PURPOSE
Neurotrophic tyrosine receptor kinase (NTRK) gene fusions encode oncogenic, chimeric tropomyosin receptor kinase (TRK) proteins. Larotrectinib, an approved TRK inhibitor, is efficacious in locally advanced or metastatic (adv/met) TRK fusion cancer. We evaluated the time from initial diagnosis to locally advanced or metastatic disease and to initiation of larotrectinib treatment as well as larotrectinib impact on disease course.
MATERIALS AND METHODS
Patients were grouped by prior lines of therapy (0, 1-2, and ≥ 3) and pre-larotrectinib duration of adv/met disease (short [< 3.5 months], medium [3.5 to < 15.7 months], and long [≥ 15.7 months]). Overall response rate (ORR), duration of response (DOR), and progression-free survival were assessed.
RESULTS
One hundred sixty-four patients were evaluated. The median time from initial diagnosis to development of locally adv/met stage was 2.1 months; the duration of pre-larotrectinib adv/met disease was 7.3 months (n = 153). In patients with 0, 1-2, and ≥ 3 prior lines of therapy, the median time from diagnosis to adv/met stage was 0.9, 1.2, and 9.4 months, and 1.5, 5.8, and 29.0 months from adv/met disease to larotrectinib initiation, respectively. Clinical outcomes were independent of line of therapy (ORR: 86%, 63%, and 80%, respectively; median DOR: 27.6, not reached, and 32.9 months), and similar across subgroups of short, medium, and long duration of pre-larotrectinib adv/met disease status (ORR: 88%, 65%, and 69%, respectively; median DOR: not reached, 27.6, and 32.9 months).
CONCLUSION
The short time from initial diagnosis to adv/met stage before larotrectinib suggests that NTRK gene fusion does not generally have a positive prognostic value. Patients on larotrectinib had high, sustained ORR, independent of number of prior therapies or duration of adv/met disease, suggesting that the effect of TRK inhibition in molecularly selected patients is independent of prior treatments or disease course.
INTRODUCTION
Neurotrophic receptor tyrosine kinase (NTRK) genes, NTRK1, 2, and 3, encode tropomyosin receptor kinase (TRK) A, B, and C subtypes that regulate neuronal development and function. Chromosomal rearrangements may result in somatic NTRK gene fusions that lead to expression of chimeric TRK fusion proteins. These fusion proteins are constitutively active or overexpressed and capable of driving oncogenesis.1 Such oncogenic TRK fusions are found at low frequency in many common pediatric and adult cancers (lung, breast, GI, melanomas, and sarcomas) but are enriched in several rare tumors, including infantile fibrosarcoma (IFS), mammary analog secretory carcinoma, secretory breast carcinoma, and cellular congenital mesoblastic nephroma.2-8
CONTEXT
Key Objective
Approval of larotrectinib as a tumor-agnostic therapy was based on favorable efficacy or safety profiles from three larotrectinib trials in patients with neurotrophic tyrosine receptor kinase (NTRK) fusion cancers. Given the heterogeneous nature of these cancers, this study explored whether a patient's disease course before larotrectinib can influence efficacy outcomes.
Knowledge Generated
Assessment of disease course before larotrectinib initiation showed rapid progression from initial diagnosis to development of locally advanced or metastatic (adv/met) disease. Evaluation of subgroups on the basis of number of prior lines of therapy or duration of adv/met disease before larotrectinib initiation showed durable responses across subgroups, including patients with 3+ prior lines of therapy and those with longest duration of adv/met disease.
Relevance
Patients enrolled in the larotrectinib trials showed rapid progression to adv/met disease before larotrectinib initiation. Treatment with larotrectinib favorably altered time to progression, independent of number of prior lines of therapy or duration of adv/met disease.
Targeting tumors that harbor NTRK gene fusions represents an emerging therapeutic strategy that has resulted in favorable clinical outcomes. TRK inhibitors have demonstrated high response rates (> 75% for larotrectinib; 57% for entrectinib) in patients with TRK fusion cancer, independent of tumor histology, and are among the first tumor-agnostic therapies to be approved in the United States and European Union.9-12
Larotrectinib is a highly selective and potent, CNS-active, orally administered, small molecule, TRK inhibitor. It is the first tumor-agnostic therapy to be approved for TRK fusion cancer, with approval on the basis of marked and durable antitumor activity and favorable safety profile observed in three phase I/II clinical trials in children, adolescents, and adults.13 The results showed a pooled investigator-assessed overall response rate (ORR) of 79%, median duration of response (DOR) of 35.2 months, and median progression-free survival (PFS) of 28.3 months in locally advanced or metastatic (adv/met) TRK fusion cancer.9 Clinical findings from TRK inhibitor trials have been practice-changing as international consensus guidelines representing US, European, and Asian oncology organizations have been developed to address diagnostic approaches and patient selection criteria to identify those expected to benefit most from TRK inhibitor therapy.14
The broad heterogeneity of cancer types that harbor NTRK fusions and high variability in the frequency of fusion events have limited the understanding of prognostic value of NTRK gene fusions in clinical course or natural history of the disease. In a small study, there was a trend to higher risk of death in patients with NTRK gene fusions compared with those without (median overall survival [OS]: 12.5 v 16.5 months), but the difference did not reach significance.15 In another comparative analysis conducted at Memorial Sloan Kettering Cancer Center (MSKCC), TRK fusion–positive cancers showed no clear evidence of having an unexpectedly favorable prognosis, compared with NTRK wild-type tumors.16
To gain greater understanding of the natural course of disease of patients enrolled in the larotrectinib studies, we evaluated the speed of progression of disease from initial diagnosis to locally adv/met stage to initiation of study treatment. Using prior line of therapy and time from adv/met stage to larotrectinib initiation as measures of disease dynamics, we determined whether clinical outcomes from larotrectinib treatment were affected by prior line of therapy or duration of adv/met disease preceding treatment initiation with larotrectinib.
