Abstract
Purpose:
Emerging evidence suggests metformin compared to sulfonylurea is associated with an 8–10% lower risk for dementia. Guidelines recommend metformin as initial diabetes treatment, but there is still the question of treatment timing. Thus, the risk of dementia associated with initiating metformin compared with not initiating or delaying treatment was examined.
Methods:
A retrospective cohort study (1996–2015) was conducted with electronic health records from Veteran Health Affairs (VHA; n=112,845) and Kaiser Permanente Washington (KPW; n=14,333) healthcare systems. Patients were age ≥ 50 years, had a hemoglobin A1c (HbA1c) between 6.5 and < 9.5 mg/dL, and did not have dementia or fills for anti-diabetic medications before cohort entry. Initiators started metformin monotherapy and non-initiators used no anti-diabetic medications in the 6- months after the first qualifying HbA1c. The primary outcome was incident dementia. Propensity scores and inverse probability of treatment weighting (IPTW) controlled for confounding in Cox proportional hazards models.
Results:
During a median follow-up of 6.2 years in VHA and 6.8 years in KPW, there were 7,547 new dementia cases in VHA and 1,090 in KPW. After IPTW, there was no association between initiation of metformin (vs. no initial treatment) and incident dementia in VHA (HR=1.04; 95%CI:0.95–1.13) or KPW (HR=0.81; 95%CI:0.51–1.28). Results did not differ by age, baseline HbA1c, or race.
Conclusions:
Results do not support initiating metformin earlier to prevent cognitive decline and, thus, may dampen enthusiasm for metformin as a potential anti-dementia drug. Randomized clinical trials could help clarify the relationship between metformin and cognitive decline.
Keywords: Diabetes, Dementia, Metformin, Pharmacoepidemiology
INTRODUCTION
Metformin is the recommended first-line treatment for type 2 diabetes (T2D) because it is effective and has few serious side effects. T2D is associated with a 70% increased risk for dementia1 and there is emerging evidence that the treatment of T2D with metformin may attenuate this risk. Several studies in animal models of dementia have found that metformin can prevent the buildup of amyloid plaques and delay the onset of memory deficits.2–6 However, studies in human populations that compared initiation of metformin treatment with any other control group have had mixed results,7 while others comparing initiation of metformin to initiation of sulfonylurea treatment suggest that metformin therapy may be associated with only a modest, 8–10% reduction in risk for dementia.8,9 It is unclear whether findings from the epidemiologic studies reflect a potential benefit from metformin treatment, potential harm from sulfonylureas, or residual confounding. Moreover, sulfonylureas are no longer a preferred first-line treatment for T2D because of the availability of safer and potentially more effective alternatives.10
Among older adults with T2D, the decision to initiate glucose-lowering therapy is influenced by the expected benefits, which may depend on life expectancy and the presence of chronic illness, as well as the risks of hypoglycemia and other adverse drug effects.11 A wide range of treatment goals based on hemoglobin A1c (HbA1c) levels have been endorsed. Because delayed initiation of treatment is not uncommon in older patients with T2D,12,13 it is possible to conduct a natural experiment evaluating the risks for dementia associated with the initiation of metformin treatment compared with no initiation of any treatment within months of reaching a glycemic threshold. We tested the hypothesis that the early initiation of metformin treatment is associated with a decreased risk of developing dementia in electronic health record (EHR) data from a large cohort of Veteran Health Affairs (VHA) patients and attempted to replicate findings in a sample of patients from a second healthcare system, Kaiser Permanente Washington (KPW). Second, we examined whether results differed by age group, race and baseline HbA1c.
METHODS
A retrospective cohort study was conducted with EHR data obtained from the VHA (10/01/1999–09/30/2015, fiscal years (FY) 2000–2015) and KPW (01/01/1996–09/30/2015) healthcare systems. The VHA cohort included patients with encounters from a nationally distributed network of all VHA hospitals and outpatient clinics, and also utilized merged Medicare enrollment database and claims data, except for Medicare Part D pharmacy data because the latter were available for only part of the study period. KPW is an integrated healthcare system providing care to patients in the Pacific Northwest. EHR data included ICD-9-CM diagnosis codes (inpatient, outpatient, Medicare), prescription fills, vital signs, lab results and demographic information. KPW data also included enrollment information. The project was approved by the Institutional Review Boards of participating institutions.
Cohort eligibility
Detailed sampling approaches are shown in Figures 1 and 2. Both sites created cohorts in a two-step process. Eligibility criteria were first applied to all HbA1c laboratory results between 6.5% and < 9.5% (blue boxes). We chose this range because these patients would be considered candidates for pharmacologic therapy, and we expected to see some variability in exposure status. The first HbA1c meeting eligibility criteria in blue boxes was considered the index HbA1c, and the date of that test was considered the “index HbA1c date”. The remaining eligibility criteria were applied to this index HbA1c (green boxes). The exposure period, or the period that defined patients as initiating metformin therapy and non-initiation, was the 6-month period after the index HbA1c. This timing for the exposure period is supported by prior literature on early or delayed metformin treatment,14 and allowed for classification of patients into substantially different groups based on metformin use. Also, this exposure window balanced the threat of misclassification of initiators as non-initiators with too short of an exposure window with the threat of left truncation of early dementia cases with too long of an exposure window. The end date of this 6-month exposure period defined Time 0 (baseline or start of follow-up, which is HbA1c index date + 6-months).
Figure 1.
