Abstract
Background:
Recurrent or refractory (R/R) classical Hodgkin lymphoma (CHL) may be cured with multiagent salvage chemotherapy followed by autologous stem cell transplant. The aim of this study is to determine the safety and efficacy of dose dense (DD) brentuximab vedotin (BV) combined with ifosfamide, carboplatin, and etoposide (ICE) chemotherapy in second line treatment of CHL.
Methods:
We conducted a single-arm, open-label phase 1/2 study of DD-BV-ICE. BV was delivered on days 1 and 8 at either 1.2 or 1.5 mg/kg IV (capped at 150 mg) with standard dosing of ICE on days 1–3 for two cycles. The primary endpoint was to determine the recommended phase 2 dose (RP2D) and complete response (CR) rate in response-evaluable patients after 2 cycles with a pre-specified target of 78%.
Findings:
Between October 16, 2014 and February 10, 2020, we enrolled 45 patients with a median age of 31 (range 20–60). The RP2D dose was determined to be BV 1.5 mg/kg IV. Objective responses and CRs occurred in 91% and 74% among all 43 efficacy evaluable patients. Grade 3–4 hematologic toxicity was common, including neutropenia (33, 73%), anemia (6, 13%), and thrombocytopenia (36, 80%). Grade 3–4 non-hematologic toxicity included febrile neutropenia/sepsis (6, 13%), elevated ALT (5, 11%), hyperglycemia (3, 7%), pulmonary embolism (2, 4%), and elevated AST (2, 4%). There was one (2%) on treatment death due to multi-system organ failure. Serious adverse events were seen in 14 (31%) of patients.
Interpretation:
DD-BV-ICE is a rapid and active salvage regimen for R/R CHL patients despite a CR rate just under the pre-specified target. Efficacy results are comparable to previously presented BV-chemo salvage combinations delivered over significantly longer durations.
Introduction
Classical Hodgkin lymphoma (CHL) is highly curable with frontline combination chemotherapy with or without consolidative radiotherapy. Success rates range from 85–90% in early-stage patients(1, 2), to 75–80%(3–5) in advanced stage patients. Patients requiring second-line therapy may still be cured with multiagent salvage chemotherapy followed by autologous stem cell transplant (ASCT)(6–8). However, up to half of patients will remain refractory to chemotherapy or relapse after ASCT. The likelihood of long-term remission following ASCT for relapsed/refractory (R/R) CHL is predicted by complete metabolic response to pre-ASCT salvage therapy(9). Importantly, patients with primary refractory CHL appear to have lower success rates with standard salvage approaches, and improved combination regimens are needed.
The anti-CD30 antibody-drug conjugate brentuximab vedotin (BV) is highly active as monotherapy in relapsed/refractory CHL. In a pivotal study, patients with CHL who relapsed after ASCT had an overall response rate (ORR) of 75%, and a CR rate of 34% occurring at a median of 12 weeks (10). However, the median PFS was 5.6 months, indicating that these responses were not durable in most patients without additional therapy(11). This has led to the study of BV sequentially or in combination in a variety of CHL treatment settings, including as first salvage. BV has been previously studied in CHL sequentially with ICE (ifosfamide, carboplatin, etoposide)(12) and in combination with bendamustine(13), ESHAP (etoposide, methylprednisolone, cytarabine, and cisplatin)(14), and nivolumab(15). Unfortunately, none of the published studies evaluated strategies to safely escalate the cumulative BV doses concurrently with standard chemotherapy to rapidly maximize the CR rate. While early results of BV in combination with ICE for first salvage chemotherapy in CHL has been presented in abstract form(16), there is no currently published data on concurrent BV with ICE chemotherapy, particularly in a patient population who have exclusively received ABVD or ABVD-like primary chemotherapy.
We hypothesized that concurrent therapy with dose-dense BV and 2 cycles of ICE would be safe, efficient, and produce high CR rates necessary for superior outcomes after ASCT. Herein we present the results of a phase I/II study of BV in combination with ICE as second line therapy for relapsed/refractory CHL.
Methods
Study Design and Participants
This was a prospective, single-arm, open-label phase 1/2 study. Patients age 18 or older with a diagnosis of first relapse or primary refractory CHL after one prior line of therapy. Prior consolidative radiation as part of frontline combined modality therapy was allowed. Patients were required to have at least one FDG-avid measurable site of at least 1 cm in longest axis. Additional inclusion criteria included Eastern Cooperative Oncology Group performance status 0–1 (performance status of 2 was allowed if due to underlying lymphoma). Adequate organ function was required as defined by absolute neutrophil count (ANC) ≥ 1,500/μL, platelets ≥ 100,000/μL, serum creatinine < 1.5 mg/dL or creatinine clearance > 60 mL/min, total bilirubin < 2 times upper limit of normal (unless due to Gilbert’s syndrome), AST/ALT < 2.5 times upper limit of normal. Key exclusion criteria included known diagnosis of HIV, pregnant/nursing women, prior malignancies within 5 years, evidence of CNS lymphoma, prior receipt of brentuximab vedotin, peripheral neuropathy grade > 1, pelvic radiation within 12 months, chemotherapy within 3 weeks, and other medical conditions that would contraindicate an aggressive chemotherapy regimen.
The phase 1 portion of the study was designed to determine the safety and tolerability of the study combination with a primary endpoint to determine a maximum tolerated dose (MTD) of BV to be administered with ICE chemotherapy. This portion was conducted with a “3+3” design with a starting dose level 1 of BV 1.2 mg/kg on days 1 and 8.
This was a single center study conducted at the University of Washington/Seattle Cancer Care Alliance in accordance with the Declaration of Helsinki and approved at the local institutional review board. Written informed consent was obtained from each patient prior to enrollment.
Procedures
Study treatment consisted of two cycles (cycle length 21 days) of combination therapy of BV with ICE chemotherapy. The administration schedule for each cycle included ICE administeration as an inpatient on days 1–3 according to previously published dosing (ifosfamide 5 g/m2 with equal amounts of mesna infused over 24 hours on day two, carboplatin area under the curve (AUC) 5 on day 2, and etoposide 100 mg/m2 on days 1–3). BV was administered as an inpatient on day 1 and outpatient on day 8 (Supplementary figure 2).
Subsequent cycles of therapy could not begin until the ANC was ≥ 1,000/μL, platelets ≥ 50,000/μL, with normal liver and kidney function as defined by the inclusion criteria. Treatment could be delayed for up to 3 weeks for toxicity, after which the patient would be removed from protocol treatment.
