Abstract
Various lineages of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) have contributed to prolongation of the Coronavirus Disease 2019 (COVID-19) pandemic. Several non-synonymous mutations in SARS-CoV-2 proteins have generated multiple SARS-CoV-2 variants. In our previous report, we have shown that an evenly uneven distribution of unique protein variants of SARS-CoV-2 is geo-location or demography-specific. However, the correlation between the demographic transmutability of the SARS-CoV-2 infection and mutations in various proteins remains unknown due to hidden symmetry/asymmetry in the occurrence of mutations. This study tracked how these mutations are emerging in SARS-CoV-2 proteins in six model countries and globally. In a geo-location, considering the mutations having a frequency of detection of at least 500 in each SARS-CoV-2 protein, we studied the country-wise percentage of invariant residues. Our data revealed that since October 2020, highly frequent mutations in SARS-CoV-2 have been observed mostly in the Open Reading Frame (ORF) 7b and ORF8, worldwide. No such highly frequent mutations in any of the SARS-CoV-2 proteins were found in the UK, India, and Brazil, which does not correlate with the degree of transmissibility of the virus in India and Brazil. However, we have found a signature that SARS-CoV-2 proteins were evolving at a higher rate, and considering global data, mutations are detected in the majority of the available amino acid locations. Fractal analysis of each protein's normalized factor time series showed a periodically aperiodic emergence of dominant variants for SARS-CoV-2 protein mutations across different countries. It was noticed that certain high-frequency variants have emerged in the last couple of months, and thus the emerging SARS-CoV-2 strains are expected to contain prevalent mutations in the ORF3a, membrane, and ORF8 proteins. In contrast to other beta-coronaviruses, SARS-CoV-2 variants have rapidly emerged based on demographically dependent mutations. Characterization of the periodically aperiodic nature of the demographic spread of SARS-CoV-2 variants in various countries can contribute to the identification of the origin of SARS-CoV-2.
Keywords: SARS-CoV-2, Invariant residues, Mutations, Relative frequency, Aperiodically periodic
1. Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the causative agent of the Coronavirus Disease (COVID-19) (Pedersen and Ho, 2020; Setti et al., 2020; Domingo et al., 2020). SARS-CoV-2 has spread rapidly and has evolved prolonging pandemic and precarious clinical entity (Health, 2020; Tapper and Asrani, 2020). Since the beginning of the pandemic, SARS-CoV-2 has increasingly accumulated various mutations leading to patterns of genomic diversity (van Dorp et al., 2020; Zhou et al., 2020). The wide SARS-CoV-2 variations were scattered across the various geo-locations, and it can underlie geographically specific etiological effects (Mercatelli and Giorgi, 2020). It was expected that these mutations could be of use to monitor the spread of the virus, and to identify sites putatively under selection as SARS-CoV-2 potentially adapts to its new human host. SARS-CoV-2 may be evolving towards higher transmissibility as it may not yet fully adapt to its human host. The most plausible mutations under putative natural selection are those which have emerged repeatedly and independently (van Dorp et al., 2020). It was reported that 198 sites in the SARS-CoV-2 genome appear to have already undergone recurrent, independent mutations (van Dorp et al., 2020). Various SARS-CoV-2 missense mutations are the key evolving factors affecting the infectivity, and virulence, and pathogenicity of the virus (Mercatelli and Giorgi, 2020). Several SARS-CoV-2 variants have significantly strengthened the infectivity (Chen et al., 2020). It was previously reported that the rate of SARS-CoV-2 mutations are relatively low compared to other RNA viruses, such as influenza virus (Kupferschmidt, 2020; Khan et al., 2020; Gómez-Carballa et al., 2020). The low SARS-CoV-2 mutation rate might relate to its proofreading ability, which is a unique embedded function of SARS-CoV-2 (Romano et al., 2020; Ogando et al., 2020). Thus far, although several mutations have been detected, SARS-CoV-2 seem not to be drifting antigenically (Yuan et al., 2021; Williams and Burgers, 2021). However, the mechanism of SARS-CoV-2 evolution or developing gain of function variations have remained unclear (Wang et al., 2021). A non-uniform mutation pattern in the viral proteins was recently reported, which has further accelerated the discussion on the question of the origin of SARS-CoV-2 (Hassan et al., 2021b).
The rapidly evolving data on mutations and various strains of SARS-CoV-2 makes it vulnerable to firmly assert whether SARS-CoV-2 results from a zoonotic emergence or from an accidental escape from a laboratory (Sallard et al., 2021; Pipes et al., 2021; Nadeau et al., 2021; Seyran et al., 2021). This issue of origin needs to be resolved because it has important consequences on the risk/benefit balance of human interactions with ecosystems, on intensive breeding of wild and domestic animals, on some laboratory practices and on scientific policy and bio-safety regulations (Sallard et al., 2021). Despite these recent investigations, several issues related to the evolutionary patterns and origin of the COVID-19 pandemic remain to be fully characterized (Liu et al., 2020, 2019; Domingo, 2021a, Domingo, 2021b; Grassberger, 1993). No direct correlation was observed in the mutation pattern of SARS-CoV-2 from the infection rate in the first and second waves of COVID-19 (Ko et al., 2021; Lv et al., 2020; Kumar et al., 2020).
In this study, the potential embedded mutation pattern of the spike (S), envelope (E), membrane (M) (Domingo, 2021b), nucleocapsid (N), ORF3a, ORF6, ORF7a, ORF7b, ORF8, and ORF10 proteins of SARS-CoV-2 are analyzed in six model countries viz. USA, UK, Brazil, Germany, India, South Africa (SA), and globally.
2. Data specifications and methods
2.1. Data
The majority of publicly available SARS-CoV-2 genomic sequences were sourced from GISAID, NCBI,and CNGB. The SARS-CoV-2 sequence data was taken from the GISAID database (Shu and McCauley, 2017). Mutation data with their respective details were collected from the CoVal database. In this study, we focused on the S, M, E, N, ORF3a, ORF6, ORF7a, ORF7b, ORF8, and ORF10 proteins of SARS-CoV-2. We also considered mutations within the protein of our interest from all geo-locations available in the CoVal database. In particular, a set of six model countries, the USA, UK, South Africa, India, Germany, and Brazil was selected.
