Abstract
Acid-fast bacilli from pediatric patients with lymphadenopathy were detected in the BACTEC radiometric system and in MB Redox broth, but not on Löwenstein Jensen medium. PCR amplification identified the isolates as Mycobacterium haemophilum, which has special nutrition requirements (iron supplements) for growth. Suitable culture medium ensures optimal recovery of this microorganism, avoiding underdiagnosis.
Mycobacterium haemophilum was first described in 1978 as the cause of cutaneous ulcerating lesions in a 51-year-old Israeli woman with Hodgkin’s disease (20). Since then, fewer than 70 confirmed cases of infection with this organism have been reported. However, recent studies indicate that it is rapidly emerging as a serious pathogen, particularly in immunocompromised patients (9, 18, 21). M. haemophilum has been associated with lesions occurring secondarily to immunosuppressive therapy after transplantation and with AIDS (4, 10–12, 16, 18, 24, 25). It has also been isolated from localized lesions in pediatric patients with cervical lymphadenopathy who otherwise had no underlying immunocompromising factors (1, 5, 18, 22).
The clinical presentation of M. haemophilum infection includes painful cutaneous lesions, multiple skin nodules, respiratory symptoms, pneumonitis, and tuberculosis-like granulomas in the lungs. Bacteremia, septic arthritis, and osteomyelitis have also been reported (9, 14, 18, 21). The disease is rare in otherwise healthy patients, in whom it can usually be successfully treated with appropriate antibiotics. However, in patients with impaired cellular immunity, the disease is chronic, disseminated, and sometimes fatal (9, 16, 21).
M. haemophilum is a strongly acid- and alcohol-fast bacillus (20) which grows optimally at 30 to 32°C; it requires an iron supplement (or ferric iron-containing compounds) such as hemin or ferric ammonium citrate for growth (3, 6, 17). The true incidence of infection with M. haemophilum may be seriously underestimated because of its distinctive growth requirements (8, 21, 23).
We describe the isolation of M. haemophilum from specimens obtained from nine children with cervical lymphadenopathy.
Patients and specimens.
Biopsy specimens of nine submandibular lymph nodes and one preauricular lymph node from nine pediatric patients (six males and three females) were examined for bacterial cultures, including culture for mycobacteria.
Media and bacterial isolates.
Direct Gram and Ziehl-Neelsen stains were applied. For the mycobacterial cultures, the specimens were inoculated onto BACTEC 460 12B radiometric broth (Becton Dickinson) and Löwenstein-Jensen (L-J)(Heipha Diagnostika Biotest, Heidelberg, Germany) media and incubated at 37°C in all cases. In five of nine cases, specimens were also directly inoculated into MR Redox broth (Heipha Diagnostika, Biotest), a new colorimetric medium for mycobacteria. In addition, subcultures of the initial growth from the clinical samples in BACTEC radiometric broth were performed concomitantly to the same medium, L-J medium, MB Redox broth, and blood agar and then incubated at 30 and 37°C.
Microorganism identification.
The following biochemical tests were carried out: niacin, nitrate reductase, semiquantitative catalase, thermostable catalase, Tween-80 hydrolysis, arylsulfatase, acetamide, benzamide, urea, nicotinamide, succinamide, allantoin, and pyrazinamide.
Gen-Probe AccuProbe tests.
AccuProbe tests (Gen-Probe, San Diego, Calif.) for M. tuberculosis, M. kansasii, and M. avium were performed according to the manufacturer’s instructions.
PCR.
Nucleic acids were isolated from mycobacteria growing in MB Redox medium according to a previously published protocol (15). Fragments of the 16S rRNA gene (rDNA) were amplified by PCR using sets of broad-range eubacterial primers combined with mycobacterial genus-specific primers (19). Further characterization to species level was performed by direct DNA cycle sequencing of the 16S rDNA amplicons (19). Sequencing reactions were carried out in triplicate to rule out any polymerase-induced errors.
Results.
The principal characteristics of the nine patients are summarized in Table 1. Direct Gram and acid-fast smears were negative in all cases. Growth of acid-fast bacilli was detected in BACTEC radiometric broth within an average of 14 days after incubation. In the five specimens that were also inoculated directly into MB Redox broth, growth of acid-fast bacilli was detected after the same incubation period. No growth was observed on L-J medium even after 10 weeks.
