Abstract
COVID-19 restrictions have led to an unprecedented global hiatus in anthropogenic activities, providing a unique opportunity to assess human impact on biological systems. Here, we describe how a national network of acoustic tracking receivers can be leveraged to assess the effects of human activity on animal movement and space use during such global disruptions. We outline variation in restrictions on human activity across Australian states and describe four mechanisms affecting human interactions with the marine environment: 1) reduction in economy and trade changing shipping traffic; 2) changes in export markets affecting commercial fisheries; 3) alterations in recreational activities; and 4) decline in tourism. We develop a roadmap for the analysis of acoustic tracking data across various scales using Australia's national Integrated Marine Observing System (IMOS) Animal Tracking Facility as a case study. We illustrate the benefit of sustained observing systems and monitoring programs by assessing how a 51-day break in white shark (Carcharodon carcharias) cage-diving tourism due to COVID-19 restrictions affected the behaviour and space use of two resident species. This cessation of tourism activities represents the longest break since cage-diving vessels started day trips in this area in 2007. Long-term monitoring of the local environment reveals that the activity space of yellowtail kingfish (Seriola lalandi) was reduced when cage-diving boats were absent compared to periods following standard tourism operations. However, white shark residency and movements were not affected. Our roadmap is globally applicable and will assist researchers in designing studies to assess how anthropogenic activities can impact animal movement and distributions during regional, short-term through to major, unexpected disruptions like the COVID-19 pandemic.
Keywords: Acoustic telemetry, Coronavirus, IMOS, National network, Monitoring, White shark, Wildlife tourism, Yellowtail kingfish
1. Introduction
The influence of human activity on natural ecosystems has become so pervasive that the epoch of overwhelming human influence has come to be known as the Anthropocene (Crutzen, 2006). Anthropogenic stressors have been identified across almost every ecosystem, including the marine environment, with putative impacts on the functional diversity of marine organisms (e.g. megafauna; Pimiento et al., 2020), dramatic changes in marine biogeography (Darling and Carlton, 2018), and a need to revise marine conservation targets in the face of climate change (Rilov et al., 2020). Yet, while there is strong evidence of anthropogenic stress, identifying and quantifying causative human impacts with the intention of developing appropriate mitigation strategies is extremely difficult given the almost overwhelming pace at which change is occurring (but see Nabe-Nielsen et al., 2018). As a result, there is a high degree of inference in attribution of different stressors on ecosystems, with varying degrees of confidence (Stock et al., 2018). The tragic circumstances of the spread of the novel zoonotic coronavirus, SARS-CoV-2, and associated COVID-19 disease have led to many and varied restrictions on human activity to curtail the spread of the virus. This disruption is unprecedented, with restrictions being enacted globally and affecting all socioeconomic sectors (Diffenbaugh et al., 2020; Le Quéré et al., 2020). Given this sudden but variable pause in many human activities (now termed ‘Anthropause’; Rutz et al., 2020) and the existence of long-term observing programs, there is the potential to apply a Before-After-Control-Impact approach to measure the effects of different anthropogenic activities on the marine environment (Conner et al., 2016).
Quantifying animal responses to human activities in the marine environment can be challenging due to the inherent difficulties of observing animals that spend their entire lives underwater, often in remote locations, and across extended time-periods. This can be particularly difficult for species undertaking large-scale movements over broad distances (e.g. Heupel et al., 2015), as regional studies are unlikely to detect changes occurring at a larger spatial scale than their specific study. However, comprehensive national and international ocean observing and animal tracking networks have been developed and deployed in many parts of the world over the last 15–20 years (Brodie et al., 2018; Cowley et al., 2017; Iverson et al., 2019; Reubens et al., 2019). These networks have been specifically designed to detect changes in animal movement behaviour in the face of changing oceans and to collect time-series over a range of spatial scales. The strength of these networks to provide long-term observations is illustrated in this period of COVID-19 restrictions during which anthropogenic activities have been restricted, since data collection has continued, enabling the research community to assess whether and how much animal movement behaviour has been affected.
In 2007, Australia initiated the Integrated Marine Observing System (IMOS, imos.org.au) Animal Tracking Facility to establish a permanent array of acoustic receivers around the country and provide a central national database to collate datasets and enable researchers to investigate individual behaviour across a broad range of taxa (Brodie et al., 2018; Hoenner et al., 2018). Here, we showcase the potential for the IMOS Animal Tracking network to measure changes in animal movement behaviour during COVID-19 restrictions. We first provide an overview of COVID-19 restrictions in Australia and describe how these have affected human activity across several industry sectors related to the marine environment. We then discuss how these changes can affect the movement behaviour of marine organisms and how these potential impacts may be assessed using different experimental designs. Finally, we use data obtained from the IMOS Animal Tracking Facility to determine whether the pause in wildlife tourism caused by COVID-19 restrictions affected the residency of white sharks (Carcharodon carcharias) and yellowtail kingfish (Seriola lalandi) at a site where regular cage-diving tourism has taken place for over 20 years.
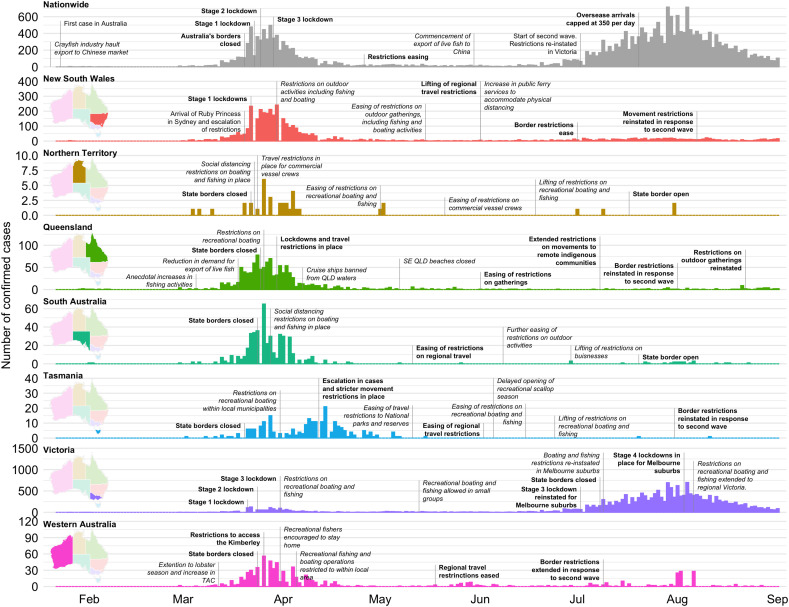
2. COVID-19 impact on marine activities in Australia
The impacts of COVID-19 affected Australia later than in many other parts of the world and initial restrictions were comparatively short-lived. Infections were largely driven by returned travellers and the risk of further community transmission was effectively controlled through enforced quarantine following entry into the country (Fig. 1 ). Light restrictions on gathering in social groups commenced in early March 2020. On the 22nd March, Public Health Orders placed heavier restrictions on gathering and movement in most jurisdictions. These controls remained in place until the 12th May 2020 when some of the national restrictions eased. This March 23–May 12 2020 period represents the core “lockdown” period for Australia, as all non-essential human movement was prohibited and remote work and study conditions were encouraged, where appropriate. During this period, several state border closures were also implemented throughout Australia to limit the spread of the virus through interstate travel from New South Wales and Victoria, where most of the infections occurred. Controls were progressively lifted through June and into July as the nation emerged from the first wave of infections. Most Australian states have since relaxed restrictions, with a focus on basic social distancing and limitations on crowd density in shops and businesses (Fig. 1). However, Victoria entered a second wave of infections in mid-June, driven by community transmission. This jurisdiction has subsequently re-enacted restrictions on movement and trade. Adjoining borders with other states were also closed.
Fig. 1.
Timeline of confirmed COVID-19 cases and subsequent restrictions on marine-related human activities within each Australian state and nationally. Policies and restrictions relating to social distancing and human movement are highlighted in bold text, while restrictions affecting marine activities are italicised. Data obtained from the Grattan coronavirus announcements tracker and Zheng et al. (2020).
Not surprisingly, the Public Health Orders and associated restrictions curtailed human activities that interacted with marine and estuarine environments. Changes in anthropogenic pressure on marine resources occurred through both direct (e.g. reduced shipping activities) and indirect (e.g. lower number of tourists in the region) impacts, with most changes in human movement observed during the core lockdown period. Altered human interaction with the marine environment occurred through several mechanisms encompassing: 1) a general contraction in global economies and reduction in trade leading to reduced shipping traffic; 2) a decline in export markets for Australian fisheries, which may have contributed to a reduction in commercial fishing activity; 3) a general reduction in recreational activities linked to restricted human movement; and 4) a reduction in tourism, particularly to regional areas where tourism-based economies precipitate periodic peaks of interaction with the marine environment.
