PURPOSE
Genomic cancer risk assessment (GCRA) is standard-of-care practice that uses genomic tools to identify individuals with increased cancer risk, enabling screening for early detection and cancer prevention interventions. GCRA is not available in most of Mexico, where breast cancer (BC) is the leading cause of cancer death and ovarian cancer has a high mortality rate.
METHODS
Guided by an implementation science framework, we piloted the Genomic Risk Assessment for Cancer Implementation and Sustainment (GRACIAS) intervention, combining GCRA training, practice support, and low-cost BRCA1/2 (BRCA) gene testing at four centers in Mexico. The RE-AIM model was adapted to evaluate GRACIAS intervention outcomes, including reach, the proportion of new patients meeting adapted National Comprehensive Cancer Network criteria who participated in GCRA. Barriers to GCRA were identified through roundtable sessions and semistructured interviews.
RESULTS
Eleven clinicians were trained across four sites. Mean pre-post knowledge score increased from 60% to 67.2% (range 53%-86%). GCRA self-efficacy scores increased by 31% (95% CI, 6.47 to 55.54; P = .02). Participant feedback recommended Spanish content to improve learning. GRACIAS promoted reach at all sites: 77% in Universidad de Guadalajara, 86% in Instituto Nacional de Cancerología, 90% in Tecnológico de Monterrey, and 77% in Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. Overall, a pathogenic BRCA variant was identified in 15.6% (195 of 1,253) of patients. All trainees continue to provide GCRA and address barriers to care.
CONCLUSION
We describe the first project to use implementation science methods to develop and deliver an innovative multicomponent implementation intervention, combining low-cost BRCA testing, comprehensive GCRA training, and practice support in Mexico. Scale-up of the GRACIAS intervention will promote risk-appropriate care, cancer prevention, and reduction in related mortality.
INTRODUCTION
Genomic cancer risk assessment (GCRA) is a standard-of-care practice using genetic and genomic tools to identify and manage individuals with inherited cancer risk.1 Despite evidence that GCRA enables earlier stage at diagnosis or prevention of cancers among BRCA1/2 (BRCA) pathogenic variant (PV) carriers, GCRA services are not well-diffused,2 particularly in low- and middle-income countries (LMICs) like Mexico, where breast cancer (BC) is the leading cancer cause of death in women and ovarian cancer also has a high rate of mortality.3-6 There are limited GCRA expertise among healthcare professionals in Mexico and barriers in access to cancer genetic testing for most of the population.
CONTEXT
Key Objective
Although genomic cancer risk assessment (GCRA) is a standard-of-care service in developed nations, access remains limited in Mexico and other low- and middle-income countries. We describe the first project to use implementation science methods to develop and deliver an innovative multicomponent implementation intervention called Genomic Risk Assessment for Cancer Implementation and Sustainment (GRACIAS), combining low-cost BRCA testing, comprehensive GCRA training, and practice support.
Knowledge Generated
Roundtables identified limitations in healthcare finance, adequately trained workforce, and population-based registries. GRACIAS resulted in increased reach and sustainment of GCRA services at four major centers in Mexico, and the yield of BRCA pathogenic variants was comparable with the yield in US high-risk clinics.
Relevance
GCRA risk stratification could inform allocation of limited resources and result in prevention of cancer. The implementation science developed for GRACIAS may help scale up dissemination of GCRA in Latin America and for application in low-resource settings, including rural communities and safety net hospitals in the United States.
Implementation of GCRA services and BRCA testing in Mexico would identify patients with hereditary cancer who may benefit from reduction of new primary cancer risk or targeted therapy7,8 and also has the potential to reduce cancer burden through cascade testing and cancer screening and prevention for at-risk family members. This may allow for better allocation of limited and cancer screening resources, in alignment with emerging health policies in Mexico.9
We implemented pro bono GCRA services to address disparities at a Hispanic-serving safety net hospital in Los Angeles10-13 and used a community-based clinical research network to collect data and learn from similar low-resource settings across the United States.10,14,15 This paper describes the development, delivery, and preliminary outcomes of a Genomic Risk Assessment for Cancer Implementation and Sustainment (GRACIAS) implementation intervention in Mexico.
Theoretical Approach
We tested the GRACIAS intervention at four Mexican clinics, guided by the Interactive Systems Framework for Dissemination and Implementation (ISF).16 The ISF helped us understand the various systems (synthesis, translation, support, and delivery systems) involved in new practice implementation and also informed our development of capacity-building tools and resources. We used the RE-AIM Framework17 to evaluate the impact of the implementation strategies.
METHODS
The process and tools used for developing and assessing the GRACIAS intervention are summarized in Figure 1 and described below.
FIG 1.