TABLE 1.
Principal characteristics of the nine study patientsa
| Patient no. | Age (yr)/sex | Clinical data | Response to PPDb (5 IU) | Pathology | Outcome |
|---|---|---|---|---|---|
| 1 | 9/F | 6 weeksc; right cervical lymphadenitis; lesion size, 2 by 2 cm | 13 | Granulomatous lymphadenitis | Cured after 6 mo |
| 2 | 1/M | 4 weeks; right preauricular lymphadenitis; lesion size, 1 by 1 cm | 6 | Fine-needle aspiration showed granulomatous lymphadenitis | Cured after 5 mo |
| 3 | 3/M | 3 weeks; left submandibular lymphadenitis; lesion size, 3 by 3 cm | 20 | Fine-needle aspiration showed granulomatous lymphadenitis | Cured after 5 mo |
| 4 | 2/M | 4 weeks; left submandibular lymphadenitis; lesion size, 3 by 3 cm | Negative | Fine-needle aspiration showed granulomatous lymphadenitis | Cured after 6 mo |
| 5 | 2/M | 1 week; left submandibular lymphadenitis; lesion size, 3 by 4 cm | 30 | Suppurative lymphadenitis | Cured after 6 mo |
| 6 | 4/F | 1 week; right submandibular lymphadenitis; lesion size, 5 by 3 cm | 14 | Suppurative lymphadenitis | Cured after 5 mo |
| 7 | 6/F | 8 weeks; left submandibular lymphadenitis; lesion size, 3 by 3 cm | 25 | Granulomatous lymphadenitis | Cured after 3 mo |
| 8 | 4/M | 6 weeks; left submandibular lymphadenitis; lesion size, 4.5 by 1.5 cm | 15 | Granulomatous lymphadenitis | Cured after 6 mo |
| 9 | 10/M | 6 weeks; right submandibular lymphadenitis; lesion size, 5 by 5 cm | Negative | Granulomatous lymphadenitis | Cured after 4 mo |
All patients were treated with cephalosporins or β-lactam antibiotics before hospitalization, without response.
PPD, purified protein derivative. The diameter of the induration (in millimeters) is listed.
Length of hospitalization.
Subcultures from the radiometric broth to the same media, MB Redox broth and L-J medium, yielded growth in both cases after 2 days of incubation at 30°C and after 3 days of incubation at 37°C. Further subcultures from each liquid medium to blood agar showed growth of acid-fast bacilli after 3 days of incubation at 30°C and after 5 days at 37°C. Cultures for all other bacteria were negative.
The only positive biochemical test was cleavage of pyrazinamide. AccuProbe tests for M. tuberculosis, M. kansasii, and M. avium were negative.
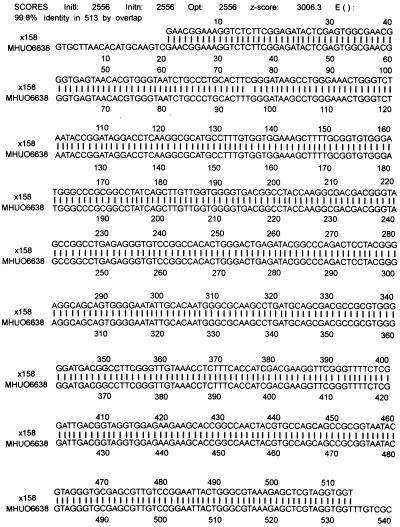
On PCR amplification, the sequence found was identical for all four tested samples. Comparison of the nucleic acids with entries in the GenBank database yielded a 99.8% agreement with the sequence of M. haemophilum (accession number U06638) (Fig. 1) and 98.5% agreement with the sequence of M. haemophilum (accession number L24800).
FIG. 1.
Comparison of the sequence of the 16S rDNAs of the isolates investigated (×158) with the sequence of M. haemophilum (strain MHUO6638). Agreement between the nucleotides is shown by the vertical lines.
Discussion.