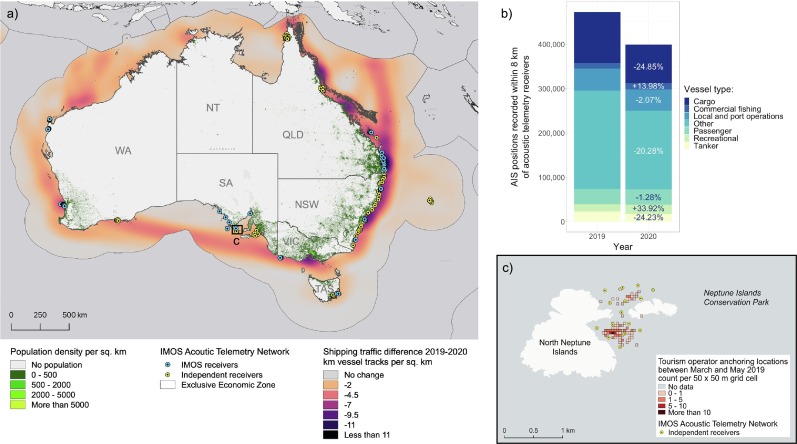
The trend of de-globalisation is likely to have accelerated in response to the COVID-19 pandemic, contributing to concomitant declines in international economic trade and investment (Nicola et al., 2020; Tokic, 2020). Countries reliant on global trade of goods manufactured in China and elsewhere were impacted when lockdowns disrupted supply chains, ceasing manufacturing activities and imports, which resulted in a reduction in shipping traffic. Nearly 44.3% of the global ocean and 77.5% of 21 national jurisdictions showed a decrease in traffic density during April 2020, with changes in marine traffic attributed to policy actions related to COVID-19 restrictions on human mobility and a reduction of consumer demand on food and trade (March et al., 2020). To quantify changes in shipping traffic around Australia during this period, we used Automatic Identification System (AIS) vessel tracking data downloaded from operations.amsa.gov.au. While the greatest impact in Australia was observed from March 2020, we extended the analysis period from January 2020 when China, Australia's largest trade partner accounting for 26% of goods and services (dfat.gov.au/geo/china/Pages/china-country-brief#:~:text=China%20is%20Australia's%20largest%20two,per%20cent%20year%20on%20year), alerted the world of COVID-19 cases. AIS data were converted to track data using vessel ID and time tags. Kernel density analysis was used to calculate km of AIS tracks per km2 using a kernel function to fit a smoothly tapered surface using 10 km grid cells and a 200 km search radius to identify broad patterns in shipping traffic across the exclusive economic zone. A difference raster map was calculated between January–June 2019 and 2020 with negative values indicating a decrease in shipping traffic (Fig. 2a). The most notable reduction in shipping traffic was on major shipping routes in the east and southern coasts and of vessel activity surrounding major ports. To provide a measure for change in human activities in relation to the IMOS Animal Tracking network, we also assessed AIS detections within 8 km of receivers in the IMOS Animal Tracking network. The 8 km radius was selected based on a study showing that cetaceans located 7–8 km from a vessel could experience sound pressure levels above the threshold for the onset of behavioural disturbance (Cominelli et al., 2020). Although arbitrary and likely to affect the range of species tagged in the IMOS Animal Tracking network in different ways, we consider this an acceptable proxy considering the limited knowledge available on vessel impacts and given the objective was to document change in human activity across the receiver network. However, we also did a sensitivity analysis to test how much changing the radius to 5 km or 15 km affected the outcomes (supplementary material Fig. S1). A 16% reduction in AIS detections was observed between 2019 and 2020 within 8 km of acoustic receivers, and this varied across vessel class. The most notable change in defined classes was observed in Cargo (−25%), Tankers (−24%) and Other vessel types (−20%), representing ~75% of AIS detections within 8 km of the IMOS receiver network (Fig. 2b). In comparison, Local and port operations (i.e. tugs, tenders, pilot vessels, enforcement, diving, dredging operations) and Passenger class vessels showed similar activities across 2019 and 2020 (−2% and −1% respectively). The sensitivity analysis showed that overall reduction in shipping activity varied from −11% (with 15 km radius) to −16% (8 km radius), with a reduction of 14% for the 5 km radius. The AIS data do not capture the full extent of vessel traffic within proximity to receivers as not all vessel classes are required to carry AIS units. This is particularly relevant for fishing and recreational vessels and may under-represent human activities for these sectors.
Fig. 2.
a) Map of the Australian IMOS Acoustic Tracking network during the COVID-19 pandemic in relation to population density and difference in vessel traffic between 2019 and 2020. Blue and yellow circles indicate the location of IMOS and researcher-owned acoustic tracking receivers, respectively. b) Changes in Automatic Identification System vessel detections within 8 km of receivers in the IMOS Animal Tracking network between January–June 2019 and 2020. Information is provided for the top six vessel classes and other classes. c) Map of North Neptune Islands, in South Australia, where white shark (Carcharodon carcharias) cage-diving tourism takes place. Yellow dots represent location of acoustic receivers; coloured raster cells show the 2019 distribution of cage-diving operators anchoring locations. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
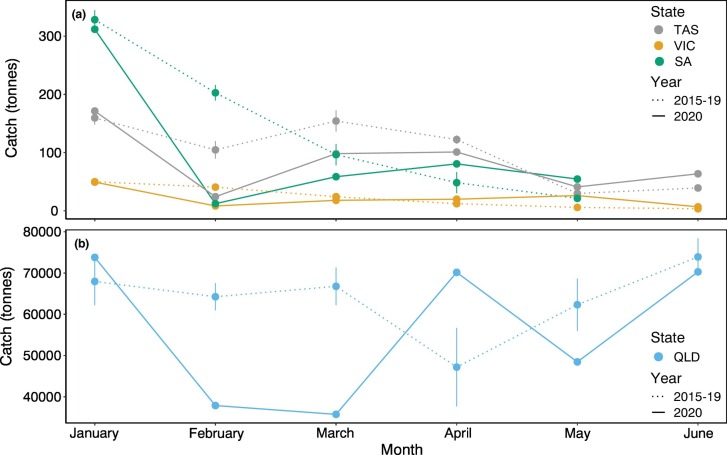
Declines in global seafood trade combined with reduced demand and ability to freight produce overseas led to decreases in the export market of some marine species caught in Australia. Southern rock lobster (Jasus edwardii) catches decreased in February and March 2020 compared to monthly averages of 2015–19 for Tasmania, Victoria, and South Australia (and April for Tasmania), with subsequent increases in May and June (Fig. 3a). Southern rock lobsters from these jurisdictions are almost exclusively exported overseas, mainly to China, Hong Kong, and Japan (85–100%), and marketed as a luxury food item (Plagányi et al., 2018). We attribute the decrease in catches in February and March to reduced demand as this was the height of the COVID-19 pandemic in China which also coincided with the Chinese New Year when demand is highest. In Queensland, coral trout (Plectropomus spp.) caught through the Queensland Reef Line Fishery were also lower in February and March 2020 compared to 2019. However, the Feb–Mar 2020 catches (~37 t) were not unusual compared to monthly catch fluctuations recorded over the preceding five years (~15–104 t, State of Queensland Department of Agriculture Fisheries, 2020) (Fig. 3b). Export of Queensland coral trout was initially hindered by limited freight flights and logistical challenges to coordinate landing of the catches within Indigenous declared areas. However, commercial fisheries were seen in Queensland as an essential service and were less affected by restrictions than in other states (T Roberts, pers. comm.).
Fig. 3.
(a) Southern rock lobster (Jasus edwardii) catches (tonnes) from Tasmania (TAS), Victoria (VIC), and South Australia (SA); and (b) Coral trout (Plectropomus spp.) catches (tonnes) from Queensland (QLD) by month from January to June. Data shown are for 2020 and five-year 2015–2019 mean (±standard error) monthly catches. Data were obtained from the Victorian Fisheries Authority (vfa.vic.gov.au/commercial-fishing/rock-lobster/interactive-stock-assessment-report), the Tasmanian Department of Primary Industries, Parks, Water and Environment (DPIPWE; dpipwe.tas.gov.au), the South Australian Research and Development Institute (SARDI; pir.sa.gov.au/research), and the State of Queensland, Department of Agriculture, Fisheries (fishnet.fisheries.qld.gov.au/Content/Public/PublicRegister.aspx, licenced under a Creative Commons Attribution 3.0 Australia creativecommons.org/licenses/by/3.0/au).