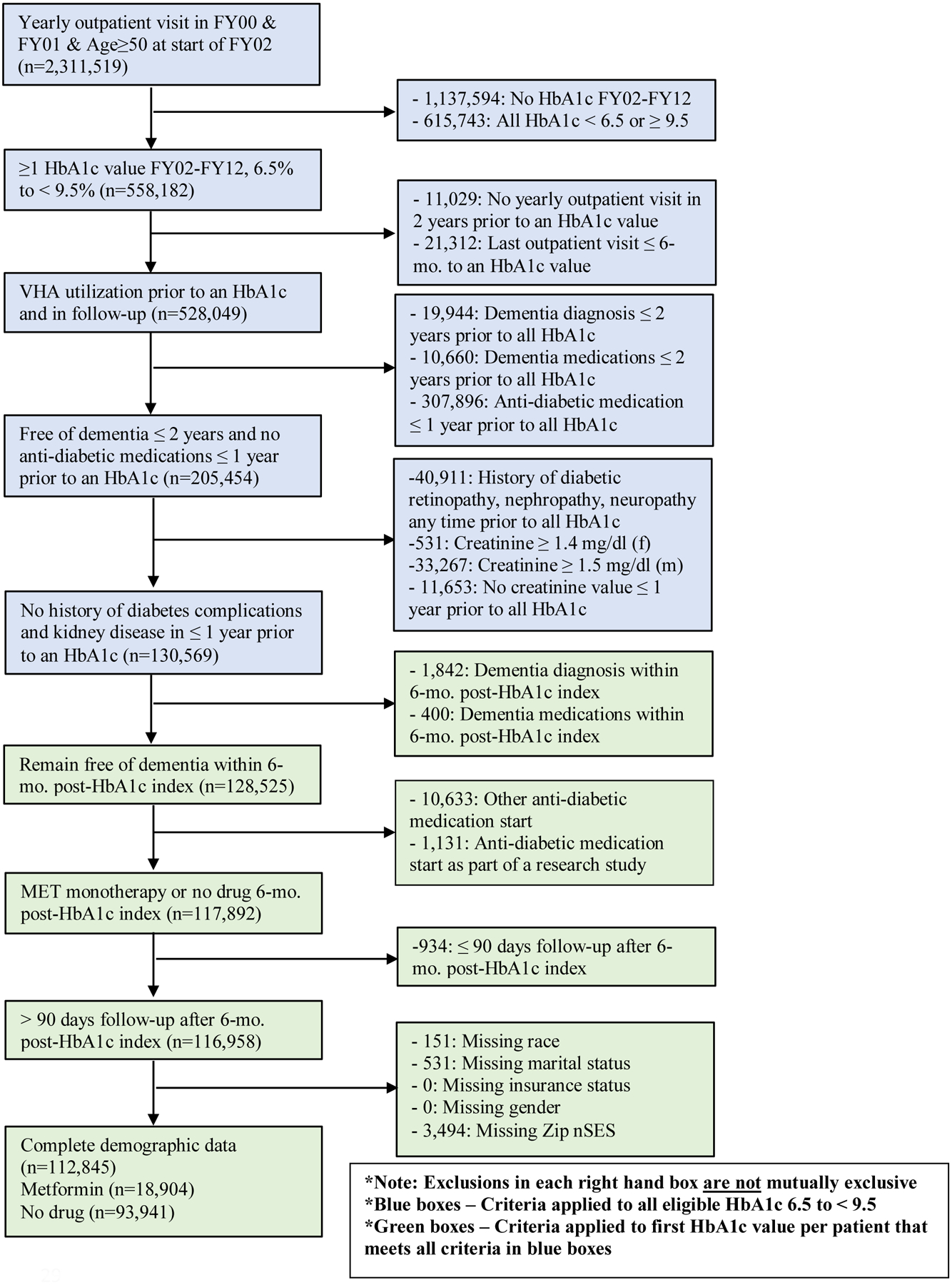
Veterans Health Administration eligibility criteria
Figure 2.
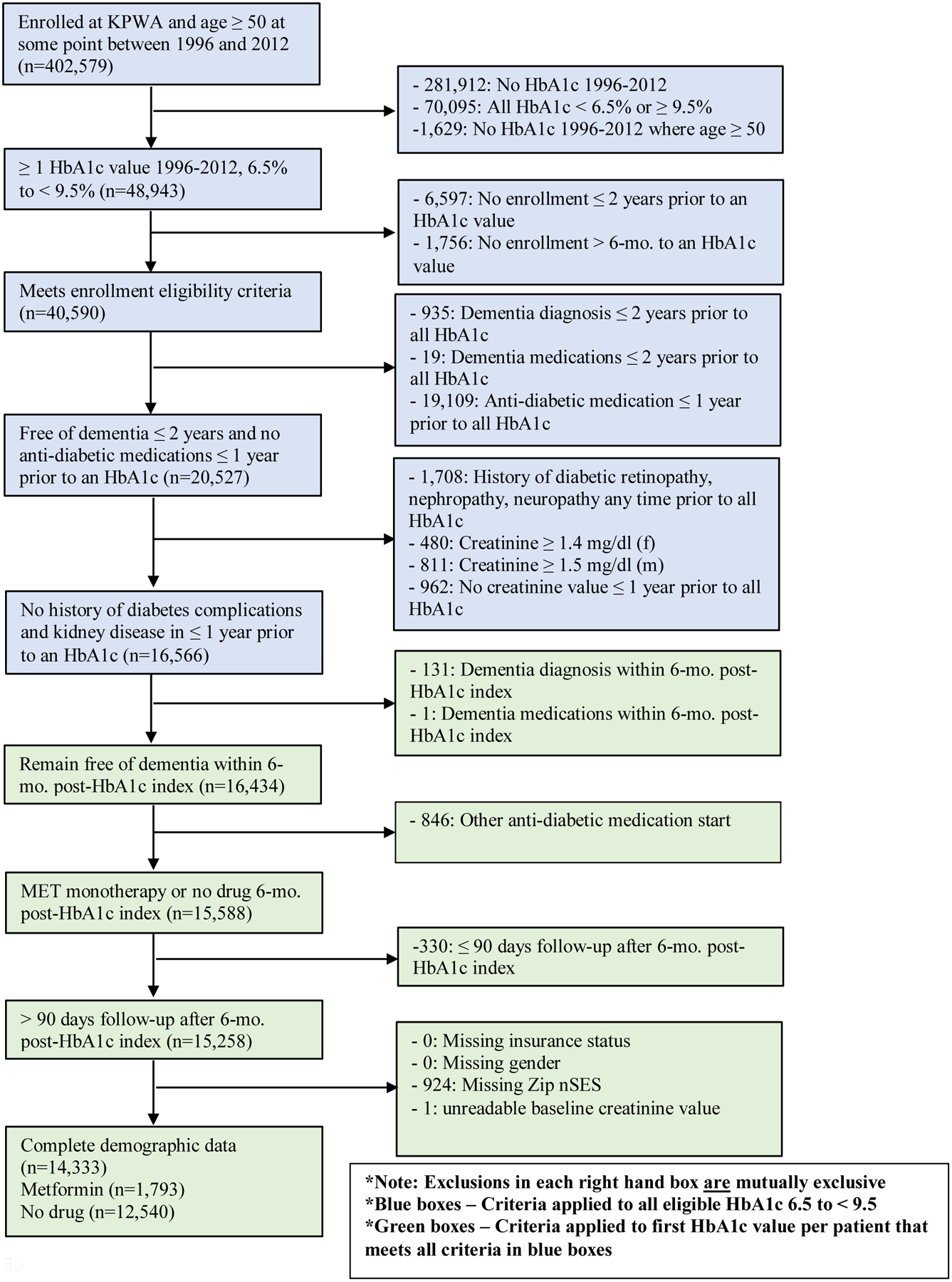
Kaiser Permanente Washington State eligibility criteria
VHA cohort selection (Figure 1).
