PURPOSE
Acute myeloid leukemia (AML) is the most prevalent acute leukemia in adults and is responsible for the majority of cancer-related mortality. In Saudi Arabia, leukemia is ranked the fifth most prevalent type of malignancy in adults. Our aim is to review existing epidemiologic data in Saudi Arabia and develop consensus guidelines for management of AML.
METHODS
We review literature related to AML epidemiology, treatment patterns, and outcomes in Saudi Arabia, as well as literature related to the current advances in AML treatment. A panel of 10 experts from eight institutions in Saudi Arabia reviewed the literature and developed a consensus statement.
RESULT
We provide an update of the available AML epidemiologic data in Saudi Arabia and describe recent developments in the diagnostic workup, risk stratification, and treatment algorithm. The consensus recommendations for the management of AML in Saudi Arabia were developed.
CONCLUSION
The recommendations are in parallel with the recent international guidelines for the diagnosis and management of AML.
INTRODUCTION
Acute myeloid leukemia (AML) is a malignant disorder of the hematopoietic stem cells and progenitor cells, characterized by the progressive clonal expansion of abnormally immature myeloid cells within the bone marrow and peripheral blood. This results in the impairment of normal hematopoiesis and progressive marrow failure, which is usually fatal if left untreated.1 The advances in AML treatment and our understanding of AML have significantly improved the survival of younger patients diagnosed with AML. However, the prognosis for elderly patients with AML is still poor, with a 1-year survival of < 30%.2,3 The current treatment landscape of AML includes induction chemotherapy, followed by a consolidation strategy using either chemotherapy or allogeneic hematopoietic cell transplantation (allo-HCT). Navigating the current landscape of new therapeutic developments for AML is a major challenge, because of the rapidly expanding volume of data generated over the past few years. In this paper, we review the current state of the art in the practices related to AML diagnosis, risk stratification, and treatment updates. We describe some of the challenges related to the management of AML in Saudi Arabia and present summary recommendations in the context of available resources and the institutional variation to optimize the management.
CONTEXT
Key Objective
Recent progress in acute myeloid leukemia (AML) understanding resulted in the approval of multiple novel agents. Navigating treatment options for patients with newly diagnosed AML has become a challenging task, primarily because of limited availability of novel agents and incomplete diagnostic workup to identify targeted mutations. Lack of disease local epidemiologic and economic data adds more complexity to inform local health care policies to prioritize options on the basis of needs. In this review, available local epidemiologic data were reviewed and compared with international figures. Also, the latest AML diagnostic and therapeutic updates were discussed.
Knowledge Generated
Multiple local challenges of AML management were identified, and potential solutions were recommended. Recommendations for workup and management of AML were generated for local guidance on the basis of available published data, local practice experience, and available resources.
Relevance
The development of local guidelines would unify the management approach of AML and optimize early patient referral.
CONSENSUS DEVELOPMENT
The panel consists of 10 members with clinical expertise in AML from eight institutions in Saudi Arabia. The first meeting of the panel was held during the Saudi Society of Blood and Marrow Transplantation meeting held in 2020. The members searched the literature and produced a consensus statement.
EPIDEMIOLOGY AND BURDEN OF AML IN SAUDI ARABIA
The global incidence rate of AML ranges from 0.9 to 2.8 per 100,000 population in males and 0.4-2.2 per 100,000 population in females.4 In 2020, 19,940 new cases were estimated in the United States alone, giving an age-adjusted annual incidence rate of 4.3 per 100,000 population and an estimated lifetime risk of developing AML of 0.5%.5 The incidence of AML is believed to be broadly similar across European countries, at 3.5 cases per 100,000 population per year.6 According to the Saudi Cancer Registry (SCR) in 2016, the age-standardized rate of leukemia was 3.6 per 100,000 in males and three per 100,000 in females. AML was diagnosed in almost 17% and 25% of male and female leukemia cases, respectively, with actual reported numbers of 59 males and 70 females.7 The incidence of AML varied slightly per region in Saudi Arabia, with the highest rate in the Eastern Region.8 AML is considered a disease of the elderly population. In the United States, the median age at diagnosis of AML ranged from 62 to 68 years.9 A similar range was observed in Europe and Canada.10,11 However, AML is more prevalent in patients younger than 60 years old in countries such as Brazil and Algeria.12,13 In Saudi Arabia, the median age at diagnosis of AML appears to be lower than that reported for the United States and Europe, although the evidence base to support the assumption is limited to single institution experiences.14,15 Although there is no published accurate estimate of AML ranking in comparison with other cancers, leukemia, in general, is a public health burden in Saudi Arabia and ranked as the fifth most prevalent type of cancer.7 Over the past 15 years, the incidence of leukemia increased steadily in the different age groups.8 There is a lack of studies investigating the economic cost and burden of AML in Saudi Arabia. However, AML is globally known to be associated with high cost and a major economic burden, primarily because of the need for prolonged hospitalization, treatment of complications, and the associated medical procedures, such as allo-HCT.16 The current local literature may not be representative of the AML epidemiology in Saudi Arabia. The only reliable source is the SCR. It should be noted that the data provided by the SCR are probably underestimated, possibly because of some peripheral hospitals not having the diagnostic capabilities to evaluate patients with suspected AML, or the patient being unfit for invasive diagnostic procedures, or only fit patients are referred and accepted in tertiary hospitals for the evaluation and management of AML and subsequently recorded in the SCR. In addition, many of the referring physicians at peripheral hospitals may not be aware of the latest advances in the treatment of AML for the unfit group, resulting in a lower referral rate to tertiary hospitals.
Panel consensus: The panel agreed that local literature may not be representative of the AML epidemiology in Saudi Arabia. The panel recommends a national and unified registry for AML to improve our understanding of the epidemiology. Also, the panel recommends outreach campaigns to peripheral hospitals to increase the awareness and the level of knowledge regarding recent advances in AML, to overcome the challenges related to the diagnosis and management of the older unfit AML population.
CHALLENGES OF AML MANAGEMENT IN SAUDI ARABIA
The panel acknowledges some unmet needs in the management of AML. Currently, the data related to the burden of the disease and local epidemiology are scarce. Although the establishment of an electronic referral system resulted in rapid access for patients with AML to a tertiary hospital for advance care, many elderly unfit patients, not eligible for allo-HCT, are persistently treated at local hospitals, which might not be equipped to treat such patients. Next-generation sequencing (NGS)–based molecular testing is not available at many hospitals treating patients with AML, compelling the hospitals to send samples to a reference laboratory outside the country, which delays the results. Another important matter is the high cost of the newer AML therapeutic agents, in addition to the lack of AML clinical trials in Saudi Arabia, which limits patients' access to novel agents. The cost of health care is another challenge in the management of AML cases. Adult patients with AML usually require prolonged hospitalization during the treatment cycles and frequent intensive care unit admission, increasing the economic burden.17
Panel consensus: The panel recommends broadening the relationship between governmental health sectors and pharmaceutical industries to increase clinical trials. Increasing the number of clinical trials, particularly industry-sponsored clinical trials, would positively affect the practice of medicine and increase the opportunity for patients to access the latest pharmaceuticals and novel compounds at an early stage. It should also indicate the suitability of some of the novel agents in our patient population, as they are under-represented in international clinical trials.