Growth factors (filgrastim, pegfilgrastim or equivalent) were mandated by the protocol. Antiemetics and prophylactic antibiotics were administered at investigator discretion per institutional standard of care. Hematopoietic stem cell mobilization and collection was permitted during cycle 2. Details and timing of mobilization and collection were deferred to the investigator per institutional standard of care. While it was anticipated that most patients would proceed with high-dose therapy and ASCT following completion of two cycles of study therapy, patients could go on to receive other therapy at the discretion of the treating physician. However, BV was no longer provided by the study after 2 cycles. In addition, post-ASCT maintenance therapy was optional and could be administered per institutional standard of care.
A status report including accrual, adverse events, and death information was reviewed by the Fred Hutchinson Cancer Research Center (FHCRC) data safety monitoring committee annually. In addition, the study was monitored regularly by the FHCRC Clinical Research Support office.
Exploratory studies were done to evaluate the microenvironment. Immunohistochemical (IHC) evaluation was performed in the University of Washington IHC laboratory on paraffin embedded tissue sections treated with antibodies to CD68, granzyme B, and CD20 (molecules previously shown to correlate with outcomes in CHL(18–20)) using an automated staining technique as described previously(21). Cells staining for CD68 and granzyme B were counted by JRF in least two representative high-power fields (tumor cell areas; at least 500 cells counted) and the percentage of positive cells as a fraction of overall cellularity was calculated. Fraction of cells staining for CD20 was estimated at low and medium power.
Paraffin-embedded biopsy specimens were analyzed for identifiable IgH or IgK sequences which were analyzed in peripherals blood samples at baseline, post-cycle 2, and post ASCT. DNA was extracted and analyzed by Adaptive Biotechnologies similar to previously published methods(22).
Outcomes
The primary endpoints of the study were to determine the RP2D of BV in combination with ICE and to determine the CR rate after completion of 2 cycles of therapy.
Secondary endpoints include safety, peripheral blood stem cell (PBSC) yield, progression-free survival (PFS), overall survival (OS), and correlative biomarkers. Additional post-hoc descriptive analyses include stratification of PFS by primary-refractory (defined as never achieving a CR to primary therapy or relapse within 3 months) vs. non-primary refractory as well as ASCT vs. no ASCT.
Statistical design
The highest potential dose tested was dose level 2 of BV 1.5 mg/kg on days 1 and 8. It was estimated that 9–12 patients would be needed to complete the phase 1 portion. A dose-limiting toxicity (DLT) was defined as any non-hematologic grade 4 or 5 adverse event (excluding asymptomatic grade 4 laboratory abnormalities), grade 3 or higher peripheral neuropathy, treatment delay of > 3 weeks following cycle 1 due to prolonged recovery from observed toxicity, or inability to complete one full cycle of therapy due to toxicity. All enrolled patients were evaluated for safety.
Once the MTD of BV was determined, enrollment was continued with a phase 2 expansion cohort to further evaluate the efficacy of this combination by treating a minimum of 36 additional patients. The primary endpoint of this portion of the study was complete response (CR) rate after two cycles of DD-BV-ICE. If the true CR rate of DD-BV-ICE is 78%, this would provide 80% power to detect a statistically-significant increase in the CR rate from an historical rate of 60%(9) based on a one-sample chi-square test with a one-sided significance level of 5%. Analyses of secondary endpoints were primarily descriptive. Patients who completed study therapy and an end of treatment response assessment were evaluable for response, including patients who had dose interruptions or modifications due to toxicity. Statistical software R version 3.6.0 was used for analyses using packages dplyr, survival, and survminer.
Responses were determined using the 2007 Revised Response Criteria for Lymphoma (Cheson et al.)(17) after completion of up to two cycles of study therapy. Additional imaging was not required by the protocol either before or after ASCT but could be performed per the discretion of the investigator. Safety assessments were performed at baseline and on day 1 and 8 of each cycle. Adverse events (AEs) were monitored through study therapy until an end of treatment visit approximately 30 days after last dose of study therapy or initiation of additional anticancer therapy (whichever was earlier). AEs were graded and defined using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. This study was registered with clinicaltrials.gov (#NCT02227199).
Role of the funding source
The study was designed by the investigators of the study with input from Seagen, Inc. The investigators were responsible for the implementation of the study, data collection, analysis, and interpretation of the data. All authors had full access to the data in the study and accept responsibility to submit for publication. The draft manuscript was composed by the first author and corresponding author and all authors contributed to reviewing and editing the final manuscript. Seagen, Inc was not directly involved in the decision to publish this data or in the process of drafting of the manuscript, but were provided a copy of the manuscript prior to submission which led to minor edits for clarification.
Results
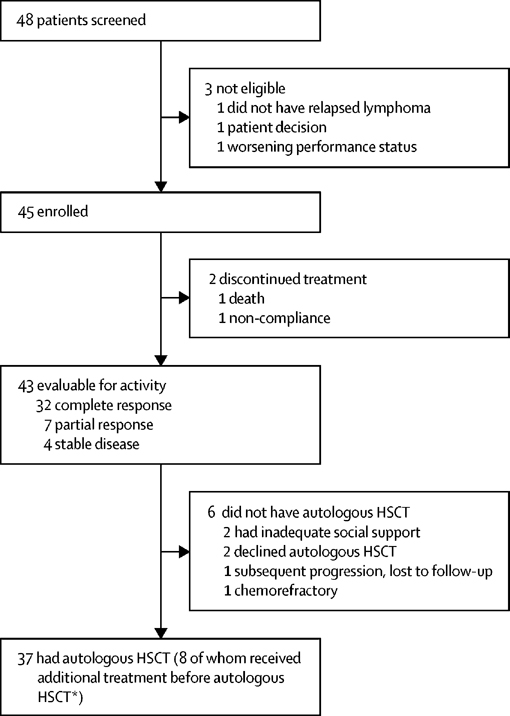
Forty-five patients were enrolled between October 16, 2014 and February 10, 2020. Three patients consented but were not enrolled due to patient decision, no confirmation of relapse, and worsening performance status. Enrollment ended after planned accrual goals were met. Patient disposition including response to therapy, receipt of ASCT, and additional lymphoma-directed therapy are outlined in figure 1. Median age was 31 (range 20–60). Nearly all patients (44, 96%) received ABVD with or without omission of bleomycin by the end of frontline therapy. Overall, 29 patients (64%) had primary refractory disease. Thirty-eight patients (84%) were at high risk of relapse with at least one of the criteria outlined in the AETHERA trial(23, 24) that included inability to achieve CR with primary therapy (15, 33%), remission duration of < 1 year (37, 82%), and extranodal sites at relapse (11, 24%). Several patients had multiple AETHERA risk factors, including 15 (33%) with 2 risk factors, and 5 (11%) with 3 risk factors. Additional patient characteristics are outlined in table 1.
