PURPOSE
To compare the efficacy and safety of 177Lu-DOTATATE plus radiosensitizing capecitabine and octreotide long-acting release (LAR) as first-line systemic therapy in advanced well-differentiated gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
MATERIALS AND METHODS
Data of consecutive patients of advanced inoperable or metastatic grade 1 or 2 GEP-NETs treated with first-line 177Lu-DOTATATE plus radiosensitizing capecitabine or octreotide LAR from September 2012 to December 2019 were collected and analyzed for response, toxicity, and survival outcomes.
RESULTS
Seventy-six patients (median age: 53 years; range 14-81 years) with treatment-naïve advanced grade 1 or 2 GEP-NETs were included. Thirty-six patients received a median cumulative dose of 27.3 GBq of 177Lu-DOTATATE intravenously at 8-12 weeks' intervals along with 1,250 mg/m2 oral capecitabine on days 0-14 of each cycle of 177Lu-DOTATATE, whereas 40 patients were administered 30 mg octreotide LAR intramuscularly every 4 weeks. Using response evaluation criteria in solid tumor 1.1, the objective response rate was 38% in the 177Lu-DOTATATE arm compared with 15% in the octreotide LAR arm (P = .025), whereas the disease control rates were 88% and 67% in 177Lu-DOTATATE and octreotide LAR arms, respectively (P = .035). The median durations of progression-free survival in the 177Lu-DOTATATE and octreotide LAR arms were 54 months and 16 months, respectively (P = .017), whereas the median overall survival was not reached and not significantly different across both the arms. Of the treatment-related adverse events, no major difference was observed in the occurrence of grade 3 or 4 toxicities between the two treatment arms.
CONCLUSION
First-line systemic 177Lu-DOTATATE plus radiosensitizing capecitabine achieved better radiologic response and longer progression-free survival compared with octreotide LAR in patients with advanced grade 1 or 2 GEP-NETs. Future randomized controlled trials are, however, required to determine the best treatment sequence for the treatment-naïve patients with advanced GEP-NETs.
INTRODUCTION
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are a rare group of malignancies that have shown an increased burden in recent times with an incidence of 3.56 per 100,000 persons.1 Treatment options for advanced inoperable or metastatic GEP-NETs are currently limited: somatostatin analogs as first-line treatment, and targeted or cytotoxic chemotherapies as second-line agents for progressive disease (PD).2,3 However, more often than not, disease progression eventually ensues and there exists a lack of therapeutic alternatives. Furthermore, the adverse effects and costs associated with these treatment modalities often limit their practical utility for patients especially in low- to middle-income countries (LMICs). In this setting, Peptide receptor radionuclide therapy (PRRT) has emerged as an attractive option with encouraging results.4-7
CONTEXT
Key Objective
177Lu-DOTATATE has been approved for patients with progressive, well-differentiated, advanced, somatostatin receptor–positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs) based on the NETTER-1 trial. However, its role as a first-line therapeutic agent vis-à-vis somatostatin analogs in treatment-naïve patients has not yet been demonstrated. In this study, we intended to retrospectively compare the efficacy and safety of 177Lu-DOTATATE (plus radiosensitizing capecitabine) versus octreotide long-acting release (LAR) as first-line systemic therapy in patients with advanced well-differentiated inoperable or metastatic GEP-NETs.
Knowledge Generated
Objective response to first-line 177Lu-DOTATATE was significantly better than octreotide LAR. Upfront 177Lu-DOTATATE also achieved considerably longer progression-free survival compared with octreotide LAR.
Relevance
The findings suggest that 177Lu-DOTATATE could be favorably initiated early in the course of advanced GEP-NETs instead of resorting to it only as a last-line measure. In view of the shorter treatment course with 177Lu-DOTATATE, this can potentially prove to be a cost-effective strategy in low- to middle-income countries.