The panel recognizes the deficit of local economic studies investigating the burden of AML. The recommendation is to implement strategies to measure the economic impact of AML and implement programs, for example, an outpatient intensive chemotherapy clinic, to reduce the prolonged hospital stay and cost burden.18
DIAGNOSIS AND RISK STRATIFICATION OF AML
Diagnostic Workup of AML
Although AML may be discovered accidentally during routine blood tests, the majority of cases present with the signs and symptoms of one or more forms of cytopenia, including symptomatic anemia, bleeding, or infection. The physical examination may reveal bruises or bleeding because of thrombocytopenia. Extramedullary disease can be observed in some patients, including CNS involvement.19 Whenever indicated, imaging such as positron emission tomography can be used to evaluate potential extramedullary disease. However, a biopsy from the suspected site is essential to confirm the disease, as multiple factors could result in a false-positive positron emission tomography scan reading, such as infections and the use of growth factor agents. Any neurologic signs or symptoms should be evaluated through a diagnostic lumbar puncture, once the blasts are reduced in the peripheral blood, and magnetic resonance imaging of the brain. Lumbar puncture can also be considered for patients presented with monocytic differentiation or high white blood cell count.20
The criteria for the diagnosis of AML depend on the number of myeloblasts within the bone marrow or peripheral blood. The 2016 revision of the WHO classification of myeloid neoplasms and acute leukemia stated that the diagnosis of AML is established when the myeloblasts represent at least 20% of the nucleated cells in the bone marrow (biopsy or aspirate) or peripheral blood smear and at least 500 bone marrow nucleated cells or 200 peripheral blood leukocytes should be counted.21 Patients with AML-related recurrent cytogenetic abnormalities, for example, t(8:21), are diagnosed as AML cases, irrespective of the blast count. The identification of the myeloid lineage of blasts is done through the visualization of Auer rods, positive myeloperoxidase staining, or, more frequently, the overexpression of myeloid markers such as CD13, CD33, or CD117 by flow cytometry.22 The presence of monocytic markers (such as CD14, CD11c, or CD64) provides evidence of monocytic differentiation of leukemic cells. Past medical history of previous exposure to radiation or chemotherapy is important to establish the diagnosis of therapy-related AML. A history of myelodysplastic syndrome or myelodysplastic and myeloproliferative neoplasm, in addition to other criteria, is also important for establishing the diagnosis of AML with myelodysplastic-related changes. The 2016 revision of the WHO classification of myeloid neoplasms and acute leukemia provides the full classification of AML.21 The diagnostic workup that informs the risk stratification and treatment approaches includes cytogenetics and molecular investigations.20,23 Various genetic testing techniques are available to characterize the genetic alterations in patients with AML. Conventional karyotyping can mainly identify numerical abnormalities, and fluorescence in situ hybridization allows for high-resolution analysis of recurrent structural chromosomal rearrangements, specific to AML. Fluorescence in situ hybridization is mandatory if conventional karyotyping failed to detect cryptic aberrations (ie, inv16), specifically in AML with normal karyotypes.24 Molecular testing for certain genetic mutations such as FLT3 (internal tandem duplication [ITD] and tyrosine kinase domain), using real-time quantitative polymerase chain reaction, must be expedited to initiate the FLT3 inhibitor. Other molecular studies to detect mutational genetic abnormalities are important for the risk stratification (TP53, NPM1, RUNX1, ASXL1, KIT and biallelic CEBPA).25 Molecular studies using a reverse transcriptase polymerase chain reaction are required for the quantification of a fusion transcript for RUNX1-RUNX1T1, CBFB-MYH11, and PMLRARA, as well as quantitative polymerase chain reaction for NPM1 mutation, at the time of diagnosis and during the treatment for measurable residual disease (MRD) monitoring. The recommendation is to perform these tests on bone marrow samples, preferably on the first bone marrow aspirate obtained to avoid sample hemodilution. The use of NGS with the common myeloid gene panel gained importance to inform the risk stratification and therapeutic options.26
Risk Stratification
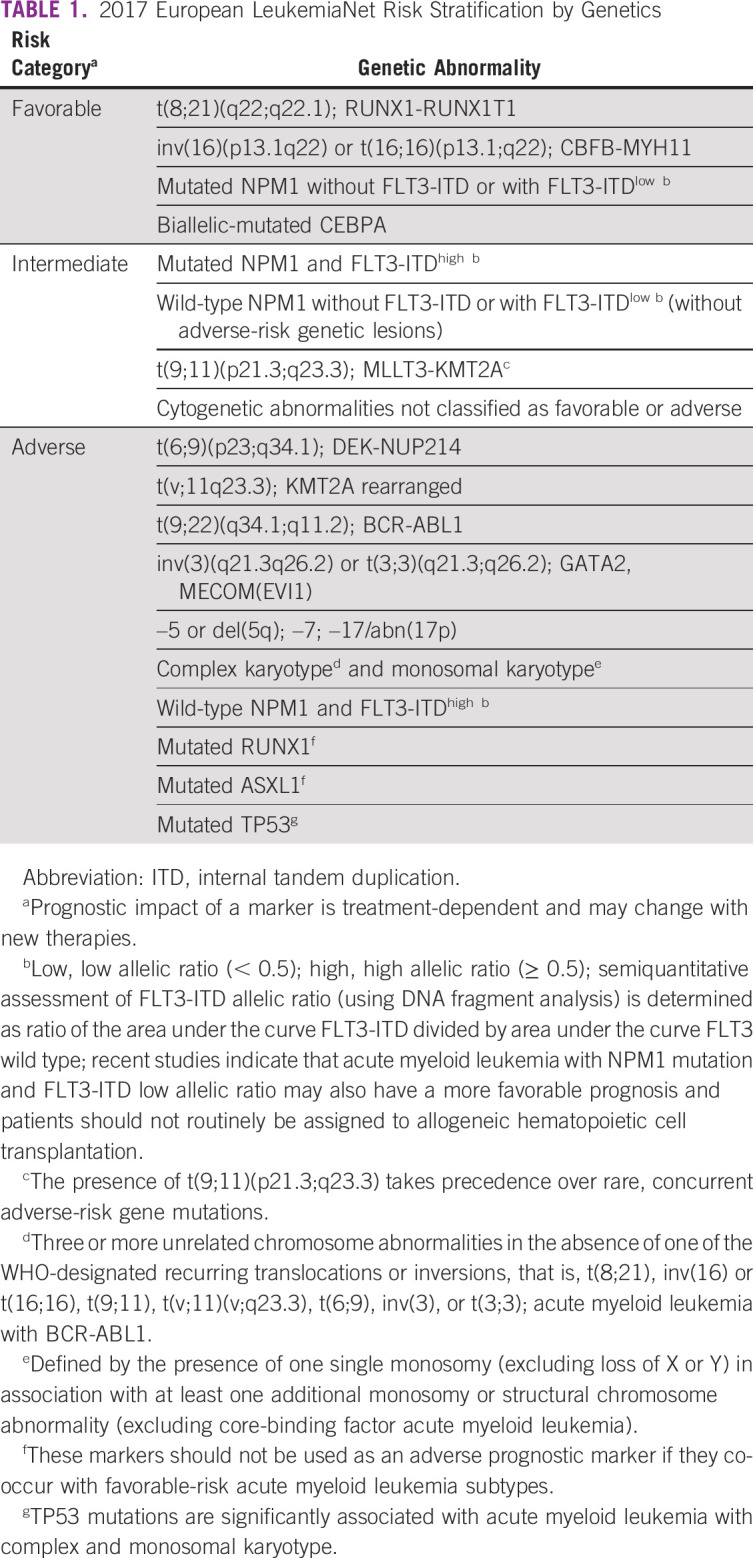
Risk stratification of AML is critical to predict the response rate and survival of each risk group, especially after the introduction of more therapeutic options. The identification of the risk groups can assist the clinician to determine the intensity of the induction therapy and the kind of postremission therapy (either chemotherapy or allo-HCT). The current body of evidence indicates that genetic abnormalities are the strongest predictors of the outcome in AML. According to the 2017 European LeukemiaNet (ELN) recommendations, AML can be stratified in favorable, intermediate, or adverse prognostic risk groups, on the basis of their genetic profile alone (Table 1).27
TABLE 1.
2017 European LeukemiaNet Risk Stratification by Genetics
In addition to cytogenetics and the molecular status, MRD is another important prognostic indicator. Literature indicates that a negative MRD is highly correlated with treatment outcomes.28,29 Methods that are currently widely applied, including multiparameter flow cytometry and reverse transcriptase polymerase chain reaction, in addition to newer technologies, including digital PCR and NGS, are emerging. The clinical use and technical aspects of MRD were recently endorsed by the ELN MRD Working Party.30
Panel consensus: Although some centers in Saudi Arabia are equipped with advanced laboratories to perform the diagnostic and molecular genetic testing, for the majority of centers this is not the case. This deficit can jeopardize the delivery of effective immediate treatment as discussed in the Management of AML section.