Figure 1: Trial profile.
HSCT =haematopoietic stem-cell transplantation. *Four patients received one cycle of brentuximab vedotin with ifosfamide, carboplatin, and etoposide; one patient received one cycle of ifosfamide, carboplatin, and etoposide; two patients received two cycles of gemcitabine, vinorelbine, and liposomal doxorubicin; and one patient received two cycles of brentuximab vedotin with bendamustine.
Table 1.
Patient characteristics
| Characteristic | N = 45 |
|---|---|
|
| |
| Age, median (range) | 31 (20–60) |
|
| |
| Male, n (%) | 16 (36%) |
|
| |
| Stage at Diagnosis, n (%) | |
| I | 3 (7%) |
| II | 24 (53%) |
| III | 6 (13%) |
| IV | 12 (27%) |
|
| |
| Stage at Enrollment, n (%) | |
| I | 6 (13%) |
| II | 22 (49%) |
| III | 6 (13%) |
| IV | 11 (24%) |
|
| |
| Primary therapy received, n (%) | |
| ABVD/AVD | 43 (96%) |
| ABVE-PC | 1 (2%) |
| Stanford V | 1 (2%) |
|
| |
| Prior consolidative radiotherapy, n (%) | 8 (18%) |
|
| |
| Baseline disease characteristics, n (%) | |
| B symptoms at diagnosis | 18 (40%) |
| Extranodal disease at diagnosis | 12 (27%) |
| Spleen involved at diagnosis | 7 (16%) |
|
| |
| Response to frontline therapy, n (%) | |
| Primary Refractory* | 29 (64%) |
| Relapse 3–12 months after treatment completion | 8 (18%) |
| Relapse > 12 months after treatment completions | 8 (18%) |
|
| |
| Relapse disease characteristics, n (%) | |
| B symptoms at relapse | 1 (2%) |
| Extranodal involvement at relapse | 11 (24%) |
| Spleen involvement at relapse | 4 (9%) |
For patients with progression within 3 months, best response (including interim PET) to frontline therapy for patients with primary refractory disease includes: PD (1 patient), PR (14 patients), and CR (14 patients)
ABVD: Doxorubicin, bleomycin, vinblastine, dacarbazine
AVD: Doxorubicin, vinblastine, dacarbazine
ABVD/AVD: No patient received AVD alone. However, this group includes patients who received ABVD alone as well as those who initiated ABVD and subsequently omitted bleomycin and finished their primary treatment with AVD
ABVE-PC: doxorubicin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide
In the phase 1 portion of the study, the first 3 patients were treated with BV at dose level 1 of 1.2 mg/kg without any DLTs. Only 1 of the next 6 treated with BV dose level 2 of 1.5 mg/kg experienced a DLT. Therefore 1.5 mg/kg was determined to be the MTD and recommended phase 2 dose. An additional 36 patients (for an overall total of 42 at the phase 2 dose) received at least one cycle of the combination therapy.
Most patients (41/45, 91%) completed both cycles of therapy. One patient experienced grade 5 multi-system organ-failure during cycle 1 that was considered treatment-related. One patient was removed from protocol due to non-compliance, and two patients omitted cycle 2 due to toxicity (grade 4 sepsis, grade 3 Sweet syndrome attributable to G-CSF). Two patients received both cycles of ICE but omitted at least one dose of BV due to toxicity. Cycle 2 was delayed in 13/41 (32%) of the remaining patients by a median of 7 days (range 6–17) due to toxicity, primarily elevated transaminases (10/13, 77%). Nearly all (44/45, 98%) patients experienced at least one treatment emergent adverse event (TEAE). Serious adverse events (SAE) were experienced by 14 (31%) patients. Peripheral neuropathy was common (16/45, 36%), but grade 2 or higher peripheral neuropathy was rare (3/45, 7%). Grade 3–4 hematologic toxicity was common, including neutropenia (73%), anemia (6, 13%), and thrombocytopenia (36, 80%). Other grade 3–4 non-hematologic toxicity included febrile neutropenia/sepsis (6, 13%), elevated ALT (5, 11%), hyperglycemia (3, 7%), pulmonary embolism (2, 4%), and elevated AST (2, 4%). Additional information on adverse events is provided in table 3.