2.2. Methods
2.2.1. Mutation search using the CoVal database
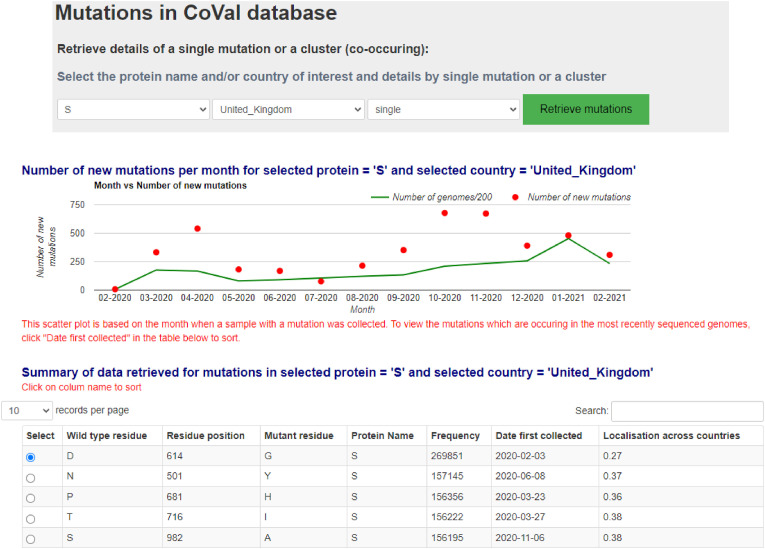
Single mutation details were retrieved from the CoVal database by searching by the name of the model country and the SARS-CoV-2 protein of interest. For example, details of all single mutations in the SARS-CoV-2 S protein from the UK were retrieved, of which a snapshot is presented in Figure 1 .
Fig. 1.
Details of a single mutation retrieval from the CoVal database.
Likewise, for other model countries and SARS-CoV-2 proteins, details of single mutations were retrieved. Prior to proceeding into the result section, some definitions are recalled and redefined for easy reading.
3. Results
Four different classes were defined on the date of the first detection and frequency of mutations in the SARS-CoV-2 proteins of our interest. Class-I contains all mutations detected in the proteins of SARS-CoV-2 across the world. Class-II contains only those mutations with a frequency more than or equal to 500 (a reasonably good frequency of a mutation detected in a geo-location) in SARS-CoV-2 in affected patients worldwide. Mutations that were detected after October 2020 belong to Class-III. Mutations with a frequency larger than 500 since October 2020 are members of Class-IV.
3.1. Invariant residues of SARS-CoV-2 proteins
Amino acid residues where no mutations were detected are termed “invariant residues”. From the CoVal database, we first found distinct residue positions of mutations, and the total number of invariant residues (r) of each type. Furthermore, the percentage of invariant residues in each protein of length (l) was determined using the formula l−r × 100.
Class-I. The percentages of invariant residues (Class-I) in SARS-CoV-2 proteins in various countries are listed in Table 1 .
Table 1.
Percentage of invariant residues (considering all mutations so far accounted) in the SARS-CoV-2 proteins in various countries.
| Countries | S | E | M | N | ORF10 | ORF3a | ORF6 | ORF7a | ORF7b | ORF8 |
|---|---|---|---|---|---|---|---|---|---|---|
| Across all countries | 14.69 | 6.67 | 24.32 | 10.74 | 0 | 1.1 | 0 | 0 | 0 | 0 |
| USA | 32.52 | 29.34 | 37.39 | 19.33 | 5.26 | 5.1 | 6.56 | 2.48 | 2.32 | 2.48 |
| UK | 33.54 | 29.34 | 42.8 | 19.57 | 7.9 | 7.63 | 6.55 | 1.65 | 0 | 4.13 |
| Germany | 61.35 | 72 | 69.82 | 46.06 | 44.74 | 24.37 | 39.35 | 71.07 | 34.99 | 27.27 |
| India | 80.83 | 80 | 88.29 | 69.21 | 57.89 | 55.64 | 77.05 | 66.11 | 55.81 | 60.33 |
| Brazil | 87.51 | 84 | 92.8 | 80.67 | 78.95 | 71.28 | 83.6 | 73.55 | 76.74 | 73.55 |
| SA | 90.49 | 90.67 | 94.14 | 82.82 | 84.21 | 77.82 | 91.8 | 90.08 | 83.72 | 82.64 |
Considering all mutations with amino acid changes in all available geo-locations, it was observed that, except for the SARS-CoV-2 structural proteins, the ORF proteins (ORF3a, ORF6, ORF7a, ORF7b, ORF8, and ORF10) possessed mutations at every residue position of the respective protein. On the other hand, it was found that an increasing order of the percentage of invariant residues in the structural proteins of SARS-CoV-2 turned out to be E (6.67) < N (10.74) < S (14.69) < M (24.32). In other words, the highest and lowest number of mutations were detected in the E and M proteins, respectively. Across six countries, the highest and lowest number of invariant residues were found in the M and ORF3a proteins, respectively. It was noted that the highest frequency of invariant residues in the E among all proteins was observed in Germany. Among all proteins, the highest number of mutations was detected in the ORF3a proteins in Germany, India, Brazil, and South Africa, whereas in the USA and UK, ORF7b possessed the highest number of mutations. Notably, it was observed that each protein in SARS-CoV-2 possessed an almost similar number of mutations in the USA and UK. Among six countries, the least amount of mutations across all proteins was found in COVID-19 patients from South Africa, whereas in the USA, the highest number of mutations in SARS-CoV-2 were detected.
An increasing-order (decreasing-order) of the six geo-locations based on the invariant residues (mutations) across all proteins was found in the USA < UK < Germany < India < Brazil < SA (reverse order).
Class-II. The percentages of invariant residues (Class-II) over the SARS-CoV-2 proteins in various countries are listed in Table 2 .
Table 2.
