hepatectomy | 177 (48%) |
0.013 1.3 (1.1-1.7) |
0.064 1.3 (1.0-1.7) |
| Operative blood loss, mL* | 645 (776) |
< 0.001 1.2 (1.1-1.4) |
< 0.001 1.3 (1.2-1.4) |
| Pringle time, minutes* | 34 (17) | 0.388 1.1 (0.9-1.2) |
0.522 1.0 (0.9-1.2) |
| Tumor rupture | 15 (4%) | 0.868 0.9 (0.5-1.7) |
0.605 1.2 (0.6-2.2) |
| Local extrahepatic invasion | 18 (5%) |
< 0.001 2.5 (1.5-4.2) |
0.014 2.0 (1.2-3.4) |
| R1 margin | 18 (5%) |
< 0.001 2.8 (1.7-4.6) |
< 0.001 3.4 (2.0-5.6) |
| Pathological data | |||
| Largest tumor, cm* | 7.6 (4.7) |
< 0.001 1.3 (1.1-1.4) |
< 0.001 1.3 (1.1-1.5) |
| <5 | 117 (32%) | ||
| 5-10 | 160 (43%) | ||
| > 10 | 93 (25%) | ||
| Multiple nodules | 101 (27%) |
< 0.001 2.2 (1.7-2.8) |
< 0.001 1.9 (1.4-2.5) |
| Differentiation | |||
| Well | 54 (15%) | ||
| Moderate (vs. well) | 220 (60%) | 0.533 1.1 (0.8-1.6) |
0.502 (0.8-1.7) |
| Poor (vs. well) | 91 (25%) | 0.190 1.3 (0.9-1.9) |
0.361 (0.8-1.9) |
| Vascular invasion | 173 (47%) |
< 0.001 1.7 (1.4-2.2) |
< 0.001 1.8 (1.4-2.4) |
| Cirrhosis | 94 (25%) |
0.007 1.4 (1.1-1.9) |
0.003 1.5 (1.2-2.1) |
| Steatosis | 141 (38%) |
0.004 0.7 (0.5-0.9) |
0.004 0.6 (0.5-0.9) |
HR hazard ratio, CI confidence interval, RFS recurrence-free survival, OS overall survival, AFP α-fetoprotein, INR international normalized ratio, AST asparate aminotransferase, NLR neutrophil to lymphocyte ratio, PLR platelet to lymphocyte ratio, LMR lymphocyte to monocyte ratio, PNI prognostic nutritional index, APRI AST to platelet ratio index, ANRI AST to neutrophil ratio index. Categorical variables are expressed as frequency (percentage). Continuous variables are expressed as mean (standard deviation).
per 1 standard deviation increase.
p-value compares hepatitis B or C or both vs. no history of viral hepatitis. Bolded p-values indicate statistical significance.
In this study, 173 (47%) had tumor MVI. Similar to patients with worse RFS and OS, those with MVI had high AFP, AST, platelets, NLR, and PLR, and lower levels of albumin, LMR, and PNI compared to those without MVI on univariate analysis (Table 2). Median RFS was 14 months for patients with MVI compared to 36 months for those without MVI (p < 0.001, Fig. 1A). Median OS was 39 months for patients with MVI compared to 82 months for patients without MVI (p < 0.001, Fig. 1B). On multivariate analysis with evaluation of all serum inflammatory indices and their individual components and with incorporation of all other preoperative clinical variables, higher level of AFP, lower level of albumin, and larger tumor size on preoperative imaging were independently associated with MVI (Table 3A).
Table 2.
