Abstract
Recent studies have revealed that cells utilize liquid–liquid phase separation (LLPS) as a mechanism in assembly of membrane-less organelles, such as RNP granules. The nucleus is a well-known membrane-bound organelle surrounded by the nuclear envelope; the nuclear pore complex on the nuclear envelope likely applies LLPS in the central channel to facilitate selective biological macromolecule exchange. Karyopherin-β family proteins exclusively pass through the central channel with cargos by dissolving the phase separated hydrogel formed by the phenylalanine-glycine (FG) repeats-containing nucleoporins. Karyopherin-βs also exhibit dissolution activity for the phase separation of cargo proteins. Many cargos, including RNA-binding proteins containing intrinsically disordered regions (IDRs), undergo phase separation; however, aberrant phase separation is linked to fatal neurodegenerative diseases. Multiple weak interactions between karyopherin-βs and phase separation-prone proteins, such as FG repeats-containing nucleoporins or IDR-containing karyopherin-β cargos, are likely to be important for passing through the nuclear pore complex and maintaining the soluble state of cargo, respectively. In this review, we discuss how karyopherin-βs regulate phase separation to function.
Keywords: karyopherin-βs, liquid–liquid phase separation, low-complexity domain, neurodegenerative disease, RNA binding proteins
Nuclear import system and nuclear pore complexes
Cells organize their components by using organelles. Genomic DNA is encapsulated and protected by the double-lipid bilayer nuclear envelope. The nucleus enables an efficient and accurate transcription and replication by regulating biological macromolecules traverse. The nucleus utilizes a nuclear pore complex (NPC) to form an octagonal structure using multiple copies of 30 different types of nucleoporins (Nups). The central channel surrounded by layered inner/outer/transmembrane rings is buried in the nuclear membrane. The rings are sandwiched by cytoplasmic ring with cytoplasmic filaments and nuclear ring with nuclear basket. The rings are constructed by structured nucleoporins. To understand structure and function of NPC, integrative structural and biophysical techniques have been applied for many decades (1–4). The nucleoporins with phenylalanine-glycine sequences, called phenylalanine-glycine (FG)-nucleoporins, construct a permeability barrier to prevent passive molecular exchange between the nucleus and cytoplasm (1, 5, 6). FG-repeat regions are intrinsically disordered and likely phase separated in the central channel (7, 8). Molecules weighing over 40-kDa cannot pass through the NPC except when bound to nuclear transport receptors that are also known as the karyopherin-β family. The karyopherin-β proteins shuttle in and out of the nucleus through the NPC to carry cargo substrates, which are mainly nuclear proteins with signal sequences that can be recognized and bound by karyopherin-β proteins. Karyopherin-βs are classified into nuclear import receptors (also known as importin-βs) and nuclear export receptors (also known as exportins) based on the direction of the transport of cargos. Nuclear import receptors carry their cargo from the cytoplasm to the nucleus, while the opposite is true for the nuclear export receptors (9). Approximately 10 family members of karyopherin-βs have been identified as nuclear import receptors in humans. Generally, karyopherin-βs have an alpha solenoidal structure that is composed of ∼20 HEAT repeats (Fig. 1A). The name of HEAT is given from four structural similar proteins, Huntingtin, elongation factor 3, protein phosphatase 2A and the yeast kinase TOR1.
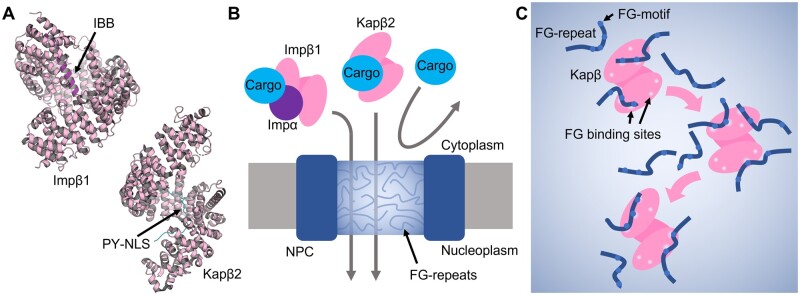
Fig. 1.
Karyopherin-βs and FG-repeats. (A) Crystal structures of importin-β (Impβ) in complex with Importin-β1 binding domain of Importin-α (IBB) (PDB ID: 1QGK) and karyopherin-β2 in complex with proline-tyrosine nuclear localization signal (PY-NLS) of hnRNPA1 (PDB ID: 2H4M). (B) Import cargos can pass through phase separated central channel exclusively with karyopherin-β. (C) Model of interactions between FG-repeats and karyopherin-β (Kapβ). Multiple weak and transient interactions dissolve phase separated FG-repeats while traversing.
Each nuclear import receptor recognizes a nuclear localization signal (NLS), which is a consistent pattern of the import cargo sequence or motif (10–13). For example, Importin-β1 (Impβ1, also known as karyopherin-β1) binds to the classical NLS (cNLS), a lysine/arginine-rich sequence, through the adapter protein importin-α (Impα). Impα binds to both the monopartite and bipartite basic clusters of the NLS. Moreover, it employs an auto-inhibitory system, that is, the importin-β binding (IBB) domain of the importin-α fills cNLS binding groove in the absence of cNLS. In contrast to Impβ1, nuclear import receptor karyopherin-β2 (Kapβ2) directly binds to its NLS, proline-tyrosine NLS (PY-NLS), which contains a C-terminal conserved proline-tyrosine sequence (14). PY-NLS consists of a basic or hydrophobic patch and a conserved arginine residue, which is followed by a proline-tyrosine sequence. The binding modes of IBB and NLS by Impβ1 and Kapβ2, respectively, have been structurally uncovered and summarized in other review articles (9, 10, 15). Not only Impα/β1 and Kapβ2 but also other karyopherin-βs that are capable of binding to multiple cargos contain conserved sequences or motifs. This broad recognition facilitates the binding and transport of a variety of cargos. The mutations in a cargo, especially in the NLS, lead to mislocalization in the cytoplasm, which cause diseases (described in the later section).