Increased somatostatin receptor (SSTR) expression in GEP-NETs, as demonstrated by high-grade uptake on SSTR scintigraphy, provides the rationale for the use of PRRT.8 The PRRT agents bind to the SSTRs on the tumor cell membrane resulting in the delivery of the beta-emitting radionuclides and thus causing cellular damage.9,10 Of the PRRTs, 177Lu-DOTATATE was recently approved for patients with progressive, advanced SSTR-positive GEP-NETs based on the NETTER-1 trial.11 However, its role as a first-line therapeutic agent vis-à-vis somatostatin analogs in treatment-naïve patients has not yet been elucidated. Here, we intended to retrospectively compare the efficacy and safety of 177Lu-DOTATATE versus octreotide long-acting release (LAR) as first-line systemic therapy in patients with advanced well-differentiated inoperable or metastatic GEP-NETs.
MATERIALS AND METHODS
Patient Population
This was a retrospective analysis of a prospectively maintained registry at a tertiary care institution in a lower- to middle-income country. Data of consecutive patients of advanced inoperable or metastatic well-differentiated GEP-NETs treated with either 177Lu-DOTATATE or octreotide LAR from September 2012 to December 2019 were collected and analyzed. All the patients had undergone an initial whole-body 68Ga-DOTANOC positron emission tomography-computed tomography (PET-CT) for evaluation of the degree of somatostatin expression and the extent of the lesions. Baseline CBC, renal function test, estimated glomerular filtration rate, and liver function test were also obtained. Serum chromogranin A levels, wherever available, were documented. Only patients with histopathologically confirmed grade 1 and 2 GEP-NETs who had tracer-avid inoperable or metastatic lesions (lesion uptake ≥ physiologic liver uptake) on 68Ga-DOTANOC PET-CT and were considered for first-line systemic treatment either with 177Lu-DOTATATE or octreotide LAR were included in this study cohort. Additional eligibility criteria included hemoglobin ≥ 8 g/dL; total leukocyte count ≥ 3,000/µL; neutrophils ≥ 1,500/µL; platelets ≥ 75,000/µL; estimated glomerular filtration rate ≥ 60 mL/min; and Eastern Cooperative Oncology Group performance ≤ 2. Patients with non or faintly SSTR-expressing lesions, second malignancies, and those on prior or concurrent antitumor medications were excluded. Patients also underwent 18F-labeled fluorodeoxyglucose PET-CT within 2 weeks of 68Ga-DOTANOC PET-CT and those patients demonstrating discordant lesion(s) were also excluded from this analysis. Patients who received treatment with either 177Lu-DOTATATE or octreotide LAR in the second-line setting after initial disease progression were also not included. The decision for treatment, either with 177Lu-DOTATATE or octreotide LAR, was based on physician and patient preference and patient's affordability. Specifically, considerations were given to the relatively short-course treatment with 177Lu-DOTATATE vis-à-vis the prolonged treatment with octreotide LAR and the potential toxicities associated with either treatment. Both the treatment modalities were provided against funding (self-pay or insurance coverage) and hence, the patients' financial status was taken into account before deciding on the course of management. Informed written consent was obtained from each patient before initiation of therapy. The study was approved by the Institutional Ethics Committee (INT/IEC/2020/000576) and was conducted as per the guidelines enshrined in the Helsinki Declaration.
Treatment Characteristics
Lutetium chloride (177LuCl3) was obtained from the Board of Radiation and Isotope Technology, Bhabha Atomic Research Centre, Mumbai, India, and DOTATATE peptide was procured from ABX (GmBH, Radeberg, Germany). Radiolabeling of DOTATATE with 177LuCl3 was then carried out in our in-house radiopharmacy as described previously.12
In the 177Lu-DOTATATE arm, approximately 6.0-7.4 GBq/cycle of 177Lu-DOTATATE was administered intravenously over 30 minutes. Patients were usually administered up to four such cycles, at 8-12 weeks' intervals unless treatment discontinuation was necessitated in view of unacceptable toxicity, disease progression, death, or unwillingness of the patient. Decision regarding the need for further treatment with additional cycles was personalized and based on interdepartmental consensus. To ensure nephroprotection, an amino-acid infusion of lysine and arginine was given over 4 hours, starting 30 minutes before the infusion of 177Lu-DOTATATE. Pretreatment with ondansetron and dexamethasone was also carried out. Patients were monitored for 24 hours for any immediate adverse event (AE). Additionally, as part of the institutional protocol, oral capecitabine was given as a radiosensitizer at a dose of 1,250 mg/m2/day from days 0 to 14 of each cycle of 177Lu-DOTATATE as followed by Ballal et al.13
In the octreotide LAR group, octreotide LAR was administered intramuscularly at a dose of 30 mg every 4 weeks for a duration of at least 6 months and treatment was continued till the time patients were willing and unacceptable toxicity, disease progression, or death occurred. In both the treatment groups, patients were allowed to receive rescue injections of short-acting octreotide subcutaneously if they experienced symptoms associated with hormonal crisis (eg, diarrhea and/or flushing).