From a base sample of 2,311,519 patients age ≥50 years old with a yearly outpatient visit in FY00 and FY01, all patients with ≥1 HbA1c value between 6.5% to <9.5% in FY02–12 (n=558,1282) were selected. We chose 2012 to allow for ≥3 years of follow-up. Patients were subsequently eligible if they had ≥1 eligible HbA1c with a yearly visit in the two years prior (to ensure adequate baseline data), ≥1 visit in the period >6 months after an HbA1c, (to ensure continued contact with the VHA system), no ICD-9-CM codes or dementia medication fills (donepezil, rivastigmine, galantamine or memantine) within 2 years prior to an HbA1c, and no anti-diabetic medication fills, diabetes complications, or creatinine values contraindicating metformin use or indicating kidney disease in the 1 year prior (n=130,569). The first HbA1c meeting the prior criteria was the index HbA1c. Patients were required to remain free of dementia and either initiate metformin monotherapy or remain free of diabetes treatment in the 6-month exposure period, and have > 90 days follow-up after Time 0 to allow accumulation of drug exposure (n=116,958). The > 90 days follow-up eligibility criteria means that patients must not have had the incident dementia outcome occur or be censored (as defined below) in the first 90 days following the start of follow-up. Patients with missing demographic data were excluded, resulting in an analytic sample of 112,845 VHA patients.
KPW cohort selection (Figure 2).
From a base sample of 402,579 patients enrolled at KPW in 1996 to 2012 and age ≥50 years old, there were 48,943 with ≥1 HbA1c between 6.5% to <9.5%. To ensure adequate availability of data, we required that a patient be enrolled in KPW for 2 years prior to and in the period >6 months after an HbA1c (n=40,590). All subsequent eligibility criteria were similar to those for VHA. Final analytic sample size for KPW was 14,333.
Variable definitions
Detailed information on variable definitions are reported in Appendix A.
Metformin use.
Metformin use was measured with prescription fills from VHA and KPW pharmacies in the 6-month exposure period following the index HbA1c and up to Time 0 or baseline. Metformin initiators started metformin monotherapy as the initial treatment in the 6-month exposure period. Non-initiators did not initiate any anti-diabetes drug treatment in the 6-month exposure period (had no anti-diabetes drug fills). Patients initiating metformin monotherapy could have started other drugs later in the 6-month exposure period or at any time during follow-up. Non-initiators during the 6-month exposure period could have started metformin or another anti-diabetic medication after the 6-month exposure period during follow-up.
Dementia.
Incident dementia was defined as ≥2 diagnostic codes for dementia (see Appendix A) on two separate days in any 12-month period.
Follow-up time.
Follow-up was defined as days from Time 0 to dementia or censoring. VHA patients were censored at last available inpatient, outpatient, or Medicare claim. KPW patients were censored at disenrollment, death, or September 30, 2015.
Covariates.
All covariates were measured at the time of or prior to the index HbA1c; thus, all covariates occur prior to the 6-month exposure period and thus, prior to metformin initiation. Sociodemographic characteristics included age, race, gender, insurance type, neighborhood socioeconomic status (nSES) and marital status (only available for VHA). For KPW, due to the high rate of missing race, an ‘unknown’ category was included to retain patients for analyses. Health insurance type was defined as VHA-only insurance versus VHA plus other insurance for the VHA. This variable is a proxy for socioeconomic status and a control for access to care outside of the VHA, as it indicates whether additional forms of insurance other than what is already provided by the VHA were used. KPW defined health insurance as having federal aid (Medicaid or Medicare) compared to commercial insurance. nSES was computed from zip code level census data and classified as low vs. high (Appendix A).15 We controlled for HbA1c index year to adjust for the increasing prevalence of metformin prescribing in the latter half of the observation time.9 Detection bias was addressed by adjusting for high (top 25th percentile) volume of health care use, which was based on the distribution of the average number of visits per month prior to HbA1c index.
Baseline creatinine was the most recent value available in the 12-months prior to HbA1c index date. Index HbA1c was categorized in 0.5% increments, ranging from 6.5 % to < 9.5% (e.g. 6.5–<7, 7–<7.5, etc.). Baseline chronic health conditions, psychiatric comorbidities, and medications were measured from the start of FY00 to HbA1c index for the VHA and during the 2 years prior to HbA1c index for KPW. Chronic physical and psychiatric health comorbidities were defined using ICD-9-CM diagnostic codes while obesity was defined using ICD-9-CM diagnostic codes and measured heights and weights (defined as body mass index ≥30 kg/m2) (Appendix A). Finally, sustained use (≥2 fills in any 6-month period) of medications that may be associated with the risk of dementia (statins, anticholinergics, NSAIDs, antihypertensives) were measured using fills in the same time period as comorbidities.
Propensity scores (PS) and inverse probability of treatment weighting (IPTW)
Baseline covariates and sociodemographic factors that may confound the association between initiating metformin and incident dementia were balanced across exposure groups using PS and IPTW. The PS was calculated using binary logistic regression, estimating the probability of metformin initiation versus no anti-diabetic medication initiation in the 6-month exposure period prior to start of follow-up given baseline covariates. Using the PS and marginal probability of exposure, which is the observed probability of metformin initiation vs. no anti-diabetic medication initiation, a stabilized weight for each patient was calculated.16,17 Stabilizing weights reduces bias associated with extreme weights due to increased variance and retains the original sample size in the analysis, thereby preserving Type I error rate.18 Stabilized weights should have a mean close to one and a maximum less than ten, thus, weights ≥ 10 were trimmed.19,20 Adequate balance of baseline covariates between exposure groups after IPTW was assessed with the standardized mean difference (SMD%), where an SMD% < 10% was considered good balance.21
Primary Analysis
All analyses were performed with SAS v9·4 (SAS Institute, Cary, NC) at a 2-tailed alpha=0.05. Analyses followed an intention-to-treat approach, where interest was given to the initial treatment effect of metformin initiation versus no anti-diabetes drug initiation in the 6-month exposure window. Unweighted bivariate analyses used chi-square tests for categorical variables, independent samples t-tests for continuous variables, and SMD% for effect size estimates for differences between groups. Cox proportional hazard models before and after IPTW calculated hazard ratios and 95% confidence intervals, overall and stratified by age. If residual bias existed after IPTW (i.e., SMD≥10% after weighting), imbalanced covariates were added to final, weighted treatment effect models.19 Effect modification by age was tested with an interaction term of age group and metformin initiation versus no anti-diabetic initiation in overall models. Weighted models used robust, sandwich-type variance estimators to calculate confidence intervals and p-values.21 The proportional hazard assumption was met for all models (p-value range: 0.10 to 0.90). Main results from VHA and KPW were combined using an inverse variance weighted fixed effects meta-analysis.22
Secondary Analyses
Secondary analyses tested whether the association of metformin initiation compared to no anti-diabetic medication initiation was different by baseline HbA1c category or white versus African-American race. These analyses were limited to the VHA cohort because of small cell sizes due to the small number of dementia cases in KPW. Cox proportional hazard models were weighted to assure balance in each stratum. We considered effect modification to be present if there was a statistically significant interaction term (p<0.05) in overall models.