Comparison of characteristics in patients based on tumor microvascular invasion (MVI).
| All patients (n=370) |
MVI present (n=173) |
MVI absent (n=197) |
P-value | |
|---|---|---|---|---|
| Demographic and history | ||||
| Age | 65 (12) | 65 (13) | 66 (11) | 0.712 |
| Male | 271 (73%) | 130 (75%) | 141 (72%) | 0.481 |
| Child Pugh class B | 7 (2%) | 5 (3%) | 2 (1%) | 0.259 |
| Primary liver disease | ||||
| Hepatitis B | 93 (25%) | 50 (29%) | 43 (22%) | 0.346# |
| Hepatitis C | 68 (18%) | 30 (17%) | 38 (19%) | |
| Hepatitis B + C | 6 (2%) | 3 (2%) | 3 (2%) | |
| Alcoholic | 40 (11%) | 16 (9%) | 24 (12%) | |
| Hemochromatosis | 6 (2%) | 0 (0%) | 6 (3%) | |
| None | 157 (42%) | 74 (43%) | 83 (42%) | |
| Metabolic syndrome | 101 (27%) | 45 (26%) | 56 (28%) | 0.641 |
| Preoperative blood tests * | ||||
| AFP, ng/mL | 7799 (33988) | 15776 (48872) | 1087 (5056) | < 0.001 |
| Neutrophil, K/mcL | 4.3 (1.4) | 4.4 (1.4) | 4.2 (1.4) | 0.075 |
| Lymphocyte, K/mcL | 1.5 (0.6) | 1.5 (0.6) | 1.6 (0.7) | 0.139 |
| Monocyte, K/mcL | 0.4 (0.2) | 0.4 (0.2) | 0.4 (0.1) | 0.523 |
| Total bilirubin, mg/dL | 0.7 (0.4) | 0.7 (0.3) | 0.8 (0.4) | 0.319 |
| INR | 1.1 (0.2) | 1.1 (0.1) | 1.1 (0.2) | 0.151 |
| Creatinine, mg/dL | 1.1 (0.4) | 1.1 (0.5) | 1.1 (0.3) | 0.457 |
| AST, units/L | 59 (50) | 66 (55) | 53 (44) | 0.001 |
| Platelet, K/mcL | 236 (106) | 249 (112) | 225 (100) | 0.026 |
| Albumin, g/dL | 4.1 (0.4) | 4.1 (0.5) | 4.2 (0.4) | 0.018 |
| NLR | 3.3 (2.1) | 3.5 (1.9) | 3.2 (2.3) | 0.014 |
| PLR | 186 (135) | 199 (133) | 173 (136) | 0.016 |
| LMR | 4.3 (2.1) | 4.1 (2.2) | 4.5 (2.0) | 0.039 |
| PNI | 48.9 (5.9) | 48.0 (6.2) | 49.7 (5.5) | 0.009 |
| APRI | 0.8 (0.8) | 0.8 (0.9) | 0.8 (0.8) | 0.119 |
| ANRI | 14.9 (13.6) | 16.0 (14.5) | 13.9 (12.7) | 0.057 |
| Preoperative imaging | ||||
| Largest tumor, cm | 7.6 (4.7) | 8.9 (5.0) | 6.6 (4.1) | < 0.001 |
| Multiple nodules | 44 (12%) | 23 (13%) | 21 (11%) | 0.520 |
MVI microvascular invasion, AFP α-fetoprotein, INR international normalized ratio, AST aspartate aminotransferase. NLR neutrophil to lymphocyte ratio, PLR platelet to lymphocyte ratio, LMR lymphocyte to monocyte ratio, PNI prognostic nutritional index, APRI AST to platelet ratio index, ANRI AST to neutrophil ratio index. Categorical variables are expressed as frequency (percentage). Continuous variables are expressed as mean (standard deviation).
per 1 standard deviation increase.
p-value compares hepatitis B or C or both vs. no history of viral hepatitis. Bolded p-values indicate statistical significance.
Fig. 1.