Table 3 –
Treatment Emergent Adverse events (n=45)
| Adverse Event: | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|
| Anemia | 38 ( 84% ) | 6 ( 13% ) | 0 ( 0% ) | 0 ( 0% ) |
| Nausea | 37 ( 82% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Elevated AST | 34 ( 76% ) | 2 ( 4% ) | 0 ( 0% ) | 0 ( 0% ) |
| Elevated ALT | 32 ( 71% ) | 5 ( 11% ) | 0 ( 0% ) | 0 ( 0% ) |
| Constipation | 24 ( 53% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Alopecia | 23 ( 51% ) | 1 ( 2% ) | 0 ( 0% ) | 0 ( 0% ) |
| Vomiting | 22 ( 49% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Fatigue | 21 ( 47% ) | 1 ( 2% ) | 0 ( 0% ) | 0 ( 0% ) |
| Headache | 21 ( 47% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Peripheral sensory neuropathy | 15 ( 33% ) | 1 ( 2% ) | 0 ( 0% ) | 0 ( 0% ) |
| Sinus Tachycardia | 15 ( 33% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Non-cardiac chest pain | 14 ( 31% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Abdominal Pain | 13 ( 29% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Diarrhea | 13 ( 29% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Rash | 12 ( 27% ) | 1 ( 2% ) | 0 ( 0% ) | 0 ( 0% ) |
| Anorexia | 12 ( 27% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Hypokalemia | 11 ( 24% ) | 3 ( 7% ) | 1 ( 2% ) | 0 ( 0% ) |
| Dyspepsia | 11 ( 24% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Myalgia | 10 ( 22% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Rhinorrhea | 10 ( 22% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Fever | 9 ( 20% ) | 1 ( 2% ) | 0 ( 0% ) | 0 ( 0% ) |
| Cough | 9 ( 20% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Pruritus | 9 ( 20% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Bone pain | 8 ( 18% ) | 1 ( 2% ) | 0 ( 0% ) | 0 ( 0% ) |
| Hypomagnesemia | 8 ( 18% ) | 1 ( 2% ) | 0 ( 0% ) | 0 ( 0% ) |
| Back pain | 8 ( 18% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Hypotension | 7 ( 16% ) | 0 ( 0% ) | 1 ( 2% ) | 0 ( 0% ) |
| Pain in extremity | 7 ( 16% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Thrombocytopenia | 6 ( 13% ) | 18 ( 40% ) | 18 ( 40% ) | 0 ( 0% ) |
| Chills | 6 ( 13% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Dizziness | 6 ( 13% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Upper respiratory infection | 6 ( 13% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Anxiety | 5 ( 11% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Arthralgia | 5 ( 11% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Infusion related reaction | 5 ( 11% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Mucositis oral | 5 ( 11% ) | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) |
| Neutropenia | 4 ( 9% ) | 7 ( 16% ) | 26 ( 58% ) | 0 ( 0% ) |
| Insomnia | 4 ( 9% ) | 1 ( 2% ) | 0 ( 0% ) | 0 ( 0% ) |
| Pain | 4 ( 9% ) | 1 ( 2% ) | 0 ( 0% ) | 0 ( 0% ) |
| Dyspnea | 3 ( 7% ) | 1 ( 2% ) | 0 ( 0% ) | 0 ( 0% ) |
| Hypocalcemia | 2 ( 4% ) | 0 ( 0% ) | 1 ( 2% ) | 0 ( 0% ) |
| Hyperglycemia | 2 ( 4% ) | 3 ( 7% ) | 0 ( 0% ) | 0 ( 0% ) |
| Enterocolitis infectious | 2 ( 4% ) | 1 ( 2% ) | 0 ( 0% ) | 0 ( 0% ) |
| Hypophosphatemia | 1 ( 2% ) | 4 ( 9% ) | 1 ( 2% ) | 0 ( 0% ) |
| Febrile neutropenia | 1 ( 2% ) | 3 ( 7% ) | 0 ( 0% ) | 0 ( 0% ) |
| Cellulitis | 1 ( 2% ) | 1 ( 2% ) | 0 ( 0% ) | 0 ( 0% ) |
| Dehydration | 1 ( 2% ) | 1 ( 2% ) | 0 ( 0% ) | 0 ( 0% ) |
| Multi-system organ failure | 0 ( 0% ) | 0 ( 0% ) | 0 ( 0% ) | 1 ( 2% ) |
| Sepsis | 0 ( 0% ) | 0 ( 0% ) | 6 ( 13% ) | 0 ( 0% ) |
| Acute kidney injury | 0 ( 0% ) | 0 ( 0% ) | 1 ( 2% ) | 0 ( 0% ) |
| Respiratory failure | 0 ( 0% ) | 0 ( 0% ) | 1 ( 2% ) | 0 ( 0% ) |
| Elevated lactate | 0 ( 0% ) | 2 ( 4% ) | 0 ( 0% ) | 0 ( 0% ) |
| Pulmonary Embolism | 0 ( 0% ) | 2 ( 4% ) | 0 ( 0% ) | 0 ( 0% ) |
| Hemorrhoids | 0 ( 0% ) | 1 ( 2% ) | 0 ( 0% ) | 0 ( 0% ) |
| Pneumonia | 0 ( 0% ) | 1 ( 2% ) | 0 ( 0% ) | 0 ( 0% ) |
Forty-three patients were evaluable for efficacy (including two patients who did not receive cycle 2 due to toxicity but were evaluable for response). Overall response to combination therapy is outlined in table 2. Overall response rate (ORR) and CR for all enrolled patients were 91% (95% CI 77.9–97.4%) and 74% (95% CI 58.8–86.5%), respectively. This CR rate was below the pre-specified CR target of 78%. Several post-hoc analyses were performed, and among the primary refractory patients, ORR and CR were 86% and 68%, respectively. ORR and CR were calculated for patients stratified by early stage (93%, 82%) vs. advanced stage (88%, 63%) at enrollment, respectively. Among 14 response-evaluable patients who never achieved a CR to primary therapy, 9 (64%) achieved a CR with DD-BV-ICE. A waterfall plot of responses to therapy is illustrated in supplemental figure 1.
Table 2 –
Response at end of study therapy
| Clinical response, n (%) [95% CI] | |||||
|---|---|---|---|---|---|
| Characteristic | CR | PR | SD | PD | ORR |
| Overall (n=43) | 32 (74%) [58.8–86.5%] |
7 (16%) [6.8–30.7%] |
4 (9%) [2.6–22.1%] |
0 (0%) [0–8.2%] |
39 (91%) [77.9%–97.4%] |
| Response to primary therapy | |||||
| Primary refractory (n=28) | 19 (68%) [47.6–84.1%] |
5 (18%) [6.1–36.9%] |
4 (14%) [4–32.7%] |
0 (0%) [0–12.3%] |
24 (86%) [67.3–96%] |
| Relapse 3–12 months (n=8) | 8 (100%) [63.1–100%] |
0 (0%) [0–36.9%] |
0 (0%) [0–36.9%] |
0 (0%) [0–36.9%] |
8 (100%) [63.1–100%] |
| Relapse > 12 months (n=7) | 5 (71%) [29–96.3%] |
2 (29%) [3.7–71%] |
0 (0%) [0–41%] |
0 (0%) [0–41%] |
7 (100%) [59–100%] |
| ASCT | |||||
| Yes(n=37) | 3 (50%) [11.8–88.2%] |
1 (17%) [0.4–64.1%] |
2 (33%) [4.3–77.7%] |
0 (0%) [0–45.9%] |
4 (67%) [22.3–95.7%] |
| No (n=6) | 29 (78%) [61.8–90.2%] |
6 (16%) [6.2–32%] |
2 (5.4%) [0.7–18.2%] |
0 (0%) [0–9.5%] |
35 (95%) [81.8–99.3%] |
Among the 43 patients who completed study therapy, 37 ultimately proceeded with ASCT. Some of these patients received additional chemotherapy at the discretion of the clinician or transplant physician prior to ASCT with the goal of deepening the clinical response or bridging prior to transplant. Post study treatment responses among these 8 patients who received additional treatment include 3 in CR, 3 with a partial response (PR), and 2 with stable disease (SD). All these patients had reduction in tumor volume with study therapy. In addition, 5 of 8 of these patients received only one additional cycle of chemotherapy prior to proceeding with ASCT. Among the 6 patients who did not receive an ASCT, two patients were in CR but did not have adequate social support for ASCT. Another two patients declined ASCT after completion of study therapy. One patient progressed after study treatment but was lost to follow up. This left only one patient who was truly chemorefractory and therefore unable to proceed with ASCT. Additional detail is outlined in Figure 1.