Restrictions on gathering size and travel between regions greatly impacted recreational activities. Popular Sydney beaches were closed in March and most states restricted access to national parks as well as recreational boating and fishing to uphold physical distancing regulations. Changes in human activity on waterways and marine environments were anticipated to have resulted in reduced vessel noise (e.g. jet skis, ski boats) (Depellegrin et al., 2020; Thomson and Barclay, 2020) and catch of marine species, particularly in states where recreational fishing was not allowed. In Queensland, recreational boating was only permitted if fishing for sustenance (i.e. deemed a component of the food supply), with required adherence to physical distancing rules. Anecdotal reports of increased fishing activity since March were likely influenced by excellent weather, school holidays, and fewer fishers per vessel. Analysis of June boat ramp survey data identified a significant increase in boat trailer numbers (index of effort) at 10 of the 42 monitored boat ramps (Fisheries Queensland, 2020), which may be biased if more boats with less people were being used to abide by social distancing rules. National analysis of AIS data indicated ~34% increase in recreational boat activity within the 8 km buffer of the Animal Tracking Facility network between January–June 2019 and 2020 (Fig. 2b). In contrast, lockdowns in Victoria prohibited recreational boating and fishing resulting in a complete cessation of these activities. While there was not a complete cessation of boating and fishing in Tasmania, vessels generally could only be launched from local municipalities given government restrictions on human movement. While there was anecdotal evidence that this resulted in an increase in urban fishing, there was a 25% decline in recreational southern rock lobster catches in the 2019–20 season compared to the previous season, which was directly attributed to COVID-19 restrictions (Lyle et al., 2020). Additionally, the opening of the Tasmanian recreational scallop fishery was delayed from April 2020 until June 2020 (Tasmanian Department of Primary Industries, Parks, Water and Environment, unpublished data). However, other states enacted limited restrictions with recreational fishing in Western and South Australia being allowed locally, if physical distancing was adhered to. Although recreational fishing activities were not restricted in NSW, other than the requirement to comply with socially distancing, the sales of recreational fishing licenses in March–April 2020 was down by ~35% compared to March–April 2019. This was followed by an increase of ~22% in recreational fishing licenses sales in May–June 2020 compared to the same period in 2019 (NSW Department of Primary Industries, unpublished data). This may reflect the impact of reduced regional tourism during the core lockdown period, followed by increased tourism afterwards, particularly due to continuing prohibitions on international travel.
Combined with the preceding bushfires, tourism in Australia suffered from a global reduction in international travel and localised restrictions caused by COVID-19. International arrivals sharply decreased with the tightening of restrictions at the end of March 2020, from 3% and 5.4% less travellers in February and March 2020 compared to 2019, which increased to 99.7% less travellers in April 2020 (Tourism Australia, 2020a). As of February 2021, international arrival in Australia is still banned. Australia's tourism sector is worth $60.8 billion to the national gross domestic product with 44% of tourist dollars spent in regional areas (2018–19, Tourism Australia, 2020b). Regional tourism in Australia diminished considerably. Even with some relaxation of border closures enabling inter-state travel, operators in Queensland-based ecotourism hubs such as Cairns and the Whitsunday Islands in the northern Great Barrier Reef experienced drastic reductions in visitor numbers. The Great Barrier Reef Marine Park Authority collects data on numbers of annual visitors to the reef through an Environmental Management Charge, providing a resource for quantifying tourism activity on the Great Barrier Reef (see gbrmpa.gov.au/our-work/reef-strategies/visitor-contributions/numbers). Finer-time scale data that exclusively capture COVID-19 were obtained from the operators. For example, data from five tourism vessels visiting reefs and islands of the Great Barrier Reef reveals tourism dropped from 80939 visitors in March–June 2019 to 9327 for March–June 2020, an 88% reduction in visitor numbers. The Whitsundays bareboat charter fleet consists of 140 boats and tourism data were obtained for a subsample of 35 boats. Boat usage dropped from 194 trips for 1791 nights at sea for the 1st of April to June 20th in 2019, to 33 trips for 325 nights for the same period in 2020, a decrease of 83% in trips and 82% in nights at sea. The operators stated that this decrease likely reflected the reduction in tourism for the entire fleet (T Rees, pers. comm.). At Lady Elliot Island, in the southern Great Barrier Reef, tourism activity ceased for the first time in 20 years between the 1st of April to June 12th 2020 due to the lockdown. This included a 100% reduction in overnight guests (~1090 per month) and day visitors (~570 per month) for that period, a 95% reduction in tour boats and airplanes operating in the area, and an 80% reduction in staff present on the island. During the period of reduced tourism activities, increased illegal fishing activity and coral damage were reported, highlighting the role of tourism in discouraging illegal fishing and boating practices (P Gash and A Gash, pers. comm.). The whale watching industry was also impacted by the COVID-19 pandemic throughout the east coast of Australia. In Hervey Bay, for example, Pacific Whale Foundation Eco Adventures experienced a 42% reduction in the number of boat tours conducted for the 2020 season, with onboard passenger capacity reduced by a further 36% due to social distancing restrictions in place (A Ellis, pers. comm.).
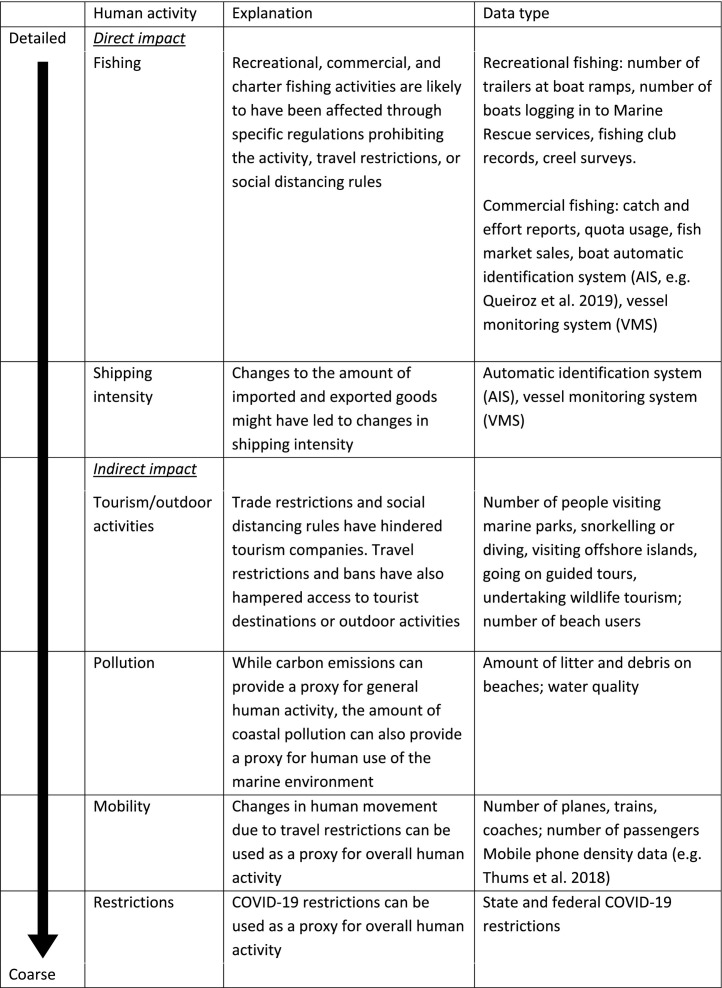
The disparity of restrictions across states (Fig. 1) illustrates the complexity of understanding how COVID-19 restrictions affected human behaviour and the importance of selecting suitable datasets when choosing to quantify changes in anthropogenic activities and appropriately infer impact on animal movement. Datasets that could be used as covariates to contextualise changes in human activity in marine environments during the COVID-19 pandemic are provided in Table 1 .
Table 1.
Potential changes in human activity due to COVID-19 restrictions grouped by direct vs. indirect impacts with an explanation of each activity and associated data type. The arrow on the left-hand side illustrates the scale at which the human activity and associated data type occurs, from detailed to coarse.
3. Experimental design considerations to assess impacts of changes in human activity
The precipitous and sudden change in human behaviour caused by the COVID-19 pandemic has resulted in what has been referred to as a Global Human Confinement Experiment (Bates et al., 2020) enabling the assessment of the impacts of human activity on biological systems. The sudden reduction in human activity, however, provides unique challenges in formulating effective experimental designs to leverage the power of monitoring marine organisms via acoustic tracking that are perhaps different to established techniques.
3.1. Before-after response
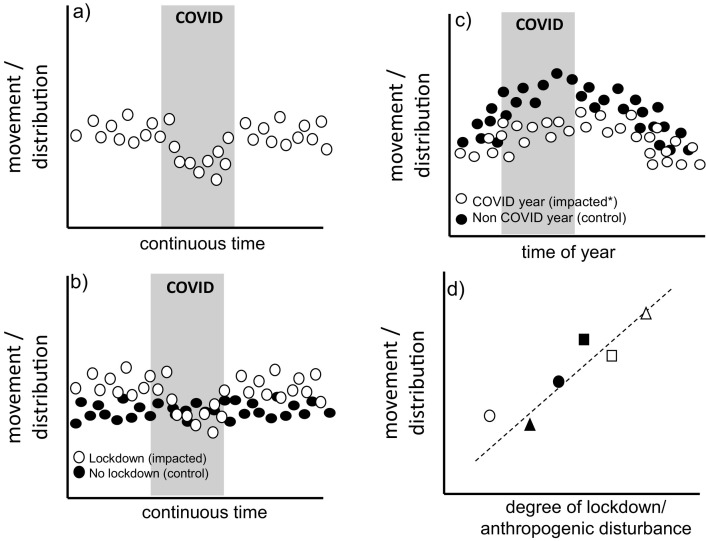
The simplest way, in logistical terms, to assess how changes in human behaviour may impact animal movement would involve using a “before, during and after” design (Fig. 4 ). In the fortuitous case where an acoustic tracking study was initiated prior to the COVID-19 pandemic, this provides an opportunity to detect immediate changes before and after restrictions were in place, assuming transmitter battery life spanned the desired period. Although such an approach is logistically simple, it suffers from potentially confounding factors. Given that restrictions on human activity lasted many months in some places, it is conceivable that changes in movement and distribution are a consequence of other environmental factors not related to COVID-19, such as seasonality and stochasticity in environmental conditions.