Percentage of invariant residues (considering all mutations having a frequency >500) in the SARS-CoV-2 proteins in various countries.
| Countries | S | E | M | N | ORF10 | ORF3a | ORF6 | ORF7a | ORF7b | ORF8 |
|---|---|---|---|---|---|---|---|---|---|---|
| Across all countries | 89.94 | 92 | 94.14 | 80.19 | 78.95 | 72 | 91.8 | 81 | 83.72 | 72.72 |
| USA | 96.54 | 97.34 | 99.55 | 92.6 | 94.74 | 92.36 | 100 | 98.35 | 97.67 | 89.26 |
| UK | 97.1 | 100 | 99.1 | 93.8 | 94.74 | 89.1 | 98.36 | 96.7 | 93.02 | 93.39 |
| Germany | 98.74 | 100 | 100 | 97.61 | 97.37 | 97.45 | 100 | 100 | 100 | 98.35 |
| India | 99.92 | 100 | 100 | 99.28 | 100 | 99.63 | 100 | 100 | 100 | 100 |
| Brazil | 99.76 | 100 | 100 | 99.04 | 100 | 100 | 98.36 | 100 | 100 | 100 |
| SA | 99.53 | 98.67 | 100 | 99.28 | 100 | 99.27 | 100 | 100 | 100 | 100 |
While we considered only those mutations with a frequency of at least 500, it was observed that the increasing order of the percentage of invariant residues in the SARS-CoV-2 structural proteins was N (80.19) < S (89.94 )< E (92) < M (94.14). Therefore, the highest and lowest frequency of mutations were detected in the N and M proteins, respectively. Across six geo-locations, the ORF3a and M proteins possessed the highest and lowest amount of amino acid changing mutations, respectively. It was further noticed that each SARS-CoV-2 protein possessed almost the same number of mutations in the USA and UK. Among six countries, the least number of mutations across all proteins is found in COVID-19 patients from the UK, whereas the highest number of mutations were detected in India.
A decreasing order of the total number of mutations in proteins at the six geo-locations was the UK > USA > Germany > SA > Brazil > India (from highest to lowest). In other words, the highest number of different mutations across all these proteins were detected in the UK, and the lowest number of mutations was observed in India. In India, Brazil, and SA, no mutation with a frequency of at least 500 (originating in these countries) was detected in any SARS-CoV-2 protein, except one or two reported most frequent deleterious mutations D614G in the S protein and Q57H in the ORF3a protein. Note that in Germany, the S, N, ORF10, ORF3a, and ORF8 proteins possessed a couple of mutations, whereas the E, M, ORF6, ORF7a, and ORF7b proteins did not have any mutations originating from Germany, with a frequency of more than 500.
Class-III. The percentages of invariant residues (Class-III) in the SARS-CoV-2 proteins in various countries are presented in Table 3 .
Table 3.
Percentage of invariant residues (considering all mutations since October 2020) in the SARS-CoV-2 proteins in various countries.
| Countries | S | E | M | N | ORF10 | ORF3a | ORF6 | ORF7a | ORF7b | ORF8 |
|---|---|---|---|---|---|---|---|---|---|---|
| Across all countries | 24.51 | 22.67 | 33.78 | 15.03 | 2.63 | 4 | 1.64 | 0.826 | 4.65 | 0.826 |
| USA | 56.72 | 56 | 64.41 | 51.55 | 18.42 | 33.1 | 32.79 | 26.45 | 13.95 | 28.92 |
| UK | 53.18 | 61.33 | 57.2 | 49.4 | 55.26 | 28.73 | 16.39 | 9.92 | 23.25 | 23.14 |
| Germany | 68.66 | 82.67 | 77.48 | 67.3 | 60.53 | 40.73 | 47.54 | 39.67 | 41.86 | 37.2 |
| India | 97.49 | 100 | 97.75 | 97.14 | 92.1 | 96 | 98.36 | 93.39 | 95.35 | 91.73 |
| Brazil | 94.58 | 94.67 | 97.75 | 94.03 | 97.37 | 90.18 | 91.8 | 90.9 | 95.35 | 88.43 |
| SA | 97.25 | 100 | 98.65 | 94.99 | 97.37 | 92.37 | 98.36 | 97.52 | 93.02 | 95.87 |
It was observed that the SARS-CoV-2 ORF3a protein was no longer a hotspot for dominant mutations. From October 2020 until June 2021, the M and ORF8 proteins owned the lowest (66.22%) and the highest (99.174%) number of mutations, respectively, across the world. Notably, in the past, the ORF3a protein possessed the highest number of mutations (Bianchi et al., 2021; Hassan et al., 2020). Currently, it seems that ORF3a mutations in the USA, UK, and elsewhere are relatively rare (less than 10%). Since October 2020, the highest number of mutations have been detected in the ORF3a protein in SA. In the UK, Germany, India, and SA, the E protein had the lowest frequency of mutations, whereas the M protein possessed several mutations amounting to the highest frequency among others in the USA and Brazil. The highest percentage of mutations were detected in the ORF8 protein, since October 2020 in Germany, India, and Brazil. The highest frequency of mutations in the USA and UK were observed in the ORF7a and ORF7b proteins, respectively.
An increasing order of the six geo-locations based on the variability of mutations in SARS-CoV-2 proteins (from October 2020 until June 2021) was India < SA < Brazil < Germany < USA < UK.
Class-IV. The percentages of invariant residues (Class-IV) in SARS-CoV-2 proteins in various countries are listed in Table 4 .
Table 4.
Percentage of invariant residues (considering all mutations since October 2020 having a frequency of >500) in the SARS-CoV-2 proteins in various countries.
| Countries | S | E | M | N | ORF10 | ORF3a | ORF6 | ORF7a | ORF7b | ORF8 |
|---|---|---|---|---|---|---|---|---|---|---|
| Across all countries | 95.68 | 96 | 98.2 | 94.51 | 92.1 | 90.91 | 96.72 | 95.04 | 88.37 | 88.43 |
| USA | 99.76 | 98.67 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| UK | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Germany | 99.6 | 100 | 100 | 98.8 | 97.37 | 100 | 100 | 100 | 100 | 100 |
| India | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Brazil | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| SA | 99.53 | 98.67 | 100 | 99.76 | 97.37 | 99.27 | 100 | 100 | 100 | 100 |
Considering all mutations having a frequency of more than 500, from October 2020 until June 2021, it was observed that the ORF7b and ORF8 proteins owned the highest frequency of various mutations, whereas the M protein contained the lowest number of mutations. In fact, all four structural proteins S, E, M, and N contain more than 94% of invariant residues. No mutations that had a frequency of more than 500 since October 2020 in the UK, India, and Brazil were found in any of the aforementioned SARS-CoV-2 proteins. In South Africa, the proteins S, E, N, ORF3a, and ORF10 owned a couple of mutations with a frequency of more than 500 detected since October 2020. The S, N, and ORF10 proteins possessed a couple of mutations of this kind in Germany, whereas only in the S and E proteins, a few mutations of this type were noticed.