The panel recommends establishing a local central reference laboratory, equipped with state-of-the-art advanced molecular technology. This will expedite testing, standardize results, and decrease the cost of external tests.
The panel recommends an algorithmic diagnostic approach for the diagnosis and classification of AML, on the basis of the 2017 WHO classification (Fig 1), as well as a workup algorithm to guide physicians, which will support risk stratification and therapeutic decisions (Fig 2).
FIG 1.
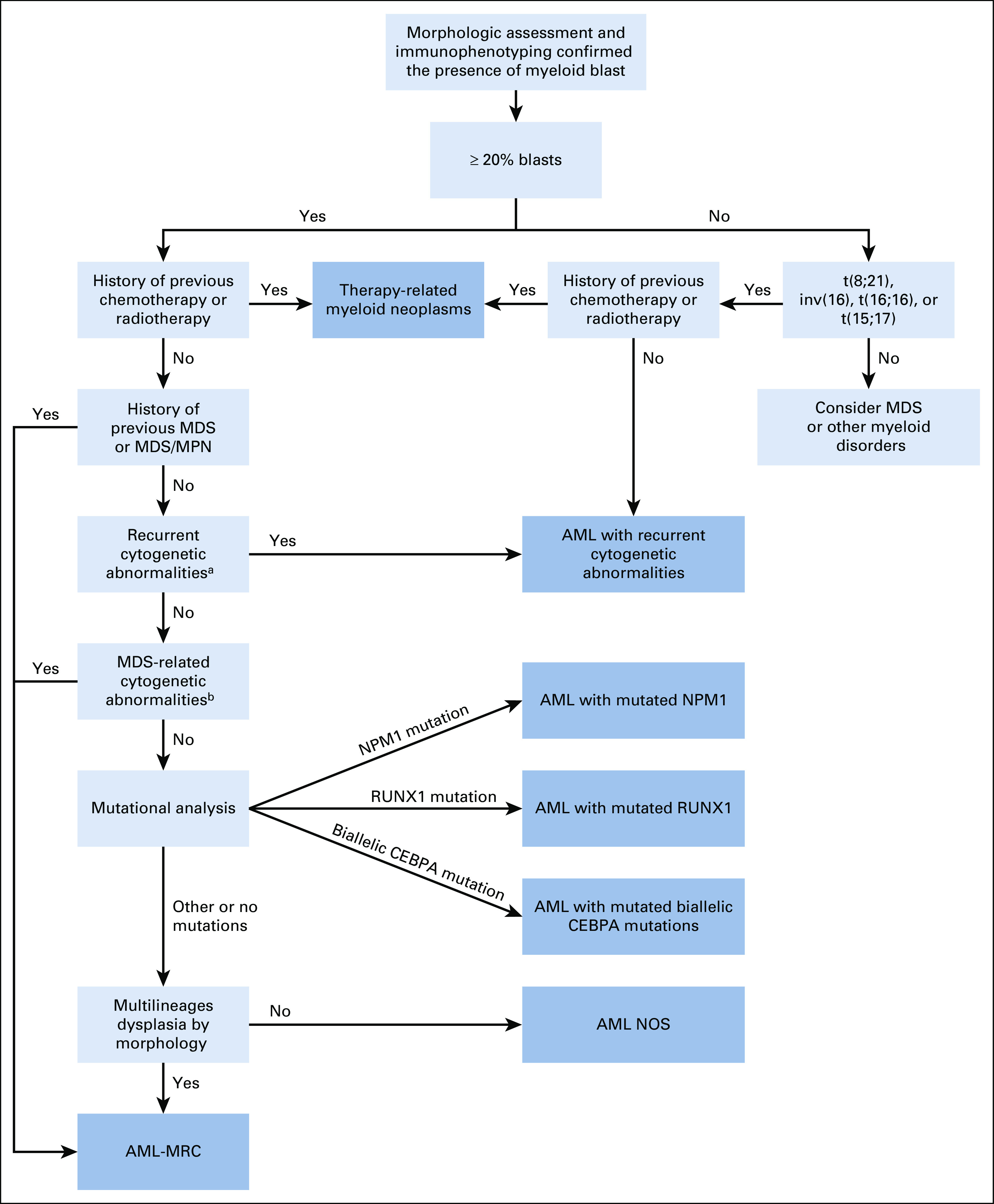
Diagnostic algorithm for AML according to the 2017 WHO classification. aRecurrent cytogenetic abnormalities: t(8;21), inv(16), t(16;16), t(15;17), t(9;11), t(6;9), inv(3), t(3;3), and t(1;22). bMDS-related cytogenetic abnormalities: Complex karyotype. Unbalanced abnormalities: –7/del(7q), –5/del(5q), i(17q)/t(17p), –13/del(13q), del(11q), del(12p)/t(12p), and idic(X)(q13). Balanced abnormalities: t(11;16), t(3;21), t(1;3), t(2;11), t(5;12), t(5;7), t(5;17), t(5;10), and t(3;5). AML, acute myeloid leukemia; AML-MRC, AML with myelodysplasia-related changes; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; NOS, not otherwise specified.
FIG 2.
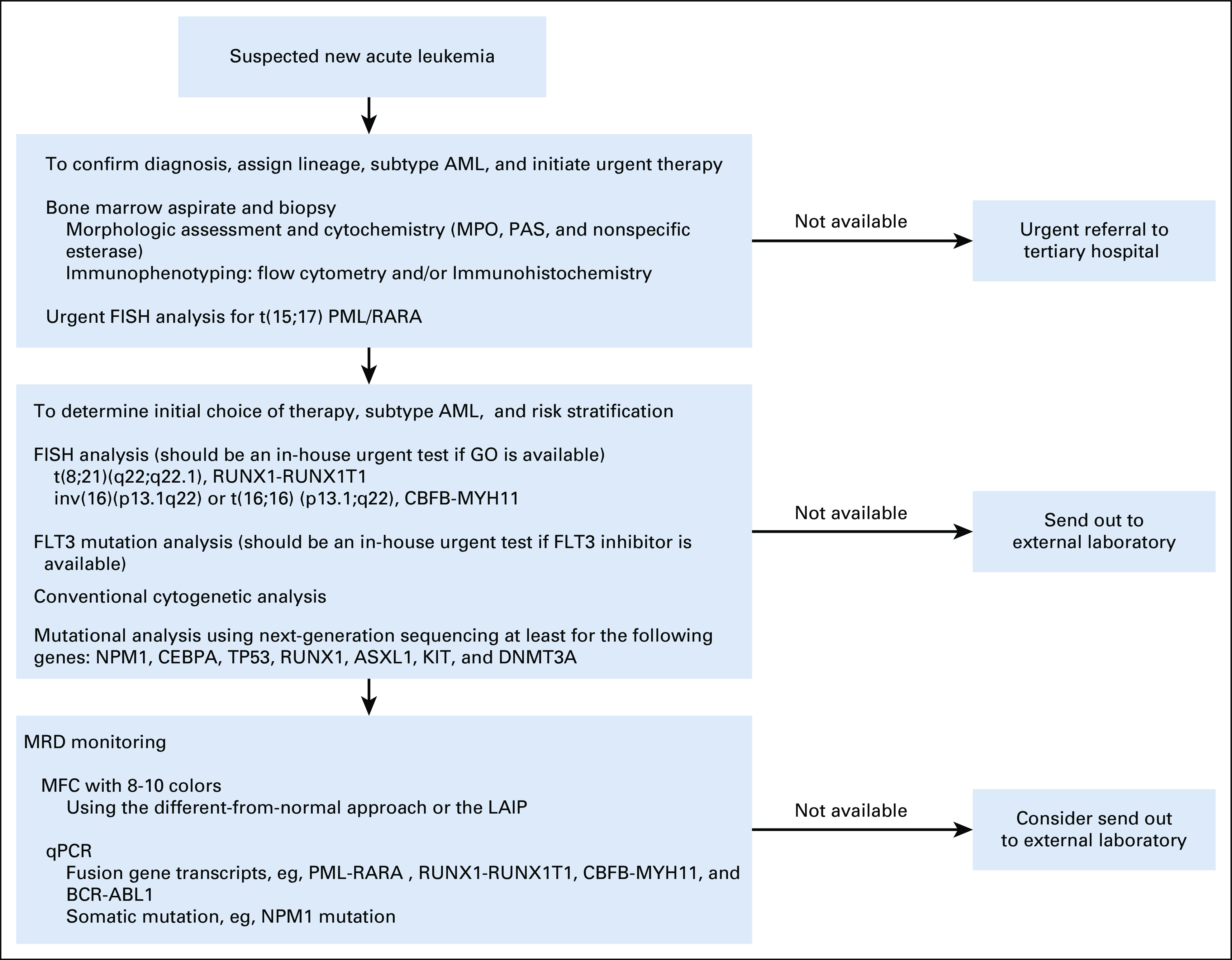
Approach to workup of AML. AML, acute myeloid leukemia; FISH, fluorescence in situ hybridization; GO, gemtuzumab ozogamicin; LAIP, leukemia-associated immunophenotype; MFC, multiparameter flow cytometry; MPO, myeloperoxidase; MRD, measurable residual disease; PAS, periodic acid Schiff; qPCR, quantitative polymerase chain reaction.