Fig. 4.
Graphical representation of the different experimental designs that can be used in conjunction with acoustic tracking data. a) before-during-after at a single site; b) before-during-after of impacted and control site (spatial control); c) before-during-after with temporal control; and d) comparative analysis.
Traditionally, impact assessments use controls to avoid confounding variables hampering the quantification of effects. The same also applies to assessing the effects of a reduction in human activity on wildlife. In the context of acoustic tracking, two distinct controls can be considered, spatial and temporal. Experimental designs that include spatial control require a similar site to the site impacted by COVID restrictions. Comparisons of the response between the two study sites is expected to remove confounding factors related to both seasonality and environmental stochasticity. Although powerful, spatial controls will be difficult to implement as a result of the requirement for near-identical study sites that only differ in their degree of the impact from COVID-19 restrictions. Given that lockdowns were mostly implemented at a national level, identification of appropriate sites not affected by COVID-19 restrictions is a challenge. Temporal controls offer an alternative to spatial controls, because they do not rely on the identification of a control site, instead using the movement or distribution of animals at the same site in other years as controls. An important consideration for this type of approach is inter-annual variability and animal tagging effort across years. Since environmental conditions in different years do not necessarily mirror one another, it is imperative that any statistical modelling uses suitable environmental covariates to reduce the confounding impact of inter-annual change. The long battery life of some acoustic tags makes temporal controls an attractive solution.
3.2. Comparative approach
Coordinated large-scale national or international acoustic tracking networks also allow for a comparative approach. In this case, multiple researchers with the same species in their receiver array can compare movement and residency metrics to assess how animals responded to varying degrees of change in human activity as a result of spatial differences in COVID-19 restrictions among regions or countries. Similar to other approaches, habitat and environmental conditions will be confounding factors of animal behaviours and, consequently, collection of environmental data at the scale of each regional array is paramount. Additionally, for widely distributed species, the potential effects of stock structure in movement patterns must also be considered.
4. Quantifying the effects of COVID-19 restrictions on the movement behaviour of marine organisms
Depending on the timing, frequency, and duration of the human activity, there are a range of ways that marine animals might respond to lockdown-related shifts in human behaviour. Acoustic tracking data, especially in the context of national or international arrays, provide an opportunity to quantify various metrics of animal behaviour, movement, and distribution (e.g. presence, residency, frequency and duration of visits, rate or extent of movement, times of departure and arrival from an area, and home ranges or activity spaces such as 2- or 3-dimensional kernel utilisation distribution size or centroid). Marine organism responses can be acute and/or chronic; with acute responses more easily detectable and changes in chronic responses more difficult to identify as they take longer to transpire. For example, it is possible that the presence of marine organisms and residency will increase quickly due to a reduction in fishing-induced mortality and boat disturbances in areas with high human population density, whereas increase in the extent and rate of their movement might take longer to occur.
Understanding the short- and long-term effect of reduction or absence of human activities on animal behaviour and movement requires an animal response be chosen that reflects the temporal and spatial scales appropriate to the human activity being assessed, including the type of activity and its timing, duration, and frequency. Responses over a small spatial scale are more likely to occur and easier to detect and attribute to specific changes in human activity types. For example, an increase in individual presence and residency is expected in response to a reduction in tourism activity at popular destinations (e.g. Great Barrier Reef, Ningaloo Reef). In contrast, large spatial scale changes such as migrations (e.g. speed or routes) will be harder to detect due to environmental variation and differences in restriction level across states; for instance aquatic activities and boating were not equally restricted across all states.
Long-term exposure to human activities may have already irreversibly changed marine organism movements and distributions, and the short duration of the drop in human activities due to COVID-19 might be too brief to see any changes in animal behaviour and movement. For instance, changes in activity spaces (e.g. for bream, snapper, yellowtail kingfish) in Sydney Harbour might be harder to detect due to long-term levels and cumulative impacts of human activities in the area (e.g. fishing, tourism, shipping, noise pollution). It is also possible, given that Australia has a relatively low human population density, that levels of human activities in Australia may have few measurable impacts on marine organism movements and distributions compared to more heavily populated coastal regions. Strict management and conservation policies in place might also be in part responsible for any lack of discernible marine organism responses to changes as they already control access to many areas.
In summary, assessing how marine organisms respond to changes in human activity types and levels is complex and requires careful study design. Having access to continuous long-term spatial data such as acoustic tracking data, especially in the context of large, national, or international networks provides an opportunity to examine marine organism movements and habitat use during this unprecedented drop in human activities levels.
5. The Integrated Marine Observing System (IMOS) Animal Tracking Facility
The IMOS Animal Tracking Facility is a permanent array of acoustic receivers around continental Australia that uses a single technology and is therefore compatible across receivers deployed nationally (Brodie et al., 2018; Fig. 2). At the outset, IMOS developed a central national database to foster collaborative research across the user community and quantify individual behaviour across a broad range of taxa (Brodie et al., 2018; Hoenner et al., 2018). The IMOS permanent array of acoustic receivers is integrated with a large number of independent, project-based, non-IMOS installations that are deployed by individual researchers and research teams to address regional research needs (Brodie et al., 2018). This means that array design is dependent upon local and temporal research objectives. For example, arrays may be deployed as curtains perpendicular to shore to monitor long-distance migrations (Heupel et al., 2006; Steckenreuter et al., 2017), deployed as gates across the entrance to a bay to examine ingress and egress to bays and harbours (Smoothey et al., 2019; Taylor et al., 2017), or may be arranged as grids within a focal area such as a fishery or marine park to examine fine-scale movement and residency within an area (Espinoza et al., 2015; Fowler et al., 2017; Pillans et al., 2014).
The broad-scale (continental) and long-term (14 years) nature of the IMOS Animal Tracking network means it is well-suited to address the question of how pauses or reductions in human activity may influence coastal marine life, because it is possible to match the scale of potential human behaviour changes to biologically meaningful changes in animal behaviour. For instance, alterations in recreational fishing or boating activity may be detectable from receiver arrays close to areas with previously high fishing intensity, which enable the research community to ask questions about fine-scale residency or space use for highly resident species, while fundamental changes due to broader scale impacts Australia-wide may be detectable for migratory species. The ability to answer these questions is enhanced by the recent development of the Animal Tracking Toolbox (ATT, Udyawer et al., 2018), a standardised framework and analytical tool-set that comprises a collection of functions to estimate metrics of detection, dispersal, short-term centres of activity, and home range from passive tracking datasets. The ATT has the advantage of being able to use standardised metrics to directly compare the same species at multiple sites; to compare multiple species tagged at a single study site; and to assess changes in activity space metrics over time, thereby providing the analytical power to rapidly address the question of whether there are detectable effects arising from COVID-19.
Unlike satellite-linked GPS tags (e.g. Iridium, ARGOS, GSM) where the acquisition of location data is near-instantaneous, acoustic receivers need to be serviced by researchers to recover acoustic tag detections. This means that any logistical delays in gaining access to field sites to download receivers or delays in uploading detection data into the collaborative IMOS Animal Tracking Facility also delays our ability to test for COVID-related changes on tagged marine fauna. This is, however, similar to many environmental data that are often delivered in delayed mode, but are necessary to account for natural variability due to changing environmental conditions. With data recently downloaded from the IMOS Animal Tracking network, we provide a case study as an example of future analyses that can be conducted across the nation, once servicing periods are reached for most receiver installations and COVID-19 restrictions for servicing are lifted.
6. Case study: residency and activity space of white sharks and yellowtail kingfish at a popular wildlife tourism site
6.1. Background
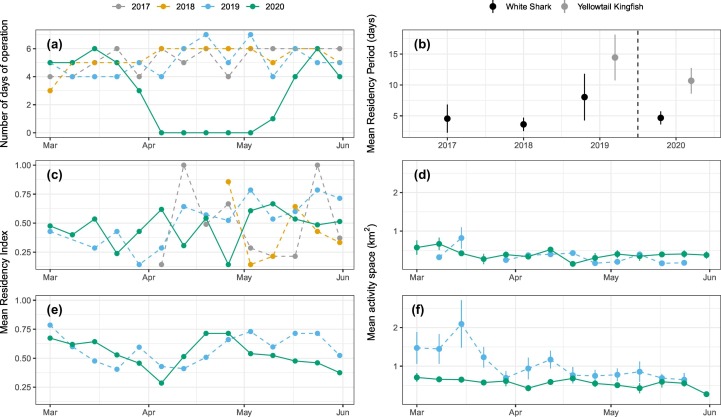
The white shark cage-diving industry began in the late 1970s in waters off the Eyre Peninsula in South Australia. The industry has been restricted to the Neptune Islands Group Marine Park located 60–70 km south of Port Lincoln since 2002, with most cage-diving activities focused at the North Neptune Islands group (Fig. 2c). After 2007, the industry expanded from two to three operators and the mean annual number of days when tours operated rose from 124 (2000–2006) to 265 (2008–2011) (Bruce and Bradford, 2013). Due to concern over the potential impact of the cage-diving industry on white sharks, the number of days operators are allowed at the Neptune Islands has been limited since 2012, with operators allowed to run trips on 10–12 days per fortnight. Over the years, operators have used the Neptune Islands relatively consistently and operated throughout the year on 227–280 days of the year (mean 256.75 ± 7.02 days; 2017–2019; Fig. 5 ). However, the COVID-19 restrictions imposed on the 22nd of March 2020 led to operators being unable to take tourists and the industry effectively shutting down until restrictions were lifted on the 12th of May 2020. As a result, no tourism boat visited the Neptune Islands for 51 days, the longest period of industry inactivity for over 12 years. We hypothesised that, during this absence of cage-diving operations: 1) animal residency would decrease due to the lack of olfactory stimulus and bait to entice animals to remain at the Neptune Islands; and 2) for individuals that remained at the Neptune Islands, activity space would increase from spending time within the close proximity of cage-diving vessel locations to swimming around the study site and islands.