3.2. Hidden self-organized self-similarity in frequency of SARS-CoV-2 mutations
The degree of fractality, fractal dimension (FD), of the normalized factor time series (NFTS) for each SARS-CoV-2 protein in a given model country was quantified (Table 5 ). The FD revealed any hidden self-organized self-similar patterns (if any) in the frequency of emerging mutations in each protein (Pilgrim and Taylor, 2018; Sánchez-Granero et al., 2012). Here the NFTS is defined as follows:
Table 5.
Fractal dimension of the normalized factor time series.
| Countries | S | E | M | N | ORF3a | ORF6 | ORF7a | ORF7b | ORF8 | ORF10 |
|---|---|---|---|---|---|---|---|---|---|---|
| All | 1.9336 | 1.7811 | 1.9167 | 1.9351 | 1.9272 | 1.916 | 1.9158 | 1.9396 | 1.9216 | 1.6707 |
| UK | 1.9496 | 1.603 | 1.9131 | 1.9352 | 1.9146 | 1.899 | 1.923 | 1.8826 | 1.9352 | 1.6439 |
| India | 1.7112 | 1.1699 | 1.2521 | 1.7539 | 1.7603 | 1.0925 | 1.4771 | 1.179 | 1.7549 | 1.179 |
| USA | 1.9484 | 1.8502 | 1.9333 | 1.9308 | 1.9271 | 1.9302 | 1.9232 | 1.9376 | 1.9156 | 1.9771 |
| SA | 1.6752 | 0.6491 | 1 | 1.585 | 1.585 | 0.5144 | 1.4594 | 0.6491 | 1.179 | 0.5144 |
| Germany | 1.8155 | 1.179 | 1.5319 | 1.8507 | 1.7984 | 1.7618 | 1.7379 | 1.3315 | 1.7297 | 1.1377 |
| Brazil | 1.8119 | 1.585 | 1.0925 | 1.7655 | 1.6705 | 1.585 | 1.2849 | 1.3219 | 1.5502 | 0.6491 |
Normalized factor time series: For each mutation (m) in a given SARS-CoV-2 protein, a normalized factor is determined based on the presence of the mutation in various geo-locations (countries). Normalized factor NF m is defined as
Here, NG m and TG m denote the number of genomes with this specific mutation (m) in this country, and the total number of genomes with this mutation (m) worldwide, respectively. It varies from 0 to 1. Here the normalized factor 0 denotes the spreading of the mutations uniformly across various geo-locations, whereas 1 denotes the detection of the mutation in a single geo-location (Gelly et al., 2011). A series of detected mutations in a given protein lead to anNFTS.
From Table 5 it was concluded that the FD of NFTS for SARS-CoV-2 proteins ranged from 0.5144 to 1.9771. This wide range of the FD depicts the hidden non-uniform distribution of the frequency in various countries. We observed that the lowest FD for each SARS-CoV-2 protein was detected in South Africa, whereas the highest fractality for all proteins were either found in the USA or UK. These model countries were ranked in increasing order based on the FD for each protein (Table 6 ).
Table 6.
Increased order (from top to bottom row) of countries based on the fractal dimension (FD) of the normalized factor time series. Identical rankings of countries based on the FD of normalized factor time series (NFTS) are highlighted in red, ochre, and green colors. Fractality of NFTS that did not follow any strict ordering are in black.
The ranking of countries based on the FD of NFTS for the S and N proteins was identical (Table 6). Likewise, an identical ranking was noticed for the SARS-CoV-2 M and ORF3a proteins. Also, the country-ranking based on the FD of NFTS for the ORF6 and ORF7b proteins were the same. Notably, the fractality of NFTS for the E and ORF7a proteins in India, Germany and Brazil did not follow any strict order.
3.3. The emergence of new SARS-COV-2 variants
To understand the emergence of new strains and which SARS-CoV-2 strain dominates, we looked at the dates of the detection of various mutations in each SARS-CoV-2 protein and their respective frequencies (in logarithmic scale) (Table 7 ).
Table 7.
Correlation of the date a mutation was first detected and its frequency (log).