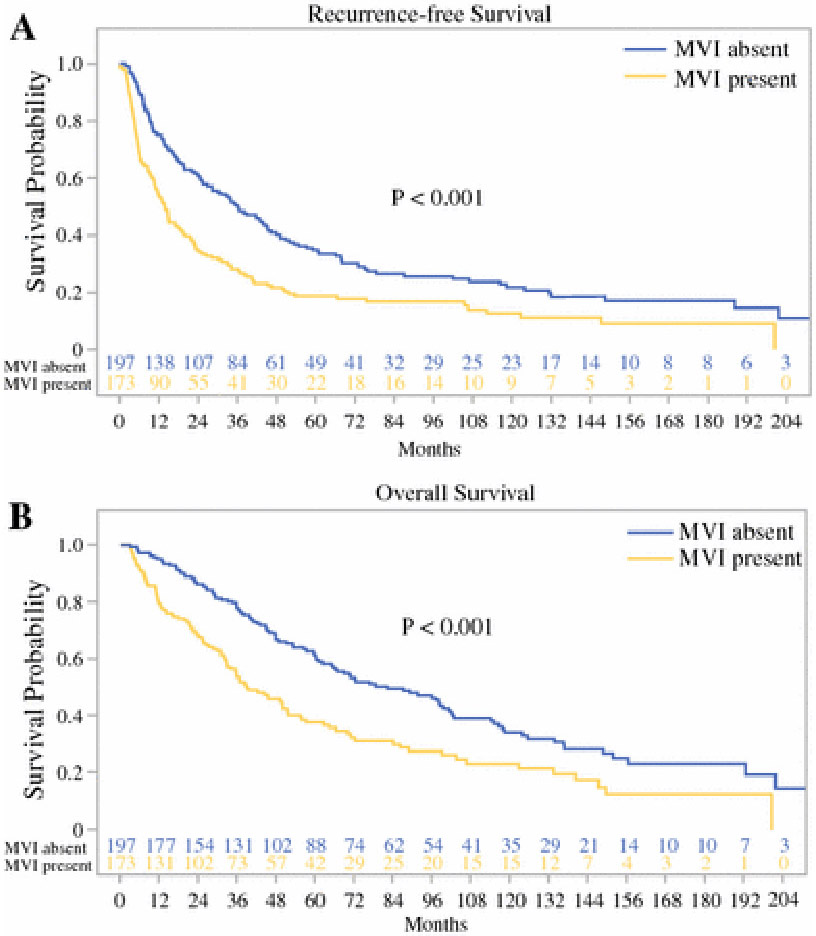
Relation of microvascular invasion (MVI) to (A) RFS and (B) OS.
Table 3.
Factors independently associated with microvascular invasion (MVI), RFS, and OS.
| A. MVI | ||
|---|---|---|
| OR (95% CI) | p-value | |
| AFP* | 5.471 (1.660-18.034) | 0.005 |
| Albumin* | 0.780 (0.609-0.999) | 0.049 |
| Radiologic tumor size* | 1.455 (1.133-1.869) | 0.003 |
| OR hazard ratio, CI confidence interval, AFP α-fetoprotein. *per 1 standard deviation increase. | ||
| B. RFS | ||
| HR (95% CI) | p-value | |
| Male | 1.502 (1.090-2.068) | 0.013 |
| AFP* | 1.303 (1.145-1.483) | < 0.001 |
| PLR* | 1.292 (1.129-1.479) | < 0.001 |
| Microvascular invasion | 1.539 (1.174-2.017) | 0.002 |
| Multiple nodules or satellites | 1.826 (1.357-2.458) | < 0.001 |
| HR hazard ratio, CI confidence interval, AFP α-fetoprotein, PLR platelet to lymphocyte ratio. *per 1 standard deviation increase. | ||
| C. OS. | ||
| HR (95% CI) | p-value | |
| Age* | 1.375 (1.171-1.614) | < 0.001 |
| Male | 1.764 (1.241-2.508) | 0.002 |
| INR* | 1.143(1.030-1.269) | 0.012 |
| PLR* | 1.235 (1.057-1.444) | 0.008 |
| Microvascular invasion | 1.763 (1.293-2.406) | < 0.001 |
| Multiple nodules or satellites | 1.474 (1.067-2.037) | 0.019 |
| Tumor size* | 1.297 (1.100-1.529) | 0.002 |
| Cirrhosis | 2.458 (1.702-3.551) | < 0.001 |
| Steatosis | 0.688 (0.497-0.952) | 0.024 |
| HR hazard ratio, CI confidence interval, INR international normalized ratio, PLR platelet to lymphocyte ratio. *per 1 standard deviation increase. | ||
In multivariate evaluation of preoperative and postoperative factors, those independently associated with worse RFS were male gender, higher level of AFP and PLR, as well as presence of MVI and multiple nodules (Table 3B). Factors independently associated with worse OS were older age, male gender, higher level of INR and PLR, MVI, and multiple nodules, larger tumor, and presence of cirrhosis but absence of steatosis (Table 3C).