Treatment End Points
68Ga-DOTANOC PET-CT was carried out for evaluation of radiologic response at 6-8 weeks after every two cycles of 177Lu-DOTATATE and at 3-4 weeks after every six cycles of octreotide LAR. The best morphologic response was assessed on the contrast-enhanced CT images using the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.14 The primary end point was the objective response rate (ORR), which was calculated as the proportion of patients achieving complete response and partial response (PR).
Secondary end points included disease control rate (DCR), best biochemical response, toxicity profile, progression-free survival (PFS), and overall survival (OS). For DCR, the proportions of complete response, PR, and stable disease on RECIST 1.1 were pooled. Biochemical response was assessed using serial serum chromogranin A levels: a ≥ 50% reduction in serum chromogranin A compared with baseline was considered as PR, whereas an increase of ≥ 25% was regarded as PD. Treatment-related toxicity was evaluated using Common Terminology Criteria for Adverse Events, version 5.0. PFS was estimated from the start of the treatment regimen till documented radiologic or clinical progression or death because of any cause. OS was estimated from the start of the treatment till the occurrence of death because of any cause.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 20.0, IBM Corp, Armonk, NY. Categorical variables were expressed as number and percentages. Fisher's exact test was used for testing significance of difference in these variables across groups. Continuous variables were expressed as median and range. Survival analysis was done using Kaplan-Meier curve method and Cox proportional-hazards model. The median duration of follow-up was estimated using the reverse Kaplan-Meier curve method. Tarone-Ware test was used to compare the survival times between the two groups. A two-tailed P value < .05 was considered as statistically significant.
RESULTS
Demographic and Clinical Characteristics
A total of seventy-six patients with advanced inoperable or metastatic well-differentiated GEP-NETs were included, comprising 36 patients in the 177Lu-DOTATATE arm and 40 patients in the octreotide LAR arm. The demographic and clinical characteristics of the patients were observed to be comparable in both the arms (Table 1). The median age of the patients was 53 years (range 14-81 years). Pancreatic neuroendocrine tumor (pNET) accounted for the majority of the primary tumors (39%) followed by midgut neuroendocrine tumor (NET; 22%). Most of the patients had grade 1 NETs (58%) and presented with metastases to liver (76%) and lymph nodes (72%). Although 177Lu-DOTATATE or octreotide LAR was administered as first-line systemic therapy in all the patients, 30 (39%) patients had also undergone prior resection of the primary tumor.
TABLE 1.
Demographic and Clinical Characteristics of Patients in the Study

In the 177Lu-DOTATATE arm, the patients received a median cumulative activity of 27.3 GBq (range 14.3-37.6 GBq) of 177Lu-DOTATATE over 2-5 cycles and 1,250 mg/m2 capecitabine per day starting from days 0 to 14 of each PRRT cycle. All the 36 patients received at least two cycles of 177Lu-DOTATATE, whereas seven (19%) patients received three cycles, 16 (44%) patients received four cycles, and three (8%) patients received five cycles. The median number of cycles was four, and the median time interval between the treatment cycles was 8 weeks. In the octreotide LAR arm, the patients were administered a median of six doses of octreotide LAR 30 mg at 4 weeks intervals. All the 40 patients received at least six doses, whereas five (12.5%) patients received nine doses and 12 (30%) patients received 12 doses of octreotide LAR.