| Countries | S | E | M | N | ORF10 | ORF3a | ORF6 | ORF7a | ORF7b | ORF8 |
|---|---|---|---|---|---|---|---|---|---|---|
| All | −0.20 | 0.01 | −0.25 | −0.31 | −0.21 | −0.25 | −0.22 | −0.14 | −0.07 | −0.27 |
| UK | −0.46 | −0.41 | −0.49 | −0.47 | −0.55 | −0.50 | −0.43 | −0.36 | −0.47 | −0.47 |
| India | −0.22 | −0.13 | −0.22 | −0.42 | −0.24 | −0.35 | 0.29 | −0.20 | −0.37 | −0.47 |
| USA | −0.51 | −0.39 | −0.47 | −0.54 | −0.55 | −0.53 | −0.46 | −0.45 | −0.50 | −0.55 |
| SA | −0.82 | −0.99 | 0.14 | −0.39 | 0.15 | −0.68 | 0.72 | 0.30 | −0.39 | −0.38 |
| Germany | −0.34 | −0.39 | −0.25 | −0.33 | −0.71 | −0.18 | −0.32 | −0.36 | −0.40 | −0.47 |
| Brazil | −0.09 | −0.06 | −0.22 | −0.22 | 0.48 | −0.31 | −0.49 | −0.37 | 0.36 | 0.11 |
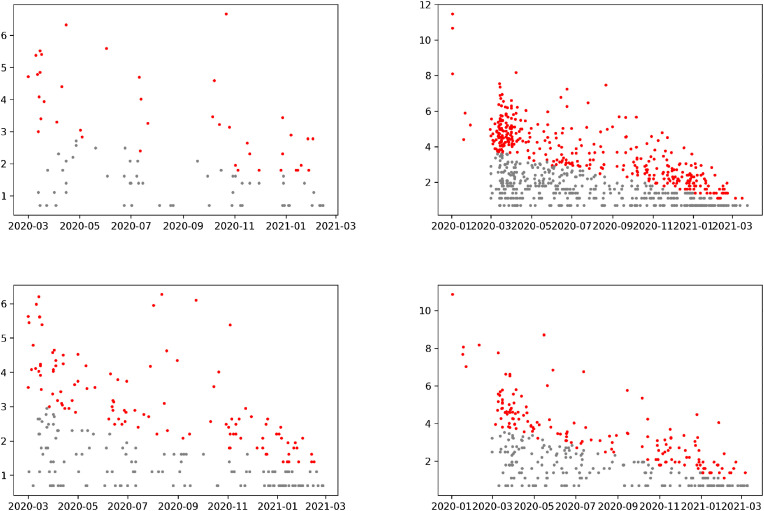
A negative correlation signifies that the early detected mutations since the beginning of the COVID-19 pandemic, are more dominant in the present scenario (Lee Rodgers and Nicewander, 1988). We noticed precisely that the pattern in the USA, UK, and Germany was that each correlation coefficient was negative (Mukaka, 2012). The similar pattern was seen in South Africa and Brazil. We detected newer ORF3a mutations that were dominant in South Africa, and some new ORF10 mutations were seen. The trend was that the newer ORF10 mutations were more dominant in Brazil. Mutations in the E and S proteins in Brazil also showed the emergence of new strains. To understand the emergence of newer variants, we plotted the date and frequencies (log) of mutations in the SARS-CoV-2 E, M, ORF3a, and ORF8 proteins (Figure 2). Outlier points (in red) in Fig. 2, Fig. 3 showed the emergence of newer mutations in the four SARS-CoV-2 proteins.
Fig. 2.
Date and frequencies (log) of mutations in E, M, ORF3a, and ORF8 proteins in the USA.
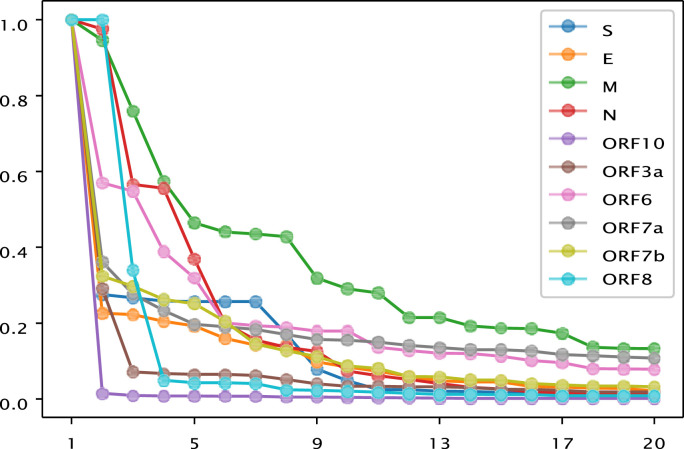
Fig. 3.
Relative frequency of the first 20 dominant mutations of S, E, M, N, ORF3a, ORF6, ORF7a, ORF7b, ORF8, and ORF10 proteins of SARS-CoV-2.
The density of outlier points in the ORF3a protein implies the emergence of new variants of SARS-CoV-2 with a significant number of missense mutations. These new SARS-CoV-2 variants would also contain various mutations in the M and ORF8 proteins, whereas it was observed that mutations in the E protein were no longer dominant.
The first twenty dominant (with regards to frequency) mutations worldwide are plotted for all proteins in Figure 3 .
It seems that the M protein had more versatile dominant mutants globally (Fig. 3). For the ORF10 protein, only one dominant mutant P10 was identified globally. Two mutants (S24, R52) dominate globally for the ORF8 protein. For the E and S proteins, there was one dominant mutant in each protein and many sub-dominant mutants. The N protein showed an intermediary pattern between the M and the ORF8, ORF10, ORF6, and ORF7a proteins and it also falls into the common pattern of a few dominant mutants and a dozen or so sub-dominant mutants.
4. Discussion and concluding remarks
We studied and tracked how mutant variants emerged in the SARS-CoV-2 S, E, M, N, ORF10, ORF3a, ORF6, ORF7a, ORF7b, ORF8, and ORF10 proteins from six model countries. While we considered all SARS-CoV-2 mutations since the beginning of the COVID-19 pandemic, it was noticed that the least number of invariant residues in each SARS- CoV-2 protein was observed in South Africa. On the other hand, prevalent missense mutations were detected in the UK and USA. Considering the mutations in each protein having a frequency of more than 500, it was found that the increasing order of the model countries based on the percentage of invariant residues is UK < USA < Germany < SA < Brazil < India. Interestingly, India had the lowest number of protein variants with high frequent mutations (frequency of more than 500). Furthermore, the ample protein variations in the USA and UK were ascribed to the presence of highly frequent mutations in each SARS-CoV-2 protein. Since October 2020, high-frequent mutations in SARS-CoV-2 have been found only in ORF7b and ORF8, worldwide. No such highly frequent mutations in any of the SARS-CoV-2 proteins were found in the UK, India, and Brazil, which does not seem to correlate with the transmutability of the virus in India and Brazil. Not every high-frequency mutation necessarily affects the life cycle of the virus.Neither ORF7b nor ORF8 proteins are essential for the virus life cycle as previously shown for the S and/or ORF3a proteins (Hassan et al., 2021a, b). Of note, the majority of Manaus (Brazil) citizens had been reinfected with the P.1 strain bearing these mutations, which may offer a further capability of immune escape (Faria et al., 2021). The combination of the E484Kand Δ140 mutations led to a complete abolition of neutralizing antibodies. Based on these observations the questionis whether there is any ethnic correlation with SARS-CoV-2 infection? Ethnic/racial disparities in the SARS-CoV-2 infection, incidence, and COVID-19 severity and deaths have been reported (Hollis et al., 2021; McCoy et al., 2020; Vahidy et al., 2020; Shoily et al., 2021; Sze et al., 2020). In the context of correlation between ethnic/racial origin and SARS-CoV-2 infections the interaction between the S protein and the ACE2-solute carrier family 6-member 19 (SLC6A19) dimer was investigated applying a quantitative dynamics cross-correlation matrix (Gupta et al., 2020). Forty-seven potential functional missense variants were identified from genomic databases within ACE2/SLC6A19/TMPRSS2, which justified genomic enrichment studies in COVID-19 patients. Although the variants occurred at ultra-low frequency, two ACE2 non-coding variants (rs4646118 and rs143185769) found in 9% of African Americans may be involved in the regulation of ACE2 expression and may contribute to an enhanced risk of COVID-19. Additionally, studies have demonstrated that ACE2 expression levels relate to the rs2285666 polymorphism significantly affecting SARS-CoV-2 susceptibility in Asian and European populations (Asselta et al., 2020; Srivastava et al., 2020). Moreover, it was discovered that SARS-CoV-2 cell entry depended on polymorphism in the ACE2, TMPRSS2, TMPRSS11A, cathepsin L (CTSL), and elastase (ELANE) genes in American, African, European, and Asian populations (Vargas-Alarcón et al., 2020).