Several cargos are exclusively imported into the nucleus by specific karyopherin-βs; however, exceptions exist; for instance, histones. Histones H1, H2A–H2B complex and H3–H4 complex are mainly imported into the nucleus by Imp-β1/Importin-7 heterodimer, Importin-9 and Importin-4, respectively; however, other importins can also support the transfer (9, 16). The imported cargo is released upon binding to the small GTPase RanGTP in the nucleoplasm. Expression patterns of karyopherin-βs in the tissues or organs are summarized in the review by Kimura and Imamoto (11). They also mapped the relationship between import cargos and karyopherin-βs using the high-throughput mass spectrometry technique (13). In terms of Kapβ2, hnRNP family proteins and transcription factors are main import cargos, suggesting that karyopherin-β2 plays a key role in transcription and mRNA maturation. Expression of Kapβ2 in brain is likely important in neurogenesis and/or circadian rhythms in mammals (17). Interestingly, nuclear import of transcription factor NSY-7 by IMB-2, which is the Kapβ2 homologue in Caenorhabditis elegans, determines the olfactory neuronal cell type (18). Thus, karyopherin-β2 regulates multiple cellular systems through nuclear import of cargos (19).
Phase separation of the FG-repeats
One-third of the nucleoporins are classified as FG-nucleoporins. They have intrinsically disordered FG-repeat regions with folded domains. The folded domains mainly form rings in the nuclear membrane, and FG-repeats mainly protrude to the inside of central channel. The concentration of FG repeats in the central channel has been estimated as 10–300 mg/ml (20). Furthermore, conserved sequence patterns of FG-repeats have been identified, such as FxFG, GLFG, PxFG and SxFG. A mesh-like network of FG-nucleoporins provides a molecular sieve for facilitating selective nuclear transport (Fig. 1B) (6, 7).
The purified FG-repeat region of one of the yeast homolog of FG-nucleoporins, Nsp1, undergoes phase separation and forms hydrogels at high concentrations (8). Upon substitution of phenylalanine with serine residues, the hydrogel formation is impaired, which suggests that pi–pi interactions between phenylalanine residues are important for driving the phase separation. The saturated FG-hydrogel prevents the influx of large macromolecules, like the tetrameric red fluorescent protein (MW > 100 kDa), while karyopherin-βs can permeate the hydrogel barrier (21). In addition to Nsp1, a species-wide study of Nup98 across various organisms, including fungi, plants, insects and mammals, revealed that the purified FG-repeat region of Nup98 rapidly forms droplets through phase separation. The droplets exclude macromolecules; however, the entry of karyopherin-βs and karyopherin-β cargo protein complexes is allowed (22, 23). Many major FG-nucleoporins, including Nup62, Nup98, Nup153 and Nup214, form droplets via liquid–liquid phase separation (LLPS) (24). Thus, a permeability barrier of the NPC is formed by the FG-repeat sequence of nucleoporin undergoing phase separation. The phase state of FG-repeats has been optimized for nuclear transport receptors. Amyotrophic lateral sclerosis (ALS)-causing C9orf72-derived proline-arginine poly-dipeptides (20 repeats of PR) bind to the central channel of NPC and block nuclear transport (25). Additionally, the phase separation property of FG-repeats of nucleoporins is important for the stability and assembly of the NPC (26, 27). FG-repeats recruit both scaffold nucleoporins and FG-nucleoporins.
Cross-β polymers, continuous intermolecular β-sheets formed by the same fragment of the protein and stack perpendicular to the fibre axis, are resulted from self-templated aggregation. Phase separation and cross-β polymer formation are closely related to each other. The NQTS-rich spacer sequence of Nsp1 forms an amyloid-like cross-β structure, which is important for formation of a selective molecular sieve (28). The cross-β-forming region contains multiple aromatic residues because of FG-repeats. The cross-β structure tends to form kinked structures, which are called low-complexity aromatic-rich kinked segments (29). These findings concur with those of a recent study on the aromatic-rich cross-β structure of RNA-binding proteins under phase separation. The toxicity of cross-β structures is still unclear; however, cells could possibly utilize functional cross-β structures that are different from those in irreversible aggregates. It should be noted that in vitro phase separation properties of FG-repeats using purified proteins may differ from the central channel of NPC in vivo. In vivo analysis of phase separation of NPC is technically difficult (30). Advanced biophysical and structural biological approaches will proof the state of FG-repeats in central channel in the feature.
Nuclear import receptors pass through the phase-separated central channel of the NPC
The mechanism by which phase-separated FG-repeats facilitate karyopherin-βs has been discussed for many decades (30). The surface properties of mobile species, including karyopherin-βs and cargos, are likely to be important (31). Hydrophobic residues, cysteine, histidine and arginine residues facilitate translocation, while lysine, aspartic acid and glutamic acid residues impede translocation. By using many GFP mutants with different surface properties, it was revealed that mutants with high passage rates exhibited high affinity for phase-separated droplets formed by purified FG-repeats. Interestingly, arginine and lysine residues exhibited opposite effects. This could be attributed to the property of planar guanidinium group of arginine residues preferably make contact with phenylalanine residues via cation-π interactions compared to lysine residues. Acidic residues on molecular surface negatively contribute to pass through NPC. Although karyopherin-βs have acidic isoelectric points, acidic regions are located inside the solenoidal structure, which are utilized for binding NLS or IBB. Two hydrophobic pockets on the surface of Impβ1 binding FG-repeats were identified by X-ray crystallography (32). These findings suggest that karyopherin-βs transiently interact with FG-repeats using multiple binding sites and partially dissolve the phase-separated gel-like structure to traverse NPC (Fig. 1C). The gel-like form was self-healing after karyopherin-βs treatment (33).
Karyopherin-βs pass through a 30-nm thick NPC within 5 ms. This fast traversing may be facilitated by multivalent and weak interactions. Multiple FG units can bind multiple sites on karyopherin-βs. Global affinity (i.e. interaction between single karyopherin-β molecule and multiple FG-repeats) increases, and the dissociation rate constant decreases, thereby increasing the number of binding sites (7, 34). Thus, individual interactions between single FG unit and single FG binding site on karyopherin-βs should have a low affinity to achieve faster traversing. Although the sequence identity among karyopherin-βs is approximately 15% or less, karyopherin-βs represent spring-like solenoidal structures using HEAT repeats. The solenoidal structures of Kapβ2 slightly shrink upon binding to RanGTP or NLSs (35), and allosteric regulation and/or fluctuation may also facilitate traversing the phase-separated central channel by FG-repeats (36, 37).
Phase separation of the cargo proteins
Intrinsically disordered regions are frequently observed in the cargo proteins of karyopherin-βs, namely nuclear proteins. The reason why nuclear proteins are enriched with the intrinsically disordered regions had been largely unknown. Biological phase separation studies shed new lights on the function of intrinsically disordered region. Numerous intrinsically disordered proteins form functional membraneless organelles (also known as biomolecular condensates) through LLPS. Various membraneless organelles are found in the nucleus including nucleoli, nuclear speckles, paraspeckles and Cajal bodies (38).