From the panel, only a few centers have the in-house capability to measure the MRD status (using molecular and/or multiparameter flow cytometry techniques).
MANAGEMENT OF AML
Patient's eligibility for intensive chemotherapy is determined on the basis of age, comorbidities, performance status, and the genetic risk profile. The German AML Cooperative Group developed a predictive model for complete remission (CR) and early death, taking into account the hematologic parameters, type of leukemia, the age, and the cytogenetics of the patient.31 Similar predictive models are available.32,33 The goal of treatment for AML is to achieve CR through the reduction of the blast count to < 5% in the bone marrow and restoration of normal hematopoiesis. The induction therapy is followed by consolidation regimens to prevent relapse and provide a durable response.
General Initial Management of AML
Around 20% of patients with AML present with hyperleukocytosis, which is associated with a high risk of mortality and morbidity.34 Patients presenting with hyperleukocytosis (WBC ≥ 100 × 109/L) can be managed with hydroxyurea and supportive measures. Leukapheresis can be used as an option for cytoreduction, in conjunction with other measures, in patients with symptoms related to hyperleukocytosis. However, the effect on the reduction of early mortality is less clear.34,35 Prompt initiation of induction chemotherapy, in fit patients, is recommended for symptomatic patients in addition to other management and supportive measures for tumor lysis syndrome, coagulopathy, infections, and bleeding.27 The use of antimicrobial prophylaxis during induction is recommended including quinolone, posaconazole, and acyclovir.36,37 It is possible, in the case of low-proliferative AML, to withhold AML-directed treatment until the results from cytogenetics and molecular testing are available, especially if this is expected to influence the choice of therapy.38 In patients with CNS involvement, intrathecal cytarabine with or without methotrexate twice weekly should be used until blast clearance from the cerebrospinal fluid.39
Panel consensus: Hydroxyurea or induction chemotherapy can be sufficient to treat AML presenting with hyperleukocytosis in the absence of apheresis service or if apheresis is expected to be delayed. Apheresis should be avoided in acute promyelocytic leukemia.
Management of Patients With Newly Diagnosed AML Fit for Intensive Chemotherapy
Induction therapy.
Seven days of standard-dose cytarabine (100-200 mg/m2/d) plus 3 days of anthracycline (7 + 3) is the standard induction regimen in patients fit for intensive chemotherapy. Daunorubicin at a dose of 60-90 mg/m2 once daily or idarubicin at a dose of 12 mg/m2 once daily for 3 days is the current anthracycline of choice, which can result in a 60%-85% remission rate in patients age < 60 years and in 40%-60% in the older patient group.40 Patients with CD33-positive AML may benefit from gemtuzumab ozogamicin (GO), which improved the survival benefit when added to the standard induction therapy in patients with favorable- or intermediate-risk cytogenetics.41 There was an improvement in the 6-year overall survival (OS) of 20.7% in the favorable risk group; however, for the intermediate risk group, the improvement was significant, but only 5.7%. The adverse risk group did not benefit from the addition of GO, and it is not recommended for this group of patients. The approved GO dose is 3 mg/m2 on days 1, 4, and 7 of induction. Alternatively, a single dose of 3 mg/m2 during the standard induction chemotherapy can also be considered.41 In FLT3-mutated AML cases, midostaurin had a significant OS improvement in comparison with the placebo, when added to the standard induction and consolidation regimens, with the median OS of 74.7 versus 25.6 months.42 Midostaurin is now recommended for FLT3-mutated (ITD and tyrosine kinase domain) AML, added to the standard induction 7 + 3 and consolidation. Sorafenib, a multikinase inhibitor, appears to improve survival in a retrospective study using a propensity score for patients with newly diagnosed FLT3-ITD AML.43 In patients with ELN adverse risk, the addition of a purine analog, cladribine, to the standard induction resulted in an improvement in OS.44 A large randomized study reported that fludarabine, cytarabine, GCSF, and idarubicin (FLAG-IDA) was associated with an improved relapse-free survival (RFS), traded with an increased treatment-related mortality.45 Although most cases of AML are de novo, secondary AML and therapy related-AML, representing 25% of all AML cases, are associated with poor outcomes. Emerging data demonstrated improved survival in older patients with secondary AML when a dual-drug liposomal formulation of cytarabine and daunorubicin in a 5:1 molar ratio (CPX-351) is used as frontline therapy.46
After induction, a bone marrow assessment at day 14 or nadir bone marrow is not recommended, because of no proven benefit.47 The response to the induction therapy should be assessed by a bone marrow aspirate and biopsy after count recovery or at day 28. Patients with a bone marrow blast ≥ 5% should receive a second induction identical to the first one or alternatively, a cytarabine-containing regimen such as FLAG-IDA. Patients in CR or CR with incomplete count recovery (CRi), after one or two inductions, should proceed with postremission therapy. However, if not in CR/CRi after two inductions, the patient should be managed as primary refractory AML.27
Postremission therapy.