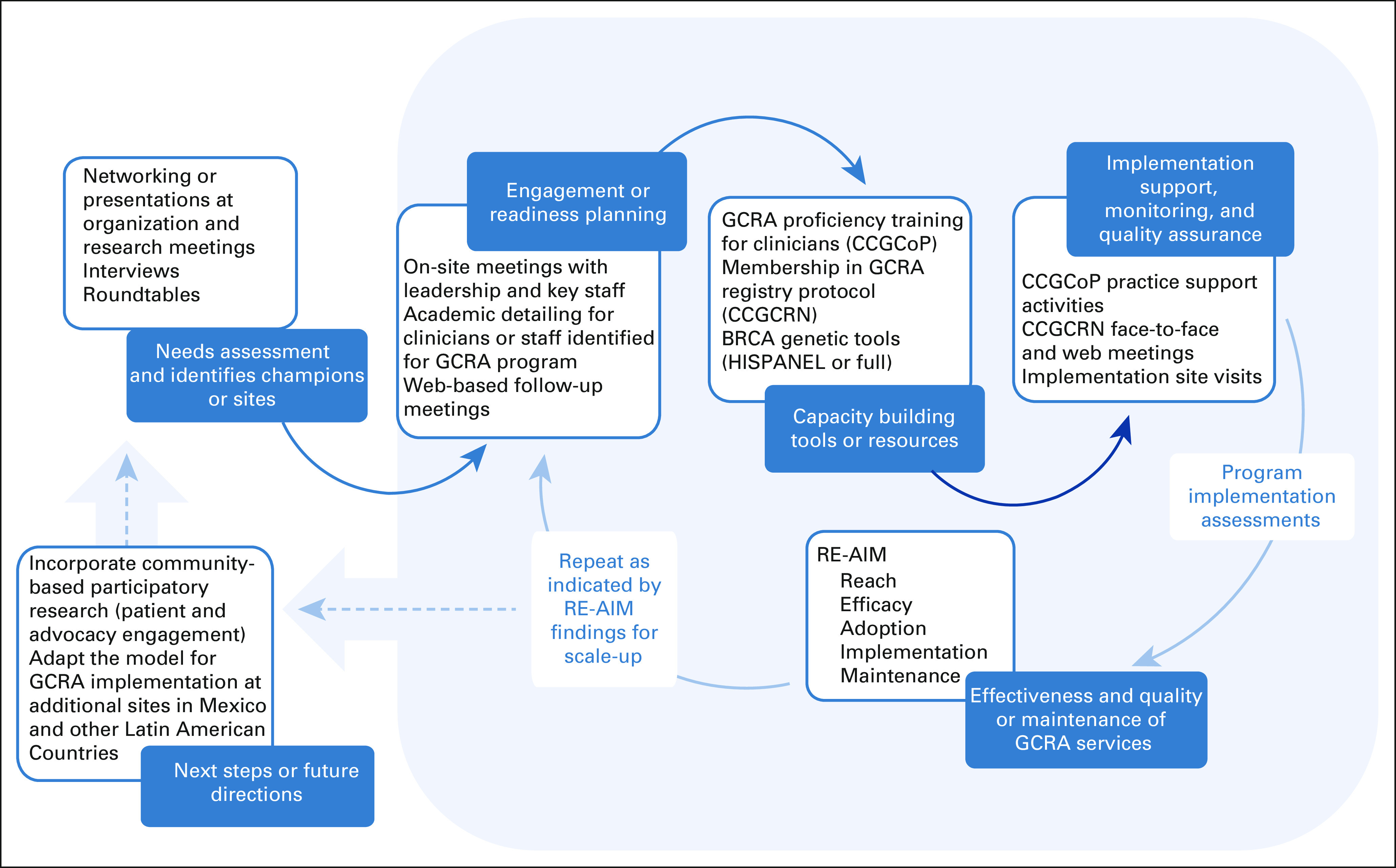
GRACIAS project. The key components and iterative process for implementing the pilot GCRA intervention in Mexico. CCGCoP, Clinical Cancer Genomics Intensive Course and Community of Practice; CCGCRN, Clinical Cancer Genomics Community Research Network; GRACIAS, Genomic Risk Assessment for Cancer Implementation and Sustainment; GCRA, genomic cancer risk assessment.
Participants and Settings
Clinician participants
Clinician scientists identified through networking at academic oncology conferences were identified as champions for delivering GCRA services at their respective Mexican health centers.
Settings
We piloted the GRACIAS intervention at Universidad de Guadalajara (UdeG), which collaborates with two public hospitals (Instituto Jalisciense de Cancerología and the OPD Hospital Civil de Guadalajara) to care for patients with cancer from Jalisco, and Instituto Nacional de Cancerología (INCan) in Mexico City, the largest center and main referral resource for specialized cancer care for the uninsured population. We subsequently added Tecnológico de Monterrey Escuela de Medicina Monterrey (TecSalud), an academic and community health system, and Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (Nutrición) in Mexico City. Nutrición is a national referral center, providing care to > 4,000 mostly uninsured patients annually.
The health system in Mexico comprises insurance for employees, public assistance for the uninsured, and a private sector. Approximately 50% of people are uninsured. All GRACIAS sites serve the uninsured population. Although some general genetics services were available before the GRACIAS intervention, this was primarily empirical cancer risk counseling on the basis of syndromic phenotypes, with limited access to BRCA testing.
Patient participants
Patient eligibility for GCRA was adapted from National Comprehensive Cancer Network (NCCN) guidelines and included a diagnosis of BC ≤ 50 years, triple-negative breast cancer, or ovarian cancer at any age.18
Ethics approval and consent to participate
Patients who received GCRA services in this study were recruited into a shared prospective human subjects Institutional Review Board (IRB; City of Hope IRB# 96144; data coordinating center)–approved cancer genetics registry protocol used across the federated Clinical Cancer Genetics Community Research Network (CCGCRN). The protocol was reviewed and approved at each partner institution (IRB# 00007348, INCan, Mexico City; IORG# IORG0006166, UdeG, Guadalajara, México). Patients provide written informed consent for collection of baseline and follow-up medical and family history and blood or saliva sample and for recontact to receive genetic test results.
Engagement and Readiness Planning
Guided by the ISF, we initially met with institution leaders and clinician stakeholders at UdeG in 2011, INCan in 2012, TecSalud in 2014, and Nutrición in 2015 to (1) assess the organizational infrastructure of each institution, including pre-existing genetic services; (2) determine the patient pools eligible for GCRA; (3) assess materials, staff, and services needed for GCRA practice; and (4) work with leadership to identify common goals and clinicians for GCRA training. We also met with two patient advocacy groups in Mexico to build awareness about the potential benefits of GCRA for patients with BC and their families and to explore their information and resource needs: CIMA,19 a nonprofit organization aimed at reducing BC mortality in Mexico and Oncoayuda,20 which is dedicated to improving quality of life among cancer survivors.