Fig. 5.
(a) Number of days/week operators were present at the Neptune Islands across 2017–2020; (b) mean residency period (days) of white sharks (Carcharodon carcharias) and yellowtail kingfish (Seriola lalandi) between March 23 to May 12 across 2017–2020; (c) mean residency index of white sharks; (d) mean activity space (km2) of white sharks; (e) mean residency index of yellowtail kingfish; (f) mean activity space (km2) of yellowtail kingfish. (c)(d)(e) and (f) are shown from March to June across 2017–2020. Error bars represent standard error.
6.2. Methods
6.2.1. Receiver deployments and shark tagging
Three VR2AR acoustic receivers (Innovasea Ltd., Halifax, Canada) have been deployed at the Neptune Islands since July 2016 using a low-profile subsurface mooring system. One VR2AR was deployed at each of the main berleying sites at the North Neptune Islands group. In 2018, an additional array of 13 VR2AR receivers were deployed, and expanded the acoustic coverage at the North Neptune Islands.
Since 2013, 130 white sharks have been externally tagged with V16—6H tags (Innovasea Ltd., Halifax, Canada) as part of the South Australian cage-diving industry monitoring program. Tags were tethered to a Domeier umbrella dart-tag head using a 10- to 15-cm-long stainless wire trace (1.6 mm diameter) and implanted in the dorsal musculature of sharks using a modified spear-gun applicator. Biases in residency estimates can be introduced by targeting specific sharks (e.g. sharks likely to remain in the Neptune Islands) or due to temporal variations in residency (e.g. sharks are more likely to remain within Neptune Islands during weaning of New Zealand fur seals). To minimise the potential impacts of these biases, tags were opportunistically deployed throughout the year.
Sixteen yellowtail kingfish (Seriola lalandi) were acoustically tagged between August 2018 and May 2019. Fish were caught using handline and put onto a paddle cradle. Seawater was circulated across the gills using a water pump to ensure continuous oxygenation during handling. A small incision (1.5–2 cm) was made along the central line of the ventral surface, anterior of the pelvic fins. A V13A or V16A tag was inserted into the body cavity. The incision was stitched using 2–3 non-continuous external sutures (3/0 Monosyn absorb violet 70 cm, needle tapercut). A plastic head conventional identification tag (Hallprint™, Hindmarsh Valley, South Australia) was inserted into the muscle below the first dorsal fin to identify tagged fish in the event of a recapture. Data was obtained from the IMOS Animal Tracking web-interface (Huveneers, 2020; Clarke et al., 2020)
6.2.2. Data analysis
For each tagged shark and fish, the number of consecutive days that individuals were present was calculated each time they entered the study area. A residency period was defined as the number of days between the first and last detection of a tagged shark, without any gaps in consecutive days of detection exceeding five days. A five-day period was selected on the basis of estimated transit times between the North and South Neptune Islands (Bruce and Bradford, 2013). Where sharks were not detected over periods of over five consecutive days, individuals were assumed to have left the Neptune Islands and any subsequent return was considered to represent a new residency period. The residency of white sharks is reported for the COVID-19 restriction period from the 23rd of March until the 12th of May 2020 and compared across years of monitoring (2017–2020).
Weekly residency and activity space were estimated for March–May 2020 for each white shark and yellowtail kingfish. Residency index is the number of days a tag is detected divided by the total number of days it could be detected (i.e. seven days for each weekly subset). Centre of activities (COA; Simpfendorfer et al., 2002) were estimated to calculate activity space for each individual within each weekly subset. Measures of activity space in this study was defined as the area within the 95% contour of the Kernel Utilization Distribution using a Brownian Bridge movement model (henceforth BB-KUD). Both the residency index and BB-KUD were calculated using the VTrack package (Udyawer et al., 2018).
We tested the effects of COVID-19 restrictions on the residency period, residency index, and activity space using a Generalised Linear Mixed-Model (GLMM) with tag ID as the random effect, year as a fixed factor and as an interaction term, and the lmer or lme function in the lme4 package (Bates et al., 2014). The error structure of GLMM corrects for non-independence of statistical units due to shared temporal structure and permits the random effects variance explained at different levels of clustering to be decomposed. The inclusion of tag ID as a random effect enabled the analysis to account for the lack of independence in behaviour within each tagged shark and fish. We accounted for temporal autocorrelation for the residency index and activity space by using an AR1 correlation error structure in the models (form = ~1|tag/week). The most appropriate transformation, statistical family, and validity of the model were determined through an examination of the distribution of the response variable and a visual inspection of the residuals for the saturated models.
6.3. Results
The reduction in tourism arising from COVID-19 restrictions had no effect on residency of white shark or yellowtail kingfish (Fig. 5b, c, e; Table 2 ), nor on white shark activity space (Fig. 5d; Table 2). However, there was a reduction in the activity space of yellowtail kingfish in 2020, coincident with the reduction in tourism (Fig. 5f; Table 2).
Table 2.
Generalised Linear Mixed-Model (GLMM) results showing the effects of COVID-19 restrictions on white shark (Carcharodon carcharias) and yellowtail kingfish (Seriola lalandi) residency index and activity space. SE = standard error; CI = confidence interval; df = degree of freedom. Significant results are highlighted in bold (Queiroz et al., 2019; Thums et al., 2018).
| Parameter | Coefficient | SE | 95% CI | t-value | df | P-value |
|---|---|---|---|---|---|---|
| White shark (residency period) | ||||||
| Intercept | 0.90 | 0.49 | −0.07,1.86 | 1.82 | 40 | 0.069 |
| COVID vs pre-COVID | −0.58 | 0.63 | −1.82,0.65 | −0.93 | 40 | 0.354 |
| Yellowtail kingfish (residency period) | ||||||
| Intercept | 3.03 | 0.47 | 2.11,3.95 | 6.43 | 50 | <0.001 |
| COVID vs pre-COVID | 0.26 | 0.57 | −0.86,1.38 | 0.45 | 50 | 0.653 |
| White shark (residency index) | ||||||
| Intercept | 0.27 | 0.15 | −0.04,0.57 | 1.75 | 77 | 0.083 |
| Week | 0.01 | 0.01 | −0.01,0.03 | 1.19 | 77 | 0.239 |
| Year (2017) | 0.41 | 0.37 | −0.32,1,14 | 1.11 | 77 | 0.27 |
| Year (2018) | −0.04 | 0.92 | −1.88,1.80 | −0.05 | 77 | 0.962 |
| Year (2019) | −0.27 | 0.32 | −0.91,0.37 | −0.85 | 77 | 0.397 |
| Week ∗ year (2017) | −0.03 | 0.02 | −0.07,0.02 | −1.25 | 77 | 0.217 |
| Week ∗ year (2018) | 0.00 | 0.05 | −0.09,0.09 | −0.03 | 77 | 0.978 |
| Week ∗ year (2019) | 0.02 | 0.02 | −0.02,0.05 | 0.99 | 77 | 0.325 |
| Yellowtail kingfish (residency index) | ||||||
| Intercept | 0.71 | 0.1 | 0.52,0.91 | 7.26 | 207 | <0.001 |
| Week | −0.01 | 0.01 | −0.02,0.00 | −2.11 | 207 | 0.036 |
| Year (2019) | −0.18 | 0.15 | −0.47,0.11 | −1.22 | 207 | 0.226 |
| Week ∗ year (2019) | 0.01 | 0.01 | 0.00,0.03 | 1.58 | 207 | 0.116 |
| White shark (activity space) | ||||||
| Intercept | 0.63 | 0.12 | 0.41,0.86 | 5.48 | 62 | <0.001 |
| Week | −0.01 | 0.01 | −0.02,0.00 | −1.60 | 62 | 0.109 |
| Year (2019) | 0.02 | 0.24 | −0.45,0.50 | 0.09 | 62 | 0.925 |
| Week ∗ year (2019) | −0.01 | 0.01 | −0.03,0.02 | −0.50 | 62 | 0.619 |
| Yellowtail kingfish (activity space) | ||||||
| Intercept | 0.78 | 0.1 | 0.58,0.99 | −1.99 | 279 | <0.001 |
| Week | 0.00 | 0.01 | −0.01,0.01 | −3.61 | 279 | 0.931 |
| Year (2019) | 0.32 | 0.12 | 0.08,0.56 | −1.36 | 279 | 0.010 |
| Week ∗ year (2019) | −0.02 | 0.01 | −0.03,-0.01 | 0.58 | 279 | 0.007 |
6.4. Discussion
COVID-19 restrictions led to a dramatic reduction in shark cage-diving operations at the Neptune Islands. During the early period of COVID-19 (28th March – 12th May), there was a complete cessation of boat visits and the longest consecutive period without cage-diving vessels present in over 20 years. Despite this significant reduction in boat visitation and associated use of food-based (mix of minced southern bluefin tuna, Thunnus maccoyii, products) and sound attractant, the residency of acoustically tagged white sharks was not measurably affected, refuting our first hypothesis. There are two plausible explanations for this result. First, white shark residency is not primarily driven by the attractants used but is linked to their natural migratory patterns (Bradford et al., 2020) and their use of the Neptune Islands to feed on pinnipeds and teleosts (Meyer et al., 2019). Alternatively, considering the intensity of cage-diving tourism with boats present at the Neptune Islands nearly every day over the last 12 years (Tanner et al., 2019), it may be that the presence of the cage-diving industry induced a more pervasive change that was not alleviated by a 1.5-month break in tourism. The similar nil change in residency behaviour of yellowtail kingfish also suggests either that the cage-diving industry does not affect residency or that any effects of near daily operations persist through a 1.5-month break in tourism. However, the migration of a tagged yellowtail kingfish from the Neptune Islands to Batemans Bay (NSW) in July–August 2020 (P Butcher and C Huveneers, unpublished data), after the cage-diving industry resumed operations, shows that the attractants used by the cage-industry does not prevent this fish from leaving the Neptune Islands and swimming at least 1600 km to NSW.