RNA-binding protein Fused in sarcoma (FUS), one of the most extensively studied in the field of phase separation, is nuclear imported by Kapβ2 (39) and is essential for the formation of the nuclear body paraspeckles (40). Under normal conditions, FUS localizes in the nucleus where it plays roles in DNA repair and transcriptional regulation (41, 42). Under stress conditions, FUS is export to the cytoplasm and is recruited into the cytoplasmic membraneless organelle stress granule (43). Therefore, LLPS of FUS is important for several cellular processes. FUS is composed by N-terminus Prion-like domain (PrLD) [also known as low complexity domain or Prion-like domain (PrLD), three RGG repeats, RNA recognition motif (RRM), zinc finger (ZnF) and PY-NLS (Fig. 2A)]. Except for RRM and ZnF, FUS is composed of intrinsically disordered regions (IDRs). PY-NLS can only form alpha helix when it bound to Kapβ2 (44). FUS protein phase separation is driven by multiple weak and promiscuous interactions (45). The PrLD, which is required for FUS LLPS, forms a reversible labile cross-β polymer (46). The cross-β structure of the fibril-forming part of SYGQ-rich region was determined by solid-state NMR. The folded fibril core composed by polar residues, which is different from the highly stable cross-β structures of the pathogenic fibril including amyloid βs and α-synuclein (46). The unstructured RGG domains also mediate FUS LLPS by cation–π interactions between the arginine residues in the RGGs and the tyrosine residues in the PrLD, which governs the saturation concentration of LLPS (47, 48). The material property of the phase separated FUS droplets are further regulated by the glycines, glutamines and serines in the PrLD (47).
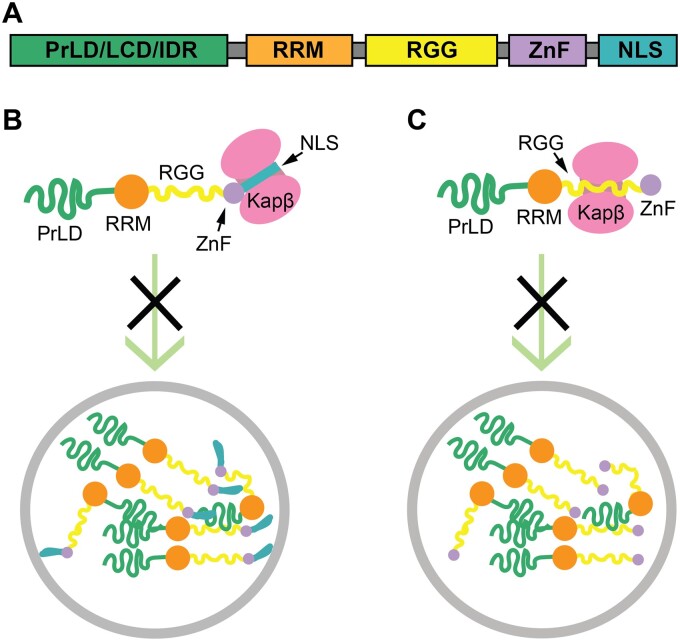
Fig. 2.
Karyopherin-βs as phase regulators. (A) Generic model of a karyopherin-β cargo protein that can undergo liquid–liquid phase separation, depicting a Prion-like Domain (PrLD), low-complexity domain (LCD), or intrinsically disordered region (IDR) and an RNA-recognition motif (RRM), a Zinc-finger domain (ZnF), an RGG/RG domain, and a nuclear localization signal (NLS). (B) Karyopherin-βs (Kapβ) can prevent the phase separation and aberrant phase transition of RBP by engaging the NLS. (C) For RBPs without NLS, karyopherin-βs can prevent their phase separation by engaging the RGG.
The other two FET proteins, EWSR1 and TAF15, which are also nuclear imported by Kapβ2 through binding of their C-terminal PY-NLS, share the domain structure and sequence features with FUS (Fig. 2A) (43). Similar to FUS, they possess the requisite sequence features to drive phase separation at low-protein concentrations and physiologically relevant salt concentrations (47). HnRNPA1 and hnRNPA2 are two other Kapβ2 cargos that can undergo LLPS. Similar to the FET proteins, hnRNPA1 and hnRNPA2 are RBPs with PrLD, RBDs (RNA-Binding domains) and PY-NLS. However, instead of a C-terminally localized basic-PY-NLSs (bPY-NLSs) which is characterized by a basic patch in the N-terminal region of the PY-NLS, hnRNPA1 and hnRNPA2 have hydrophobic-PY-NLSs (hPY-NLSs) that locate in the PrLD, which could directly mediate LLPS (14, 49, 50).
cNLS-containing karyopherin-β cargos can also undergo LLPS. For example, TDP-43, which is nuclear imported by Impα/β1 complex, forms membraneless organelles in both the nucleus and the cytoplasm (43, 51, 52). In addition to the C-terminal PrLD, LLPS of TDP-43 is also mediated by the oligomerization of N-terminal domain (53). Moreover, NLS of TDP-43 is also a PAR-Binding domain, which mediates TDP-43 LLPS thought interactions with PAR (54).
The nuclear import of several of the SR-rich proteins, including ASF/SF2 and SC35 (SRSF2), is mediated by a specific karyopherin-β, termed transportin-SR (55). The arginine/serine (RS)-rich domains in these proteins function as NLS that is important for their localization to the nucleus, as well as the nuclear speckles, which are phase separated nuclear condensates that functions in RNA splicing.
Dysregulation of phase separation in neurodegenerative diseases
While LLPS is essential for the formation of beneficial condensates, dysregulated phase separation can be detrimental. Indeed, the natural tendency of PrLD-containing RNA-binding proteins to engage in promiscuous interactions promotes LLPS and also renders them prone to aberrant phase transition. During aberrant phase transition, phase separated condensates with dynamic liquid-like property mature into a less dynamic gel-like state, and eventually forms solid-like fibrillary aggregates, which is a key pathological feature in several devastating neurodegenerative diseases (50, 56–60). For example, TDP-43 was identified as the major component of the proteinaceous inclusions present in ∼97% ALS and ∼45% FTD patients (61). Noteworthy, TDP-43 pathology has also been observed in an increasing spectrum of other neurodegenerative disorders, including Alzheimer’s disease, Huntington’s disease and Parkinson’s disease. FUS is present in the cytoplasmic aggregates of ∼1% ALS and ∼9% FTD cases (61). And in all FTD-FUS subtypes, FET (FUS, EWSR1 and TAF15) proteins were found to co-accumulate in FUS-positive cytoplasmic inclusions (62, 63). hnRNPA1 is a predominantly nuclear RBP that can mislocalize to cytoplasmic inclusions in the degenerating tissues of multisystem proteinopathy (MSP) patients (64).