Radiologic Response
Two patients were lost to follow-up after two cycles in the 177Lu-DOTATATE arm, and best radiologic response using RECIST 1.1 could be assessed in a total of 74 patients. The ORR was 38% in the 177Lu-DOTATATE arm versus 15% in the octreotide LAR arm (P = .025), whereas the DCR was 88% in the 177Lu-DOTATATE arm versus 67% in the octreotide LAR arm (P = .035). The results pertaining to the radiologic responses are summarized in Table 2.
TABLE 2.
Best Radiologic Responses of Patients in the Study as Evaluated With RECIST 1.1

Biochemical Response
Among the 76 patients, serial serum chromogranin A levels were available only for 20 patients: 12 patients in the 177Lu-DOTATATE arm and eight patients in the octreotide LAR arm. A ≥ 50% reduction in serum chromogranin A levels was observed in 5/12 (42%) patients in the 177Lu-DOTATATE arm compared with 3/8 (37%) patients in the octreotide LAR arm (P = 1.000). Biochemical progression was noted in a total of 5/20 patients: three patients in the 177Lu-DOTATATE arm and two patients in the octreotide LAR arm.
Survival Analysis and Follow-Up
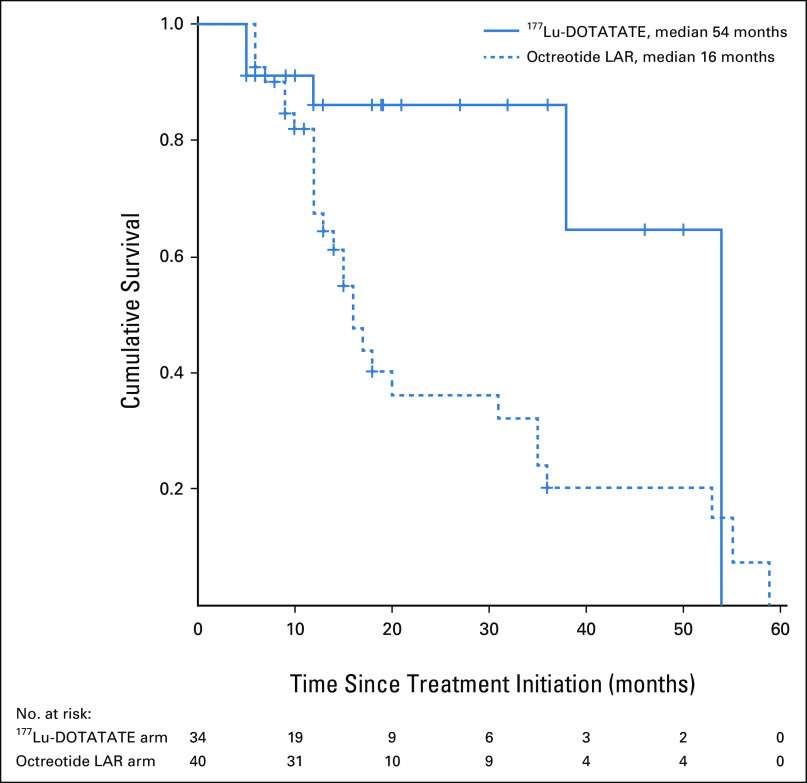
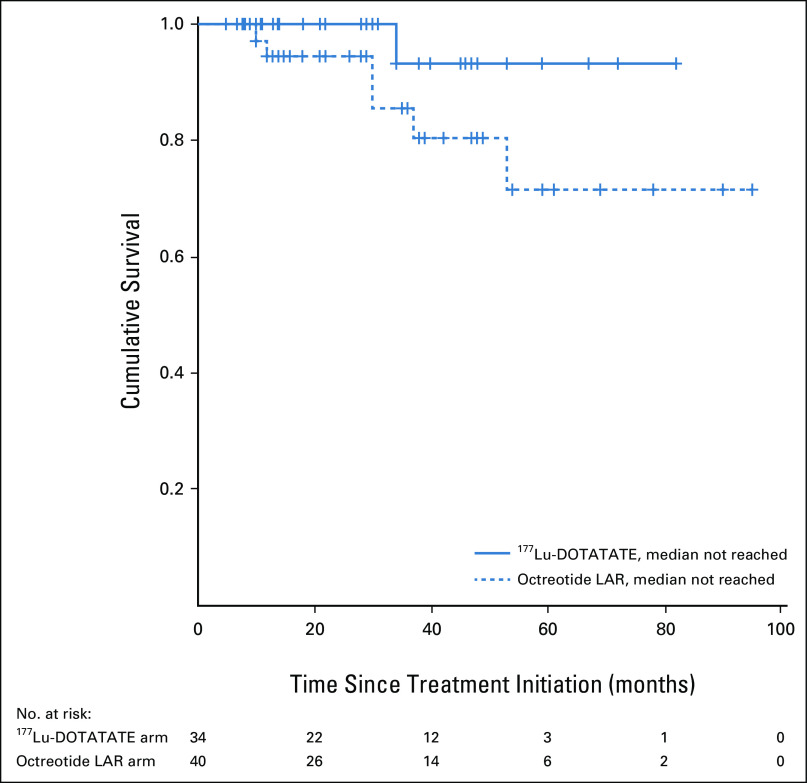
The patients in this study cohort had a median follow-up duration of 34 months (range 6-95 months). During the course of follow-up, a total of 34 events of disease progression or death had occurred in the study cohort: six events in the 177Lu-DOTATATE arm and 28 events in