Capacity Building Tools or Resources
Informed by our initial visits to UdeG and INCan, we adapted the following established resources to incorporate into the GRACIAS intervention:
The Clinical Cancer Genomics Intensive Course and Community of Practice
Since its inception in 2001, this award-winning21 NCI-funded (R25CA171998) initiative combines distance learning, face-to-face workshops, and ongoing web-based practice support for GCRA clinicians.22,23 More than 1,200 clinicians from all 50 US states and 27 countries completed the course22-25 and became members of the Clinical Cancer Genomics Intensive Course and Community of Practice (CCGCoP). Adaptations: Funding from the Breast Cancer Research Foundation and Avon (grant No. 02-2013-044) supported the participation of Mexican physicians.
The CCGCRN
The CCGCRN is a consortium of 45 community-based and academic oncogenetic practices. The shared registry protocol collects family history, biospecimens for genomic testing, and clinical outcomes data and allows clinicians to provide clinically actionable research results to patients. Progeny Web (Progeny Software LLC, Delray Beach, FL) is the CCGCRN relational database and serves as a pedigree drawing tool for clinical use at all participating sites. Adaptations: Registry consent and protocol documents were translated to Spanish and approved by the IRB for each site according to institutional policies and national regulations, including Mexico's Federal Commission for Protection against Sanitary Risk to obtain a permit for batch shipment of patient biospecimens.
Low-cost BRCA genetic testing
We developed a 115-PV BRCA panel (HISPANEL) that identifies recurrent BRCA PVs among Hispanics for $20 US dollars per case.26-28 HISPANEL-negative patients are screened with full BRCA gene sequencing with a researcher-designed next-generation sequencing assay and for copy number variants in BRCA1 by multiplex ligation–dependent probe amplification (MRC-Holland, Amsterdam, the Netherlands). No BRCA2 copy number variants were detected among 1,627 participants (manuscript under review). HISPANEL (77% sensitivity) and full BRCA sequencing were used sequentially as a low-cost29 approach to support GCRA (Fig 2).
FIG 2.
Low-cost BRCA genetic testing process. CNV, copy number variant; MFM, Mexican founder mutation; MLPA, multiplex ligation-dependent probe amplification; NGS, next‑generation sequencing; PCR, polymerase chain reaction; USD, US dollars.
Implementation Support, Monitoring, and Quality Assurance
We conducted the following activities to provide expert guidance, assess quality, and help identify and address barriers to implementing and sustaining GCRA services.
Clinical practice and research support
Clinicians who completed GCRA training were provided access to practice support resources of the CCGCoP (eg, web-based discussion boards and interprofessional case review conferences) and met with the research team quarterly via web conference and once in person at annual CCGCRN meetings to promote networking and collaborative research.
Implementation site visits
Site visits were conducted by research team leaders to understand the processes, facilitators, and barriers to GCRA services, assess and provide feedback to site leaders and administrators on the quality and fidelity of GCRA services, and provide academic detailing to raise awareness and promote referral to GCRA services.
Roundtable Forums
Two roundtable discussion forums were conducted by the research team. The first was in March 2014 in the City of Hope campus with Latin American physicians to consider the state of GCRA services in Latin America. The second was in January 2020 in Mexico City with participants from the GRACIAS pilot sites and candidate GCRA implementation sites, focused on context-specific themes. The sessions were conducted in Spanish and recorded, transcribed, and translated into English for thematic analysis by three bilingual cancer genetics clinician-researchers.
Effectiveness and Quality or Maintenance of GCRA Services
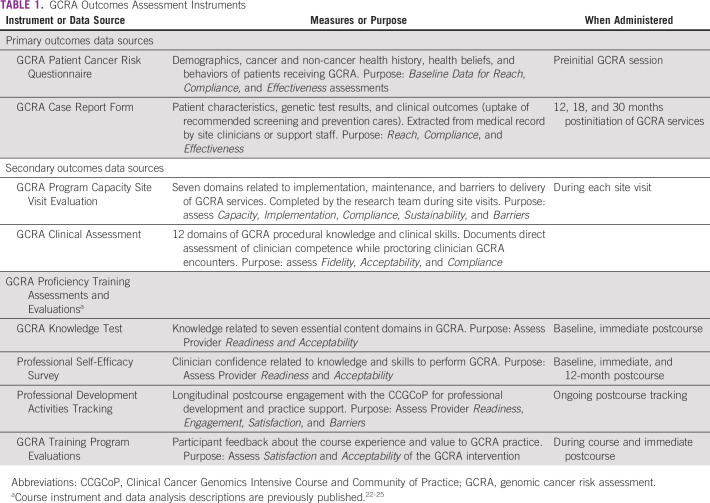
Table 1 shows the instruments used to document the progress and outcomes of the intervention.
TABLE 1.
GCRA Outcomes Assessment Instruments
Data Analysis
The RE-AIM model was adapted to provide systematic evaluation of GRACIAS intervention outcomes as follows: Reach, the proportion of the target patient population (anonymized data on new patient registrations at participating sites, meeting adapted NCCN criteria) vs. profile of enrolled patients referred for GCRA; Effectiveness, the number of patients who completed GCRA and received BRCA test results and the number identified as BRCA carriers; Adoption, the number of organizations and providers who engage in the facilitation of GCRA; Implementation, successful delivery of GCRA practice (measured by observation of genetics consultations and informed consent process, patient pedigree, clinical characteristics, and genetic test results documentation); and Maintenance, the extent to which the delivery is sustained over time. Identification of barriers to GCRA implementation was obtained through semistructured interviews and feedback from clinicians, administrators, and staff during site visits and meetings.