Several mechanisms were proposed that could lead to dysregulation of phase transition of karyopherin cargoes in neurodegenerative diseases. First of all, genetic mutations in the PrLD can alter phase behaviour of the RBP. In fact, ALS/FTD-causing mutations are clustered in the PrLDs of TDP-43, FUS, EWSR1, TAF15, hnRNPA1 and hnRNPA2. These mutations affect different aspects of phase behaviour of these proteins (65). For example, G335D in TDP-43 increases the LLPS propensity by enhancing the α-helical forming propensity of residues 320–343 in the disordered region (66). On the other hand, G156E mutation in FUS PrLD does not change the LLPS propensity of FUS. Instead, it alters the physical properties of the condensates by promoting gelation (67). In addition, D262V mutation in hnRNPA1 and D290V in hnRNPA2 accelerates the aberrant phase transition and fibrillization of these RBPs by introduces a potent steric zipper into the PrLD (64). Second, nucleocytoplasmic transport defects, which have been observed in many neurodegenerative diseases, can lead to accumulation and alleviated concentration of karyopherin-β cargoes in the cytoplasm, which promotes phase separation and aberrant phase transition of these cargo proteins (68, 69). For FUS and EWSR1, the nucleocytoplasmic transport defects can be caused by many of the disease-causing mutations clustered in the PY-NLS (65). In addition to altering the localization of the karyopherin-β cargoes, these mutations can also alter the material state of the phase separated condensates, which further promote aberrant phase transition (44). Even in cases where no apparent mutations in RBP NLSs are observed, cytoplasmic mislocalization of karyopherin-β cargoes has been observed. For example, mislocalization and aggregation of WT TDP-43 in the cytoplasm is a pathological hallmark of C9orf72-mediated ALS and FTD, which is the most common genetic cause in both familial ALS and FTD (70, 71). C9orf72-mediated ALS and FTD are characterized by a GGGGCC (G4C2) hexanucleotide repeat expansion in the gene C9orf72, which can be translated via repeat-associated non-AUG translation into five distinct dipeptide repeats (poly-GA, -GP, -GR, -PA and -PR). Multiple evidence suggests that R-rich DPRs (i.e. poly-GR and -PR) may impair nucleocytoplasmic transport and hence cause karyopherin-β cargo mislocalization in C9-ALS/FTD to the cytoplasm and promote their LLPS and aberrant phase transition (70, 71). Finally, LLPS and aberrant phase transition of TDP-43 and other karyopherin-β cargoes can be promoted by direct binding of the R-rich DPRs to these intrinsically disordered proteins with PrLD/LCD (72).
Karyopherin-βs as a phase regulators
Phase separation of RBPs can be modulated by pH, temperature, crowding agent and salt concentration. In cell, phase separation of RBPs can be regulated by ATP, RNA, PAR and chaperone proteins, such as hsp104 and hsp70 (73). Recently, we and others reported that in addition to their canonical function as nuclear transporters, karyopherin-βs can also function as chaperones and protein disaggregases to modulate the phase separation of RBPs (43, 74–76). Because of self-association tendency, the purified RBPs were difficult to handle in solution; however, we found that the RBPs became soluble in the presence of karyophrin-βs. Then, we sought beyond the nuclear import function of karyopherin-βs. It has been shown that nuclear import factors can function as molecular chaperones to prevent the aggregation of basic nuclear proteins in the cytoplasm (77), our results further demonstrated that they can also regulate phase separation of RBPs and reverse aberrant RBP phase transition. For example, Kapβ2 can prevent the phase separation and aberrant phase transition of PY-NLS containing RBPs, including FUS, EWSR1, TAF15, hnRNPA1 and hnRNPA2 (43,76). In addition, Kapβ2 can also reverse the formation of liquid droplets, solid-like hydrogel, and fibrils of FUS, hnRNPA1 and hnRNPA2. Mechanistic studies of the chaperone and disaggregase activity of Kapβ2 against FUS phase separation revealed that tight binding between Kapβ2 and the PY-NLS is essential for its activity (Fig. 2B) (43, 76). As a result, disease-causing mutations in FUS PY-NLS that disrupt NLS-Kapβ2 binding, such as P525L and R495X, impair nuclear transport, as well as the chaperon function of Kapβ2, leading to dysregulation of FUS LLPS in cell. Kapβ2 also engages other regions across FUS, including the PrLD and RGG domains that mediate FUS LLPS (43, 76). Therefore, Kapβ2 can also modulate FUS LLPS by directly blocking residues that are important for FUS LLPS, such as tyrosines in the PrLD, and arginines in the RGG domains. Kapβ2 also weakly engages the RNA-binding domains in FUS, such as the RRM and ZnF, and competes with RNA for FUS binding. Combining with the observation that RNA concentration can regulate FUS LLPS, these results indicate that Kapβ2 could also regulate FUS LLPS by altering FUS-RNA interaction. In addition, arg-methylation in the FUS RGG also suppresses FUS LLPS and SG partitioning, and Kapβ2 has higher affinity for hypomethylated FUS (74, 75), adding another layer of regulation for FUS LLPS through post-translational modification. Interestingly, in FTD-FUS patients, arg-methylation of FUS is lost and Kapβ2 is observed in cytoplasmic FUS inclusions, indicating that the chaperone activity of Kapβ2 is impaired (74, 75).
The activity of Kapβ2 as a chaperon in cell has been demonstrated in different cellular model of FUS and hnRNPA1 proteinopathy. Kapβ2 expression prevents and reverses FUS accumulation in stress granules and increases cell viability (43). Kapβ2 expression also restores the nuclear localization of FUS, and the nuclear function of FUS in RNA processing (43). Moreover, even modest overexpression of Kapβ2 restores the liquid property of FUS RNP granule and rescues the impaired protein synthesis in axon terminals caused by hypomethylated FUS assemblies (75). In addition, in a Drosophila model of FUS-ALS, where lifespan is reduced by expressing FUS R521H in the motor neuron, co-expression of Kapβ2 suppressed FUS toxicity (43). Similar rescue activity was reported in a Drosophila model where muscle degeneration was induced by MSP-linked hnRNPA2 D290V (43). Thus, Kapβ2 can suppress RBP toxicity in diverse settings.