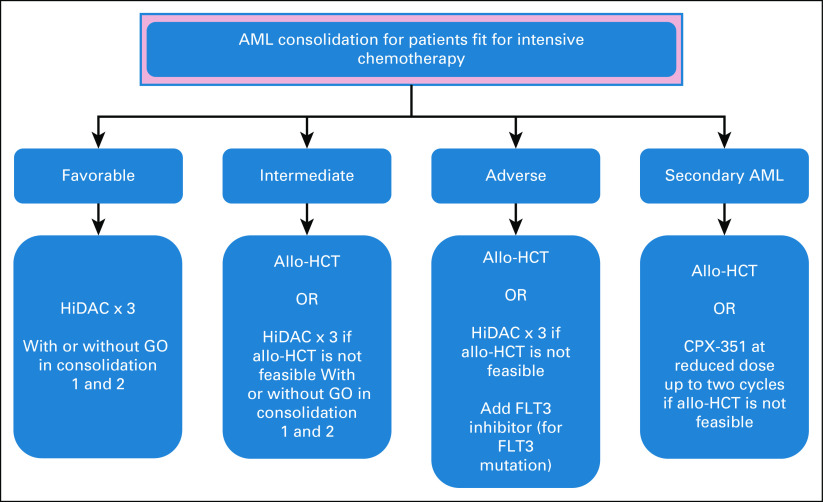
After the achievement of remission after induction, patients with an ELN favorable risk should proceed with chemotherapy consolidation only. However, patients with an intermediate or adverse risk should be consolidated with allo-HCT because of the poor outcome associated with chemotherapy-based consolidation in this group.27
Consolidation chemotherapy
Patients with favorable risk should be consolidated with chemotherapy using high-dose cytarabine (HiDAC) 1.5-3 g/m2 (every 12 hours in days 1, 3, and 5) for three cycles in patients age < 60 years or 1-1.5 g/m2 for patients age 60 years and above.20 The addition of GO to consolidation cycles 1 and 2 can be considered, although no clear benefit is observed.48 In core-binding factor AML-associated KIT mutation, chemotherapy consolidation alone may be associated with a poor outcome, particularly in t(8;21).49,50 However, emerging data indicate that the absence of molecular MRD may mitigate the poor outcome associated with a KIT mutation and the need for allo-HCT.28 Patients with an FLT3 mutation who could not proceed to allo-HCT can also receive HiDAC consolidation with the addition of midostaurin.42 Other risk category patients should also receive HiDAC if there is no suitable donor or the patient is not fit for allo-HCT. CPX-351 with a reduced dose can be used for consolidation after a successful induction with CPX-351.46 Autologous transplant resulted in improved RFS in selected patient groups, without an OS advantage. The role of autologous transplant in AML is not yet clear.51,52
Allogeneic hematopoietic cell transplantation
Allo-HCT is a potentially curative modality for patients with AML. Patients with AML comprise the majority of patients who receive allo-HCT, particularly after the major advances in conditioning regimen and the use of alternative donors.53-55 A growing body of evidence supports the efficacy of allo-HCT in patients with ELN intermediate-risk and adverse-risk cytogenetics, with no survival benefit observed in patients with favorable-risk cytogenetics.56 In the SWOG and Eastern Cooperative Oncology Group trial, the 5-year survival of the group who received allo-HCT was 44%, compared with 13% for the group who received autologous in the subgroup of patients with unfavorable cytogenetics. However, no difference was detected between the autologous HCT group and the chemotherapy consolidation group. Similar findings were reported for patients with intermediate-risk cytogenetics.57 Insufficient data are available to evaluate the use of allo-HCT in the first remission for patients with AML and favorable-risk cytogenetics, outside a clinical trial.58 Allo-HCT is recommended for patients in first CR with ELN intermediate or adverse risk, who are fit with age ≤ 75 years.27 Selecting a fit patient is an important step to ensure a successful outcome after the transplant. The use of hematopoietic cell transplantation comorbidity index can predict nonrelapse mortality and OS post–allo-HCT.33 The donor pool is expanding because of the improved outcome after the use of alternative donor (cord blood and haploidentical transplant).54,55 The first donor choice is an HLA-matched sibling donor followed by an unrelated matched donor. In Saudi Arabia, the chance of finding a matched sibling donor is almost 60% in comparison with < 30% in western countries, because of the large family size in the former.59,60 An alternative donor can be considered for patients without an HLA-matched donor. The use of myeloablative conditioning regimen resulted in lower relapse and an improved outcome in comparison with reduced-intensity conditioning (RIC), but with increased toxicity.61 The optimal conditioning regimen is not yet defined; however, younger (age ≤ 55 years) and fit patients should receive myeloablative conditioning.
Panel consensus: 7 + 3 remains the treatment of choice. If available, GO and midostaurin should be added for favorable-risk and FLT3-mutated AML, respectively (Fig 3). Day 14 marrow can be considered at physician discretion. Postremission therapy should be based on risk stratification according to the algorithm in Figure 4.
FIG 3.
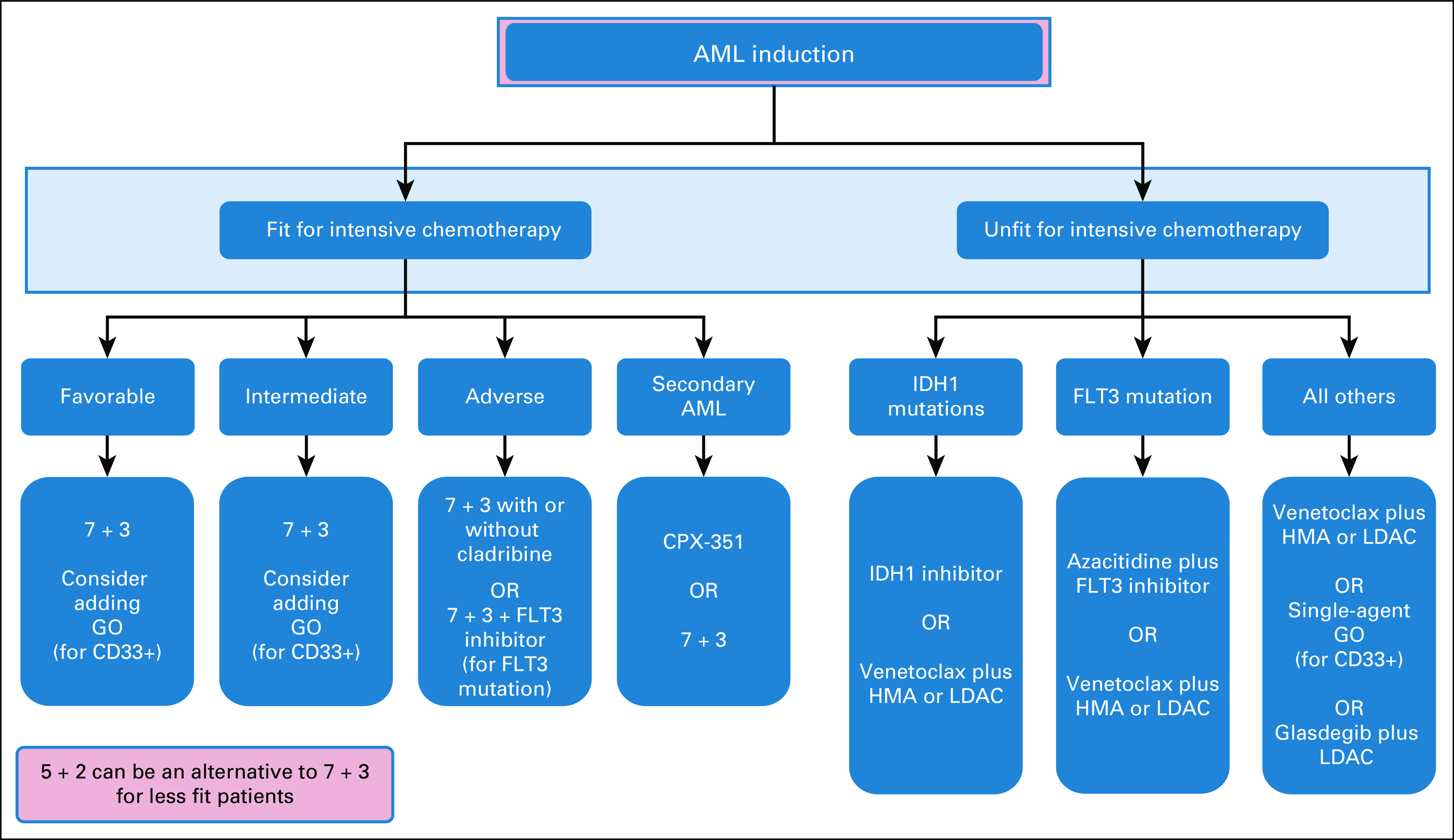
Treatment algorithm for first-line therapy for patients with newly diagnosed acute myeloid leukemia. 7 + 3, 7 days of standard-dose cytarabine plus 3 days of idarubicin or daunorubicin; AML, acute myeloid leukemia; CPX-351, liposomal daunorubicin and cytarabine; GO, gemtuzumab ozogamicin; HMA, hypomethylation agent; LDAC, low-dose cytarabine.
FIG 4.
Treatment algorithm for acute myeloid leukemia consolidation therapy for patients fit for intensive therapy. Allo-HCT, allogeneic hematopoietic cell transplantation; AML, acute myeloid leukemia; CPX-351, liposomal daunorubicin and cytarabine; GO, gemtuzumab ozogamicin; HiDAC, high-dose cytarabine (1.5-3 g/m2).