Survey data were entered into Microsoft Excel 2013 (Microsoft, Redmond, WA) and audited for accuracy. Descriptive statistics were computed for demographic, yes or no, and rating scale items. Quantitative data were analyzed using Fisher's test or χ2 statistics, with a two-sided P value of < .05 considered significant (SPSS 14.0 New York). Paired t-test assessed baseline to postcourse changes in physician knowledge. Coding and thematic analysis of open-ended responses were conducted by three researchers through a series of iterations. Qualitative findings were triangulated with quantitative outcomes to increase the depth and support the validity of quantitative findings.30
RESULTS
Training Outcomes
Eleven healthcare professionals (six geneticists, two oncologists, one gynecologist, one general physician, and one genetic laboratory clinician) age 26-35 years, with 1-5 years of clinical practice experience, participated in the CCGCoP training from 2011 to 2017. The mean knowledge score increased from 60% (range 53%-75%) at baseline to 67.2% (range 53%-86%) postcourse. Two participants did not demonstrate significant increase in knowledge score and self-reported challenges with written English language proficiency. Open-ended feedback also suggested that content in Spanish would improve the learning experience. Professional self-efficacy (SE) scores related to GCRA knowledge and skills increased by 29.4% (95% CI, 3.29 to 16.3; P = .005). The median self-efficacy increased 0.69 points, from 3.73 at baseline (range 1.94-4.54) to 4.42 (range 4.03-4.78) postcourse.
RE-AIM Outcomes
Reach and effectiveness
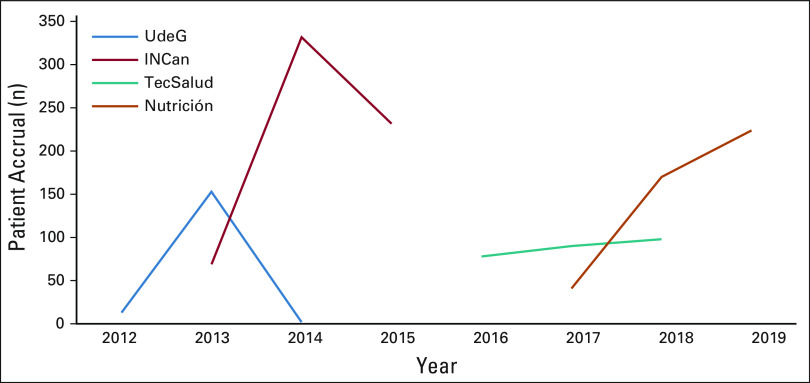
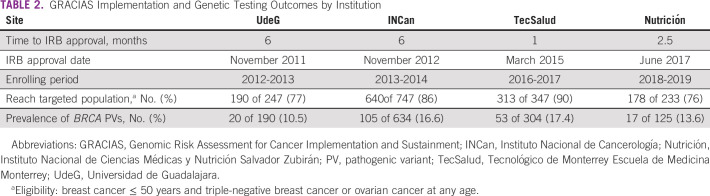
A 2-year window of patient accrual for each site is depicted in Figure 3 and summarized in Table 2. At UdeG, 190 of 247 patients were accrued in the first year of implementation in 2012 (77% reach), with 20 BRCA PVs detected among 190 tested (10.5%). UdeG accrual was subsequently interrupted because of personnel limitations (the only GCRA-trained clinician went out on personal leave) and protracted regulatory challenges with Federal Commission for the Protection against Sanitary Risk. GCRA accrual at INCan was 640 of 747 patients from 2013 through 2014 (86% reach), with 105 BRCA PVs detected among 634 tested (16.6%). Interim analysis demonstrated that reach at INCan was significantly greater than that at UdeG (P = .0013).
FIG 3.
Two-year window of patient accrual for each site. INCan, Instituto Nacional de Cancerología; Nutrición, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán; TecSalud, Tecnológico de Monterrey Escuela de Medicina Monterrey; UdeG, Universidad de Guadalajara.
TABLE 2.
GRACIAS Implementation and Genetic Testing Outcomes by Institution
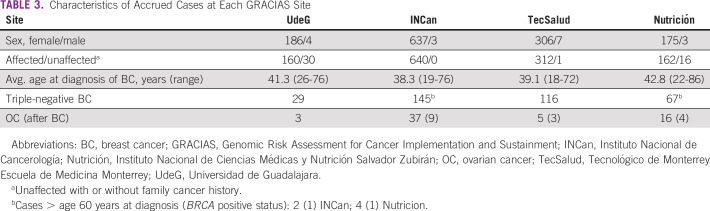
Differences between the two sites included more trained personnel at INCan (five) versus UdeG (one) and an earlier site visit at INCan (after 1 year of GCRA services v after three years at UdeG), wherein academic detailing by study leaders increased awareness of the new GCRA services, and clinical proctoring and site assessments facilitated process improvements. A 20% increase in accruals followed each site visit. Consequently, we incorporated annual site visits for all new sites. Accrual at TecSalud from 2016 to 2017 was 313 of 347 patients (90%), with 53 BRCA PVs detected among 304 tested (17.4%), and 178 of 233 patients were accrued at Nutrición from 2018 through 2019 (76%), with 17 BRCA PVs detected among 125 tested (13.6%). Table 3 summarizes the characteristics of the accrued patients at the respective sites, with greatest yield of BRCA PVs at sites (INCan and TecSalud) with younger age and more patients affected with cancer; two of six patients over 60 years with triple-negative breast cancer had a BRCA1 PV. All sites met or exceeded the benchmark of 6%-15% yield reported in published studies of high-risk clinics using NCCN testing criteria.7,28,31-35
TABLE 3.