For RBPs with cNLS, such as TDP-43, the phase separation and aggregation can be prevented and reversed by its cognate import receptor Impα/β (43). Recent studies also demonstrated that karyopherin-βs can regulate the LLPS of arg-riched proteins without NLS. For example, Kapβ2 can still interact with ALS-causing FUS mutant FUS R495X, which lacks the PY-NLS, and inhibits the LLPS of FUS R495X (39). This interaction is mediated by the RGG2-ZnF-RGG3 segment of FUSR495X binding to the PY-NLS binding site of Kapβ2 (Fig. 2C) (39). Similarly, Kapβ2 recognizes an RG/RGG-rich region in the cold-inducible RNA-binding protein (CIRBP) and regulates the nuclear localization, phase separation and stress granule recruitment of CIRBP (78). Kapβ2 uses the overlapping sites to engage the RG/RGG region of CIRBP and FUS PY-NLS. Another R-rich region, the RSY-rich region of CIRBP is recognized by both Kapβ2 and karyopherin-β TNPO3 (transportin-SR2), which also regulates CIRBP phase separation and localization (78). Similarly, Kapβ2 or Impβ1 suppress poly(GR)-induced formation of TDP-43 condensates by sequestering poly(GR), instead of direct interacting with TDP-43 (72). Interaction of Kapβ2 and Impβ1 with R-rich DPRs also suppresses RNA-mediated condensation of poly(GR) in vitro (72). Given the highly abundant of RG/RGG rich region in human proteome and their contribution in RBP LLPS, these studies suggest that karyopherin-βs likely function broadly to regulate LLPS of RBP.
Perspective
We discussed karyopherin-βs beyond the nuclear import function. Phase separation study of biological macromolecules provided a new point of view for protein regulation. Phase regulation by karyopherin-βs is important in both passing through NPC and maintaining soluble state of cargos. karyopherin-βs can be developed as a new category of chaperon. Multiple weak interactions promote unwinding phase separated proteins. The interactions seem competing while passing through NPC as karyopherin-β and cargo complex. It remains unclear how karyopherin-βs manage the interactions. It also remains unclear whether chaperone activity for phase separated proteins is common in other karyopherin-β families. Further molecular basis study may pave the way to understand the mechanisms.
Funding
T.Y. was supported by the JSPS KAKENHI (grant number 19K16060), AMED Practical Research Project for Rare/Intractable Diseases (grant number 21ek0109558h0001) and Uehara Memorial Foundation. L.G. was supported by the Dr. Ralph and Marian Falk Medical Research Trust, Frick Foundation for ALS Research, the National Institute of General Medical Sciences (grant R35GM138109).
Conflict of Interest
None declared.
Abbreviations
- cNLS
classical NLS
- FG
phenylalanine-glycine
- FUS
fused in sarcoma
- IBB
importin-β binding
- IDRs
intrinsically disordered regions
- Impβ1
Importin-β1
- LCD
low-complexity domain
- LLPS
liquid–liquid phase separation
- MSP
multisystem proteinopathy
- NLS
nuclear localization signal
- NPC
nuclear pore complex
- Nups
nucleoporins
- PrLD
Prion-like domain.
References
- 1.Strambio-De-Castillia C., Niepel M., Rout M.P. (2010) The nuclear pore complex: bridging nuclear transport and gene regulation. Nat. Rev. Mol. Cell Biol. 11, 490–501 [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Martinez J., Rout M.P. (2012) A jumbo problem: mapping the structure and functions of the nuclear pore complex. Curr. Opin. Cell Biol. 24, 92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoelz A., Glavy J.S., Beck M. (2016) Toward the atomic structure of the nuclear pore complex: when top down meets bottom up. Nat. Struct. Mol. Biol. 23, 624–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez-Martinez J., Rout M.P. (2021) One ring to rule them all? Structural and functional diversity in the nuclear pore complex. Trends Biochem. Sci. 46, 595–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raices M., D'Angelo M.A. (2012) Nuclear pore complex composition: a new regulator of tissue-specific and developmental functions. Nat. Rev. Mol. Cell Biol. 13, 687–699 [DOI] [PubMed] [Google Scholar]
- 6.Hampoelz B., Andres-Pons A., Kastritis P., Beck M. (2019) Structure and assembly of the nuclear pore complex. Annu. Rev. Biophys. 48, 515–536 [DOI] [PubMed] [Google Scholar]
- 7.Lemke E.A. (2016) The multiple faces of disordered nucleoporins. J. Mol. Biol. 428, 2011–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frey S., Richter R.P., Gorlich D. (2006) FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science. 314, 815–817 [DOI] [PubMed] [Google Scholar]
- 9.Chook Y.M., Süel K.E. (2011) Nuclear import by karyopherin-βs: recognition and inhibition. Biochim. Biophys. Acta Mol. Cell Res. 1813, 1593–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu D., Farmer A., Chook Y.M. (2010) Recognition of nuclear targeting signals by Karyopherin-β proteins. Curr. Opin. Struct. Biol. 20, 782–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura M., Imamoto N. (2014) Biological significance of the importin-β family-dependent nucleocytoplasmic transport pathways. Traffic. 15, 727–748 [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto Y., Yamada K., Yoneda Y. (2016) Importin α: a key molecule in nuclear transport and non-transport functions. J. Biochem. 160, 69–75 [DOI] [PubMed] [Google Scholar]
- 13.Kimura M., Morinaka Y., Imai K., Kose S., Horton P., Imamoto N. (2017) Extensive cargo identification reveals distinct biological roles of the 12 importin pathways. Elife. 6, 1–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee B.J., Cansizoglu A.E., Süel K.E., Louis T.H., Zhang Z., Chook Y.M. (2006) Rules for nuclear localization sequence recognition by karyopherinβ2. Cell. 126, 543–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soniat M., Chook Y.M. (2015) Nuclear localization signals for four distinct Karyopherin-β nuclear import systems. Biochem. J. 468, 353–362 [DOI] [PubMed] [Google Scholar]