M. haemophilum has been described as a human pathogen in less than 70 confirmed cases within the last 20 years. According to the published studies, affected patients can be divided into two broad categories. The main risk group consists of patients who are severely immunocompromised and in whom M. haemophilum occurs as an opportunistic infection. Indeed, the earliest reports documented infection in persons with either lymphoma or renal transplants (2, 13, 20). Today, patients with AIDS are the largest reported group with this infection (7, 9, 21), and bone marrow transplant recipients have been added to the list of individuals at risk (24). Indeed, any condition resulting in marked suppression of cell-mediated immunity is likely to predispose patients to M. haemophilum infection. As reported in previous papers, material from superficial lesions, including lymph nodes and joint fluid, as well as deep-tissue and bone infections, should also be considered.
The second risk group category consists of immunocompetent children in whom M. haemophilum infection induces cervical and perihilar lymphadenitis, clinically similar to that induced by infection with M. avium complex, M. tuberculosis, and M. scrofulaceum (21). M. haemophilum adenitis has been reported in children in Australia, Canada, and the United States (1, 5, 18, 22). More recently, these nine sporadic cases detected at our Center, within a period of 1 year, support the assumption that M. haemophilum infection may occur more frequently than reported in the medical literature. The true incidence of M. haemophilum may be underestimated either because it is not reported or because some laboratories have not changed their routine procedures for some specimens to include a broth with an iron supplement to be incubated at 30°C.
REFERENCES
- 1.Armstrong K L, James R W, Dawson D J, Francis P W, Masters B. Mycobacterium haemophilum causing perihilar or cervical lymphadenitis in healthy children. J Pediatr. 1992;121:202–205. doi: 10.1016/s0022-3476(05)81188-6. [DOI] [PubMed] [Google Scholar]
- 2.Branger B, Gouby A, Oules R, Balducci J P, Mourad G, Fourcade J, et al. Mycobacterium haemophilum and Mycobacterium xenopi associated infection in a renal transplant patient. Clin Nephrol. 1985;23:46–49. [PubMed] [Google Scholar]
- 3.Damato J J, Collins M T. Radiometric studies with gas-liquid and thin-layer chromatography for rapid demonstration of hemin dependence and characterization of Mycobacterium haemophilum. J Clin Microbiol. 1984;20:515–518. doi: 10.1128/jcm.20.3.515-518.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis B R, Brumbach J, Sanders W J, Wolinsky E. Skin lesions caused by Mycobacterium haemophilum. Ann Intern Med. 1982;97:723–724. doi: 10.7326/0003-4819-97-5-723. [DOI] [PubMed] [Google Scholar]
- 5.Dawson D J, Blacklock Z M, Kane D W. Mycobacterium haemophilum causing lymphadenitis in an otherwise healthy child. Med J Aust. 1981;2:289–290. doi: 10.5694/j.1326-5377.1981.tb128321.x. [DOI] [PubMed] [Google Scholar]
- 6.Dawson D J, Jennis F. Mycobacteria with a growth requirement for ferric ammonia citrate, identified as Mycobacterium haemophilum. J Clin Microbiol. 1980;11:190–192. doi: 10.1128/jcm.11.2.190-192.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dever L L, Martin J W, Seaworth B, Jorgensen J I I. Varied presentations and responses to treatment of infections caused by Mycobacterium haemophilum in patients with AIDS. Clin Infect Dis. 1992;14:1195–1200. doi: 10.1093/clinids/14.6.1195. [DOI] [PubMed] [Google Scholar]
- 8.Fischer L J, Quinn F D, White E H, King C H. Intracellular growth and cytotoxicity of Mycobacterium haemophilum in a human epithelial cell line (Hec-1-B) Infect Immun. 1996;64:269–276. doi: 10.1128/iai.64.1.269-276.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiehn T E, White M. Mycobacterium haemophilum: an emerging pathogen. Eur J Clin Microbiol Infect Dis. 1994;13:925–931. doi: 10.1007/BF02111493. [DOI] [PubMed] [Google Scholar]