For the preoperative level of PLR, the optimal cutoff associated with prognosis was determined to be 275 for RFS and 298 for OS. Patients with a preoperative level of PLR above 275 had significantly worse RFS compared to those with lower PLR (median RFS of 30 months vs. 6.5 months, p = 0.007, Fig. 2A). Similarly, patients with a preoperative level of PLR above 298 had significantly worse RFS compared to those with lower PLR (median OS of 66 months vs. 31 months, p = 0.018, Fig. 2B). Notably, although patients with MVI had higher level of PLR as a continuous variable or as dichotomized by the optimal cutoff of above 275 (p= 0.012) on univariate analysis, PLR was not independently associated with the presence of MVI on multivariate analysis.
Fig. 2.
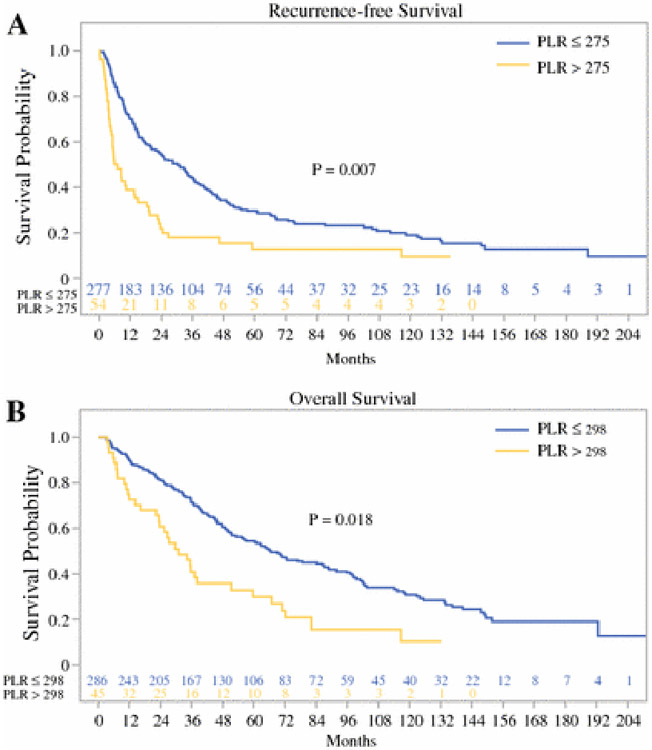
Relation of platelet to lymphocyte ratio (PLR) to (A) RFS and (B) OS.
DISCUSSION
To our knowledge, this is the largest western series to comprehensively evaluate the utility of preoperative serum inflammatory indices and their individual components to predict MVI, RFS and OS in patients following resection of HCC. While multiple inflammatory markers were associated with MVI, RFS, and OS on univariate analysis, only albumin and PLR were independently associated with MVI and survival on multivariate analysis.