- 16.Bernardes N.E., Chook Y.M. (2020) Nuclear import of histones. Biochem. Soc. Trans. 48, 2753–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato M., Mizoro Y., Atobe Y., Fujimoto Y., Yamaguchi Y., Fustin J.-M., Doi M., Okamura H. (2011) Transportin 1 in the mouse brain: appearance in regions of neurogenesis, cerebrospinal fluid production/sensing, and circadian clock. J. Comp. Neurol. 519, 1770–1780 [DOI] [PubMed] [Google Scholar]
- 18.Alqadah A., Hsieh Y.W., Xiong R., Lesch B.J., Chang C., Chuang C.F. (2019) A universal transportin protein drives stochastic choice of olfactory neurons via specific nuclear import of a sox-2-activating factor. Proc. Natl. Acad. Sci. U S A. 116, 25137–25146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mboukou A., Rajendra V., Kleinova R., Tisné C., Jantsch M.F., Barraud P. (2021) Transportin-1: a nuclear import receptor with moonlighting functions. Front. Mol. Biosci. 8, 638149–638118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt H.B., Görlich D. (2016) Transport selectivity of nuclear pores, phase separation, and membraneless organelles. Trends Biochem. Sci. 41, 46–61 [DOI] [PubMed] [Google Scholar]
- 21.Frey S., Görlich D. (2007) A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell. 130, 512–523 [DOI] [PubMed] [Google Scholar]
- 22.Hülsmann B.B., Labokha A.A., Görlich D. (2012) The permeability of reconstituted nuclear pores provides direct evidence for the selective phase model. Cell. 150, 738–751 [DOI] [PubMed] [Google Scholar]
- 23.Schmidt H.B.R., Görlich D. (2015) Nup98 FG domains from diverse species spontaneously phase-separate into particles with nuclear pore-like permselectivity. Elife. 4, 1–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labokha A.A., Gradmann S., Frey S., Hülsmann B.B., Urlaub H., Baldus M., Görlich D. (2013) Systematic analysis of barrier-forming FG hydrogels from Xenopus nuclear pore complexes. EMBO J. 32, 204–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi K.Y., Mori E., Nizami Z.F., Lin Y., Kato M., Xiang S., Wu L.C., Ding M., Yu Y., Gall J.G., McKnight S.L. (2017) Toxic PR n poly-dipeptides encoded by the C9orf72 repeat expansion block nuclear import and export. Proc. Natl. Acad. Sci. U S A. 114, E1111–E1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onischenko E., Tang J.H., Andersen K.R., Knockenhauer K.E., Vallotton P., Derrer C.P., Kralt A., Mugler C.F., Chan L.Y., Schwartz T.U., Weis K. (2017) Natively unfolded FG repeats stabilize the structure of the nuclear pore complex. Cell. 171, 904–917.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konishi H.A., Yoshimura S.H. (2020) Interactions between non-structured domains of FG- and non-FG-nucleoporins coordinate the ordered assembly of the nuclear pore complex in mitosis. FASEB J. 34, 1532–1545 [DOI] [PubMed] [Google Scholar]
- 28.Ader C., Frey S., Maas W., Schmidt H.B., Görlich D., Baldus M. (2010) Amyloid-like interactions within nucleoporin FG hydrogels. Proc. Natl. Acad. Sci. U S A. 107, 6281–6285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes M.P., Sawaya M.R., Boyer D.R., Goldschmidt L., Rodriguez J.A., Cascio D., Chong L., Gonen T., Eisenberg D.S. (2018) Atomic structures of low-complexity protein segments reveal kinked b sheets that assemble networks. Science. 359, 698–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayama R., Rout M.P., Fernandez-Martinez J. (2017) The nuclear pore complex core scaffold and permeability barrier: variations of a common theme. Curr. Opin. Cell Biol. 46, 110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frey S., Rees R., Schünemann J., Ng S.C., Fünfgeld K., Huyton T., Görlich D. (2018) Surface properties determining passage rates of proteins through nuclear pores. Cell. 174, 202–217.e9 [DOI] [PubMed] [Google Scholar]
- 32.Bayliss R., Littlewood T., Stewart M. (2000) Structural basis for the interaction between FxFG nucleoporin repeats and importin-β in nuclear trafficking. Cell. 102, 99–108 [DOI] [PubMed] [Google Scholar]
- 33.Frey S., Görlich D. (2009) FG/FxFG as well as GLFG repeats form a selective permeability barrier with self-healing properties. EMBO J. 28, 2554–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AramburuI.V., and , Lemke E.A. (2017) Floppy but not sloppy: Interaction mechanism of FG-nucleoporins and nuclear transport receptors. Semin. Cell Dev. Biol. 68, 34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cansizoglu A.E., Chook Y.M. (2007) Conformational heterogeneity of karyopherinβ2 is segmental. Structure. 15, 1431–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshimura S.H., Kumeta M., Takeyasu K. (2014) Structural mechanism of nuclear transport mediated by importin β and flexible amphiphilic proteins. Structure. 22, 1699–1710 [DOI] [PubMed] [Google Scholar]
- 37.Yoshimura S.H., Hirano T. (2016) HEAT repeats - versatile arrays of amphiphilic helices working in crowded environments? J. Cell Sci. 129, 3963–3970 [DOI] [PubMed] [Google Scholar]
- 38.Mitrea D.M., Kriwacki R.W. (2016) Phase separation in biology; functional organization of a higher order Short linear motifs - the unexplored frontier of the eukaryotic proteome. Cell Commun. Signal. 14, 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez A., Mannen T., Çağatay T., Fujiwara A., Matsumura H., Niesman A.B., Brautigam C.A., Chook Y.M., Yoshizawa T. (2021) Mechanism of karyopherin-β2 binding and nuclear import of ALS variants FUS(P525L) and FUS(R495X). Sci. Rep. 11, 3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hennig S., Kong G., Mannen T., Sadowska A., Kobelke S., Blythe A., Knott G.J., Iyer K.S., Ho D., Newcombe E.A., Hosoki K., Goshima N., Kawaguchi T., Hatters D., Trinkle-Mulcahy L., Hirose T., Bond C.S., Fox A.H. (2015) Prion-like domains in RNA binding proteins are essential for building subnuclear paraspeckles. J. Cell Biol. 210, 529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W.Y., Pan L., Su S.C., Quinn E.J., Sasaki M., Jimenez J.C., MacKenzie I.R.A., Huang E.J., Tsai L.H. (2013) Interaction of FUS and HDAC1 regulates DNA damage response and repair in neurons. Nat. Neurosci. 