We collected information on peripheral blood stem cell (PBSC) mobilization for all patients who had this performed at our center. This included patients who ultimately did not proceed with ASCT but excluded patients with personal or financial reasons for receiving their ASCT outside of our institution. We obtained information on collection for 36 patients, of whom 30 (83%) were able to collect at least 5 × 106 CD34+ cells/kg. ASCT was still performed in 5 of 6 (83%) of the remaining patients, with the other patient ultimately not proceeding with ASCT due to inadequate social support and not because of inadequate cell collection. Only 5 of 36 (14%) patients received plerixafor during PBSC mobilization. One patient required a second collection, with both collections being performed with growth factors (GCSF) and plerixafor. BEAM (carmustine, etoposide, cytarabine, melphalan) was the most common conditioning regimen (81%), followed by total body irradiation (TBI) + cyclophosphamide (11%), TEAM (thiotepa, etoposide, cytarabine, melphalan) (3%), TMB (thiotepa, melphalan, busulfan) (3%), and one patient (3%) with an unknown conditioning regimen. Median time from end of treatment PET to ASCT was 21 days (range 9–111 days). All patients successfully engrafted and the engraftment dates were available for 33 patients. Median time to neutrophil and platelet engraftment were 14 and 10 days, respectively.
Additional anti-lymphoma therapy (including maintenance) for patients achieving a remission was not mandated per protocol and decisions were left to the individual treating physician. Most patients (23/37, 63%) received either consolidative radiation and/or maintenance systemic therapy. Most patients received BV monotherapy (19/37, 52%) for a median of 9 cycles (range 1–16). Most BV monotherapy maintenance patients (11/19, 58%) discontinued maintenance due to toxicity. Two patients (5%) received 8 doses of BV + nivolumab on a different trial protocol. Post ASCT radiotherapy was administered in 2/37 (5%) patients, one of whom then went on to receive BV monotherapy maintenance. Among the 4 patients who did not receive an ASCT for either personal or social reasons, 2 received additional chemotherapy as maintenance (one received BV + bendamustine, the other two additional cycles of ICE). Both of these patients remain in remission without any subsequent evidence of progression. Two other patients were in a CR after study treatment and have received no additional therapy and remain in remission 28 and 60 months after last chemotherapy treatment, respectively.
With a median follow up of 3.1 years (IQR 1.7–4.1 years), the estimated 2-year OS and PFS were 97.8% (95% CI, 93.6–100%) and 80.4% (95% CI, 68.9–93.7%), respectively. Only two patients who were enrolled on the study subsequently died – one patient had a treatment-related death after cycle 1 of treatment, and another died over 2 years after treatment completion of complications related to secondary myelodysplastic syndrome and allogeneic transplant. There were no lymphoma-related deaths observed over the course of the study. In figure 2 we present Kaplan-Meier curves for both OS and PFS. We also stratified the PFS curves by all patients and those who received ASCT. The sample size as well as the ad hoc nature of this analysis precluded a formal analysis. However, the PFS curves for all patients was very similar to those who received an ASCT. Two of 8 patients who received additional therapy immediately following DD-BV-ICE and then went to ASCT eventually relapsed as compared to 3 of 29 who did not receive additional therapy.
Figure 2:
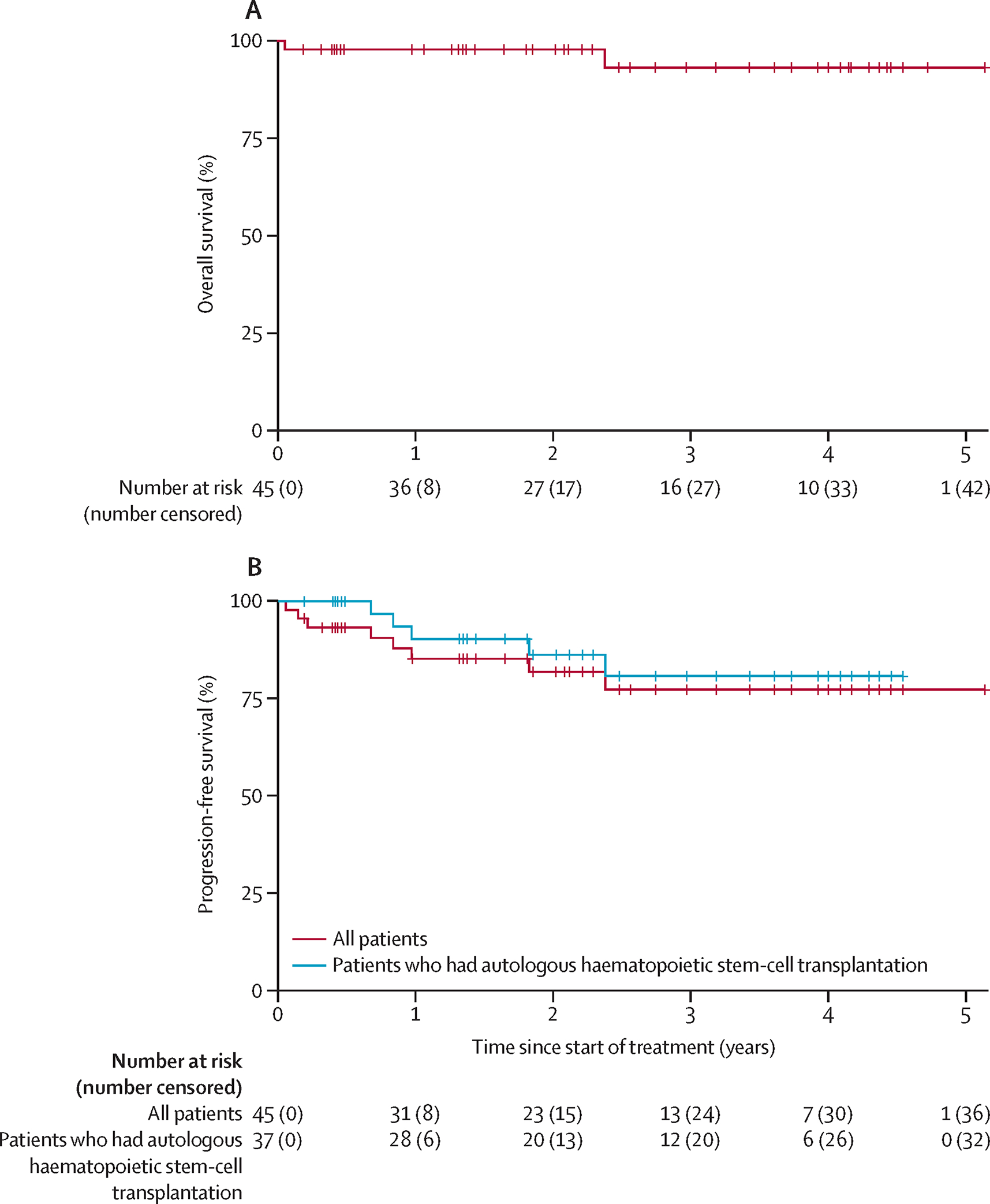
(A) Overall survival in all patients and (B) progression-free survival in all patients versus those who received autologous haematopoietic stem-cell transplantation
Proportions of CD68+ macrophages, granzyme+ cytotoxic T cells, and CD20+ background B cells by immunohistochemistry from either initial diagnosis or at relapse specimens were similar between those who achieved CR and those who did not in the initial cohort of 27 patients in the trial (Patients enrolled August 2014 to August 2017).