the octreotide LAR arm. The estimated median PFS in the 177Lu-DOTATATE arm was 54 months (95% CI: not reached) compared with 16 months (95% CI, 13.0 to 18.9 months) in the octreotide LAR arm (P = .017; Fig 1). The univariate hazard ratio (HR) for disease progression or death with 177Lu-DOTATATE versus octreotide LAR was 0.34 (95% CI, 0.14 to 0.82). A total of seven deaths were observed, one in the 177Lu-DOTATATE arm and six in the octreotide LAR arm. The median OS durations were not reached and not significantly different across both the arms (P = .116; Fig 2). On multivariate analysis, baseline patient characteristics such as age, sex, grade of tumor, and prior surgical history did not significantly affect the PFS and OS. However, a favorable outcome with respect to PFS was observed for the 177Lu-DOTATATE arm compared with the octreotide LAR arm (HR, 0.18; 95% CI, 0.05 to 0.67). The foregut primary tumors were also seen to have a favorable outcome with respect to the PFS (HR, 0.12; 95% CI, 0.02 to 0.56; Table 3).
FIG 1.
Kaplan-Meier curves for progression-free survival (PFS) in patients with advanced gastroenteropancreatic neuroendocrine tumors treated with first-line 177Lu-DOTATATE or octreotide LAR. LAR, long-acting release.
FIG 2.
Kaplan-Meier curves for overall survival (OS) in patients with advanced gastroenteropancreatic neuroendocrine tumors treated with first-line 177Lu-DOTATATE or octreotide LAR. LAR, long-acting release.
TABLE 3.