At first glance, the lack of effect on white shark activity space appears to contradict earlier research showing that the cage-diving industry influences the fine-scale spatial distribution of white sharks (Huveneers et al., 2013). However, this points to the importance of appropriate temporal and spatial scales. White shark positions in the present study were estimated using centres of activities that require binning detections over a time period (typically 30–60 min) (Simpfendorfer et al., 2002). This results in a limited number of location estimates and this constrains the calculation of weekly BB-KUD. At the Neptune Islands, operators do not use fixed moorings but instead anchor at different locations on every visit (Fig. 2c). This means that weekly BB-KUDs that include different daily core areas are unlikely to detect real but fine-scale changes in white shark distribution. Sampling methodology that allows position estimates to be captured at a higher spatial and temporal resolution, e.g. using the Vemco Positioning System (Roy et al., 2014), could improve our ability to detect potential changes in shark movements and space use at finer temporal scales (i.e. hours, days), but this study demonstrates that weekly white shark activity space is not affected by cage-diving tourism.
In contrast, the weekly activity space of yellowtail kingfish was greater during cage-diving operations compared to the same period in 2020. This reduction in the area of an individual's activity space in the absence of boat operations suggests that yellowtail kingfish movement increases when boats arrive at the site as the fish are attracted to the boats and move around and between operators and anchor points. This is a strong effect and shows a rapid response to changes in human activities at this site, and points to the need to measure effects at biologically meaningful scales. Detections from yellowtail kingfish were however limited to two years (2019 and 2020), affecting our ability to account for interannual differences due to environmental conditions.
This case study illustrates how acoustic tracking can be used to quantify potential impacts of anthropogenic activity (i.e. tourism) on marine animal behaviour and space use. By focusing on enforced reductions in human activities caused by COVID-19 restrictions, we show how animal responses to shifts in human disturbance are complex and can vary between species and across scales. In this case, although white shark movement patterns were seemingly unaffected by changes to the cage-diving industry, tagged yellowtail kingfish decreased the extent of their activity space. The spatial and temporal scale at which the analysis is undertaken is critical to the ability to detect change and needs to be tailored to the behavioural ecology of the taxa in question. For example, while we could not detect any changes in the weekly activity space of white sharks, this might be a function of resolution and the cage-diving industry might well affect their distribution if estimated on a shorter temporal scale, e.g. daily activity space. Importantly, we were able to assess whether residency and movement behaviour was affected by the cessation of wildlife tourism thanks to the existing monitoring of the cage-diving industry and the IMOS Animal Tracking Facility. Had the acoustic receivers not already been installed, we would not have been able to deploy them in time to collect ‘before’ data prior to the COVID-19 restrictions being implemented and tourism decreasing. The existing monitoring of white shark residency, collecting both tracking and human-use data, also enabled us to compare data to previous years and account for natural inter-annual variability. Our example showcases the benefit of long-term observing programs, like IMOS, to record changes from rapid and unexpected disruptions like those brought by COVID-19 or other disturbance events (e.g. marine heat wave; Oliver et al., 2017).
7. Limitations
As discussed, the ability to detect changes in animal behaviour as a result of COVID-19 restrictions is dependent on a number of factors, including a sufficient sample of tagged animals spanning the period pre (baseline) vs. during lockdown vs. post lockdown (recovery) with arrays preferably deployed in areas more affected by lockdowns (i.e. our treatment group) vs. those less affected (i.e. our control group). However, there are a number of other factors that need to be accounted for when investigating disturbance impacts using passive acoustic tracking. Furthermore, as a tagged organism is only detected when it swims within the range of a receiver, the accuracy by which movement behaviours and space utilisation are represented and the ecological inferences made are dependent on the density, arrangement and extent of these acoustic receivers (Dwyer et al., 2015; Heupel et al., 2006). It is therefore critical that the arrangement of receivers is kept consistent throughout the study period and extent of the acoustic array is large enough to capture the fate of animals as they move in response to anthropogenic or natural stimuli.
Temporal and spatial variability in the performance of acoustic receivers at their deployment site is also important (Huveneers et al., 2016). Temporal variability in environmental conditions not related to COVID-19 that may increase the amount of background noise may result in false positives (type I error) or false negatives (type II error) of an effect of COVID-19 on area use, rather than some other environmental feature. For example, reduced anthropogenic noise next to a receiver due to decreased ship traffic (Thomson and Barclay, 2020) may increase the detection range of receivers, potentially causing an upward bias on the number of transmissions detected.
Finally, while acoustic tracking could provide researchers with the long-term spatially-explicit data necessary to detect changes in the behaviour, abundance, and distribution of organisms, whether these changes affect animal fitness (e.g. reduced fecundity, increased mortality) is mostly unknown. For example, increased anthropogenic disturbance may cause animals to use riskier environments that increase predation risk (Wittmer et al., 2007), or behavioural changes due to human disturbance may reduce the time and energy allocated to important activities such as foraging, resting, or breeding (Barnett et al., 2016; Frid and Dill, 2002; Gallagher and Huveneers, 2018). Further studies are needed to determine how short-term behavioural changes in response to anthropogenic disturbance translate into long-term, biologically significant impacts on individual fitness, performance and community composition. Such information is of paramount importance to truly understand the impacts of anthropogenic disturbances on animal populations.
8. Conclusion
The various restrictions imposed to control COVID-19 infections has led to unprecedented changes in anthropogenic activities worldwide. In Australia, changes to aquatic activities have been complex and regionally-dependent, with effects on marine organisms difficult to identify. Some impacts to marine organisms are unlikely to be identified within weeks to months, only becoming detectable several years later. Environmental stochasticity will also add to the challenges of reliably being able to infer causation between COVID-19 restrictions and changes to animal movement behaviour, highlighting the importance of sustained observing programs.
Here, we have broadly described some of the impacts of COVID-19 restrictions on human activities in Australia and outlined metrics that can be used to measure changes in animal movement behaviour. Experimental design considerations are provided in the hope that it will assist others using acoustic tracking to assess whether aquatic organisms have been affected by changes in anthropogenic activities during COVID-19 reductions, or more broadly how acoustic tracking can be used to quantify the effects of human disturbances on animal movement behaviour.
National acoustic tracking networks, like the IMOS Acoustic Tracking Facility, provide unique infrastructure and datasets to assess the effects of COVID-19 on aquatic organisms at different locations or spatial scales while enabling a range of temporal and spatial comparison. Only with the existence of long-term, robust monitoring infrastructure are we able to disentangle the complex interaction between cumulative impacts, chronic long-term impacts (e.g. climate change, coastal development, commercial fishing) and short-term, acute impacts (e.g. noise pollution, heat waves, seismic surveys, oil spills), which may drive step changes in systems (e.g. trophic cascades).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors thank Sam Wines for collation of AIS data and kernel density processing, Phil McDowall, James Van Den Broek, Josh Dennis, Lauren Meyer, Chloe Roberts, Christine Barry, and Josh Davey for help deploying and recovering acoustic receivers at the Neptune Islands, and the cage-diving industry for logistic support. Tagging of white sharks and yellowtail kingfish was conducted under Flinders University Animal Welfare Committee approvals (E398, E464, and E467) and research permits from the Department for Environment and Water (Q26292, Q26612, MR-00132). We thank SARDI – Aquatic Sciences for providing rock lobster catch data from South Australia.
Funding
Data were sourced from Australia’s Integrated Marine Observing System (IMOS) – IMOS is enabled by the National Collaborative Research Infrastructure strategy (NCRIS). It is operated by a consortium of institutions as an unincorporated joint venture, with the University of Tasmania as Lead Agent. Funding for the white shark monitoring program was provided by the Department for Environment and Water. Funding for the yellowtail kingfish tagging was provided by the Holsworth Wildlife Research Endowment grants and the Biological Society of South Australia.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.biocon.2021.108995.