Analysis of the first 19 available paraffin-embedded biopsy specimens detected an identifiable immunoglobulin sequence in 9 (47%) of specimens. Only 1/14 (7%) of pre-treatment peripheral blood specimens detected an identifiable sequence. No post-cycle 2 (n=13) or post ASCT (n=3) peripheral blood specimens were able to detect an identifiable sequence.
Discussion
To our knowledge this is the first study to examine the safety and efficacy of concurrent BV combined with ICE chemotherapy in an almost exclusively ABVD or ABVD-like treatment CHL population and the first to evaluate high-cumulative doses of BV with short course chemotherapy with the goal to rapidly augment the CR rate in transplant eligible patients. This design was in part based on the observation from the initial pivotal trial of single agent BV that the cumulative doses at the median time to CR of 12 weeks were 5.4–7.2 mg/kg. Our phase II dose delivered 6mg/kg over 6 weeks. This regimen led to a high ORR and CR rate of 91% and 74%, respectively, in all evaluable patients despite the inclusion of a high proportion of patients at high risk of poor outcomes including nearly two-thirds of the patients with primary refractory disease (ORR and CR rates remained high at 86% and 68%, respectively). The majority of evaluable patients (37/43, 86%) proceeded with ASCT, with most patients achieving a durable remission.
The safety profile for dose dense concurrent BV-ICE was similar to previously published platinum salvage regimens with the exception of peripheral neuropathy and AST/ALT changes. Peripheral neuropathy was common, though grade 2 or higher peripheral neuropathy was rare (3/45, 7%). Lower rates of grade 2 or higher peripheral neuropathy is likely attributable to the limited duration of BV use (only 4 doses) with this regimen. We noted that most patients (89%) experienced an elevation in transaminases including 12% with grade 3 or higher elevation. This was reversible in all patients but did lead to treatment delay of cycle 2 in 10/45 (22%) of patients based on protocol-specified hold parameters. One patient died from multisystem organ failure without a clear sign of infection which was possibly related to the treatment. Treatment-related deaths with platinum-based salvage are uncommon but have been described in previous publications for both Hodgkin and non-Hodgkin lymphomas(6, 14, 25, 26). We cannot rule out the role of BV in augmenting the known toxicity of platinum-based salvage. Fortunately, no other patients developed this syndrome while on study therapy. However, this illustrates the potential risks of an intensive regimen like DD-BV-ICE, and providers should have an in-depth discussion with patients on the risks and benefits of various salvage chemotherapy options in the context of age and other comorbidities. We do not feel there is sufficient data to support the use of this regimen in patients over age 60 as we did not enroll any subjects in this age group.
The prespecified CR target of 78% was based on an expected accrual of less than half with primary refractory disease or early relapse, and this study did not meet that target. However, the actual accrual suggested that patients and physicians preferentially referred their higher risk patients to this study. The regimen appeared very active across all patients though CR rates were numerically lower in primary refractory versus in non-primary refractory disease (69% vs. 87%), impacting top line results. While most patients were primary refractory (64%), the majority were also early stage at enrollment (62%). Direct statistical comparison is limited by small sample size, though these competing risk factors likely serve to balance the overall results.
While our primary endpoint was CR rate after completion of 2 cycles of DD-BV-ICE, we realize that the PFS and OS secondary endpoints are affected by downstream therapy including maintenance. Unfortunately, this has complicated several similar phase II studies for second line CHL treatment. A study with BV and bendamustine included 25 of 40 patients that went on to receive BV maintenance(13). In an abstract of sequential nivolumab + ICE(27), the authors noted that the majority of patients post-ASCT went on a separate BV + nivolumab maintenance study. While clean data would be ideal, these similar phase II studies in this setting suffer from similar challenges. We believe that transparency is important in order to guide cross study comparisons and allow for better counseling of patients, so we have shared all the available data relevant to these secondary endpoints.
Cross trial comparisons across other BV-based salvage regimens for CHL are also challenging due to differences in patient composition, definitions of efficacy evaluation, and treatment intent. Our results were based on only 2 cycles (6 weeks) of study treatment, the shortest such treatment prospectively evaluated. Other BV-based regimens including BV-nivolumab(15), sequential BV-ICE(12), and BV-bendamustine(13) all allowed for treatment for up to 12–24 weeks depending on the study. While not available for review in manuscript form, a concurrent BV-ICE study from LYSA (The Lymphoma Study Association)(16) included nearly half of patients who had relapsed after BEACOPP and administered therapy over 3 cycles followed by a 4th cycle of BV alone. While we anticipate that prolonged use of DD-BV-ICE based on our study may not be well tolerated, it is likely that the CR rate would increase with additional cycles. It is notable that the small subset of patients in our study that did not go on to transplant had a similarly excellent outcome with no relapses to date, similar to the results by LaCasce and colleagues(28). In our opinion, these data are sufficiently compelling to prospectively evaluate augmented salvage regimens in comparison to transplant.