Characteristics of Accrued Cases at Each GRACIAS Site
Adoption
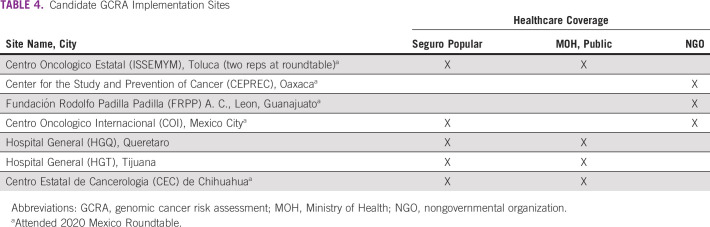
Beyond the four GRACIAS sites reported here, we have engaged seven additional candidate sites representing a cross section of clinical practices supported by Ministry of Health or other public health insurance or nongovernmental organizations (Table 4). The candidate sites include communities with largely indigenous populations.
TABLE 4.
Candidate GCRA Implementation Sites
The clinician roundtable sessions were held after GRACIAS implementation at the first two sites (UdeG and INCan) in March 2014 and again after implementation at the next two sites (TecSalud and Nutrición) in January 2020. The first was attended by 15 physicians from Mexico (including the clinician champions from the first two GRACIAS sites), Brazil, Colombia, Peru, and Puerto Rico. Findings revealed that clinicians from each country shared common barriers to GCRA implementation, including (1) lack of patient and provider knowledge about GCRA, (2) lack of providers with training or expertise to deliver GCRA, (3) lack of insurance coverage and high patient out-of-pocket costs for GCRA testing, (4) insufficient institutional and government infrastructure or policy to support GCRA programs, and (5) the need to publish GCRA outcomes and develop an evidence base documenting efficacy in their own populations to influence healthcare policy toward building GCRA services.36
The second roundtable was attended by 16 clinicians representing eight sites in major population centers in Mexico (including the four pilot GRACIAS sites) to explore the current status of GCRA services in Mexico, barriers to GCRA practice, and potential solutions. Clinician champions from five of the seven candidate sites also participated, demonstrating a high level of commitment to adopt GCRA. Findings paralleled the issues noted in the first session (eg, lack of insurance coverage and high patient out-of-pocket costs) and revealed additional concerns, based in part on political changes directly affecting public health finance and the termination of Mexico's signature safety net program (Seguro Popular). The session was conducted in the backdrop of active civil protest by physicians and patients with pediatric cancer and their families regarding new limitations in access to treatment.37,38 Participants cited the need for trained GCRA clinicians outside the capital city, particularly in underserved areas. GRACIAS participants reflected that their experience with the program was integral to implementation of GCRA in their centers.
Implementation and maintenance
Proctoring of GCRA sessions during site visits revealed appropriate (1) documentation of family history data as multigeneration pedigrees, (2) delivery of genetic counseling and informed consent, (3) interpretation and disclosure of genetic test results, and (4) provision of risk-appropriate management recommendations and referrals for high-risk care. Participating sites created practical adaptations to the protocol, such as transitioning from handwritten to direct entry of pedigrees and risk factor data into the electronic database during the GCRA session, leveraging clinician fellows to triage GCRA patients and administer clinic questionnaires, and addressing challenges with patient literacy and inefficiencies with the postal system. Overall, there was fidelity of laboratory sample handling across sites, but common challenges included correct tube selection, costs for supplies, sample storage, support staff, and shipping.
CCGCoP practice support resources were used by clinicians from all GRACIAS sites, who presented and received feedback on GCRA cases during CCGCoP web-based case conferences, and 73% (8 of 11) of site clinicians have attended one or more of the City of Hope annual cancer genomics update conferences (range 1-3). Genetic test results have been incorporated into newly established multidisciplinary tumor boards at each site, wherein hereditary case management is discussed among oncologists, surgeons, gynecologists, and psychologists.
Academic detailing conducted by the research team during site visits enhanced stature, awareness, and institutional support for their GCRA programs. Site champions continue academic detailing. For example, the Nutrición site incorporates content about GCRA in grand rounds at local hospitals and in their residency program to increase patient referrals, enhance awareness of the program, and increase the pool of clinicians interested in GCRA.
On the basis of continuous follow-up from 2012 to 2020, all 11 clinicians who received GCRA training continue to provide GCRA services. The UdeG geneticist completed training in 2011 and initiated GCRA in 2012. After the UdeG team was encouraged to train additional clinicians, a genetics intern completed GCRA training in 2016, enabling more sustained services. Independent of the pilot program since 2016, five of the seven trained clinicians, and three additional geneticists, continue to practice GCRA at INCan. Two clinicians left INCan to initiate GCRA services at other institutions; one recruited a geneticist who completed the GCRA training, and they started a new program at TecSalud. All four sites remain active after GRACIAS implementation, with a range of continuous GCRA activities of 2-8 years (average 5.5 years).
DISCUSSION
Although GCRA is a standard-of-care service in the United States and other developed nations,1,39 access remains limited in Mexico and other LMICs. The introduction of the ISF16 to identify systems and support capacity building, and RE-AIM17 to evaluate progress and outcomes, provided a systematic framework for the GRACIAS intervention. To our knowledge, this is the first time that dissemination and implementation methods have been used to promote GCRA services.
The GRACIAS intervention resulted in increased reach and sustainment of GCRA services at all sites. The yield of BRCA PVs across all four sites was comparable with the yield in high-risk clinics in the United States that follow NCCN guidelines, with the greatest yield at sites with younger age at diagnosis and more patients affected with cancer.7,28,40
More patients were seen for GCRA and the programs were better sustained in the centers where more clinicians were trained and that had earlier GRACIAS site visits, suggesting that these components are helpful for developing workforce depth and institutional commitment. Site visits may influence the allocation of resources and help inform strategies to address barriers. Although the recorded course modules allow learners with limited English language proficiency to view the curriculum repeatedly and at their own pace, a Spanish-language version of the course and outcomes assessments would be an enhancement. Despite language challenges, site visits revealed high competency and quality in the delivery of GCRA services. Post-training engagement with the CCGCoP and the integration of GCRA testing outcomes at multidisciplinary tumor boards enhance the quality of post-GCRA care and increase adoption and engagement by allied subspecialties.