Interestingly, hypoalbuminemia was associated with MVI in this study. Low serum level of albumin alone and as part of PNI have been associated with worse survival in patients following resection of HCC in prior studies, but not independently associated with MVI.3,11,12 Albumin is a negative acute-phase protein and therefore it decreases in inflammatory states.27 Hypoalbuminemia may also be due to impaired liver synthesis because of underlying liver disease.28 Although 98% of patients had Child Pugh class A, liver function as stratified by albumin-bilirubin (ALBI) calculation revealed that 74% of our patients were ALBI grade 1, 23% were grade 2, and 1% were grade 329. Thus, low albumin in association with MVI and aggressive tumor biology likely reflects an underlying inflammatory and hypermetabolic state from cancer-induced cachexia as well as some degree of hepatic dysfunction.29,30
A high level of PLR was independently associated with worse RFS and OS in this study, consistent with several prior studies in HCC patients considered for resection and liver transplantation.10,31,32 An elevated PLR results from an increased level of platelets and/or decreased level of lymphocytes. Platelets can promote angiogenesis by releasing vascular endothelial growth factor and they can protect tumor cells from cytolysis by natural killer cells and promote metastasis.33,34 On the other hand, lymphocytes comprise the adaptive, anti-tumoral immune response, and thus their reduction has been shown to promote hepatocarcinogenesis and to be associated with worse prognosis35-37.
Although both platelets and lymphocytes are integral parts of the interaction between cancer cells and immune cells, several studies also showed conflicting results in the prognostic utility of PLR12,38. It is important to note that most of these studies were evaluated in patients treated in Asia. One explanation could be that a majority of HCC patients in Asia has chronic hepatitis B leading to liver cirrhosis and concomitant thrombocytopenia, and thus, perhaps inflammation-mediated thrombocytosis may play a smaller role in Asian patients with HCC39,40. In contrast, many patients treated at Western centers have metabolic syndrome and liver steatosis, which may cause chronic inflammation in addition to cancer-related inflammation41-43.
Although steatosis-related inflammation can be tumorigenic, the presence of any steatosis was associated with improved overall survival in our study. This is likely because the presence of any liver steatosis did not result in steatohepatitis and we included not only patients with severe steatosis (4%), but also any mild (71%) or moderate (26%) steatosis as per the Kleiner-Brunt histologic scoring system. In addition, we operated on more patients with steatosis (38%) than with cirrhosis (25%), and the majority of patients with any steatosis (76%) lacked cirrhosis in our cohort. Many of our patients were also overweight and obese, with 60% having BMI ≥ 25% and 22% having BMI ≥ 30%.
In addition to PLR as a prognostic factor for both RFS and OS in patients following resection of HCC, other prognostic factors included MVI, multiple nodules, and male gender, consistent with previous reports2,3,5. In addition, higher level of AFP was also independently associated with worse RFS, whereas older age, higher INR, larger tumor size, and cirrhosis were independently associated with worse OS2,3,5.
A limitation of this study is that it is retrospective and from a single institution with its associated biases. Preoperative serum inflammatory markers were either not performed in 8 to 12% of patients within 1 month preoperatively or were excluded given an acute inflammatory or infectious state. All patients included had a normal WBC within 1 month of surgery in order to eliminate effects of acute inflammatory changes and to capture cancer-related inflammatory changes. Although our findings may be applicable to those in the Western centers, its applicability to patients treated in other parts of world may be limited. However, we are working on external validation with our international collaborators.
In conclusion, of a plethora of preoperative serum inflammatory markers evaluated, only albumin was independently associated with MVI, and PLR was independently associated with RFS and OS. Further evaluation of these readily applicable serum markers should be performed on a larger sample size and compared between diverse patient populations.
Synopsis:
This study presents a comprehensive evaluation of six preoperative serum inflammatory markers in predicting outcomes after resection of hepatocellular carcinoma. Lower serum albumin was associated with microvascular invasion whereas higher platelet to lymphocyte ratio was associated with recurrence-free and overall survival.
Grant support:
This work was supported in part by NIH/NCI P30 CA008748 Cancer Center Support Grant.
Footnotes
Disclosure: The authors report no conflicts of interest in this study.