Management of Patients With Newly Diagnosed AML Not Fit for Intensive Chemotherapy
Elderly patients are characterized by a poor performance status, high frequency of medical comorbidities, higher probability for high-risk cytogenetics, and a poor response to standard induction chemotherapy. In large registries, patients who are older than 65 years did not experience a notable improvement in their outcomes and they had a high rate of treatment-related mortality.62 A recent retrospective study indicated a survival advantage with hypomethylating agents (HMAs) over intensive chemotherapy for elderly patients.63 For patients with newly diagnosed AML, not fit for intensive chemotherapy, the use of azacitidine (75 mg/m2 once daily for 7 days) may offer a survival advantage over conventional care.64,65 More recently, the oral agent venetoclax (BCL2 inhibitor) emerged as a novel agent to treat AML. The addition of venetoclax to HMA or low-dose cytarabine (LDAC) in patients with AML ineligible for intensive chemotherapy had promising results in phase II studies supporting its approval.66 In the phase III trials, venetoclax plus azacitidine had a higher remission rate compared with azacitidine monotherapy (66% v 28%, P < .001) and a longer OS.67 In combination with LDAC, venetoclax also had a higher remission rate of 48% compared with 13% for LDAC as a single agent.68 For patients started on HMA monotherapy, the response assessment should be at least after 2-3 cycles and therapy should continue until disease progression, usually four to six cycles are required before the patient could be deemed not benefiting from therapy. Other nonintensive treatment strategies include low-dose cytarabine plus glasdegib, single-agent GO, and IDH-targeted inhibitors, enasidenib or ivosidenib.20 FLT3-ITD–positive older AML patients may benefit from the addition of sorafenib to azacitidine on the basis of a phase II study with a response rate of 46%.69
Patients who are in remission after a nonintensive approach should be evaluated for RIC allo-HCT. Previous retrospective studies indicated that RIC allo-HCT resulted in a 2-year survival of 40%-60% and a nonrelapse mortality of 20%.53,70 In addition, RIC allo-HCT was associated with a lower rate of relapse than patients who received autologous hematopoietic cell transplantation or standard chemotherapy.70,71
Panel consensus: The panel recommends nonintensive therapy for unfit patients on the basis of mutational status and drug availability according to the algorithm in Figure 3.
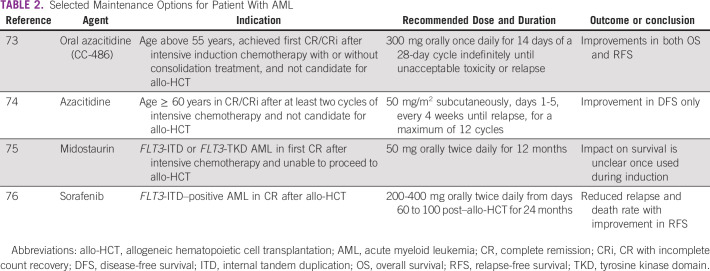
Maintenance Therapy
Multiple agents have been tested recently to define the role of maintenance therapy after intensive chemotherapy or allo-HCT.72 Few agents that showed promising outcome are summarized in Table 2.73-76 The benefit of HMA maintenance after allo-HCT is still unclear.72
TABLE 2.
Selected Maintenance Options for Patient With AML
Management of Relapsed and Refractory AML
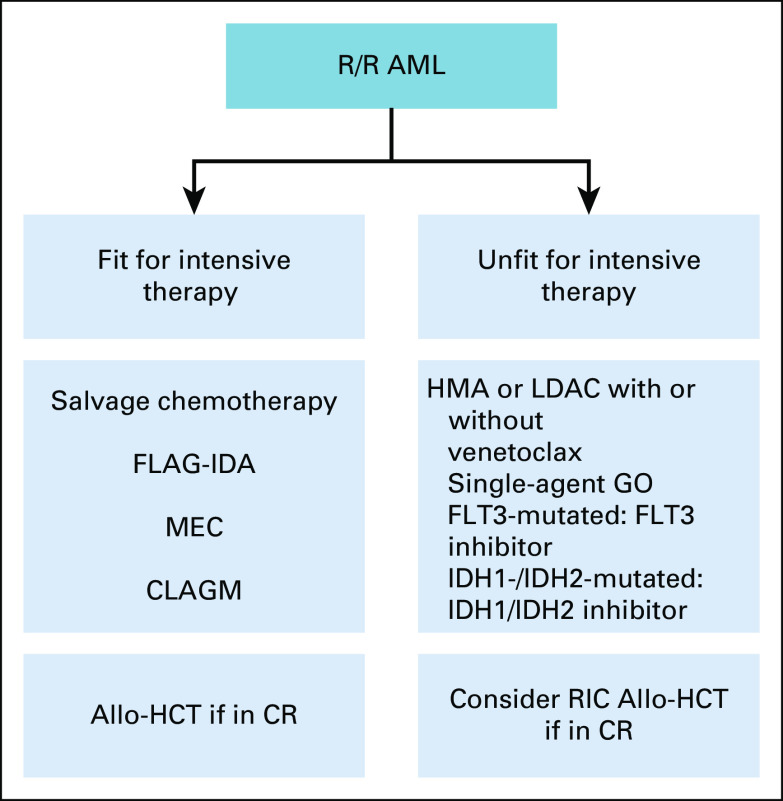
Depending on the underlying ELN risk category, 50% to 70% of patients with AML will eventually relapse after achieving remission.77 The management of relapsed or refractory (R/R) AML is challenging, with a reported cure rate in some patient groups of < 10%.78 A prognostic scoring index can be used to predict the long-term outcome after relapse and to select candidates for salvage therapy.78 Many salvage chemotherapies have been studied in R/R AML, including FLAG-IDA, MEC (mitoxantrone, etoposide, and cytarabine), or cladribine-based (cladribine, cytarabine, and GCSF) with an overall CR/CRi rate up to 50%.79,80 In medically fit patients, intensive chemotherapy can be administrated to achieve a second CR, followed by allo-HCT, which can result in 30% long-term survival.81 Patients with primary refractory AML or who relapsed within 6 months after the first CR represent a major challenge. The use of sequential transplant-conditioning regimens may provide a chance of cure for this AML patient group.82 Patients with AML who relapsed after allo-HCT have a long-term OS as low as 4%, depending on the time of relapse after transplant.79 The therapeutic options at this stage include azacitidine followed by donor lymphocyte infusion, or a second allo-HCT, if remission has been achieved. A growing number of clinical trials investigated novel agents in patients with R/R AML, demonstrating promising results. Gilteritinib, an oral selective FLT3 inhibitor, was recently approved for FLT3-mutated R/R AML, after a phase III study showed significant improvement in survival compared with salvage chemotherapy (median OS 9.3 months v 5.6 months).83 In addition, relapsed patients not fit for intensive therapy can be treated with venetoclax in combination with HMA or LDAC, on the basis of retrospective data that produced an overall response rate of 40%.84 Other novel agents include ivosidenib and enasidenib for patients with an IDH1 and IDH2 mutation, respectively.85,86 GO has been approved for R/R AML on the basis of a phase II study showing a median RFS of 11.6 months87
Panel consensus: For patients with R/R AML, the treatment should be based on the patient's fitness for intensive chemotherapy according to the algorithm in Figure 5.
FIG 5.
Treatment algorithm for R/R acute myeloid leukemia. Allo-HCT, allogeneic hematopoietic cell transplantation; AML, acute myeloid leukemia; CLAGM, cladribine, cytarabine, GCSF, and mitoxantrone; CR, complete remission; FLAG-IDA, fludarabine, cytarabine, GCSF, and idarubicin; GO, gemtuzumab ozogamicin; HMA, hypomethylation agent; LDAC, low-dose cytarabine; MEC, mitoxantrone, etoposide, and cytarabine; RIC, reduced-intensity conditioning; R/R, relapsed or refractory.
In conclusion, the recent approval of multiple novel AML therapies increased the complexity of management decisions. Limited availability of in-house molecular diagnostics often delay the identification of patients who could benefit from approved targeted therapies. Understanding the disease epidemiology and economic burden is crucial to inform health care policy to prioritize area of unmet needs. Globally, cost-effectiveness issues and limited access to clinical trials prevent many centers from implementing currently approved therapies for their patients.
ACKNOWLEDGMENT
We would like to thank the following people for their contribution to this manuscript: Dr Susanna Wright for language editing and Ms Irma Noor Caya for secretarial services.