RESULTS
Table 1 includes sample characteristics. Mean age of VHA patients was 62.6 years (±9.0) and the majority were male (96.8%), white (81.9%), and married (60.0%). Average age for KPW was 63.7 years (±9.8), most of the sample was white (82.1%), and about half were male. Average index HbA1c was 6.9 (±0.5) for VHA, and 7.1 (±0.7) for KPW.
Table 1.
Characteristics of patients ≥50 years old and free of dementia with an HbA1c between 6.5 and < 9.5 from the Veterans Health Administration (VHA; FY2002 to FY2012) and Kaiser Permanente Washington (KPW; 1996–2012) health care systemsa
| Covariates, mean(±sd) or n(%)b | VHA (n=112,845) | KPW (n=14,333) |
|---|---|---|
| Index year | ||
| 1996–1998 | - | 3653 (25.5) |
| 1999–2000 | - | 1629 (11.4) |
| 2001–2002 | - | 1457 (10.2) |
| 2002–2004 | 13479 (11.9) | - |
| 2003–2004 | - | 1539 (10.7) |
| 2005–2006 | 36461 (32.3) | 1941 (13.5) |
| 2007–2008 | 26859 (23.8) | 1639 (11.4) |
| 2009–2012 | - | 2475 (17.3) |
| 2009–2010 | 22676 (20.1) | - |
| 2011–2012 | 13370 (11.9) | - |
| Sociodemographic-related | ||
| Age (y) | 62.6 (±9.0) | 63.7 (±9.8) |
| Age category | ||
| 50–64 y | 65532 (58.1) | 8232 (57.4) |
| 65–74 y | 33167 (29.4) | 3711 (25.9) |
| ≥ 75 y | 14146 (12.5) | 2390 (16.7) |
| Male gender | 109220 (96.8) | 6971 (48.6) |
| Race | ||
| White | 92425 (81.9) | 10542 (73.6) |
| Black | 18206 (16.1) | 695 (4.9) |
| Asian | -- | 1183 (8.3) |
| Other | 2214 (2.0) | 415 (2.9) |
| Unknown | -- | 1498 (10.5) |
| Race (excluding unknown) | ||
| White | -- | 10542 (82.1) |
| Black | -- | 695 (5.4) |
| Asian | -- | 1183 (9.2) |
| Other | -- | 415 (3.2) |
| Missing race data | 0 (0) | 1498 (10.5) |
| Married | 68404 (60.6) | - |
| Low nSESc | 56422 (50.0) | 7065 (49.3) |
| VHA only insurance | 47440 (42.0) | - |
| Medicaid/Medicare | - | 6060 (42.3) |
| High healthcare utilization | 28211 (25.0) | 3559 (24.8) |
| Diabetes-related | ||
| HbA1c value | 6.9 (±0.5) | 7.1 (±0.7) |
| HbA1c category | ||
| 6.5 to < 7.0 | 80255 (71.1) | 8028 (56.0) |
| 7.0 to < 7.5 | 19204 (17.0) | 2996 (20.9) |
| 7.5 to < 8.0 | 7048 (6.3) | 1488 (10.4) |
| 8.0 to < 8.5 | 3237 (2.9) | 881 (6.2) |
| 8.5 to < 9.0 | 1920 (1.7) | 569 (4.0) |
| 9.0 to < 9.5 | 1181 (1.1) | 371 (2.6) |
| Creatinine value | 1.0 (±0.2) | 0.9 (±0.2) |
| Other comorbidities | ||
| Obesity | 60277 (53.4) | 6370 (44.4) |
| Hypertension | 101308 (89.8) | 7708 (53.8) |
| Hyperlipidemia | 96157 (85.2) | 4563 (31.8) |
| Stroke | 7319 (6.5) | 244 (1.7) |
| Ischemic heart disease | 54731 (48.5) | 2283 (15.9) |
| Congestive heart failure | 19594 (17.4) | 859 (6.0) |
| Atrial fibrillation | 16219 (14.4) | 939 (6.6) |
| Traumatic brain injury | 6074 (5.4) | 163 (1.1) |
| Vitamin B12 deficiency | 5390 (4.8) | 119 (0.8) |
| Psychiatric and substance comorbidities | ||
| Depression | 26943 (23.9) | 543 (3.8) |
| PTSD | 17198 (15.2) | 41 (0.3) |
| Other anxietyd | 13253 (11.7) | 337 (2.4) |
| Bipolar disorder | 7187 (6.4) | 168 (1.2) |
| Schizophrenia | 5662 (5.0) | 31 (0.2) |
| Nicotine abuse/dependence | 56414 (50.0) | 2182 (15.2) |
| Alcohol abuse/dependence | 15627 (13.9) | 241 (1.7) |
| Illicit drug abuse/dependence | 7889 (7.0) | 66 (0.5) |
| Other medications e | ||
| Statins | 77221 (68.4) | 3699 (25.8) |
| Anticholinergic drugs | 45758 (40.6) | 3170 (22.1) |
| NSAIDs | 57696 (51.1) | 2729 (19.0) |
| Antihypertensive drugs | 98087 (86.9) | 8875 (61.9) |
FY = fiscal year; HbA1c = hemoglobin A1c; nSES = neighborhood socioeconomic status; NSAID = nonsteroidal anti-inflammatory drug; PTSD = posttraumatic stress disorder.
VHA measures covariates from start of FY00 to index HbA1c while KPW measures from 2 years prior and up to index HbA1c.
Low nSES = top 50th percentile of factor score from 7 measures obtained from 2009–2013 5-year census estimates from the American Community Survey.
Other anxiety disorders = panic disorder, obsessive-compulsive disorder, social phobia, generalized anxiety disorder, anxiety NOS.
Other medications associated with dementia = sustained use on or before index HbA1c (≥2 fills in a 6-mo period).
Among VHA metformin initiators in the 6-month exposure period, 44.7% later used a sulfonylurea, 17.2% insulin and 7.8% another type of anti-diabetic drug. In KPW, these proportions were: 35.3% sulfonylurea, 20.3% insulin, and 0.7% other drugs. Among VHA drug non-initiators during the 6-month exposure period, 40.0% later used metformin, 25.7% a sulfonylurea, 9.1% insulin and 3.7% other drug. At KPW these proportions were 43.0% metformin, 33.5% sulfonylurea, 17.6% insulin, and 0.5% other drug. Among the non-initiators in the exposure period who later used metformin during follow-up, 80.0% in the VHA and 88.2% in KPW started metformin more than a year after the index HbA1c. Mean duration of metformin use for metformin initiators from initiation to end of follow-up was 1489 (±1059) days in VHA and 1680 (±1322) days in KPW. Among non-initiators, including those who never started metformin in follow-up, mean duration of metformin use was 462 (±820) days in VHA and 659 (±1174) in KPW.