Furthermore, it was observed that the percentage of invariant residues was inversely proportional to the FD of NFTS of any SARS-CoV-2 protein. In other words, the FD near 2 of an NFTS associated witha SARS-CoV-2 protein signifies wide variations of the protein. So, the widest variations in each SARS-CoV-2 protein variant were observed in the USA and UK since October 2020. On the contrary, the second wave of the COVID-19 infection rate has seen a slight upward trend in Brazil and India, quite significantly. The emerging SARS-CoV-2 strains are expected to contain prevalent mutations in the ORF3a, M, and ORF8 proteins (Table 6). We have found that SARS-CoV-2 proteins were evolving at a high rate considering global data mutations detected in the majority of the available amino acid locations. It was found that the most prominent variants across the globe have one mutation in all proteins. Fractal analysis of NFTS of each protein showed a periodically aperiodic emergence of dominant variants for SARS-CoV-2 protein mutations across various countries. This hidden periodically aperiodic phenomenon of mutations has a strong similarity with forest-fire models(Grassberger, 1993, Mukaka, 2012), where periodic emergence of fire happens after a brief period of non-activity (Drossel and Schwabl, 1992). This possibly distances SARS-CoV-2 from other beta-coronaviruses. Furthermore, it was noticed that there is a negative correlation between the frequency of a variant and when it was first recorded in country-wise data. This signifies that older variants are by and large more dominant, although there were many notable exceptions, and certain variants have quickly become dominant in the last couple of months.
The origin of SARS-CoV-2 has continued to receive much attention even after almost two years since the outbreak. Recombinant events and inevitable mutations keep developing during the pandemic (Danchin and Timmis, 2020). As a consequence of Muller's ratchet, mutations will deplete the genome in its cytosine content, which leads to attenuation of the virus (Muller, 1964). However, this process is counteracted by recombination, which can occur when different coronaviruses or mutants/variants co-infect the same host, creating an enormous evolutionary landscape for viruses to explore (Joffrin et al., 2020). It has been postulated that SARS-CoV-2 might have been active well before the outbreak in Wuhan and either through zoonosis or accidental release from a laboratory, mutations in the SARS-CoV-2 S protein dramatically increased its transmissibility leading to the COVID-19 pandemic (Platto et al., 2021). For this reason and to limit the emergence and impact on the pandemic, it is of utmost importance to evaluate SARS-CoV-2 mutants/variants in the light of the origin of SARS-CoV-2 (Otto et al., 2021).
In conclusion, the periodically aperiodic nature of the spread of mutant SARS-CoV-2 proteins is a unique feature of SARS-CoV-2 among beta-coronaviruses, which can contribute to the identification of the origin of SARS-CoV-2.
Authors’ contributions
SSH designed the study. SSH, PB, KL, and VNU contributed to the implementation of the research, to the analysis of the results. SSH and PB wrote the initial draft of the manuscript. SSH, KL, VNU, EMR, PPC, ASA, GKA, AAAA, TMAE, DB, PA, KT, MT, AL, GC, and SPS reviewed and edited. GP, BDU, WBC, and NGB provided constructive reviews and suggestions. All authors read the final version and approve.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We gratefully acknowledge the authors from laboratories responsible for obtaining the specimens and submitting sequence data, shared via the GISAID Initiative, on which this research is based.