Multivariate Analysis of Potential Prognostic Factors Affecting Survival Outcomes
Toxicity
The most commonly encountered symptomatic adverse effect in the 177Lu-DOTATATE arm was nausea or vomiting, which was mainly attributable to the amino-acid infusion and promptly relieved after its completion. Other common symptomatic adverse effects associated with 177Lu-DOTATATE were diarrhea, fatigue, weight gain, and anorexia. Among the patients in the octreotide LAR arm, common symptomatic AEs reported were fatigue, abdominal pain, and diarrhea. Majority of these toxicities were grade 1 or 2 events. Among the laboratory parameters, grade 1 or 2 anemia was the most commonly observed treatment-related AE (20/36 patients, 56%) in the 177Lu-DOTATATE recipients. Leukopenia of any grade was observed in 12 (33%) of these patients, whereas thrombocytopenia was reported in 10 (28%) patients. Serious hematologic AEs viz. grade 3 or 4 anemia, leukopenia, and neutropenia were observed in one (3%) patient each in the 177Lu-DOTATATE arm. Although there was no reported case of grade 3 or 4 nephrotoxicity, two (6%) patients experienced grade 3 or 4 hepatotoxicity in the form of raised serum bilirubin levels. By contrast, only a single (3%) patient in the octreotide LAR arm experienced grade 3 hyperbilirubinemia; however, there were no reported cases of grade 3 or 4 hematologic toxicity or nephrotoxicity in this group. The grade 3 or 4 hematologic and nonhematologic toxicities were observed to be transient in most patients with values normalizing between 10 and 20 weeks after therapy. Two (6%) patients had capecitabine-related grade 2 palmar-plantar erythrodysesthesia syndrome and were subsequently discontinued from capecitabine. Long-term AEs such as myelodysplastic syndrome and leukemia were not observed in the 177Lu-DOTATATE arm till the time of follow-up. The toxicity profiles of both the treatment regimens are summarized in Table 4.
TABLE 4.
Toxicity Profile of Patients in the Study as Evaluated With CTCAE v5.0
DISCUSSION
To the best of our knowledge, studies with head-to-head comparison of 177Lu-DOTATATE and octreotide LAR in the first-line setting for advanced NETs are currently lacking. In our single-institution experience, treatment with 177Lu-DOTATATE plus radiosensitizing capecitabine achieved significantly better ORR (38% v 15%) and progression-free survival (54 months v 16 months) compared with octreotide LAR with no major additional toxicity. The findings support the use of 177Lu-DOTATATE as a first-line treatment modality over long-acting somatostatin analogs for advanced inoperable or metastatic grade 1 or 2 GEP-NETs.
In the landmark NETTER trial, patients of advanced midgut NETs postprogression with standard octreotide LAR treatment were treated with 177Lu-DOTATATE plus standard-dose octreotide LAR or high-dose octreotide LAR alone. The 177Lu-DOTATATE arm achieved significantly better radiologic response and PFS compared with the best supportive care alone, following which 177Lu-DOTATATE was accorded approval for this indication.11 Treatment guidelines, thereafter, have focused on initial disease stabilization with somatostatin analogs and advocate shifting to PRRT only in case of PD.15 However, the results of this study showed excellent radiologic and survival outcomes with 177Lu-DOTATATE even in the first-line setting. The findings suggest that 177Lu-DOTATATE could be safely initiated early in the course of the disease with potential long-term beneficial effects in contrast to the current approach of resorting to it as a last-line measure. Given the much longer PFS observed with 177Lu-DOTATATE in the upfront setting in this study, such patients can then be reasonably kept on clinical and radiologic follow-up and started on octreotide LAR only after disease progression. Alternately, 177Lu-DOTATATE could be given upfront as an induction therapy to achieve a certain degree of disease remission, following which octreotide LAR could be commenced as maintenance therapy.
Octreotide LAR was approved for first-line use in advanced NETs based on the PROMID trial, which showed significantly longer PFS in the octreotide LAR group compared with placebo (14.3 months v 6 months). However, the radiologic response rates were marginal with only one out of 42 patients in the octreotide LAR group achieving an objective response and the treatment benefit was largely limited to disease stabilization.16 Our observations in the octreotide LAR arm are thus consistent with the results of this trial.