The distribution of baseline covariates by treatment group is shown in Table 2. In the VHA, small differences were found in HbA1c index year for metformin initiators versus non-initiators (SMD<10%) while in KPW there were large differences especially in the earlier and later index years. In both VHA and KPW, younger age, higher HbA1c, lower creatinine, and obesity were positively associated with metformin initiation while prevalence of ischemic heart disease, congestive heart failure and atrial fibrillation were negatively associated with metformin initiation. In the VHA, all psychiatric and substance use comorbidities were positively associated with metformin initiation while for KPW, only smoking was positively associated with initiation. Anticholinergic and NSAID medication use were positively associated with metformin initiation in the VHA while statin medication use was associated with initiation in KPW.
Table 2.
Characteristics of patients ≥50 years old and free of dementia with an HbA1c between 6.5 and < 9.5 from the Veterans Health Administration (VHA; FY2002 to FY2012) and Kaiser Permanente Washington (KPW; 1996–2012) health care systems, by metformin initiation status in 6-months after HbA1c index, before inverse probability of treatment weightinga
| VHA (n=112,845) | KPW (n=14,333) | |||||||
|---|---|---|---|---|---|---|---|---|
| Covariates, mean(±sd) or n(%)b | Non-initiators (n=93,941) | MET Initiators (n=18,904) | p-value | SMD %c | Non-initiators (n=12,540) | MET Initiators (n=1,793) | p-value | SMD %c |
| Index year | <.0001 | <.0001 | ||||||
| 1996–1998 | - | - | - | - | 3526 (28.1) | 127 (7.1) | −57.5% | |
| 1999–2000 | - | - | - | - | 1529 (12.2) | 100 (5.6) | −23.4% | |
| 2001–2002 | - | - | - | - | 1323 (10.6) | 134 (7.5) | −10.8% | |
| 2002–2004 | 11591 (12.3) | 1888 (10.0) | −7.5% | - | - | - | ||
| 2003–2004 | - | - | - | 1320 (10.5) | 219 (12.2) | 5.3% | ||
| 2005–2006 | 30475 (32.4) | 5986 (31.7) | −1.7% | 1643 (13.1) | 298 (16.6) | 9.9% | ||
| 2007–2008 | 21953 (23.4) | 4906 (26.0) | 6.0% | 1291 (10.3) | 348 (19.4) | 25.8% | ||
| 2009–2012 | - | - | - | 1908 (15.2) | 567 (31.6) | 39.5% | ||
| 2009–2010 | 18739 (20.0) | 3937 (20.8) | 2.2% | - | - | - | ||
| 2011–2012 | 11183 (11.9) | 2187 (11.6) | −1.0% | - | - | - | ||
| Sociodemographic-related | ||||||||
| Age (y) | 63.2 (±9.1) | 59.5 (±7.8) | <.0001 | −43.5% | 64.3 (±9.9) | 59.9 (±7.8) | <.0001 | −49.0% |
| Age category | ||||||||
| 50–64 y | 51741 (55.1) | 13791 (73.0) | 37.9% | 6896 (55.0) | 1336 (74.5) | 41.7% | ||
| 65–74 y | 29114 (31.0) | 4053 (21.4) | <.0001 | −21.9% | 3360 (26.8) | 351 (19.6) | <.0001 | −17.2% |
| ≥ 75 y | 13086 (13.9) | 1060 (5.6) | −28.3% | 2284 (18.2) | 106 (5.9) | −38.5% | ||
| Male gender | 90990 (96.9) | 18230 (96.4) | .003 | −2.4% | 6093 (48.6) | 878 (49.0) | .764 | 0.8% |
| Race | ||||||||
| White | 76735 (81.7) | 15690 (83.0) | 3.5% | 9229 (73.6) | 1313 (73.2) | −0.8% | ||
| Black | 15336 (16.3) | 2870 (15.2) | .0001 | −3.1% | 616 (4.9) | 79 (4.4) | −2.4% | |
| Asian | - | - | - | 1039 (8.3) | 144 (8.0) | .611 | −0.9% | |
| Other | 1870 (2.0) | 344 (1.8) | −1.3% | 362 (2.9) | 53 (3.0) | 0.4% | ||
| Unknown | - | - | - | 1294 (10.3) | 204 (11.4) | 3.4% | ||
| Married | 57601 (61.3) | 10803 (57.2) | <.0001 | −8.5% | - | - | - | |
| Low nSESd | 46677 (49.7) | 9745 (51.6) | <.0001 | 3.7% | 6116 (48.8) | 949 (52.9) | .001 | 8.3% |
| VHA only insurance | 37858 (40.3) | 9582 (50.7) | <.0001 | 21.0% | - | - | - | |
| Medicaid/Medicare | - | - | - | 5573 (44.4) | 487 (27.2) | <.0001 | −36.6% | |
| High healthcare utilization | 23108 (24.6) | 5103 (27.0) | <.0001 | 5.5% | 3177 (25.3) | 382 (21.3) | .0002 | −9.5% |
| Diabetes-related | ||||||||
| HbA1c value | 6.8 (±0.4) | 7.2 (±0.7) | <.0001 | 72.5% | 7.0 (±0.6) | 7.7 (±0.8) | <.0001 | 97.6% |
| HbA1c category | ||||||||
| 6.5 to < 7.0 | 72042 (76.7) | 8213 (43.5) | −72.2% | 7623 (60.8) | 405 (22.6) | −84.0% | ||
| 7.0 to < 7.5 | 14162 (15.1) | 5042 (26.7) | 28.8% | 2641 (21.1) | 355 (19.8) | −3.1% | ||
| 7.5 to < 8.0 | 4396 (4.7) | 2652 (14.0) | <.0001 | 32.5% | 1163 (9.3) | 325 (18.1) | <.0001 | 26.0% |