Another important characteristic of the intervention is the potential for further dissemination of GCRA services at other sites by GRACIAS-trained clinicians. Two of the four sites have developed some capacity for research-based BRCA testing. However, broader access to GCRA and genetic testing and the full spectrum of risk-appropriate interventions will require national policy change.
Preliminary observations from CCGCRN protocol outcomes highlighted creative strategies used by the new GCRA programs to include risk-reduction surgeries in the cancer treatment plan or use nongovernmental organization resources, philanthropic support, and interinstitutional agreements to manage BRCA PV carriers. Formal evaluation of post-GCRA preventive, surveillance, and cascade testing outcomes is ongoing and part of future studies.
BC advocacy groups were enthusiastic about GCRA; however, some members expressed concerns about competition for limited resources for BC screening. They emphasized the need to educate patients and policymakers about the benefits of GCRA.
This study identified limitations in healthcare finance, adequately trained workforce, and population-based registries in Mexico. Currently, only physicians provide genetic services in Mexico, as the profession of genetic counseling does not exist, and other midlevel providers are not involved. These barriers were also reflected in the roundtable sessions and previously reported in other LMICs.41-43 Post-GCRA clinical outcomes are recorded in Progeny Web by the on-site team annually for the GRACIAS registry, which compensates in part for the lack of population-based cancer registries. Jointly authored peer-reviewed publications and poster presentations (n = 16) illuminate the status of GCRA in Latin America44 and the genetic epidemiology of hereditary BC in Mexico.45,46
The GRACIAS components were demonstrably successful in the pilot project and helped elucidate factors influencing implementation of GCRA in Mexico. Next steps and needs for scale-up include the following:
Identify candidate sites representing a cross section of practice settings in other states; GCRA activities were recently initiated in Chiapas (one of the most ethnically diverse and underserved regions), and we engaged candidate sites in Chihuahua and Querétaro.
Increase the GCRA workforce through interprofessional CCGCoP training, including mid-level providers—changes in Mexican healthcare policy are needed to integrate mid-level providers into GCRA.
Create a Spanish-language version of the course curriculum, as well as knowledge and skills outcomes instruments, to optimize training and better assess respective outcomes.
Study the impact of, and explore alternate distance-mediated approaches to, site visits, which are time-, cost-, and labor-intensive.
Create interventions to enhance cascade testing and access to risk-appropriate care in partnership with key stakeholders such as site champions, administrators, and public health officials and advocates.
Promote national policy change necessary to support economical clinical-grade testing services necessary to sustain GCRA services in Mexico.
In conclusion, to our knowledge, we describe the first project to use implementation science methods to develop and deliver an innovative multicomponent implementation intervention, combining low-cost BRCA testing, comprehensive GCRA training, and practice support. Scale-up of the GRACIAS dissemination and implementation intervention will result in measurable prevention of BC and reduction in related mortality. Furthermore, the implementation science developed in this project will help establish a framework for further dissemination of GCRA and other clinical services in Latin America and for application in low-resource settings, including rural communities and safety net hospitals in the United States.
ACKNOWLEDGMENT
We are grateful for the excellent project assistance of Kevin Tsang, BS; Sharon Sand, BA; and Gloria Nunez, MPH. We thank Lenny Gallardo, MD, for her work implementing GCRA at INCan. We thank breast cancer survivor, Dr Sandra Balladares, for her advocacy and support.
Kathleen R. Blazer
Research Funding: Pfizer
Yanin Chavarri-Guerra
Research Funding: Roche
Travel, Accommodations, Expenses: Pfizer
Cynthia Villarreal Garza
Consulting or Advisory Role: Roche, Novartis, Pfizer, Lilly
Speakers' Bureau: Roche, Myriad Genetics, Novartis
Research Funding: AstraZeneca, Roche
Travel, Accommodations, Expenses: Roche, MSD Oncology, Pfizer
Bita Nehoray
Consulting or Advisory Role: Invitae
Travel, Accommodations, Expenses: Invitae
Adrian Daneri-Navarro
Patents, Royalties, Other Intellectual Property: On December 09, 2020, my university requested PCT/IB2020/061716: NANOPARTÍCULAS PARA EL TRATAMIENTO DEL CÁNCER
Rosa Maria Álvarez
Consulting or Advisory Role: Foundation Medicine
Speakers' Bureau: AstraZeneca
Jeffrey N. Weitzel
Speakers' Bureau: AstraZeneca
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
PRIOR PRESENTATION
Presented at the American Association for Cancer Research (AACR) 2016 Annual Meeting, New Orleans, LA, April 16-20, 2016.
SUPPORT
Supported by The Breast Cancer Research Foundation Grants No. 19-172 and 20-172 (J.N.W.); Avon Foundation of Women Grant No. 02-2013-044 (J.N.W.); Conquer Cancer Research Professorship in Breast Cancer Disparities (J.N.W.); and Award No. R25CA171998 (PIs: K.R.B. and J.N.W.) from the National Cancer Institute of the National Institutes of Health, which supports the Clinical Cancer Genomics Community of Practice initiative that provided cancer genomics proficiency training for this project. The Clinical Cancer Genomics Community Research Network was supported in part by RC4CA153828 (PI: J.N.W.) from the National Cancer Institute and the Office of the Director, National Institutes of Health. HISPANEL genetic testing was developed in part through support from grant No. RSGT-09-263-01-CCE from the American Cancer Society (J.N.W.). The work was also supported in part by a supplement award to NCI P30CA033572, titled: Strengthening capacity to promote genomic cancer risk assessment dissemination and implementation research and translation among underserved populations in the Americas, and some of the BRCA sequencing was performed in the Functional Genomics Core.