Mohsen Alzahrani
Honoraria: Takeda, Novartis
Consulting or Advisory Role: Takeda
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Bader Alahmari, Mohsen Alzahrani, Turki Alwasaidi, Ayman Alhejazi, Mohammed Bakkar, Amal Al Behainy, Mansour Radwi, Ahmed Alaskar
Administrative support: Ahmed Alaskar
Collection and assembly of data: Bader Alahmari, Mohsen Alzahrani, Nawal Al Shehry, Osamah Tawfiq, Mohammed Bakkar, Ahmed Alaskar
Data analysis and interpretation: Bader Alahmari, Mohsen Alzahrani, Osamah Tawfiq, Turki Alwasaidi, Mohammed Bakkar, Ahmed Alaskar
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Mohsen Alzahrani
Honoraria: Takeda, Novartis
Consulting or Advisory Role: Takeda
No other potential conflicts of interest were reported.
REFERENCES
- 1.Short NJ, Rytting ME, Cortes JE: Acute myeloid leukaemia. Lancet 392:593-606, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juliusson G Lazarevic V Hörstedt AS, et al. : Acute myeloid leukemia in the real world: Why population-based registries are needed. Blood 119:3890-3899, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyers J Yu Y Kaye JA, et al. : Medicare fee-for-service enrollees with primary acute myeloid leukemia: An analysis of treatment patterns, survival, and healthcare resource utilization and costs. Appl Health Econ Health Policy 11:275-286, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Miranda-Filho A Piñeros M Ferlay J, et al. : Epidemiological patterns of leukaemia in 184 countries: A population-based study. Lancet Haematol 5:e14-e24, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Cancer stat facts: Leukemia—acute myeloid leukemia (AML). https://seer.cancer.gov/statfacts/html/amyl.html
- 6.Visser O Trama A Maynadié M, et al. : Incidence, survival and prevalence of myeloid malignancies in Europe. Eur J Cancer 48:3257-3266, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Saudi Cancer Registry : Cancer Incidence Report 2016. National Health Information Center, 2020. https://nhic.gov.sa/eServices/Documents/2016.pdf [Google Scholar]
- 8.Bawazir A Al-Zamel N Amen A, et al. : The burden of leukemia in the Kingdom of Saudi Arabia: 15 years period (1999-2013). BMC Cancer 19:703, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song X Peng Y Wang X, et al. : Incidence, survival, and risk factors for adults with acute myeloid leukemia not otherwise specified and acute myeloid leukemia with recurrent genetic abnormalities: Analysis of the Surveillance, Epidemiology, and End Results (SEER) database, 2001-2013. Acta Haematol 139:115-127, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Shysh AC Nguyen LT Guo M, et al. : The incidence of acute myeloid leukemia in Calgary, Alberta, Canada: A retrospective cohort study. BMC Public Health 18:94, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith A Howell D Patmore R, et al. : Incidence of haematological malignancy by sub-type: A report from the Haematological Malignancy Research network. Br J Cancer 105:1684-1692, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capra M Vilella L Pereira WV, et al. : Estimated number of cases, regional distribution and survival of patients diagnosed with acute myeloid leukemia between 1996 and 2000 in Rio Grande do Sul, Brazil. Leuk Lymphoma 48:2381-2386, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Bekadja MA Hamladji RM Belhani M, et al. : A population-based study of the epidemiology and clinical features of adults with acute myeloid leukemia in Algeria: Report on behalf of the Algerian Acute Leukemia Study Group. Hematol Oncol Stem Cell Ther 4:161-166, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Abuelgasim KA Albuhayri B Munshi R, et al. : Impact of age and induction therapy on outcome of 180 adult patients with acute myeloid leukemia; retrospective analysis and literature review. Leuk Res Rep 14:100206, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alabdulwahab AS Elsayed HG Sherisher MA, et al. : AML in Saudi Arabia: Analysis according to the European LeukaemiaNet 2017 cytogenetic classification. Clin Lymphoma Myeloma Leuk 20:e212-e220, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Redaelli A Botteman MF Stephens JM, et al. : Economic burden of acute myeloid leukemia: A literature review. Cancer Treat Rev 30:237-247, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Halpern AB Culakova E Walter RB, et al. : Association of risk factors, mortality, and care costs of adults with acute myeloid leukemia with admission to the intensive care unit. JAMA Oncol 3:374-381, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mabrey FL Gardner KM Shannon Dorcy K, et al. : Outpatient intensive induction chemotherapy for acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood Adv 4:611-616, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganzel C Manola J Douer D, et al. : Extramedullary disease in adult acute myeloid leukemia is common but lacks independent significance: Analysis of patients in ECOG-ACRIN Cancer Research Group trials, 1980-2008. J Clin Oncol 34:3544-3553, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tallman MS Wang ES Altman JK, et al. : Acute myeloid leukemia, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 17:721-749, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Arber DA Orazi A Hasserjian R, et al. : The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127:2391-2405, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Hasserjian RP: Acute myeloid leukemia: Advances in diagnosis and classification. Int J Lab Hematol 35:358-366, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Heuser M Ofran Y Boissel N, et al. : Acute myeloid leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 31:697-712, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Gonzales PR, Mikhail FM: Diagnostic and prognostic utility of fluorescence in situ hybridization (FISH) analysis in acute myeloid leukemia. Curr Hematol Malig Rep 12:568-573, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Haferlach T, Schmidts I: The power and potential of integrated diagnostics in acute myeloid leukaemia. Br J Haematol 188:36-48, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bacher U Shumilov E Flach J, et al. : Challenges in the introduction of next-generation sequencing (NGS) for diagnostics of myeloid malignancies into clinical routine use. Blood Cancer J 8:113, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Döhner H Estey E Grimwade D, et al. : Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129:424-447, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jourdan E Boissel N Chevret S, et al. : Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood 121:2213-2223, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Freeman SD Hills RK Virgo P, et al. : Measurable residual disease at induction redefines partial response in acute myeloid leukemia and stratifies outcomes in patients at standard risk without NPM1 mutations. J Clin Oncol 36:1486-1497, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuurhuis GJ Heuser M Freeman S, et al. : Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood 131:1275-1291, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krug U Röllig C Koschmieder A, et al. : Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: A web-based application for prediction of outcomes. Lancet 376:2000-2008, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Walter RB Othus M Borthakur G, et al. : Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: A novel paradigm for treatment assignment. J Clin Oncol 29:4417-4423, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorror ML Storer BE Fathi AT, et al. : Development and validation of a novel acute myeloid leukemia-composite model to estimate risks of mortality. JAMA Oncol 3:1675-1682, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Röllig C, Ehninger G: How I treat hyperleukocytosis in acute myeloid leukemia. Blood 125:3246-3252, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Stahl M Shallis RM Wei W, et al. : Management of hyperleukocytosis and impact of leukapheresis among patients with acute myeloid leukemia (AML) on short- and long-term clinical outcomes: A large, retrospective, multicenter, international study. Leukemia 34:3149-3160, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J Zhou M Xu JY, et al. : Comparison of antifungal prophylaxis drugs in patients with hematological disease or undergoing hematopoietic stem cell transplantation: A systematic review and network meta-analysis. JAMA Netw Open 3:e2017652, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taplitz RA Kennedy EB Bow EJ, et al. : Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update. J Clin Oncol 36:3043-3054, 2018 [DOI] [PubMed] [Google Scholar]
- 38.Röllig C Kramer M Schliemann C, et al. : Does time from diagnosis to treatment affect the prognosis of patients with newly diagnosed acute myeloid leukemia? Blood 136:823-830, 2020 [DOI] [PubMed] [Google Scholar]
- 39.Döhner H Estey EH Amadori S, et al. : Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115:453-474, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Döhner H, Weisdorf DJ, Bloomfield CD: Acute myeloid leukemia. N Engl J Med 373:1136-1152, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Hills RK Castaigne S Appelbaum FR, et al. : Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol 15:986-996, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stone RM Mandrekar SJ Sanford BL, et al. : Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 377:454-464, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sasaki K Kantarjian HM Kadia T, et al. : Sorafenib plus intensive chemotherapy improves survival in patients with newly diagnosed, FLT3-internal tandem duplication mutation-positive acute myeloid leukemia. Cancer 125:3755-3766, 2019 [DOI] [PubMed] [Google Scholar]