| 8.0 to < 8.5 | 1789 (1.9) | 1448 (7.7) | 27.2% | 557 (4.4) | 324 (18.1) | 44.2% | ||
| 8.5 to < 9.0 | 990 (1.1) | 930 (4.9) | 22.9% | 356 (2.8) | 213 (11.9) | 35.2% | ||
| 9.0 to < 9.5 | 562 (0.6) | 619 (3.3) | 19.5% | 200 (1.6) | 171 (9.5) | 35.2% | ||
| Creatinine value | 1.1 (±0.2) | 1.0 (±0.2) | <.0001 | −18.9% | 1.0 (±0.2) | 0.9 (±0.2) | <.0001 | −26.4% |
| Other comorbidities | ||||||||
| Obesity | 47925 (51.0) | 12352 (65.3) | <.0001 | 29.4% | 5313 (42.4) | 1057 (58.9) | <.0001 | 33.6% |
| Hypertension | 84366 (89.8) | 16942 (89.6) | .441 | −0.6% | 6684 (53.3) | 1024 (57.1) | .003 | 7.7% |
| Hyperlipidemia | 80162 (85.3) | 15995 (84.6) | .011 | −2.0% | 3890 (31.0) | 673 (37.5) | <.0001 | 13.8% |
| Stroke | 6232 (6.6) | 1087 (5.8) | <.0001 | −3.7% | 216 (1.7) | 28 (1.6) | .622 | −1.3% |
| Ischemic heart disease | 46389 (49.4) | 8342 (44.1) | <.0001 | −10.5% | 2074 (16.5) | 209 (11.7) | <.0001 | −14.1% |
| Congestive heart failure | 16873 (18.0) | 2721 (14.4) | <.0001 | −9.7% | 803 (6.4) | 56 (3.1) | <.0001 | −15.5% |
| Atrial fibrillation | 14167 (15.1) | 2052 (10.9) | <.0001 | −12.6% | 864 (6.9) | 75 (4.2) | <.0001 | −11.9% |
| Traumatic brain injury | 5053 (5.4) | 1021 (5.4) | .902 | 0.1% | 144 (1.2) | 19 (1.1) | .740 | −0.9% |
| Vitamin B12 deficiency | 4657 (5.0) | 733 (3.9) | <.0001 | −5.3% | 100 (0.8) | 19 (1.1) | .252 | 2.7% |
| Psychiatric and substance comorbidities | ||||||||
| Depression | 21398 (22.8) | 5545 (29.3) | <.0001 | 15.0% | 472 (3.8) | 71 (4.0) | .685 | 1.0% |
| PTSD | 13381 (14.2) | 3817 (20.2) | <.0001 | 15.8% | 32 (0.3) | 9 (0.5) | .067 | 4.0% |
| Other anxietye | 10757 (11.5) | 2496 (13.2) | <.0001 | 5.3% | 287 (2.3) | 50 (2.8) | .191 | 3.2% |
| Bipolar disorder | 5608 (6.0) | 1579 (8.4) | <.0001 | 9.3% | 139 (1.1) | 29 (1.6) | .061 | 4.4% |
| Schizophrenia | 4523 (4.8) | 1139 (6.0) | <.0001 | 5.4% | 25 (0.2) | 6 (0.3) | .249 | 2.6% |
| Nicotine dependence/smoker | 46163 (49.1) | 10251 (54.2) | <.0001 | 10.2% | 1862 (14.8) | 320 (17.8) | .001 | 8.1% |
| Alcohol abuse/dependence | 12432 (13.2) | 3195 (16.9) | <.0001 | 10.3% | 214 (1.7) | 27 (1.5) | .536 | −1.6% |
| Illicit drug abuse/dependence | 6218 (6.6) | 1671 (8.8) | <.0001 | 8.3% | 55 (0.4) | 11 (0.6) | .306 | 2.4% |
| Other medications f | ||||||||
| Statins | 64341 (68.5) | 12880 (68.1) | .335 | −0.8% | 3168 (25.3) | 531 (29.6) | <.0001 | 9.8% |
| Anticholinergic drugs | 37148 (39.5) | 8610 (45.6) | <.0001 | 12.2% | 2776 (22.1) | 394 (22.0) | .876 | −0.4% |
| NSAIDs | 46725 (49.7) | 10971 (58.0) | <.0001 | 16.7% | 2389 (19.1) | 340 (19.0) | .929 | −0.2% |
| Antihypertensive drugs | 81594 (86.9) | 16493 (87.3) | .147 | 1.2% | 7731 (61.6) | 1144 (63.8) | .079 | 4.5% |
FY = fiscal year; MET=metformin; HbA1c = hemoglobin A1c; nSES = neighborhood socioeconomic status; NSAID = nonsteroidal anti-inflammatory drug; PTSD = posttraumatic stress disorder.
VHA measures covariates from start of FY00 to index HbA1c while KPW measures from 2 years prior and up to index HbA1c.
SMD% = standardized mean difference percentage comparing metformin initiation to non-initiation; SMD% >10 was considered a large difference.
low nSES = top 50th percentile of factor score from 7 measures obtained from 2009–2013 5-year census estimates from the American Community Survey.
Other anxiety disorders = panic disorder, obsessive-compulsive disorder, social phobia, generalized anxiety disorder, anxiety
Other medications associated with dementia = sustained use on or before index HbA1c (≥ 2 fills in a 6-mo period).
The overall median duration of follow up was 6.2 (IQR=4.0–8.5) years in VHA and 6.8 (IQR=3.8–10.8) years in KPW. Median follow-up time among metformin initiators was 6.4 (IQR=4.3–8.6) years in VHA and 5.7 (IQR=3.6–8.9) years in KPW. Median follow-up time for non-initiators was 6.1 (IQR=3.9–8.5) years in VHA and 7.0 (IQR=3.8–11.3) years in KPW. There were 7,547 new dementia cases at VHA and 1,090 at KPW during the follow-up period. The unadjusted incidence rate of dementia for metformin initiation compared with non-initiation in VHA was 8.2/1000PY and 11.4/1000PY respectively, and for KPW the incidence rates were 4.0/1000PY and 10.6/1000PY respectively (Table 3).
Table 3.