References
- Asselta R., Paraboschi E.M., Mantovani A., Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging (N Y) 2020;12:10087. doi: 10.18632/aging.103415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M., Borsetti A., Ciccozzi M., Pascarella S. SARS-CoV-2 ORF3a: mutability and function. Int. J. Biol. Macromol. 2021;170:820–826. doi: 10.1016/j.ijbiomac.2020.12.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wang R., Wang M., Wei G.W. Mutations strengthened SARS-CoV-2 infectivity. J. Mol. Biol. 2020;432:5212–5226. doi: 10.1016/j.jmb.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danchin A., Timmis K. SARS-CoV-2 variants: relevance for symptom granularity, epidemiology, immunity (herd, vaccines), virus origin and containment? Environ. Microbiol. 2020;22:2001–2006. doi: 10.1111/1462-2920.15053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo J.L. 2021. SARS-CoV-2/COVID-19: Natural or Laboratory Origin? Environ. Res. 201, 111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo J.L. What We Know and What We Need to Know about the Origin of SARS-CoV-2. Environ Res. 2021;200 doi: 10.1016/j.envres.2021.111785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo J.L., Marqués M., Rovira J. Influence of airborne transmission of SARS-CoV-2 on COVID-19 pandemic. a review. Environ. Res. 2020;188:109861. doi: 10.1016/j.envres.2020.109861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorp L., Richard D., Tan C.C., Shaw L.P., Acman M., Balloux F. No evidence for increased transmissibility from recurrent mutations in SARS-CoV-2. Nat. Commun. 2020;11:1–8. doi: 10.1038/s41467-020-19818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossel B., Schwabl F. Self-organized critical forest-fire model. Phys. Rev. Lett. 1992;69:1629. doi: 10.1103/PhysRevLett.69.1629. [DOI] [PubMed] [Google Scholar]
- Faria N.R., Mellan T.A., Whittaker C., Claro I.M., Candido D.d.S., Mishra S., Crispim M.A., Sales F.C., Hawryluk I., McCrone J.T., et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021;372:815–821. doi: 10.1126/science.abh2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelly J.C., Joseph A.P., Srinivasan N., de Brevern A.G. iPBA: A tool for protein structure comparison using sequence alignment strategies. Nucleic Acids Res. 2011;39:W18–W23. doi: 10.1093/nar/gkr333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Carballa A., Bello X., Pardo-Seco J., Martinón-Torres F., Salas A. Mapping genome variation of SARS-CoV-2 worldwide highlights the impact of COVID-19 super-spreaders. Genome Res. 2020;30:1434–1448. doi: 10.1101/gr.266221.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassberger P. On a self-organized critical forest-fire model. J. Phys. Math. Gen. 1993;26:2081. [Google Scholar]
- Gupta R., Charron J., Stenger C.L., Painter J., Steward H., Cook T.W., Faber W., Frisch A., Lind E., Bauss J., et al. SARS-CoV-2 (COVID-19) structural and evolutionary dynamicome: insights into functional evolution and human genomics. J. Biol. Chem. 2020;295:11742–11753. doi: 10.1074/jbc.RA120.014873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan S.S., Pal Choudhury P., Basu P., Jana S.S. Molecular conservation and differential mutation on ORF3a gene in Indian SARS-CoV-2 genomes. Genomics. 2020;112:3226–3237. doi: 10.1016/j.ygeno.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan S.S., Kodakandla V., Redwan E.M., Lundstrom K., Choudhury P.P., Mohamed Abd El-Aziz T., Takayama K., Kandimalla R., Lal A., Serrano-Aroca Á., et al. bioRxiv; 2021. An Issue of Concern: Unique Truncated ORF8 Protein Variants of SARS-CoV-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan S.S., Kodakandla V., Redwan E.M., Lundstrom K., Pal Choudhury P., Serrano-Aroca Á., Kumar Azad G., Aljabali A.A.A., Palú G., Mohamed Abd El-Aziz T, et al. Non-uniform aspects of SARS-CoV-2 intraspecies evolution reopen questions on its origin. Preprints. 2021 doi: 10.20944/preprints202106.0472.v1. 2021060472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health T.L.P. COVID-19 puts societies to the test. The Lancet. Publ. Health. 2020;5:e235. doi: 10.1016/S2468-2667(20)30097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis N.D., Li W., Van Dyke M.E., Njie G.J., Scobie H.M., Parker E.M., Penman-Aguilar A., Clarke K.E. Racial and ethnic disparities in incidence of SARS-CoV-2 infection, 22 US states and DC, January 1–October 1, 2020. Emerg. Infect. Dis. 2021;27:1477. doi: 10.3201/eid2705.204523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffrin L., Goodman S.M., Wilkinson D.A., Ramasindrazana B., Lagadec E., Gomard Y., Le Minter G., Dos Santos A., Schoeman M.C., Sookhareea R., et al. Bat coronavirus phylogeography in the Western Indian Ocean. Sci. Rep. 2020;10:1–11. doi: 10.1038/s41598-020-63799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.I., Khan Z.A., Baig M.H., Ahmad I., Farouk A.E., Song Y.G., Dong J.J. Comparative genome analysis of novel coronavirus (SARS-CoV-2) from different geographical locations and the effect of mutations on major target proteins: an in silico insight. PloS One. 2020;15 doi: 10.1371/journal.pone.0238344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko K., Nagashima S., E B., Ouoba S., Akita T., Sugiyama A., Ohisa M., Sakaguchi T., Tahara H., Ohge H., et al. Molecular characterization and the mutation pattern of SARS-CoV-2 during first and second wave outbreaks in Hiroshima, Japan. PloS One. 2021;16 doi: 10.1371/journal.pone.0246383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Verma H., Singhvi N., Sood U., Gupta V., Singh M., Kumari R., Hira P., Nagar S., Talwar C., et al. Comparative genomic analysis of rapidly evolving SARS-CoV-2 reveals mosaic pattern of phylogeographical distribution. mSystems. 2020;5 doi: 10.1128/mSystems.00505-20. e00505–e00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt K. 2020. The pandemic virus is slowly mutating. but does it matter? [DOI] [PubMed] [Google Scholar]
- Lee Rodgers J., Nicewander W.A. Thirteen ways to look at the correlation coefficient. Am. Statistician. 1988;42:59–66. [Google Scholar]