AUTHOR CONTRIBUTIONS
Conception and design: Kathleen R. Blazer, Alejandro Mohar, Maria Fernandez, Jeffrey N. Weitzel
Financial support: Jeffrey N. Weitzel
Administrative support: Bita Nehoray, Rosa Mejia, Jeffrey N. Weitzel
Provision of study materials or patients: Yanin Chavarri-Guerra, Adrian Daneri-Navarro, Dione Aguilar, Rosa Mejia, Jeffrey N. Weitzel
Collection and assembly of data: Kathleen R. Blazer, Cynthia Villarreal Garza, Alejandro Mohar, Adrian Daneri-Navarro, Azucena del Toro, Dione Aguilar, Jazmin Arteaga, Rosa Maria Álvarez, Rosa Mejia, Danielle Castillo, Jeffrey N. Weitzel
Data analysis and interpretation: Kathleen R. Blazer, Yanin Chavarri-Guerra, Cynthia Villarreal Garza, Bita Nehoray, Alejandro Mohar, Josef Herzog, Maria Fernandez, Jeffrey N. Weitzel
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kathleen R. Blazer
Research Funding: Pfizer
Yanin Chavarri-Guerra
Research Funding: Roche
Travel, Accommodations, Expenses: Pfizer
Cynthia Villarreal Garza
Consulting or Advisory Role: Roche, Novartis, Pfizer, Lilly
Speakers' Bureau: Roche, Myriad Genetics, Novartis
Research Funding: AstraZeneca, Roche
Travel, Accommodations, Expenses: Roche, MSD Oncology, Pfizer
Bita Nehoray
Consulting or Advisory Role: Invitae
Travel, Accommodations, Expenses: Invitae
Adrian Daneri-Navarro
Patents, Royalties, Other Intellectual Property: On December 09, 2020, my university requested PCT/IB2020/061716: NANOPARTÍCULAS PARA EL TRATAMIENTO DEL CÁNCER
Rosa Maria Álvarez
Consulting or Advisory Role: Foundation Medicine
Speakers' Bureau: AstraZeneca
Jeffrey N. Weitzel
Speakers' Bureau: AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1.Weitzel JN Blazer KR MacDonald DJ, et al. : Genetics, genomics and cancer risk assessment: State of the art and future directions in the era of personalized medicine. CA Cancer J Clin 61:327-359, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrissey E Wandersman A Seybolt D, et al. : Toward a framework for bridging the gap between science and practice in prevention: A focus on evaluator and practitioner perspectives. Eval Program Plann 20:367-377, 1997 [Google Scholar]
- 3.Ferlay J Steliarova-Foucher E Lortet-Tieulent J, et al. : Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer 49:1374-1403, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Knaul FM Arreola-Ornelas H Velazquez E, et al. : El costo de la atención médica del cáncer mamario: el caso del Instituto Mexicano del Seguro Social [The health care costs of breast cancer: The case of the Mexican Social Security Institute]. Salud Publica de Mexico 51:s286-s295, 2009. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 5.Mohar A Bargallo E Ramirez MT, et al. : Recursos disponibles para el tratamiento del cáncer de mama en México [Available resources for the treatment of breast cancer in Mexico]. Salud Publica Mex 51:s263-s269, 2009. (suppl 2) [PubMed] [Google Scholar]
- 6.Graniel CML Rincón DG Enciso AG, et al. : Consenso Mexicano de Cancer Epitelial de Ovario. Reto diagnostico y terapeutico (Mexican Consensus Epithelial Ovarian Cancer. Diagnostic and therapeutic challenge). Gaceta Mexicana de Oncología 5:6-7, 2006 [Google Scholar]
- 7.Weitzel JN Blazer KR MacDonald DJ, et al. : Genetics, genomics, and cancer risk assessment: State of the art and future directions in the era of personalized medicine. CA Cancer J Clin. 61:327-359, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tung NM Boughey JC Pierce LJ, et al. : Management of hereditary breast cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology guideline. J Clin Oncol 38:2080-2106, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Secretaría de Salud : Para la prevención, diagnóstico, tratamiento, control y vigilancia epidemiológica del cáncer de mama. Diaro Oficial de la Federación. Mexico, Ministry of Health, 2011 [Google Scholar]
- 10.Ricker C Lagos V Feldman N, et al. : If we build it…will they come?—Establishing a cancer genetics services clinic for an underserved predominantly Latina cohort. J Genet Couns 15:505-514, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Ricker CN Hiyama S Fuentes S, et al. : Beliefs and interest in cancer risk in an underserved Latino cohort. Prev Med 44:241-245, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Lagos VI Blazer KR Viveros L, et al. : Impact of Genetic Cancer Risk Assessment on Cancer Screening and Prevention Behaviors in an Underserved Predominantly Latina Population. San Francisco, CA, National Latino Cancer Summit, 2008 [Google Scholar]
- 13.Lagos VI Perez MA Ricker CN, et al. : Social-cognitive aspects of underserved Latinas preparing to undergo genetic cancer risk assessment for hereditary breast and ovarian cancer. Psychooncology 17:774-782, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Komenaka IK Nodora JN Madlensky L, et al. : Participation of low-income women in genetic cancer risk assessment and BRCA 1/2 testing: The experience of a safety-net institution. J Community Genet 7:177-183, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weitzel JN Robson M Pasini B, et al. : A comparison of bilateral breast cancers in BRCA carriers. Cancer Epidemiol Biomarkers Prev 14:1534-1538, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Wandersman A Duffy J Flaspohler P, et al. : Bridging the gap between prevention research and practice: The interactive systems framework for dissemination and implementation. Am J Community Psychol 41:171-181, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Glasgow RE, Vogt TM, Boles SM: Evaluating the public health impact of health promotion interventions: The RE-AIM framework. Am J Public Health 89:1322-1327, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NCCN : Genetic/Familial High-Risk Assessment: Colorectal, NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Fort Washington, PA, National Comprehensive Cancer Network, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Fundación CIMA : https://www.cimafundacion.org/