We performed several correlative studies including IHC assessments CD68+ macrophages, granzyme+ cytotoxic T cells, and CD20+ background B cells as well as attempting to track tumor specific immunoglobulin sequences in peripheral blood. Unfortunately, our IHC analysis did not appear to correlate with outcomes, and we were unable to identify a unique immunoglobulin sequence in the vast majority of tumor samples that could be tracked in peripheral blood. Since the design of our study, circulating tumor DNA (ctDNA) has emerged as a potential prognostic marker in CHL(29), but our banked samples were not compatible to consider such an analysis.
There are several limitations to our study. First, patients were enrolled from a single institution with experience in delivering high-intensity treatments. Second, PFS endpoints may be confounded by subsequent therapies including transplant and maintenance. Importantly, late relapses were rare regardless of post study treatment and sustained remissions were achieved in >75% of patients. Since all patients had reduction in disease burden after DD-BV-ICE, we are unable to determine the role of BV maintenance in patients who received BV-based salvage.
In conclusion, dose-dense BV-ICE is a rapid, effective first salvage regimen for relapsed/refractory CHL. Efficacy results seen in our study are comparable to previously published BV-chemo salvage combinations often delivered over longer durations with similar caveats of heterogenous post-study treatments/maintenance. The safety and efficacy profile, particularly in primary-refractory patients, supports its use in select patients with first relapse CHL.
Supplementary Material
Research in Context.
Evidence before this study
At the time of the study design brentuximab vedotin (BV) given every 3 weeks had been approved in the United States for use in classical Hodgkin Lymphoma recurrent after autologous transplantation or in those who were transplant ineligible after 2 prior lines of therapy. Weekly dosing of single agent BV had also been published in 2012 showing that this approach was feasible and effective. The most commonly used second line regimen for HL at that time was ifosfamide, carboplatin, etoposide (ICE) achieving complete remissions (CR) in 50–60% of patients. We queried pubmed in October 2013 using search terms brentuximab vedotin, Hodgkin lymphoma and chemotherapy and found no results with BV-chemotherapy combinations. We hypothesized that combining ICE chemotherapy with dose-dense BV in the second line setting could safely and rapidly improve CR rates and lead to better post-transplant outcomes and, therefore, conducted this trial.
Added value of this study
This study provides the first prospective evidence that BV can be safely combined with the most commonly employed second line multi-agent chemotherapy regimen in the US for CHL as well as to our knowledge the only data indicating that weekly dose-dense administration with chemotherapy is feasible. Historical controls prior to the approval of brentuximab vedotin show a 50–60% complete response rate to second line therapy with ICE chemotherapy. Other combinations with PD1 inhibitors or chemotherapy that have been published since our study was designed suggest a CR rate of 60–74%. Our study demonstrated that dose-dense BV-ICE is a rapid-acting and highly effective salvage chemotherapy regimen with virtually all eligible patients ultimately proceeding with autologous transplant. High CR rates were also seen in primary refractory patients, which historically had inferior outcomes. This is the first dose-dense brentuximab combination salvage chemotherapy regimen for transplant-eligible patients
Implications of all the available evidence
These results provide an important, rapidly administered second line option for patients with relapsed/refractory HL and highlight the favorable results with high CR rates seen even in primary refractory patients with all the caveats of cross-trial comparisons. In the absence of randomized data, dose-dense BV-ICE may serve as an attractive salvage chemotherapy option, particularly for young, fit patients.
Acknowledgements
We would like to acknowledge the patients who participated in this study as well as the clinical research team who helped implement this study. This work was supported by research funding from Seagen Inc, (formerly Seattle Genetics, Inc) as well as support from the Lymphoma Research Foundation Clinical Research Mentoring Program and Clinical Research Career Development Award, NIH/NCI Cancer Center Support Grant P30 CA015704, K24 CA184039 and generous philanthropic support from numerous individuals and families.
Declaration of Interests
SDS has received research funding from Acerta Pharma BV, AstraZeneca, Bayer, Beigene, De Novo Biopharma, Genentech, Incyte, Merck, and Portola Pharmaceuticals. SDS has received consulting fees from Astra Zeneca, Millenium/Takeda. RCL has received research funding from Seagen Inc. RDC has received research funding from Amgen, Merck, Pfizer, and Vanda Pharmaceuticals. RDC has received honoraria and travel payment from Pfizer for an educational lecture. RDC is on a Data Safety Monitoring Board for a prospective clinical trial by Pepromene Bio. RDC’s spouse is an employee of Seagen, Inc and has stock/stock options in Seagen, Inc. MSS has research funding from Mustang Bio, Celgene, Bristol Myers, Squibb, Pharmacyclics, Gilean, Genentech, Abbvie, TG Therapeutics, Beigne, Astra Zeneca, Sunesis, Atara Bioptherapeutics, and GenMab. MSS has consulting fees from Abbvie, Genentech, Astra Zeneca, Sound Biologics, Pharmacyclics, Beigene, Bristol Myers Squibb, Morphosys, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, Eli Lilly, and Atara Bioptherapeutics. AKG has received research funding from the NIH/NCI, Seagen, Inc, Bristol Myers Squibb, Pharmacyclics, Gilean, Genentech, Astra Zeneca, Pfizer, Teva, Takeda, Acrotec, IgM, I-Mab, Agios, and Merck. AKG has received consulting fees from Abbvie, Genentech, Janssen, Astra Zeneca, Pharmacyclics, Bristol Myers Squibb, Amgen, Morphosys, TG Therapeutics, Kite Pharma, Adaptive, Seagen, Icn, Epizyme, Kite, Gilead, ADCT, Incyte, Karyopharm, Actinium, Asana Bio, Nurix, and Apteva. AKG has participated on a Data Safety Monitoring Board for ADCT and Janssen. AJC has received research funding from Janssen, Abbvie, Harpoon, Nektar, Bristol Myers Squibb, and Sanofi. AJC has received consulting fees from Janssen, EUSA, GSK, Abbvie, Sanofi, and Cellectar. AJC has stock/stock options in Doximity.
Funding:
Seagen, Inc, Lymphoma Research Foundation, NIH/NCI Cancer Center Support Grant P30 CA015704, K24 CA184039 and generous philanthropic support from numerous individuals and families.