16, 1383–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X., Arai S., Song X., Reichart D., Du K., Pascual G., Tempst P., Rosenfeld M.G., Glass C.K., Kurokawa R. (2008) Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 454, 126–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo L., Kim H.J., Wang H., Monaghan J., Freyermuth F., Sung J.C., O'Donovan K., Fare C.M., Diaz Z., Singh N., Zhang Z.C., Coughlin M., Sweeny E.A., DeSantis M.E., Jackrel M.E., Rodell C.B., Burdick J.A., King O.D., Gitler A.D., Lagier-Tourenne C., Pandey U.B., Chook Y.M., Taylor J.P., Shorter J. (2018) Nuclear-import receptors reverse aberrant phase transitions of RNA-binding proteins with prion-like domains. Cell. 173, 677–692.e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Z.C., Chook Y.M. (2012) Structural and energetic basis of ALS-causing mutations in the atypical proline–tyrosine nuclear localization signal of the Fused in Sarcoma protein (FUS). Proc. Natl. Acad. Sci. U S A. 109, 12017–12021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boeynaems S., Alberti S., Fawzi N.L., Mittag T., Polymenidou M., Rousseau F., Schymkowitz J., Shorter J., Wolozin B., Van Den Bosch L., Tompa P., Fuxreiter M. (2018) Protein phase separation: a new phase in cell biology. Trends Cell Biol. 28, 420–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray D.T., Kato M., Lin Y., Thurber K.R., Hung I., McKnight S.L., Tycko R. (2017) Structure of FUS protein fibrils and its relevance to self-assembly and phase separation of low-complexity domains. Cell. 171, 615–627.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J., Choi J.M., Holehouse A.S., Lee H.O., Zhang X., Jahnel M., Maharana S., Lemaitre R., Pozniakovsky A., Drechsel D., Poser I., Pappu R.V., Alberti S., Hyman A.A. (2018) A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell. 174, 688–699.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Y., Currie S.L., Rosen M.K. (2017) Intrinsically disordered sequences enable modulation of protein phase separation through distributed tyrosine motifs. J. Biol. Chem. 292, 19110–19120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin E.W., Thomasen F.E., Milkovic N.M., Cuneo M.J., Grace C.R., Nourse A., Lindorff-Larsen K., Mittag T. (2021) Interplay of folded domains and the disordered low-complexity domain in mediating hnRNPA1 phase separation. Nucleic Acids Res. 49, 2931–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Molliex A., Temirov J., Lee J., Coughlin M., Kanagaraj A.P., Kim H.J., Mittag T., Taylor J.P. (2015) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell. 163, 123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gasset-Rosa F., Lu S., Yu H., Chen C., Melamed Z., Guo L., Shorter J., Da Cruz S., Cleveland D.W. (2019) Cytoplasmic TDP-43 de-mixing independent of stress granules drives inhibition of nuclear import, loss of nuclear TDP-43, and cell death. Neuron. 102, 339–357.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu H., Lu S., Gasior K., Singh D., Vazquez-Sanchez S., Tapia O., Toprani D., Beccari M.S., Yates J.R., Da Cruz S., Newby J.M., Lafarga M., Gladfelter A.S., Villa E., Cleveland D.W. (2021) HSP70 chaperones RNA-free TDP-43 into anisotropic intranuclear liquid spherical shells. Science. 371, eabb4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang A., Conicella A.E., Schmidt H.B., Martin E.W., Rhoads S.N., Reeb A.N., Nourse A., Ramirez Montero D., Ryan V.H., Rohatgi R., Shewmaker F., Naik M.T., Mittag T., Ayala Y.M., Fawzi N.L. (2018) A single N‐terminal phosphomimic disrupts TDP‐43 polymerization, phase separation, and RNA splicing. EMBO J. 37, e97452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGurk L., Gomes E., Guo L., Mojsilovic-Petrovic J., Tran V., Kalb R.G., Shorter J., Bonini N.M. (2018) Poly(ADP-Ribose) prevents pathological phase separation of TDP-43 by promoting liquid demixing and stress granule localization. Mol. Cell. 71, 703–717.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kataoka N., Bachorik J.L., Dreyfuss G. (1999) Transportin-SR, a nuclear import receptor for SR proteins. J. Cell Biol. 145, 1145–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murakami T., Qamar S., Lin J.Q., Schierle G.S.K., Rees E., Miyashita A., Costa A.R., Dodd R.B., Chan F.T.S., Michel C.H., Kronenberg-Versteeg D., Li Y., Yang S.P., Wakutani Y., Meadows W., Ferry R.R., Dong L., Tartaglia G.G., Favrin G., Lin W.L., Dickson D.W., Zhen M., Ron D., Schmitt-Ulms G., Fraser P.E., Shneider N.A., Holt C., Vendruscolo M., Kaminski C.F., St George-Hyslop P. (2015) ALS/FTD mutation-induced phase transition of FUS liquid droplets and reversible hydrogels into irreversible hydrogels impairs RNP granule function. Neuron. 88, 678–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel A., Lee H.O., Jawerth L., Maharana S., Jahnel M., Hein M.Y., Stoynov S., Mahamid J., Saha S., Franzmann T.M., Pozniakovski A., Poser I., Maghelli N., Royer L.A., Weigert M., Myers E.W., Grill S., Drechsel D., Hyman A.A., Alberti S. (2015) A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell. 162, 1066–1077 [DOI] [PubMed] [Google Scholar]
- 58.Lin Y., Protter D.S.W., Rosen M.K., Parker R. (2015) Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol. Cell. 60, 208–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elbaum-Garfinkle S., Brangwynne C.P. (2015) Liquids, fibers, and gels: the many phases of neurodegeneration. Dev. Cell. 35, 531–532 [DOI] [PubMed] [Google Scholar]
- 60.Jawerth L., Fischer-Friedrich E., Saha S., Wang J., Franzmann T., Zhang X., Sachweh J., Ruer M., Ijavi M., Saha S., Mahamid J., Hyman A.A., Jülicher F. (2020) Protein condensates as aging Maxwell fluids. Science 370, 1317–1323 [DOI] [PubMed] [Google Scholar]