Dementia incidence rates (cumulative % and per 1000 person-years (PY)), overall and by age for patients ≥50 years old in VHA and KPW who did vs. did not initiate metformin within 6-months after HbA1c index
| VHA (n=112,845) | KPW (n=14,333) | |||||
|---|---|---|---|---|---|---|
| Age group | Total n | Dementia events, n(%) | Incidence rate per 1000PY | Total n | Dementia events, n(%) | Incidence rate per 1000PY |
| All ages | ||||||
| Overall | 112,845 | 7,547 (6.7%) | 10.8/1000PY | 14,333 | 1,090 (7.6%) | 9.9/1000PY |
| Non-initiators | 93,941 | 6,561 (7.0%) | 11.4/1000PY | 12,540 | 1,044 (8.3%) | 10.6/1000PY |
| MET Initiators | 18,904 | 986 (5.2%) | 8.2/1000PY | 1,793 | 46 (2.6%) | 4.0/1000PY |
| Age 50–64 | ||||||
| Overall | 65,532 | 1,608 (2.5%) | 3.8/1000PY | 8,232 | 127 (1.5%) | 2.0/1000PY |
| Non-initiators | 51,741 | 1,282 (2.5%) | 3.8/1000PY | 6,896 | 114 (1.7%) | 2.1/1000PY |
| MET Initiators | 13,791 | 326 (2.4%) | 3.6/1000PY | 1,336 | 13 (1.0%) | 1.5/1000PY |
| Age 65–74 | ||||||
| Overall | 33,167 | 3,330 (10.0%) | 16.6/1000PY | 3,711 | 406 (10.9%) | 13.2/1000PY |
| Non-initiators | 29,114 | 2,875 (9.9%) | 16.4/1000PY | 3,360 | 389 (11.6%) | 13.7/1000PY |
| MET Initiators | 4,053 | 455 (11.2%) | 18.1/1000PY | 351 | 17 (4.8%) | 7.1/1000PY |
| Age ≥ 75 | ||||||
| Overall | 14,146 | 2,609 (18.4%) | 36.3/1000PY | 2,390 | 557 (23.3%) | 34.9/1000PY |
| Non-initiators | 13,086 | 2,404 (18.4%) | 36.2/1000PY | 2,284 | 541 (23.7%) | 35.2/1000PY |
| Initiators | 1,060 | 205 (19.3%) | 37.3/1000PY | 106 | 16 (15.1%) | 26.0/1000PY |
VHA = Veterans Health Administration; KPW = Kaiser Permanente Washington; PY=person-years; MET=metformin
IPTW balanced all baseline covariates, both overall and in each age stratum, in the VHA (all SMD<10%). However, in KPW, index year, age group, insurance, HbA1c, creatinine, and obesity did not balance between exposure groups in the overall sample. There were also a number of other variables not balancing in analyses stratifying by age for KPW (see Appendix B). Thus, when carrying out statistical modeling of dementia risk in KPW, the non-balanced variables were included as additional adjustment variables in weighted models. In the VHA sample, stabilized weights ranged from 0.21 to 9.01 with a mean of 1.00 (±0.38). In KPW, after trimming six weights due to values >10, stabilized weights ranged from 0.14 to 9.86 with a mean of 0.98 (±0.44).
Results from Cox proportional hazard models are shown in Table 4. Age stratified results were not calculated for KPW because in some age strata, there were few dementia cases (<20) and/or a large imbalance for some covariates. After controlling for confounding in weighted model 2, metformin initiation versus non-initiation was not associated with dementia risk in the VHA (HR=1.04; 95%CI=0.95–1.13) or KPW (HR=0.81; 95%CI=0.51–1.28). In analyses stratified by age in the VHA, there was no association of metformin initiation versus non-initiation and dementia in any age stratum. Meta-analysis combining results from VHA and KPW yielded a HR of 1.02 (95%CI=0.90–1.16) comparing metformin initiation versus no initiation of anti-diabetic treatment.
Table 4.
Results from Cox proportional hazards models estimating the association of metformin initiation vs. no anti-diabetic medication initiation and incident dementia, overall and stratified by age for patients ≥50 years old
| Model 1 – Crude1 MET initiation vs. non-initiation | Model 2–Weighted MET initiation vs. non-initiation | |||
|---|---|---|---|---|
| Age group | VA patients (n=112,845) | KPW patients2 (n=14,333) | VA patients3 (n=112,845) | KPW patients2, 4 (n=14,333) |
| All ages | 0.72 (0.67–0.77) | 0.46 (0.34–0.62) | 1.04 (0.95–1.13) | 0.81 (0.51–1.28) |
| Age 50–64 years | 0.95 (0.85–1.08) | - | 0.98 (0.84–1.13) | - |
| Age 65–74 years | 1.09 (0.98–1.20) | - | 1.06 (0.94–1.20) | - |
| Age ≥75 years | 1.02 (0.89–1.18) | - | 1.05 (0.89–1.25) | - |
| MET*Age, p-value 5 | p=0.233 | - | p=0.642 | - |
Note: VHA=Veterans Health Administration; KPW=Kaiser Permanente Washington; MET=Metformin; HR=hazard ratio; CI=confidence interval
Unweighted data
KPW: No age-stratified results due to small number of dementia cases
Inverse probability of treatment weighted data with robust, sandwich-type variance estimators
KPW: Weighted model + non-balanced variables (age, Index year, federal insurance, baseline HbA1c, baseline creatinine, obesity)
Test interaction of Metformin*Age – assess whether age specific hazard ratios are different
Bold text indicates statistically significant hazard ratio
Secondary analyses by HbA1c category and white versus African-American race are shown in Appendix C and Appendix D. All variables balanced and proportional hazards assumptions were met in each stratum. Results showed that there was no association between metformin initiation and incident dementia in any stratum and no differences in effect by baseline HbA1c or race.
DISCUSSION
In two large integrated health systems with robust electronic health and pharmacy dispensing data, we observed no difference in the risk of incident dementia between patients who initiated metformin compared with those who did not start any anti-diabetic medication within 6-months after an eligible HbA1c. Although previous studies have indicated that initiation of metformin compared to a sulfonylurea is associated with a lower risk for dementia,8,9,23 our results extend this research by addressing a more relevant clinical question of whether metformin initiation compared to treatment delay is associated with risk for dementia. These findings did not differ by age, race, or baseline HbA1c. In fact, there was no association of metformin initiation versus non-initiation and dementia in any age stratum, suggesting that in older adults, the early initiation of metformin therapy for T2D is unlikely to substantially reduce the risk of dementia. Also, while there were substantial differences in index HbA1c between groups, there were no differences in treatment effect by index HbA1c. A previous study reported that early metformin treatment, defined as a prescription fill within 6-months of diabetes onset, compared to delayed metformin treatment was associated with a greater likelihood of good glycemic control, more weight loss and lower risk of treatment intensification.14 If poor glycemic control results in a higher risk of dementia, we would expect delayed initiation of treatment for T2D to be associated with a greater risk for dementia. The current results and our previous findings demonstrating no change in the association after controlling for average monthly glycemic burden and hypoglycemic events after metformin initiation9,23 suggest that any potential relationship between metformin and dementia risk is unlikely to be explained by glycemic control.
Because our goals did not include contrasting metformin to sulfonylurea initiation, it is not possible to directly compare the current study to previous research indicating metformin compared to sulfonylurea is associated with lower risk of dementia8,9,23. However, our previous studies taken together with the present results encourage speculation that sulfonylurea could be a risk factor for dementia. Other possibilities include unmeasured confounding associated with starting a medication versus delaying. Patients who seek medication as a preferred treatment as compared to delaying medication, potentially in exchange for lifestyle interventions or due to poor self-care, could differ on factors associated with incident dementia.