Appendix A. Supplementary data
Sensitivity analysis testing how much changing the radius around each acoustic receiver from 5 km to 15 km affected the change in vessel presence in proximity to acoustic receivers (see section 2. COVID-19 impact on marine activities in Australia).
References
- Barnett A., Payne N.L., Semmens J.M., Fitzpatrick R. Ecotourism increases the field metabolic rate of whitetip reef sharks. Biol. Conserv. 2016;199:132–136. [Google Scholar]
- Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. arXiv preprint. 2014 arXiv:1406.5823. [Google Scholar]
- Bates A.E., Primack R.B., Moraga P., Duarte C.M. COVID-19 pandemic and associated lockdown as a “global human confinement experiment” to investigate biodiversity conservation. Biological Conservation. 2020;248 doi: 10.1016/j.biocon.2020.108665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford R., Patterson T., Rogers P., McAuley R., Mountford S., Huveneers C., Robbins R., Fox A., Bruce B. Evidence of diverse movement strategies and habitat use by white sharks, Carcharodon carcharias, off southern Australia. Mar. Biol. 2020;167:1–12. [Google Scholar]
- Brodie S., Lédée E.J.I., Heupel M.R., Babcock R.C., Campbell H.A., Gledhill D.C., Hoenner X., Huveneers C., Jaine F.R.A., Simpfendorfer C.A., Taylor M.D., Udyawer V., Harcourt R.G. Continental-scale animal tracking reveals functional movement classes across marine taxa. Sci. Rep. 2018;8:3717. doi: 10.1038/s41598-018-21988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce B.D., Bradford R.W. The effects of shark cage-diving operations on the behaviour and movements of white sharks, Carcharodon carcharias, at the Neptune Islands, South Australia. Mar. Biol. 2013;160:889–907. [Google Scholar]
- Clarke T., Whitmarsh S., Huveneers C. Flinders University; 2020. Kingfish in South Australia. (Available: Integrated Marine Observing System. Animal Tracking Database. https://animaltracking.aodn.org.au. Accessed: 2020-10-01) [Google Scholar]
- Cominelli S., Halliday W.D., Pine M.K., Hilliard R.C., Lawson J.W., Duman N.I., Devillers R. Vessel noise in spatially constricted areas: modeling acoustic footprints of large vessels in the Cabot Strait, eastern Canada. Ocean Coastal Management. 2020;194:105255. [Google Scholar]
- Conner M.M., Saunders W.C., Bouwes N., Jordan C. Evaluating impacts using a BACI design, ratios, and a Bayesian approach with a focus on restoration. Environmental Monitoring Assessment. 2016;188:555. doi: 10.1007/s10661-016-5526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley P., Bennett R., Childs A., Murray T. Reflection on the first five years of South Africa’s Acoustic Tracking Array Platform (ATAP): status, challenges and opportunities. Afr. J. Mar. Sci. 2017;39:363–372. [Google Scholar]
- Crutzen P. In: Earth System Science in the Anthropocene. Ehlers E., Krafft T., editors. Springer; Berlin, Germany: 2006. The "anthropocene"; pp. 13–18. [Google Scholar]
- Darling J.A., Carlton J.T. A framework for understanding marine cosmopolitanism in the Anthropocene. Front. Mar. Sci. 2018;5:293. doi: 10.3389/fmars.2018.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depellegrin D., Bastianini M., Fadini A., Menegon S. The effects of COVID-19 induced lockdown measures on maritime settings of a coastal region. Science of the Total Environment. 2020;740 doi: 10.1016/j.scitotenv.2020.140123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffenbaugh N.S., Field C.B., Appel E.A., Azevedo I.L., Baldocchi D.D., Burke M., Burney J.A., Ciais P., Davis S.J., Fiore A.M. The COVID-19 lockdowns: a window into the earth system. Nature Reviews Earth Environmental Toxicology. 2020:1–12. [Google Scholar]
- Dwyer R.G., Campbell H.A., Irwin T.R., Franklin C.E. Does the telemetry technology matter? Comparing estimates of aquatic animal space-use generated from GPS-based and passive acoustic tracking. Marine Freshwater Research. 2015;66:654–664. [Google Scholar]
- Espinoza M., Heupel M.R., Tobin A.J., Simpfendorfer C.A. Residency patterns and movements of grey reef sharks (Carcharhinus amblyrhynchos) in semi-isolated coral reef habitats. Mar. Biol. 2015;162:343–358. [Google Scholar]
- Fisheries Queensland . Department of Agriculture and Fisheries; Brisbane: 2020. What Was the Reason for the Increased Boating Activity in April and May 2020. [Google Scholar]
- Fowler A., Huveneers C., Lloyd M. Insights into movement behaviour of snapper (Chrysophrys auratus, Sparidae) from a large acoustic array. Mar. Freshw. Res. 2017;68:1438–1453. [Google Scholar]
- Frid A., Dill L. Human-caused disturbance stimuli as a form of predation risk. Conservation Ecology. 2002:6. [Google Scholar]
- Gallagher A.J., Huveneers C.P. Emerging challenges to shark-diving tourism. Mar. Policy. 2018;96:9–12. [Google Scholar]
- Heupel M.R., Semmens J.M., Hobday A.J. Automated acoustic tracking of aquatic animals: scales, design and deployment of listening station arrays. Mar. Freshw. Res. 2006;57:1–13. [Google Scholar]
- Heupel M.R., Simpfendorfer C.A., Espinoza M., Smoothey A.F., Tobin A., Peddemors V. Conservation challenges of sharks with continental scale migrations. Front. Mar. Sci. 2015;2:12. [Google Scholar]
- Hoenner X., Huveneers C., Steckenreuter A., Simpfendorfer C., Tattersall K., Jaine F., Atkins N., Babcock R., Brodie S., Burgess J., Campbell H., Heupel M., Pasquer B., Proctor R., Taylor M.D., Udyawer V., Harcourt R. Australia’s continental-scale acoustic tracking database and its automated quality control process. Scientific Data. 2018;5:170206. doi: 10.1038/sdata.2017.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huveneers C. Flinders University, Department for Environment and Water; 2020. White shark cage-diving industry monitoring. (Available: Integrated Marine Observing System. Animal Tracking Database. https://animaltracking.aodn.org.au. Accessed: 2020-10-01) [Google Scholar]
- Huveneers C., Rogers P.J., Beckmann C., Semmens J.M., Bruce B.D., Seuront L. The effects of cage-diving activities on the fine-scale swimming behaviour and space use of white sharks. Marine Biology. 2013;160(11):2863–2875. [Google Scholar]
- Huveneers C., Simpfendorfer C.A., Kim S., Semmens J., Hobday A.J., Pederson H., Stieglitz T., Vallee R., Webber D., Heupel M.R., Peddemors V., Harcourt R.G. The influence of environmental parameters on the performance and detection range of acoustic receivers. Methods Ecol. Evol. 2016;7:825–835. [Google Scholar]
- Iverson S.J., Fisk A.T., Hinch S.G., Mills Flemming J., Cooke S.J., Whoriskey F.G. The ocean tracking network: advancing frontiers in aquatic science and management. Canadian Journal of Fisheries Aquatic Sciences. 2019;76:1041–1051. [Google Scholar]
- Le Quéré C., Jackson R.B., Jones M.W., Smith A.J., Abernethy S., Andrew R.M., De-Gol A.J., Willis D.R., Shan Y., Canadell J.G. Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement. Nature Climate Change. 2020:1–7. [Google Scholar]
- Lyle J., Ewing F., Ewing G., Tracey S. 2020. Tasmanian Recreational Rock Lobster and Abalone Fisheries: 2019–20 Fishing Season. [Google Scholar]
- March D., Metcalfe K., Tintoré J., Godley B. 2020. Tracking the Global Reduction of Marine Traffic during the COVID-19 Pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer L., Pethybridge H., Beckmann C., Bruce B., Huveneers C. The impact of wildlife tourism on the foraging ecology and nutritional condition of an apex predator. Tour. Manag. 2019;75:206–215. [Google Scholar]
- Nabe-Nielsen J., van Beest F.M., Grimm V., Sibly R.M., Teilmann J., Thompson P.M. Predicting the impacts of anthropogenic disturbances on marine populations. Conserv. Lett. 2018;11 [Google Scholar]