- Liu P., Chen W., Chen J.P. Viral metagenomics revealed Sendai virus and Coronavirus infection of Malayan pangolins (Manis javanica) Viruses. 2019;11:979. doi: 10.3390/v11110979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Jiang J.Z., Wan X.F., Hua Y., Li L., Zhou J., Wang X., Hou F., Chen J., Zou J., et al. Are pangolins the intermediate host of the 2019 novel coronavirus (SARS-CoV-2)? PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L., Li G., Chen J., Liang X., Li Y. Vol. 11. Front. Microbiol. 11; 2020. Comparative Genomic Analyses Reveal Specific Mutation Pattern between Human Coronavirus SARS-CoV-2 and Bat-CoV RaTG13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy J., Wambier C.G., Vano-Galvan S., Shapiro J., Sinclair R., Ramos P.M., Washenik K., Andrade M., Herrera S., Goren A. Racial variations in COVID-19 deaths may be due to androgen receptor genetic variants associated with prostate cancer and androgenetic alopecia. Are anti-androgens a potential treatment for COVID-19? J. Cosmet. Dermatol. 2020;19:1542–1543. doi: 10.1111/jocd.13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercatelli D., Giorgi F.M. Geographic and genomic distribution of SARS-CoV-2 mutations. Front. Microbiol. 2020;11:1800. doi: 10.3389/fmicb.2020.01800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaka M.M. A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012;24:69–71. [PMC free article] [PubMed] [Google Scholar]
- Muller H.J. The relation of recombination to mutational advance. Mutat. Res. Fund Mol. Mech. Mutagen. 1964;1:2–9. doi: 10.1016/0027-5107(64)90047-8. [DOI] [PubMed] [Google Scholar]
- Nadeau S.A., Vaughan T.G., Scire J., Huisman J.S., Stadler T. The origin and early spread of SARS-CoV-2 in Europe. Proc. Natl. Acad. Sci. Unit. States Am. 2021;118 doi: 10.1073/pnas.2012008118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogando N.S., Zevenhoven-Dobbe J.C., van der Meer Y., Bredenbeek P.J., Posthuma C.C., Snijder E.J. The enzymatic activity of the NSP14 exoribonuclease is critical for replication of MERS-CoV and SARS-CoV-2. J. Virol. 2020;94 doi: 10.1128/JVI.01246-20. e01246–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto S.P., Day T., Arino J., Colijn C., Dushoff J., Li M., Mechai S., Van Domselaar G., Wu J., Earn D.J., et al. The origins and potential future of SARS-CoV-2 variants of concern in the evolving COVID-19 pandemic. Curr. Biol. 2021;31:R918–R929. doi: 10.1016/j.cub.2021.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S.F., Ho Y.C. SARS-CoV-2: A storm is raging. J. Clin. Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgrim I., Taylor R. IntechOpen; 2018. Fractal Analysis of Time-Series Data Sets: Methods and Challenges, in: Fractal Analysis. [Google Scholar]
- Pipes L., Wang H., Huelsenbeck J.P., Nielsen R. Assessing uncertainty in the rooting of the SARS-CoV-2 phylogeny. Mol. Biol. Evol. 2021;38:1537–1543. doi: 10.1093/molbev/msaa316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platto S., Wang Y., Zhou J., Carafoli E. History of the COVID-19 pandemic: origin, explosion, worldwide spreading. Biochem. Biophys. Res. Commun. 2021;538:14–23. doi: 10.1016/j.bbrc.2020.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano M., Ruggiero A., Squeglia F., Maga G., Berisio R. A structural view of SARS-CoV-2 RNA replication machinery: RNA synthesis, proofreading and final capping. Cells. 2020;9:1267. doi: 10.3390/cells9051267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallard E., Halloy J., Casane D., Decroly E., van Helden J. Tracing the origins of SARS-CoV-2 in coronavirus phylogenies: a review. Environ. Chem. Lett. 2021:1–17. doi: 10.1007/s10311-020-01151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Granero M., Fernández-Martinez M., Trinidad-Segovia J. Introducing fractal dimension algorithms to calculate the hurst exponent of financial time series. The European Physical Journal B. 2012;85:1–13. [Google Scholar]
- Setti L., Passarini F., De Gennaro G., Barbieri P., Perrone M.G., Borelli M., Palmisani J., Di Gilio A., Torboli V., Fontana F., et al. SARS-CoV-2 RNA found on particulate matter of Bergamo in northern Italy: first evidence. Environ. Res. 2020;188:109754. doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyran M., Pizzol D., Adadi P., El-Aziz T.M., Hassan S.S., Soares A., Kandimalla R., Lundstrom K., Tambuwala M., Aljabali A.A., et al. Questions concerning the proximal origin of SARS-CoV-2. J. Med. Virol. 2021;93:1204. doi: 10.1002/jmv.26478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoily S.S., Ahsan T., Fatema K., Sajib A.A. Disparities in COVID-19 severities and casualties across ethnic groups around the globe and patterns of ACE2 and PIR variants. Infect. Genet. Evol. 2021;92:104888. doi: 10.1016/j.meegid.2021.104888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y., McCauley J. Gisaid: global initiative on sharing all influenza data–from vision to reality. Euro Surveill. 2017;22:30494. doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Bandopadhyay A., Das D., Pandey R.K., Singh V., Khanam N., Srivastava N., Singh P.P., Dubey P.K., Pathak A., et al. Genetic association of ACE2 rs2285666 polymorphism with COVID-19 spatial distribution in India. Front. Genet. 2020;11:1163. doi: 10.3389/fgene.2020.564741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze S., Pan D., Nevill C.R., Gray L.J., Martin C.A., Nazareth J., Minhas J.S., Divall P., Khunti K., Abrams K.R., et al. EClinicalMedicine; 2020. Ethnicity and Clinical Outcomes in COVID-19: a Systematic Review and Meta-Analysis; p. 100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper E.B., Asrani S.K. COVID-19 pandemic will have a long-lasting impact on the quality of cirrhosis care. J. Hepatol. 2020;73:441–445. doi: 10.1016/j.jhep.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahidy F.S., Nicolas J.C., Meeks J.R., Khan O., Pan A., Jones S.L., Masud F., Sostman H.D., Phillips R., Andrieni J.D., et al. Racial and ethnic disparities in SARS-CoV-2 pandemic: analysis of a COVID-19 observational registry for a diverse us metropolitan population. BMJ open. 2020;10 doi: 10.1136/bmjopen-2020-039849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Alarcón G., Posadas-Sánchez R., Ramırez-Bello J. Variability in genes related to sars-cov-2 entry into host cells (ace2, tmprss2, tmprss11a, elane, and ctsl) and its potential use in association studies. Life Sci. 2020;260:118313. doi: 10.1016/j.lfs.2020.118313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang D., Zhang L., Sun W., Zhang Z., Chen W., Zhu A., Huang Y., Xiao F., Yao J., et al. Intra-host variation and evolutionary dynamics of SARS-CoV-2 populations in COVID-19 patients. Genome Med. 2021;13:1–13. doi: 10.1186/s13073-021-00847-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T.C., Burgers W.A. SARS-CoV-2 evolution and vaccines: cause for concern? The Lancet Respiratory Medicine. 2021;9:333–335. doi: 10.1016/S2213-2600(21)00075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M., Huang D., Lee C.C.D., Wu N.C., Jackson A.M., Zhu X., Liu H., Peng L., van Gils M.J., Sanders R.W., et al. Structural and functional ramifications of antigenic drift in recent SARS-CoV-2 variants. Science. 2021;373:818–823. doi: 10.1126/science.abh1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]