Strengths of our study include the use of high quality electronic health data for patients who receive all types of care within the same system. This reduces the loss of information which can occur when patients seek care from providers in different health networks and see different providers as insurance coverage changes. The merged VHA-Medicare data is a unique resource for tracking Veterans healthcare, and VHA is the only national health care system in the United States. To our knowledge, this is the first study to focus on the most clinically relevant question, that is, how dementia risk relates to timing of metformin initiation. Our design allowed for proper temporal ordering of exposure and outcome and we controlled for a large number of confounding variables. Last, the long observation period gave us better ability to detect rare outcomes, i.e. incident dementia.
Our study should be interpreted in light of several limitations. Because we relied on claims data to identify dementia, it is possible that some patients with dementia were missed and that others with qualifying diagnosis codes did not have a confirmed dementia diagnosis. If such misclassification were non-differential by treatment group, we would expect our results to be biased toward the null. Differential misclassification may have occurred if early initiators of metformin had increased contact with the healthcare system and were more likely to have their dementia recognized than patients not starting any medication; the resulting bias may have obscured a protective effect of early treatment. Although we controlled for many potential confounding variables, unmeasured confounding is possible and may impact our findings. Some of the subgroups defined by age, race and baseline HbA1c were small, which may have contributed to broader confidence intervals and limited our ability to detect effect modification. Lastly, our results may not generalize to demographic groups and geographic regions not represented in the current study.
Conclusions:
To our knowledge, our study is the first to examine the relationship between earlier initiation of metformin (vs. not initiating treatment) and the risk of dementia. Based on our findings, currently there is not compelling evidence to suggest that patients or physicians should consider starting metformin earlier than they otherwise would have with the goal of delaying cognitive decline. There is now a substantial body of literature including many observational studies examining the association between metformin use and dementia, using varied study designs and methods that have produced mixed results. It is suggested that if there is still enthusiasm about metformin’s potential to prevent dementia, the best next step would be a randomized clinical trial.
Supplementary Material
KEY POINTS.
Metformin vs. sulfonylurea treatment in patients with diabetes lowers dementia risk but timing of first line treatment may be important.
A retrospective cohort study in Veterans Health Administration and Kaiser Permanente data tested whether initiating metformin compared to not initiating or delaying treatment reduces dementia risk.
Starting metformin early in the course of diabetes compared to not initiating or delaying anti-diabetic treatment was not associated with dementia.
In the context of existing studies indicating metformin vs. sulfonylurea use is associated with lower risk for dementia, our results raise the possibility that sulfonylurea is a risk factor for cognitive decline.
ACKNOWLEDGEMENTS
Funding:
This work was supported by the National Institute on Aging [NIA grant number: R21 AG055604]. This material is the result of work supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans’ Hospital.
Role of funding sources:
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Data Access, Responsibility, and Analysis: Dr. Scherrer and Ms. Salas had full access to VHA data. Dr. Dublin had full access to KPW data. Dr. Scherrer and Ms. Salas take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interests and financial disclosures: Dr. Floyd has consulted for Shionogi Inc.
Publisher's Disclaimer: Disclaimers: The views expressed in this report do not necessarily reflect those of the Veterans Health Administration.
REFERENCES
- 1.Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J Diabetes Investig. 2013;4(6):640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen F, Dong RR, Zhong KL, et al. Antidiabetic drugs restore abnormal transport of amyloid-beta across the blood-brain barrier and memory impairment in db/db mice. Neuropharmacology. 2016;101:123–136. [DOI] [PubMed] [Google Scholar]
- 3.Kickstein E, Krauss S, Thornhill P, et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci U S A. 2010;107(50):21830–21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mousavi SM, Niazmand S, Hosseini M, et al. Beneficial Effects of Teucrium polium and Metformin on Diabetes-Induced Memory Impairments and Brain Tissue Oxidative Damage in Rats. Int J Alzheimers Dis. 2015;2015:493729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rotermund C, Machetanz G, Fitzgerald JC. The Therapeutic Potential of Metformin in Neurodegenerative Diseases. Front Endocrinol (Lausanne). 2018;9:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Gallagher D, DeVito LM, et al. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11(1):23–35. [DOI] [PubMed] [Google Scholar]
- 7.Campbell JM, Stephenson MD, de Courten B, Chapman I, Bellman SM, Aromataris E. Metformin Use Associated with Reduced Risk of Dementia in Patients with Diabetes: A Systematic Review and Meta-Analysis. J Alzheimers Dis. 2018;65(4):1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orkaby AR, Cho K, Cormack J, Gagnon DR, Driver JA. Metformin vs sulfonylurea use and risk of dementia in US veterans aged >/=65 years with diabetes. Neurology. 2017;89(18):1877–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scherrer JF, Salas J, Floyd JS, Farr SA, Morley JE, Dublin S. Metformin and Sulfonylurea Use and Risk of Incident Dementia Mayo Clinic Proceedings, In Press. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2019. Diabetes care. 2019;42(Suppl 1):S90–S102. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association. 12. Older Adults: Standards of Medical Care in Diabetes-2019. Diabetes care. 2019;42(Suppl 1):S139–S147. [DOI] [PubMed] [Google Scholar]
- 12.Eurich DT, McAlister FA, Blackburn DF, et al. Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review. BMJ. 2007;335(7618):497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morley JE, Sinclair A. Individualising treatment for older people with diabetes. Lancet. 2013;382(9890):378–380. [DOI] [PubMed] [Google Scholar]
- 14.Romanelli RJ, Chung S, Pu J, Nimbal V, Zhao B, Palaniappan L. Comparative effectiveness of early versus delayed metformin in the treatment of type 2 diabetes. Diabetes Res Clin Pract. 2015;108(1):170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roblin DW. Validation of a neighborhood SES index in a managed care organization. Med Care. 2013;51(1):e1–8. [DOI] [PubMed] [Google Scholar]
- 16.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analysis with observational databases. Medical Care. 2007;45:S103–S107. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika Trust. 1983;70:41–55. [Google Scholar]
- 18.Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measure covariate balance to test causal associations in psychological research. Psychol Methods. 2010;15:234–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sturmer T, Wyss R, Glynn RJ, Brookhart MA. Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental designs. Journal of Internal Medicine. 2014;275:570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin P, Stuart E. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Statistics in Medicine. 2015;34:3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dominguez Islas C, Rice KM. Addressing the estimation of standard errors in fixed effects meta-analysis. Stat Med. 2018;37(11):1788–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scherrer JF, Morley JE, Salas J, Floyd JS, Farr SA, Dublin S. Association between metformin initiation and incident dementia among African-American and white Veterans Health Affairs patients. Annals of Family Medicine, In Press. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