REFERENCES
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. The Lancet. 2012;379(9822):1245–1255. [DOI] [PubMed] [Google Scholar]
- 2.Cho CS, Gonen M, Shia J, et al. A novel prognostic nomogram is more accurate than conventional staging systems for predicting survival after resection of hepatocellular carcinoma. Journal of the American College of Surgeons. February2008;206(2):281–291. [DOI] [PubMed] [Google Scholar]
- 3.Shim JH, Jun MJ, Han S, et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Annals of surgery. May2015;261(5):939–946. [DOI] [PubMed] [Google Scholar]
- 4.Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. June2014;146(7):1691–1700 e1693. [DOI] [PubMed] [Google Scholar]
- 5.Ang SF, Ng ES, Li H, et al. The Singapore Liver Cancer Recurrence (SLICER) Score for relapse prediction in patients with surgically resected hepatocellular carcinoma. PloS one. 2015;10(4):e0118658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. March192010;140(6):883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mano Y, Shirabe K, Yamashita Y, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Annals of surgery. August2013;258(2):301–305. [DOI] [PubMed] [Google Scholar]
- 8.Lu SD, Wang YY, Peng NF, et al. Preoperative Ratio of Neutrophils to Lymphocytes Predicts Postresection Survival in Selected Patients With Early or Intermediate Stage Hepatocellular Carcinoma. Medicine. February2016;95(5):e2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen J, He L, Li C, et al. Prognostic nomograms for patients with resectable hepatocelluar carcinoma incorporating systemic inflammation and tumor characteristics. Oncotarget. December062016;7(49):80783–80793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma W, Zhang P, Qi J, et al. Prognostic value of platelet to lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. Sci Rep. October142016;6:35378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu SJ, Lin YX, Ye H, Li FY, Xiong XZ, Cheng NS. Lymphocyte to monocyte ratio and prognostic nutritional index predict survival outcomes of hepatitis B virus-associated hepatocellular carcinoma patients after curative hepatectomy. Journal of surgical oncology. May202016. [DOI] [PubMed] [Google Scholar]
- 12.Chan AW, Chan SL, Wong GL, et al. Prognostic Nutritional Index (PNI) Predicts Tumor Recurrence of Very Early/Early Stage Hepatocellular Carcinoma After Surgical Resection. Annals of surgical oncology. December2015;22(13):4138–4148. [DOI] [PubMed] [Google Scholar]
- 13.Shen SL, Fu SJ, Chen B, et al. Preoperative aspartate aminotransferase to platelet ratio is an independent prognostic factor for hepatitis B-induced hepatocellular carcinoma after hepatic resection. Annals of surgical oncology. November2014;21(12):3802–3809. [DOI] [PubMed] [Google Scholar]
- 14.Ji F, Liang Y, Fu SJ, et al. A novel and accurate predictor of survival for patients with hepatocellular carcinoma after surgical resection: the neutrophil to lymphocyte ratio (NLR) combined with the aspartate aminotransferase/platelet count ratio index (APRI). BMC cancer. 2016;16(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji F, Liang Y, Fu SJ, et al. A novel and accurate predictor of survival for patients with hepatocellular carcinoma after surgical resection: the neutrophil to lymphocyte ratio (NLR) combined with the aspartate aminotransferase/platelet count ratio index (APRI). BMC cancer. February222016;16:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji F, Fu S, Guo Z, et al. Prognostic significance of preoperative aspartate aminotransferase to neutrophil ratio index in patients with hepatocellular carcinoma after hepatic resection. Oncotarget. November012016;7(44):72276–72289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan KM, Groeschl RT, Turaga KK, et al. Neutrophil-to-lymphocyte ratio as a predictor of outcomes for patients with hepatocellular carcinoma: a Western perspective. Journal of surgical oncology. February2014;109(2):95–97. [DOI] [PubMed] [Google Scholar]
- 18.Parisi I, Tsochatzis E, Wijewantha H, et al. Inflammation-based scores do not predict post-transplant recurrence of hepatocellular carcinoma in patients within Milan criteria. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. November2014;20(11):1327–1335. [DOI] [PubMed] [Google Scholar]