- 44.Holowiecki J Grosicki S Giebel S, et al. : Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: A multicenter, randomized phase III study. J Clin Oncol 30:2441-2448, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Burnett AK Russell NH Hills RK, et al. : Optimization of chemotherapy for younger patients with acute myeloid leukemia: Results of the medical research council AML15 trial. J Clin Oncol 31:3360-3368, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Cortes JE Goldberg SL Feldman EJ, et al. : Phase II, multicenter, randomized trial of CPX-351 (cytarabine:daunorubicin) liposome injection versus intensive salvage therapy in adults with first relapse AML. Cancer 121:234-242, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terry CM Shallis RM Estey E, et al. : Day 14 bone marrow examination in the management of acute myeloid leukemia. Am J Hematol 92:1079-1084, 2017 [DOI] [PubMed] [Google Scholar]
- 48.Burnett AK Hills RK Milligan D, et al. : Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: Results of the MRC AML15 trial. J Clin Oncol 29:369-377, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Allen C Hills RK Lamb K, et al. : The importance of relative mutant level for evaluating impact on outcome of KIT, FLT3 and CBL mutations in core-binding factor acute myeloid leukemia. Leukemia 27:1891-1901, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Ishikawa Y Kawashima N Atsuta Y, et al. : Prospective evaluation of prognostic impact of KIT mutations on acute myeloid leukemia with RUNX1-RUNX1T1 and CBFB-MYH11. Blood Adv 4:66-75, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vellenga E van Putten W Ossenkoppele GJ, et al. : Autologous peripheral blood stem cell transplantation for acute myeloid leukemia. Blood 118:6037-6042, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Cornelissen JJ Versluis J Passweg JR, et al. : Comparative therapeutic value of post-remission approaches in patients with acute myeloid leukemia aged 40-60 years. Leukemia 29:1041-1050, 2015 [DOI] [PubMed] [Google Scholar]
- 53.Gupta V, Tallman MS, Weisdorf DJ: Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: Myths, controversies, and unknowns. Blood 117:2307-2318, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Ruggeri A Labopin M Sanz G, et al. : Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia 29:1891-1900, 2015 [DOI] [PubMed] [Google Scholar]
- 55.Ciurea SO Zhang MJ Bacigalupo AA, et al. : Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood 126:1033-1040, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koreth J Schlenk R Kopecky KJ, et al. : Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: Systematic review and meta-analysis of prospective clinical trials. JAMA 301:2349-2361, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slovak ML Kopecky KJ Cassileth PA, et al. : Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: A Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 96:4075-4083, 2000 [PubMed] [Google Scholar]
- 58.Aldoss I, Pullarkat V: Therapy-related acute myeloid leukemia with favorable cytogenetics: Still favorable? Leuk Res 36:1547-1551, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Jawdat DM Al Saleh S Sutton P, et al. : Chances of finding an HLA-matched sibling: The Saudi experience. Biol Blood Marrow Transplant 15:1342-1344, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Rosenmayr A Pointner-Prager M Mitterschiffthaler A, et al. : What are a patient's current chances of finding a matched unrelated donor? Twenty years' central search experience in a small country. Bone Marrow Transplant 47:172-180, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Scott BL Pasquini MC Logan BR, et al. : Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol 35:1154-1161, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kantarjian H O'Brien S Cortes J, et al. : Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer 106:1090-1098, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Talati C Dhulipala VC Extermann MT, et al. : Comparisons of commonly used front-line regimens on survival outcomes in patients aged 70 years and older with acute myeloid leukemia. Haematologica 105:398-406, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dombret H Seymour JF Butrym A, et al. : International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 126:291-299, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fenaux P Mufti GJ Hellström-Lindberg E, et al. : Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol 28:562-569, 2010 [DOI] [PubMed] [Google Scholar]
- 66.Wei AH Strickland SA Jr Hou JZ, et al. : Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: Results from a phase Ib/II study. J Clin Oncol 37:1277-1284, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DiNardo CD Jonas BA Pullarkat V, et al. : Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 383:617-629, 2020 [DOI] [PubMed] [Google Scholar]
- 68.Wei AH Montesinos P Ivanov V, et al. : Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: A phase 3 randomized placebo-controlled trial. Blood 135:2137-2145, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ravandi F Alattar ML Grunwald MR, et al. : Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood 121:4655-4662, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herr AL Labopin M Blaise D, et al. : HLA-identical sibling allogeneic peripheral blood stem cell transplantation with reduced intensity conditioning compared to autologous peripheral blood stem cell transplantation for elderly patients with de novo acute myeloid leukemia. Leukemia 21:129-135, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Farag SS Maharry K Zhang MJ, et al. : Comparison of reduced-intensity hematopoietic cell transplantation with chemotherapy in patients age 60-70 years with acute myelogenous leukemia in first remission. Biol Blood Marrow Transplant 17:1796-1803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Molica M Breccia M Foa R, et al. : Maintenance therapy in AML: The past, the present and the future. Am J Hematol 94:1254-1265, 2019 [DOI] [PubMed] [Google Scholar]
- 73.Wei AH Döhner H Pocock C, et al. : The QUAZAR AML-001 maintenance trial: Results of a phase III international, randomized, double-blind, placebo-controlled study of CC-486 (oral formulation of azacitidine) in patients with acute myeloid leukemia (AML) in first remission. Blood 134, 2019. (suppl 2; abstr LBA-3) [Google Scholar]
- 74.Huls G Chitu DA Havelange V, et al. : Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood 133:1457-1464, 2019 [DOI] [PubMed] [Google Scholar]
- 75.Larson RA Mandrekar SJ Sanford BL, et al. : An analysis of maintenance therapy and post-midostaurin outcomes in the international prospective randomized, placebo-controlled, double-blind trial (CALGB 10603/RATIFY [Alliance]) for newly diagnosed acute myeloid leukemia (AML) patients with FLT3 mutations. Blood Adv 130:145, 2017 [Google Scholar]
- 76.Burchert A Bug G Fritz LV, et al. : Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN). J Clin Oncol 38:2993-3002, 2020 [DOI] [PubMed] [Google Scholar]
- 77.Röllig C Bornhäuser M Thiede C, et al. : Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: Evaluation of the proposed reporting system. J Clin Oncol 29:2758-2765, 2011 [DOI] [PubMed] [Google Scholar]
- 78.Breems DA Van Putten WL Huijgens PC, et al. : Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol 23:1969-1978, 2005 [DOI] [PubMed] [Google Scholar]
- 79.Thol F Schlenk RF Heuser M, et al. : How I treat refractory and early relapsed acute myeloid leukemia. Blood 126:319-327, 2015 [DOI] [PubMed] [Google Scholar]
- 80.Wrzesień-Kuś A Robak T Lech-Marańda E, et al. : A multicenter, open, non-comparative, phase II study of the combination of cladribine (2-chlorodeoxyadenosine), cytarabine, and G-CSF as induction therapy in refractory acute myeloid leukemia—A report of the Polish Adult Leukemia Group (PALG). Eur J Haematol 71:155-162, 2003 [DOI] [PubMed] [Google Scholar]
- 81.Kurosawa S Yamaguchi T Miyawaki S, et al. : Prognostic factors and outcomes of adult patients with acute myeloid leukemia after first relapse. Haematologica 95:1857-1864, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmid C Schleuning M Schwerdtfeger R, et al. : Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood 108:1092-1099, 2006 [DOI] [PubMed] [Google Scholar]
- 83.Perl AE Martinelli G Cortes JE, et al. : Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med 381:1728-1740, 2019 [DOI] [PubMed] [Google Scholar]
- 84.Bewersdorf JP Giri S Wang R, et al. : Venetoclax as monotherapy and in combination with hypomethylating agents or low dose cytarabine in relapsed and treatment refractory acute myeloid leukemia: A systematic review and meta-analysis. Haematologica 105:2659-2663, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.DiNardo CD Stein EM de Botton S, et al. : Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med 378:2386-2398, 2018 [DOI] [PubMed] [Google Scholar]
- 86.Stein EM DiNardo CD Pollyea DA, et al. : Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 130:722-731, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taksin AL Legrand O Raffoux E, et al. : High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: A prospective study of the alfa group. Leukemia 21:66-71, 2007 [DOI] [PubMed] [Google Scholar]