Footnotes
Data Sharing
No individual patient data will be available. A copy of the protocol is available upon request to the corresponding author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johnson P, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. The New England journal of medicine. 2015;372(17):1598–607. [DOI] [PubMed] [Google Scholar]
- 2.Andre MPE, Girinsky T, Federico M, Reman O, Fortpied C, Gotti M, et al. Early Positron Emission Tomography Response-Adapted Treatment in Stage I and II Hodgkin Lymphoma: Final Results of the Randomized EORTC/LYSA/FIL H10 Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35(16):1786–94. [DOI] [PubMed] [Google Scholar]
- 3.Gordon LI, Hong F, Fisher RI, Bartlett NL, Connors JM, Gascoyne RD, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(6):684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson P, Federico M, Kirkwood A, Fosså A, Berkahn L, Carella A, et al. Adapted Treatment Guided by Interim PET-CT Scan in Advanced Hodgkin’s Lymphoma. The New England journal of medicine. 2016;374(25):2419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connors JM, Jurczak W, Straus DJ, Ansell SM, Kim WS, Gallamini A, et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. The New England journal of medicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moskowitz CH, Bertino JR, Glassman JR, Hedrick EE, Hunte S, Coady-Lyons N, et al. Ifosfamide, carboplatin, and etoposide: a highly effective cytoreduction and peripheral-blood progenitor-cell mobilization regimen for transplant-eligible patients with non-Hodgkin’s lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17(12):3776–85. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett NL, Niedzwiecki D, Johnson JL, Friedberg JW, Johnson KB, van Besien K, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin’s lymphoma: CALGB 59804. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2007;18(6):1071–9. [DOI] [PubMed] [Google Scholar]
- 8.Kuruvilla J, Nagy T, Pintilie M, Tsang R, Keating A, Crump M. Similar response rates and superior early progression-free survival with gemcitabine, dexamethasone, and cisplatin salvage therapy compared with carmustine, etoposide, cytarabine, and melphalan salvage therapy prior to autologous stem cell transplantation for recurrent or refractory Hodgkin lymphoma. Cancer. 2006;106(2):353–60. [DOI] [PubMed] [Google Scholar]
- 9.Moskowitz CH, Matasar MJ, Zelenetz AD, Nimer SD, Gerecitano J, Hamlin P, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non–cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119(7):1665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(18):2183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gopal AK, Chen R, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood. 2015;125(8):1236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moskowitz AJ, Schöder H, Yahalom J, McCall SJ, Fox SY, Gerecitano J, et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin’s lymphoma: a non-randomised, open-label, single-centre, phase 2 study. The Lancet Oncology. 2015;16(3):284–92. [DOI] [PubMed] [Google Scholar]
- 13.LaCasce AS, Bociek RG, Sawas A, Caimi P, Agura E, Matous J, et al. Brentuximab vedotin plus bendamustine: a highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Sanz R, Sureda A, de la Cruz F, Canales M, Gonzalez AP, Pinana JL, et al. Brentuximab vedotin and ESHAP is highly effective as second-line therapy for Hodgkin lymphoma patients (long-term results of a trial by the Spanish GELTAMO Group). Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2019;30(4):612–20. [DOI] [PubMed] [Google Scholar]
- 15.Herrera AF, Moskowitz AJ, Bartlett NL, Vose JM, Ramchandren R, Feldman TA, et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2018;131(11):1183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stamatoullas A, Ghesquieres H, Clement filliatre L, Quittet P, Morschhauser F, Ribrag V, et al. Brentuximab Vedotin in First Refractory/Relapsed Classical Hodgkin Lymphoma Patients Treated By Chemotherapy (ICE) before Autologous Transplantation. Final Analysis of Phase II Study. Blood. 2019;134(Supplement_1):132-. [Google Scholar]
- 17.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(5):579–86. [DOI] [PubMed] [Google Scholar]
- 18.Panico L, Tenneriello V, Ronconi F, Lepore M, Cantore N, Dell’Angelo AC, et al. High CD20+ background cells predict a favorable outcome in classical Hodgkin lymphoma and antagonize CD68+ macrophages. Leukemia & lymphoma. 2015;56(6):1636–42. [DOI] [PubMed] [Google Scholar]
- 19.Asano N, Oshiro A, Matsuo K, Kagami Y, Ishida F, Suzuki R, et al. Prognostic significance of T-cell or cytotoxic molecules phenotype in classical Hodgkin’s lymphoma: a clinicopathologic study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(28):4626–33. [DOI] [PubMed] [Google Scholar]
- 20.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. The New England journal of medicine. 2010;362(10):875–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravanpay AC, Fromm JR, Edlefsen KL, Martin P, Chesnut R. The first report of human primary thoracic spine mast cell sarcoma: a case report. Clin Neuropathol. 2018;37(1):28–35. [DOI] [PubMed] [Google Scholar]
- 22.Wu D, Emerson RO, Sherwood A, Loh ML, Angiolillo A, Howie B, et al. Detection of minimal residual disease in B lymphoblastic leukemia by high-throughput sequencing of IGH. Clin Cancer Res. 2014;20(17):4540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sweetenham J, Walewski J, Nademanee AP, Masszi T, Agura E, Holowiecki J, et al. Updated Efficacy and Safety Data from the AETHERA Trial of Consolidation with Brentuximab Vedotin after Autologous Stem Cell Transplant (ASCT) in Hodgkin Lymphoma Patients at High Risk of Relapse. Blood. 2015;126(23):3172-. [Google Scholar]
- 24.Moskowitz CH, Walewski J, Nademanee A, Masszi T, Agura E, Holowiecki J, et al. Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood. 2018;132(25):2639–42. [DOI] [PubMed] [Google Scholar]
- 25.Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(27):4184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crump M, Kuruvilla J, Couban S, MacDonald DA, Kukreti V, Kouroukis CT, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(31):3490–6. [DOI] [PubMed] [Google Scholar]
- 27.Herrera AF, Chen RW, Palmer J, Tsai N-C, Mei M, Popplewell LL, et al. PET-Adapted Nivolumab or Nivolumab Plus ICE As First Salvage Therapy in Relapsed or Refractory Hodgkin Lymphoma. Blood. 2019;134(Supplement_1):239-.31076442 [Google Scholar]
- 28.LaCasce AS, Bociek RG, Sawas A, Caimi P, Agura E, Matous J, et al. Three-year outcomes with brentuximab vedotin plus bendamustine as first salvage therapy in relapsed or refractory Hodgkin lymphoma. British journal of haematology. 2020;189(3):e86–e90. [DOI] [PubMed] [Google Scholar]
- 29.Spina V, Bruscaggin A, Cuccaro A, Martini M, Di Trani M, Forestieri G, et al. Circulating tumor DNA reveals genetics, clonal evolution, and residual disease in classical Hodgkin lymphoma. Blood. 2018;131(22):2413–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.