- 19.Kinoshita A, Onoda H, Imai N, et al. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer. September042012;107(6):988–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. Journal of the American College of Surgeons. November2003;197(5):753–758. [DOI] [PubMed] [Google Scholar]
- 21.Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. Journal of hepatology. 2003;38(2):200–207. [DOI] [PubMed] [Google Scholar]
- 22.Hirokawa F, Hayashi M, Asakuma M, Shimizu T, Inoue Y, Uchiyama K. Risk factors and patterns of early recurrence after curative hepatectomy for hepatocellular carcinoma. Surgical oncology. March2016;25(1):24–29. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Peralvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Annals of surgical oncology. January2013;20(1):325–339. [DOI] [PubMed] [Google Scholar]
- 24.Zheng J, Kuk D, Gonen M, et al. Actual 10-Year Survivors After Resection of Hepatocellular Carcinoma. Annals of surgical oncology. May2017;24(5):1358–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piscaglia F, Svegliati-Baroni G, Barchetti A, et al. Clinical patterns of hepatocellular carcinoma (hcc) in non alcoholic fatty liver disease (NAFLD): A multicenter prospective study. Hepatology. November242015. [DOI] [PubMed] [Google Scholar]
- 26.Hastie T, Tibshirani R, Friedman JH. The elements of statistical learning : data mining, inference, and prediction. 2nd ed. New York, NY: Springer; 2009. [Google Scholar]
- 27.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. The New England journal of medicine. February111999;340(6):448–454. [DOI] [PubMed] [Google Scholar]
- 28.Rothschild MA, Oratz M, Schreiber SS. Serum albumin. Hepatology. Mar-Apr 1988;8(2):385–401. [DOI] [PubMed] [Google Scholar]
- 29.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. February202015;33(6):550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vazeille C, Jouinot A, Durand JP, et al. Relation between hypermetabolism, cachexia, and survival in cancer patients: a prospective study in 390 cancer patients before initiation of anticancer therapy. Am J Clin Nutr. March292017. [DOI] [PubMed] [Google Scholar]
- 31.Kaida T, Nitta H, Kitano Y, et al. Preoperative platelet-to-lymphocyte ratio can predict recurrence beyond the Milan criteria after hepatectomy for patients with hepatocellular carcinoma. Hepatology research : the official journal of the Japan Society of Hepatology. November022016. [DOI] [PubMed] [Google Scholar]
- 32.Xia W, Ke Q, Wang Y, et al. Predictive value of pre-transplant platelet to lymphocyte ratio for hepatocellular carcinoma recurrence after liver transplantation. World journal of surgical oncology. February182015;13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer research. March151999;59(6):1295–1300. [PubMed] [Google Scholar]
- 34.Meikle CK, Kelly CA, Garg P, Wuescher LM, Ali RA, Worth RG. Cancer and Thrombosis: The Platelet Perspective. Front Cell Dev Biol. 2016;4:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma C, Kesarwala AH, Eggert T, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. March102016;531(7593):253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sideras K, Biermann K, Verheij J, et al. PD-L1, Galectin-9 and CD8+ tumor-infiltrating lymphocytes are associated with survival in hepatocellular carcinoma. Oncoimmunology. 2017;6(2):e1273309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabrielson A, Wu Y, Wang H, et al. Intratumoral CD3 and CD8 T-cell Densities Associated with Relapse-Free Survival in HCC. Cancer immunology research. May2016;4(5):419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y, Si G, Zhu F, et al. Prognostic role of platelet to lymphocyte ratio in hepatocellular carcinoma: a systematic review and meta-analysis. Oncotarget. April042017;8(14):22854–22862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choo SP, Tan WL, Goh BK, Tai WM, Zhu AX. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer. September132016. [DOI] [PubMed] [Google Scholar]
- 40.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. Journal of clinical gastroenterology. July2013;47 Suppl:S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. November2014;60(5):1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. June12016;122(11):1757–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park EJ, Lee JH, Yu GY, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. January222010;140(2):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]