- 61.Ling S.C., Polymenidou M., Cleveland D.W. (2013) Converging mechanisms in als and FTD: disrupted RNA and protein homeostasis. Neuron. 79, 416–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.MacKenzie I.R.A., Neumann M. (2012) FET proteins in frontotemporal dementia and amyotrophic lateral sclerosis. Brain Res. 1462, 40–43 [DOI] [PubMed] [Google Scholar]
- 63.Mackenzie I.R.A., Rademakers R., Neumann M. (2010) TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 9, 995–1007 [DOI] [PubMed] [Google Scholar]
- 64.Kim H.J., Kim N.C., Wang Y.D., Scarborough E.A., Moore J., Diaz Z., MacLea K.S., Freibaum B., Li S., Molliex A., Kanagaraj A.P., Carter R., Boylan K.B., Wojtas A.M., Rademakers R., Pinkus J.L., Greenberg S.A., Trojanowski J.Q., Traynor B.J., Smith B.N., Topp S., Gkazi A.S., Miller J., Shaw C.E., Kottlors M., Kirschner J., Pestronk A., Li Y.R., Ford A.F., Gitler A.D., Benatar M., King O.D., Kimonis V.E., Ross E.D., Weihl C.C., Shorter J., Taylor J.P. (2013) Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 495, 467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harrison A.F., Shorter J. (2017) RNA-binding proteins with prion-like domains in health and disease. Biochem. J. 474, 1417–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Conicella A.E., Dignon G.L., Zerze G.H., Schmidt H.B., D'Ordine A.M., Kim Y.C., Rohatgi R., Ayala Y.M., Mittal J., Fawzi N.L. (2020) TDP-43 α-helical structure tunes liquid–liquid phase separation and function. Proc. Natl. Acad. Sci. U S A. 117, 5883–5894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rhine K., Makurath M.A., Liu J., Skanchy S., Lopez C., Catalan K.F., Ma Y., Fare C.M., Shorter J., Ha T., Chemla Y.R., Myong S. (2020) ALS/FTLD-linked mutations in FUS glycine residues cause accelerated gelation and reduced interactions with wild-type FUS. Mol. Cell. 80, 666–681.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim H.J., Taylor J.P. (2017) Lost in transportation: nucleocytoplasmic transport defects in ALS and other neurodegenerative diseases. Neuron. 96, 285–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jovičić A., Paul J.W., Gitler A.D. (2016) Nuclear transport dysfunction: a common theme in amyotrophic lateral sclerosis and frontotemporal dementia. J. Neurochem. 138, 134–144 [DOI] [PubMed] [Google Scholar]
- 70.Renton A.E., Majounie E., Waite A., Simón-Sánchez J., Rollinson S., Gibbs J.R., Schymick J.C., Laaksovirta H., van Swieten J.C., Myllykangas L., Kalimo H., Paetau A., Abramzon Y., Remes A.M., Kaganovich A., Scholz S.W., Duckworth J., Ding J., Harmer D.W., Hernandez D.G., Johnson J.O., Mok K., Ryten M., Trabzuni D., Guerreiro R.J., Orrell R.W., Neal J., Murray A., Pearson J., Jansen I.E., Sondervan D., Seelaar H., Blake D., Young K., Halliwell N., Callister J.B., Toulson G., Richardson A., Gerhard A., Snowden J., Mann D., Neary D., Nalls M.A., Peuralinna T., Jansson L., Isoviita V.M., Kaivorinne A.L., Hölttä-Vuori M., Ikonen E., Sulkava R., Benatar M., Wuu J., Chiò A., Restagno G., Borghero G., Sabatelli M., Heckerman D., Rogaeva E., Zinman L., Rothstein J.D., Sendtner M., Drepper C., Eichler E.E., Alkan C., Abdullaev Z., Pack S.D., Dutra A., Pak E., Hardy J., Singleton A., Williams N.M., Heutink P., Pickering-Brown S., Morris H.R., Tienari P.J., Traynor B.J; ITALSGEN Consortium. (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., Boxer A.L., Baker M., Rutherford N.J., Nicholson A.M., Finch N.A., Flynn H., Adamson J., Kouri N., Wojtas A., Sengdy P., Hsiung G.-Y.R., Karydas A., Seeley W.W., Josephs K.A., Coppola G., Geschwind D.H., Wszolek Z.K., Feldman H., Knopman D.S., Petersen R.C., Miller B.L., Dickson D.W., Boylan K.B., Graff-Radford N.R., Rademakers R. (2011) Article Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron 72, 245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hutten S., Usluer S., Bourgeois B., Simonetti F., Odeh H.M., Fare C.M., Czuppa M., Hruska-Plochan M., Hofweber M., Polymenidou M., Shorter J., Edbauer D., Madl T., Dormann D. (2020) Nuclear import receptors directly bind to arginine-rich dipeptide repeat proteins and suppress their pathological interactions. Cell Rep. 33, 108538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Darling A.L., Shorter J. (2021) Combating deleterious phase transitions in neurodegenerative disease. Biochim. Biophys. Acta. Mol. Cell Res. 1868, 118984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hofweber M., Hutten S., Bourgeois B., Spreitzer E., Niedner-Boblenz A., Schifferer M., Ruepp M.D., Simons M., Niessing D., Madl T., Dormann D. (2018) Phase separation of FUS is suppressed by its nuclear import receptor and arginine methylation. Cell. 173, 706–719.e13 [DOI] [PubMed] [Google Scholar]
- 75.Qamar S., Wang G.Z., Randle S.J., Ruggeri F.S., Varela J.A., Lin J.Q., Phillips E.C., Miyashita A., Williams D., Ströhl F., Meadows W., Ferry R., Dardov V.J., Tartaglia G.G., Farrer L.A., Kaminski Schierle G.S., Kaminski C.F., Holt C.E., Fraser P.E., Schmitt-Ulms G., Klenerman D., Knowles T., Vendruscolo M., St George-Hyslop P. (2018) FUS phase separation is modulated by a molecular chaperone and methylation of arginine cation-π interactions. Cell. 173, 720–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshizawa T., Ali R., Jiou J., Fung H.Y.J., Burke K.A., Kim S.J., Lin Y., Peeples W.B., Saltzberg D., Soniat M., Baumhardt J.M., Oldenbourg R., Sali A., Fawzi N.L., Rosen M.K., Chook Y.M. (2018) Nuclear import receptor inhibits phase separation of FUS through binding to multiple sites. Cell. 173, 693–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jäkel S., Mingot J.-M., Schwarzmaier P., Hartmann E., Görlich D. (2002) Importins fulfil a dual function as nuclear import receptors and cytoplasmic chaperones for exposed basic domains. EMBO J. 21, 377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bourgeois B., Hutten S., Gottschalk B., Hofweber M., Richter G., Sternat J., Abou-Ajram C., Göbl C., Leitinger G., Graier W.F., Dormann D., Madl T. (2020) Nonclassical nuclear localization signals mediate nuclear import of CIRBP. Proc. Natl. Acad. Sci. USA. 117, 8503–8514 [DOI] [PMC free article] [PubMed] [Google Scholar]