Another limiting factor to the practical use of octreotide LAR is its cost effectiveness, especially in the setting of LMICs. Unlike 177Lu-DOTATATE, which has a fixed number of treatment cycles (usually 4-6), treatment with octreotide LAR has no definite end point regarding treatment completion. This has important ramifications, especially in LMICs, wherein, despite the relatively higher per-cycle cost of 177Lu-DOTATATE compared with octreotide LAR, the net treatment-related expenses for all the cycles with octreotide LAR can far exceed those with 177Lu-DOTATATE. Given the higher response rates and longer PFS associated with 177Lu-DOTATATE, its use as first-line treatment can prove to be a cost-effective strategy in this setting. Although cost-effectiveness studies with octreotide LAR are currently unavailable, similar analysis for 177Lu-DOTATATE showed an incremental cost-effectiveness ratio of £62,158 per quality-adjusted life-year (QALY) in midgut NET. This was in contrast to the incremental cost-effectiveness ratio of £199,233 per QALY reported for everolimus, which is quite similar to octreotide LAR, and has no defined end point for treatment completion.17 The effectiveness of 177Lu-DOTATATE over everolimus in the progressive pancreatic neuroendocrine tumors was also established in a recent comparative meta-analysis with 177Lu-DOTATATE showing significantly better radiologic and survival outcomes.18
Although, there was no major difference in the occurrence of grade 3 or 4 toxicities in both of the treatment arms, the overall frequency of hematologic toxicity was expectedly higher in the 177Lu-DOTATATE recipients. However, these were mostly grade 1 or 2 events and were seen to resolve within few weeks after therapy with no requirement for any additional management. Furthermore, long-term adverse effects such as myelodysplastic syndrome were not observed in this study cohort. The findings are notable, given that radiosensitizing chemotherapy in the form of low-dose capecitabine was concomitantly administered to the patients in the 177Lu-DOTATATE arm. The results suggest that the use of low-dose capecitabine could have contributed in a synergistic manner to the better treatment outcomes observed in the 177Lu-DOTATATE arm by virtue of its radiosensitizing action, but without increasing the associated risks.13,19-21 However, this needs to be further validated in future prospective trials.
Health-related quality of life is an important parameter to be considered before initiating any treatment. Although octreotide LAR has been proven to have a high degree of safety, a long duration of treatment could eventually lead to significant deterioration in the quality of life of the patients.22,23 By contrast, studies with 177Lu-DOTATATE have reported significant improvement in the patients' global health status, physical and social functioning, and mitigation of physical symptoms even after a limited number of treatment cycles.24,25 Future studies comparing the two treatment modalities should include health-related quality of life as a study end point so as to generate more evidence in this direction.
This study has a number of limitations. The relatively small number of patients included in the two treatment arms, nonrandomized treatment allocation, and retrospective analysis limit the strength of our observations. Furthermore, assessment of health-related quality of life was not carried out. Follow-up chromogranin A levels were not available in majority of the patients owing to financial constraints. Nevertheless, given the rarity of the disease, the current study serves as a proof of concept exploring the potential benefit of 177Lu-DOTATATE over somatostatin analogs as upfront therapy in advanced GEP-NETs, especially in a lower- to middle-income country. Adequately powered randomized controlled trials with head-to-head comparison of 177Lu-DOTATATE and octreotide LAR would help further validate the results of this study and determine the best treatment option for the treatment-naïve patients with advanced GEP-NETs.
AUTHOR CONTRIBUTIONS
Conception and design: Swayamjeet Satapathy, Bhagwant R. Mittal, Ashwani Sood, Rakesh Kapoor, Rajesh Gupta
Administrative support: Bhagwant R. Mittal
Provision of study materials or patients: Bhagwant R. Mittal, Apurva Sood, Rakesh Kapoor, Rajesh Gupta, Divya Khosla
Collection and assembly of data: Swayamjeet Satapathy, Ashwani Sood, Apurva Sood, Rakesh Kapoor, Rajesh Gupta, Divya Khosla
Data analysis and interpretation: Swayamjeet Satapathy, Ashwani Sood, Rakesh Kapoor