- 20.Oncoayuda : https://oncoayuda.org
- 21.American Society of Human Genetics : ASHG Honors Kathleen Blazer & Jeffrey N. Weitzel with the Arno Motulsky-Barton Childs Award for Excellence in Human Genetics Education, Rockville, MD, ASHG News, 2019 [Google Scholar]
- 22.Blazer KR MacDonald DJ Ricker C, et al. : Outcomes from intensive training in genetic cancer risk counseling for clinicians. Genet Med 7:40-47, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Blazer KR MacDonald DJ Culver JO, et al. : Personalized cancer genetics training for personalized medicine: Improving community-based healthcare through a genetically literate workforce. Genet Med 13:832-840, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blazer K: Examining the Use of Distance-Mediated Case Conferencing for Case-Based Training in Clinical Cancer Genetics [thesis]. UCLA, Los Angeles, CA, 2010 [Google Scholar]
- 25.Blazer KR Christie C Uman GC, et al. : Impact of web-based case conferencing on cancer genetics training outcomes for community-based clinicians. J Cancer Educ 27:217-225, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weitzel JN Buys SS Sherman WH, et al. : Reduced mammographic density with use of a gonadotropin-releasing hormone agonist-based chemoprevention regimen in BRCA1 carriers. Clin Cancer Res 13:654-658, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Weitzel JN Clague J Martir-Negron A, et al. : Prevalence and type of BRCA mutations in Hispanics undergoing genetic cancer risk assessment in the southwestern United States: A report from the Clinical Cancer Genetics Community Research Network. J Clin Oncol 31:210-216, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weitzel JN Lagos V Blazer KR, et al. : Prevalence of BRCA mutations and founder effect in high-risk Hispanic families. Cancer Epidemiol Biomarkers Prev 14:1666-1671, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Villarreal-Garza C Alvarez-Gómez RM Pérez-Plasencia C, et al. : Significant clinical impact of recurrent BRCA1 and BRCA2 mutations in Mexico. Cancer 121:372-378, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Creswell JW: Mixed Methods Procedures, Research Design Qualitative, Quantitative, and Mixed Method Approaches (ed 2). Thousand Oaks, CA, Sage Publications, 2003, pp 208-225 [Google Scholar]
- 31.Abugattas J Llacuachaqui M Allende YS, et al. : Prevalence of BRCA1 and BRCA2 mutations in unselected breast cancer patients from Peru. Clin Genet 88:371-375, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodríguez AO Llacuachaqui M Pardo GG, et al. : BRCA1 and BRCA2 mutations among ovarian cancer patients from Colombia. Gynecol Oncol 124:236-243, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Dutil J Golubeva VA Pacheco-Torres AL, et al. : The spectrum of BRCA1 and BRCA2 alleles in Latin America and the Caribbean: A clinical perspective. Breast Cancer Res Treat 154:441-453, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment: Breast, Ovarian, And Pancreatic. Version 1.2020—December 4, 2019. 2019 [Google Scholar]
- 35.Beck AC Yuan H Liao J, et al. : Rate of BRCA mutation in patients tested under NCCN genetic testing criteria. Am J Surg 219:145-149, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chavez T Nehoray B Obregon-Tito A, et al. : Exploring the Climate, Barriers, and Possible Approaches to Implementing Genetic Cancer Risk Assessment in Latin America: A Roundtable Discussion, 7th AACR Conference on the Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Underserved. San Antonio, TX, 2014
- 37.Oré D: “Cancer Does Not Wait”: Children's Medicine Shortage Stokes Anger in Mexico, Emerging Markets. Reuters, 2020 [Google Scholar]
- 38.Mcdonnell P, Sanchez S: In Mexico, Parents Protest Lack of Cancer Drugs for Their Children. Los Angeles, CA, Los Angeles Times: World & Nation, 2020 [Google Scholar]
- 39.MacDonald DJ, Blazer KR, Weitzel JN: Extending comprehensive cancer center expertise in clinical cancer genetics and genomics to diverse communities: The power of partnership. J Natl Compr Cancer Netw 8:615-624, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chávarri-Guerra Y Marcum CA Hendricks CB, et al. : Breast cancer associated pathogenic variants among women 61 years and older with triple negative breast cancer. J Geriatr Oncol, 2020. doi: 10.1016/j.jgo.2020.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frenk J, Gomez-Dantes O, Knaul FM: The democratization of health in Mexico: Financial innovations for universal coverage. Bull World Health Organ 87:542-548, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okonkwo QL Draisma G der Kinderen A, et al. : Breast cancer screening policies in developing countries: A cost-effectiveness analysis for India. J Natl Cancer Inst 100:1290-1300, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Zheng Y Walsh T Gulsuner S, et al. : Inherited breast cancer in Nigerian women. J Clin Oncol 36:2820-2825, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chavarri-Guerra Y, Blazer KR, Weitzel JN: Genetic cancer risk assessment for breast cancer in Latin America. Rev Invest Clin 69:94-102, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villarreal-Garza C Weitzel JN Llacuachaqui M, et al. : The prevalence of BRCA1 and BRCA2 mutations among young Mexican women with triple-negative breast cancer. Breast Cancer Res Treat 150:389-394, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villarreal-Garza CM Weitzel JN Sifuentes E, et al. : Founder effect and a high prevalence of BRCA1 mutations among young Mexican triple-negative breast cancer (TNBC) patients, ASCO 50th Annual Meeting 2014. Science & Society. Chicago, IL