- Nicola M., Alsafi Z., Sohrabi C., Kerwan A., Al-Jabir A., Iosifidis C., Agha M., Agha R. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int. J. Surg. 2020;78:185. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver E.C.J., Benthuysen J.A., Bindoff N.L., Hobday A.J., Holbrook N.J., Mundy C.N., Perkins-Kirkpatrick S.E. The unprecedented 2015/16 Tasman Sea marine heatwave. Nat. Commun. 2017;8:16101. doi: 10.1038/ncomms16101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillans R.D., Bearham D., Boomer A., Downie R., Patterson T.A., Thomson D.P., Babcock R.C. Multi year observations reveal variability in residence of a tropical demersal fish, Lethrinus nebulosus: implications for spatial management. PLoS One. 2014;9 doi: 10.1371/journal.pone.0105507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimiento C., Leprieur F., Silvestro D., Lefcheck J., Albouy C., Rasher D., Davis M., Svenning J.-C., Griffin J. Functional diversity of marine megafauna in the Anthropocene. Science Advances. 2020;6 doi: 10.1126/sciadv.aay7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagányi É.E., McGarvey R., Gardner C., Caputi N., Dennis D., de Lestang S., Hartmann K., Liggins G., Linnane A., Ingrid E. Overview, opportunities and outlook for Australian spiny lobster fisheries. Reviews in Fish Biology Fisheries. 2018;28:57–87. [Google Scholar]
- Queiroz N., Humphries N.E., Couto A., Vedor M., da Costa I., Sequeira A.M.M., Mucientes G., Santos A.M., Abascal F.J., Abercrombie D.L., Abrantes K., Acuña-Marrero D., Afonso A.S., Afonso P., Anders D., Araujo G., Arauz R., Bach P., Barnett A., Bernal D., Berumen M.L., Bessudo Lion S., Bezerra N.P.A., Blaison A.V., Block B.A., Bond M.E., Bonfil R., Bradford R.W., Braun C.D., Brooks E.J., Brooks A., Brown J., Bruce B.D., Byrne M.E., Campana S.E., Carlisle A.B., Chapman D.D., Chapple T.K., Chisholm J., Clarke C.R., Clua E.G., Cochran J.E.M., Crochelet E.C., Dagorn L., Daly R., Cortés D.D., Doyle T.K., Drew M., Duffy C.A.J., Erikson T., Espinoza E., Ferreira L.C., Ferretti F., Filmalter J.D., Fischer G.C., Fitzpatrick R., Fontes J., Forget F., Fowler M., Francis M.P., Gallagher A.J., Gennari E., Goldsworthy S.D., Gollock M.J., Green J.R., Gustafson J.A., Guttridge T.L., Guzman H.M., Hammerschlag N., Harman L., Hazin F.H.V., Heard M., Hearn A.R., Holdsworth J.C., Holmes B.J., Howey L.A., Hoyos M., Hueter R.E., Hussey N.E., Huveneers C., Irion D.T., Jacoby D.M.P., Jewell O.J.D., Johnson R., Jordan L.K.B., Jorgensen S.J., Joyce W., Keating Daly C.A., Ketchum J.T., Klimley A.P., Kock A.A., Koen P., Ladino F., Lana F.O., Lea J.S.E., Llewellyn F., Lyon W.S., MacDonnell A., Macena B.C.L., Marshall H., McAllister J.D., McAuley R., Meÿer M.A., Morris J.J., Nelson E.R., Papastamatiou Y.P., Patterson T.A., Peñaherrera-Palma C., Pepperell J.G., Pierce S.J., Poisson F., Quintero L.M., Richardson A.J., Rogers P.J., Rohner C.A., Rowat D.R.L., Samoilys M., Semmens J.M., Sheaves M., Shillinger G., Shivji M., Singh S., Skomal G.B., Smale M.J., Snyders L.B., Soler G., Soria M., Stehfest K.M., Stevens J.D., Thorrold S.R., Tolotti M.T., Towner A., Travassos P., Tyminski J.P., Vandeperre F., Vaudo J.J., Watanabe Y.Y., Weber S.B., Wetherbee B.M., White T.D., Williams S., Zárate P.M., Harcourt R., Hays G.C., Meekan M.G., Thums M., Irigoien X., Eguiluz V.M., Duarte C.M., Sousa L.L., Simpson S.J., Southall E.J., Sims D.W. Global spatial risk assessment of sharks under the footprint of fisheries. Nature. 2019;572:461–466. doi: 10.1038/s41586-019-1444-4. [DOI] [PubMed] [Google Scholar]
- Reubens J., Verhelst P., van der Knaap I., Wydooghe B., Milotic T., Deneudt K., Hernandez F., Pauwels I. The need for aquatic tracking networks: the permanent Belgian acoustic receiver network. Animal Biotelemetry. 2019;7:1–6. [Google Scholar]
- Rilov G., Fraschetti S., Gissi E., Pipitone C., Badalamenti F., Tamburello L., Menini E., Goriup P., Mazaris A.D., Garrabou J. A fast-moving target: achieving marine conservation goals under shifting climate and policies. Ecol. Appl. 2020;30 doi: 10.1002/eap.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R., Beguin J., Argillier C., Tissot L., Smith F., Smedbol S., De-Oliveira E. Testing the VEMCO positioning system: spatial distribution of the probability of location and the positioning error in a reservoir. Animal Biotelemetry. 2014;2:1–7. [Google Scholar]
- Rutz C., Loretto M.-C., Bates A.E., Davidson S.C., Duarte C.M., Jetz W., Johnson M., Kato A., Kays R., Mueller T. COVID-19 lockdown allows researchers to quantify the effects of human activity on wildlife. Nature Ecology Evolution. 2020;4:1156–1159. doi: 10.1038/s41559-020-1237-z. [DOI] [PubMed] [Google Scholar]
- Simpfendorfer C.A., Heupel M.R., Hueter R.E. Estimation of short-term centers of activity from an array of omnidirectional hydrophones and its use in studying animal movements. Can. J. Fish. Aquat. Sci. 2002;59:23–32. [Google Scholar]
- Smoothey A.F., Lee K.A., Peddemors V.M. Long-term patterns of abundance, residency and movements of bull sharks (Carcharhinus leucas) in Sydney harbour, Australia. Sci. Rep. 2019;9:1–16. doi: 10.1038/s41598-019-54365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- State of Queensland Department of Agriculture Fisheries 2020. https://fishnet.fisheries.qld.gov.au/Content/Public/PublicRegister.aspx
- Steckenreuter A., Hoenner X., Huveneers C., Simpfendorfer C., Buscot M.J., Tattersall K., Babcock R., Heupel M., Meekan M., van den Broek J., McDowall P., Peddemors V., Harcourt R. Optimising the design of large-scale acoustic telemetry curtains. Mar. Freshw. Res. 2017;68:1403–1413. [Google Scholar]
- Stock A., Crowder L.B., Halpern B.S., Micheli F. Uncertainty analysis and robust areas of high and low modeled human impact on the global oceans. Conserv. Biol. 2018;32:1368–1379. doi: 10.1111/cobi.13141. [DOI] [PubMed] [Google Scholar]
- Tanner J., Bailleul F., Bryars S., Doubell M., Foster N., Gaylard S., Gillanders B., Goldsworthy S., Huveneers C., James C., Jones A.R., Maher J., Nursey-Bray M., van Ruth P., Ward T.M. 2019. Potential Social, Economic and Ecological Indicators for Integrated Ecosystem Assessment of Spencer Gulf. (Goyder Institute for Water Research Technical Report Series) [Google Scholar]
- Taylor M.D., Laffan S.W., Fairfax A.V., Payne N.L. Finding their way in the world: using acoustic telemetry to evaluate relative movement patterns of hatchery-reared fish in the period following release. Fish. Res. 2017;186:538–543. [Google Scholar]
- Thomson D.J., Barclay D.R. Real-time observations of the impact of COVID-19 on underwater noise. The Journal of the Acoustical Society of America. 2020;147:3390–3396. doi: 10.1121/10.0001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thums M., Fernández-Gracia J., Sequeira A.M., Eguíluz V.M., Duarte C.M., Meekan M.G. How big data fast tracked human mobility research and the lessons for animal movement ecology. Front. Mar. Sci. 2018;5:21. [Google Scholar]
- Tokic D. Long-term consequences of the 2020 coronavirus pandemics: historical global-macro context. Journal of Corporate Accounting Finance. 2020;31:9–14. [Google Scholar]
- Tourism Australia International market performance statistics. 2020. https://www.tourism.australia.com/en/markets-and-stats/tourism-statistics/international-market-performance.html
- Tourism Australia The value of tourism. 2020. https://www.tourism.australia.com/content/dam/assets/document/1/c/1/k/n/2241527.pdf
- Udyawer V., Dwyer R.G., Hoenner X., Babcock R.C., Brodie S., Campbell H.A., Harcourt R.G., Huveneers C., Jaine F.R.A., Simpfendorfer C.A., Taylor M.D., Heupel M.R. A standardised framework for analysing animal detections from automated tracking arrays. Animal Biotelemetry. 2018;6:17. [Google Scholar]
- Wittmer H.U., McLellan B.N., Serrouya R., Apps C.D. Changes in landscape composition influence the decline of a threatened woodland caribou population. J. Anim. Ecol. 2007;76:568–579. doi: 10.1111/j.1365-2656.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- Zheng Q., Jones F.K., Leavitt S.V., Ung L., Labrique A.B., Peters D.H., Lee E.C., Azman A.S. HIT-COVID, a global database tracking public health interventions to COVID-19. Scientific Data. 2020;7:1–8. doi: 10.1038/s41597-020-00610-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity analysis testing how much changing the radius around each acoustic receiver from 5 km to 15 km affected the change in vessel presence in proximity to acoustic receivers (see section 2. COVID-19 impact on marine activities in Australia).