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Dasari A Shen C Halperin D, et al. : Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumours in the United States. JAMA Oncol 3:1335-1342, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cives M, Strosberg JR: Gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin 68:471-487, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Pavel M O'Toole D Costa F, et al. : ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology 103:172-185, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Bodei L Cremonesi M Grana CM, et al. : Peptide receptor radionuclide therapy with 177Lu-DOTATATE: The IEO phase I-II study. Eur J Nucl Med Mol Imaging 38:2125-2135, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Ezziddin S Attassi M Yong-Hing CJ, et al. : Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumours after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med 55:183-190, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Brabander T Van der Zwan WA Teunissen JJ, et al. : Long-term efficacy, survival, and safety of [177Lu-DOTA0, Tyr3] octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumours. Clin Cancer Res 23:4617-4624, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Zandee WT Brabander T Blažević A, et al. : Symptomatic and radiological response to 177Lu-DOTATATE for the treatment of functioning pancreatic neuroendocrine tumours. J Clin Endocrinol Metab 104:1336-1344, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Hofman MS, Lau WE, Hicks RJ: Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: Clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics 35:500-516, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Van Der Zwan WA Bodei L Mueller-Brand J, et al. : GEP–NETs update: Radionuclide therapy in neuroendocrine tumours. Eur J Endocrinol 172:R1-R8, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Hirmas N, Jadaan R, Al-Ibraheem A: Peptide receptor radionuclide therapy and the treatment of gastroentero-pancreatic neuroendocrine tumors: Current findings and future perspectives. Nucl Med Mol Imaging 52:190-199, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strosberg J El-Haddad G Wolin E, et al. : Phase 3 trial of 177Lu-DOTATATE for midgut neuroendocrine tumours. N Engl J Med 376:125-135, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satapathy S Mittal BR Sood A, et al. : Peptide receptor radionuclide therapy as first-line systemic treatment in advanced inoperable/metastatic neuroendocrine tumors. Clin Nucl Med 45:e393-e399, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Ballal S Yadav MP Damle NA, et al. : Concomitant 177Lu-DOTATATE and capecitabine therapy in patients with advanced neuroendocrine tumours: A long-term-outcome, toxicity, survival, and quality-of-life study. Clin Nucl Med 42:e457-466, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA Therasse P Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Herrera-Martínez AD Hofland J Hofland LJ, et al. : Targeted systemic treatment of neuroendocrine tumours: Current options and future perspectives. Drugs 79:21-42, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinke A Muller HH Schade-Brittinger C, et al. : Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID study group. J Clin Oncol 27:4656-4663, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Mujica-Mota R Varley-Campbell J Tikhonova I, et al. : Everolimus, Lutetium-177 DOTATATE and sunitinib for advanced, unresectable or metastatic neuroendocrine tumours with disease progression: A systematic review and cost-effectiveness analysis. Health Technol Assess 22:1-326, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satapathy S, Mittal BR: 177Lu-DOTATATE peptide receptor radionuclide therapy versus everolimus in advanced pancreatic neuroendocrine tumors: A systematic review and meta-analysis. Nucl Med Commun 40:1195-1203, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Claringbold PG Brayshaw PA Price RA, et al. : Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur J Nucl Med Mol Imaging 8:302-311, 2011 [DOI] [PubMed] [Google Scholar]
- 20.van Essen M Krenning EP Kam BL, et al. : Report on short-term side effects of treatments with 177Lu-octreotate in combination with capecitabine in seven patients with gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 35:743-748, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kesavan M, Claringbold PG, Turner JH: Hematological toxicity of combined 177Lu-octreotate radiopeptide chemotherapy of gastroenteropancreatic neuroendocrine tumors in long-term follow-up. Neuroendocrinology 99:108-117, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Bornschein J Drozdov I Malfertheiner P, et al. : Safety and tolerability issues. Expert Opin Drug Saf 8:755-768, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Rinke A Neary MP Eriksson J, et al. : Health-related quality of life for long-acting octreotide versus placebo in patients with metastatic midgut neuroendocrine tumors in the phase 3 PROMID trial. Neuroendocrinology 109:141-151, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Marinova M Mücke M Mahlberg L, et al. : Improving quality of life in patients with pancreatic neuroendocrine tumor following peptide receptor radionuclide therapy assessed by EORTC QLQ-C30. Eur J Nucl Med Mol Imaging 45:38-46, 2018 [DOI] [PubMed] [Google Scholar]
- 25.Strosberg J Wolin E Chasen B, et al. : Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177Lu-DOTATATE in the phase III NETTER-1 trial. J Clin Oncol 36:2578-2584, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]