- 10.Kiehn T E, White M, Pursell K J, Boone N, Tsivitis M, Brown A E, Polsky B, Armstrong D. A cluster of four cases of Mycobacterium haemophilum infection. Eur J Clin Microbiol Infect Dis. 1993;12:114–118. doi: 10.1007/BF01967586. [DOI] [PubMed] [Google Scholar]
- 11.Krisjansson M, Bieluch V M, Byeff P D. Mycobacterium haemophilum infection in immunocompromised patients: case reports and review of the literature. Rev Infect Dis. 1991;13:906–910. doi: 10.1093/clinids/13.5.906. [DOI] [PubMed] [Google Scholar]
- 12.Males B M, Timothy E, West T E, Bartholomew W R. Mycobacterium haemophilum infection in a patient with acquired immunodeficiency syndrome. J Clin Microbiol. 1987;25:186–190. doi: 10.1128/jcm.25.1.186-190.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mezo A, Jennis F, McCarthy S W, Dawson D J. Unusual mycobacteria in 5 cases of opportunistic infections. Pathology. 1979;11:377–384. doi: 10.3109/00313027909059014. [DOI] [PubMed] [Google Scholar]
- 14.Plemmons R M, McAllister C K, Garces M C, Ward R L. Osteomyelitis due to Mycobacterium haemophilum in a cardiac transplant patient: case report and analysis of interaction among clarithromycin, rifampin, and cyclosporine. Clin Infect Dis. 1997;24:995–997. doi: 10.1093/clinids/24.5.995. [DOI] [PubMed] [Google Scholar]
- 15.Reischl U, Pulz M, Ehret W, Wolf K. PCR-based detection of mycobacteria in sputum samples using a simple and reliable DNA extraction protocol. BioTechniques. 1994;17:844–845. [PubMed] [Google Scholar]
- 16.Rogers P L, Walker R E, Lane H C, Witebsky F G, Kovacs J A, Parrillo J E, Masur H. Disseminated Mycobacterium haemophilum infection in two patients with the acquired immunodeficiency syndrome. Am J Med. 1988;84:640–642. doi: 10.1016/0002-9343(88)90150-7. [DOI] [PubMed] [Google Scholar]
- 17.Ryan C G, Dwyer B W. New characteristics of Mycobacterium haemophilum. J Clin Microbiol. 1983;18:976–977. doi: 10.1128/jcm.18.4.976-977.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saubolle M A, Kiehn T E, White M H, Rudinsky N F, Armstrong D. Mycobacterium haemophilum: microbiology and expanding clinical and geographic spectra of disease in humans. Clin Microbiol Rev. 1996;9:435–447. doi: 10.1128/cmr.9.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroeder K H, Naumann L, Kroppenstedt R M, Reischl U. Mycobacterium bassicum sp. nov., a new rapidly growing thermophilic mycobacterium. Int J Syst Bacteriol. 1997;47:86–91. doi: 10.1099/00207713-47-1-86. [DOI] [PubMed] [Google Scholar]
- 20.Sompolinsky D, Lagziel A, Naveh D, Yankielevitzt T. Mycobacterium haemophilum sp. nov., a new pathogen of humans. Int J Syst Bacteriol. 1978;28:67–75. [Google Scholar]
- 21.Straus W L, Ostroff S M, Jernigan D B, Kiehn T E, Sordillo E M, Armstrong D, Boone N, Schneider N, Kilburn J O, Silcox V A, LaBombardi V, Good R C. Clinical and epidemiologic characteristics of Mycobacterium haemophilum, an emerging pathogen in immunocompromised patients. Ann Intern Med. 1994;120:119–125. doi: 10.7326/0003-4819-120-2-199401150-00004. [DOI] [PubMed] [Google Scholar]
- 22.Thibert L, Level F, Martineau B. Two cases with Mycobacterium haemophilum infection in Canada. J Clin Microbiol. 1990;28:621–623. doi: 10.1128/jcm.28.3.621-623.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tonjum T, Welty D B, Jantzen E, Small P L. Differentiation of Mycobacterium ulcerans, M. marinum, and M. haemophilum: mapping of their relationships to M. tuberculosis by fatty acid profile analysis, DNA-DNA hybridization, and 16S rRNA gene sequence analysis. J Clin Microbiol. 1998;36:918–925. doi: 10.1128/jcm.36.4.918-925.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White M H, Papadopoulos E B, Small T N, Kiehn T E, Armstrong D. Mycobacterium haemophilum infections in bone marrow transplant recipients. Transplantation. 1995;60:957–960. [PubMed] [Google Scholar]
- 25.Zappe C H, Barlow D, Zappe H, Bolton I J, Roditi D, Steyn L M. 16S rRNA sequence analysis of an isolate of Mycobacterium haemophilum from a heart transplant patient. J Med Microbiol. 1995;43:189–191. doi: 10.1099/00222615-43-3-189. [DOI] [PubMed] [Google Scholar]