MATERIALS AND METHODS
Study Design and Participants
Data for this analysis were pooled from three trials (NCT02122913, NCT02637687, and NCT02576431), as previously described, but on the basis of independent review committee rather than investigator assessment of response.9 Briefly, eligible patients were 1 month or older and had locally adv/met solid tumors, previously received standard therapy (if available), and adequate organ function. TRK fusion positivity was determined by locally obtained molecular testing. All studies were conducted in accordance with the standard of good clinical practice, the Declaration of Helsinki, and all applicable country and local regulations. Protocols were approved by institutional review boards or independent ethics committees at each site, and all patients provided written informed consent (or assent by parents and guardians of minor patients) before study initiation.
Procedures
Larotrectinib was given orally on continuous 28-day schedules at doses starting from 100 mg twice daily (twice a day) for adolescent and adult patients, as previously described.9 Most pediatric patients received 100 mg/m2 per dose, up to a maximum of 100 mg, twice a day. Larotrectinib was administered until disease progression, study withdrawal, or occurrence of an unacceptable level of adverse events.
Outcomes
In this ad hoc analysis, the duration between initial diagnosis and locally adv/met stage and the duration between adv/met stage and larotrectinib initiation were determined collectively and by individual tumor type. Subgroups by prior lines of therapy (0, 1-2, or ≥ 3) and evenly distributed subpopulations on the basis of time from adv/met stage to larotrectinib initiation (short [< 3.5 months], medium [3.5 to < 15.7 months], and long [≥ 15.7 months]) were evaluated for responses, DOR, and PFS, as previously described.9 Responses were assessed by an independent review committee. Patients with missing time-to-adv/met stage data were excluded. The data cutoff was July 15, 2019.
Statistical Analysis
Post hoc analyses were prepared using descriptive statistics, using Kaplan-Meier methodology for time-to-event variables. Patients were classified into time from adv/met stage to larotrectinib initiation using a tercile split. For sensitivity analyses, additional analyses were performed that excluded patients with IFS and pediatric patients.
RESULTS
Baseline Disease Characteristics and Clinical Course of Disease Before Larotrectinib Treatment
A total of 164 patients were included in the analysis. Data on the analysis of time from initial diagnosis to development of locally adv/met stage and/or to treatment initiation were missing for 11 patients (Data Supplement). To gain a fuller understanding of the disease progression dynamics of patients before enrollment, we evaluated patient history to determine the median time from initial diagnosis to adv/met stage of disease and the duration of adv/met disease before larotrectinib.
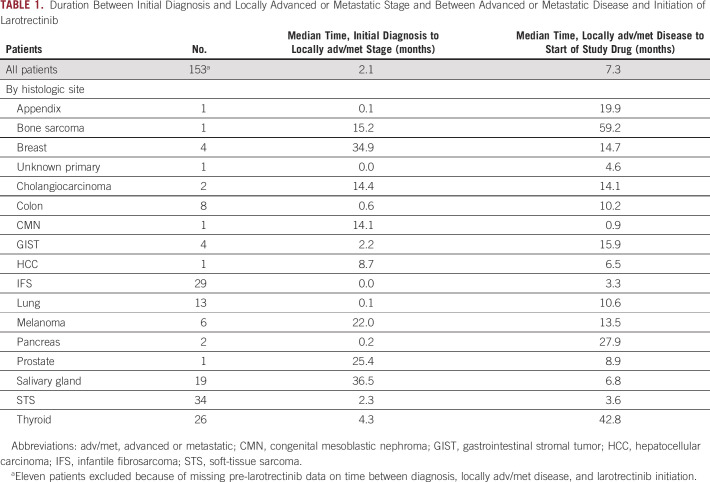
Among 153 patients representing 19 different tumor types in the larotrectinib trials, 74.4% had metastatic disease; the median time from initial diagnosis to locally adv/met stage was 2.1 months, and the median duration of pre-larotrectinib adv/met disease was 7.3 months. The median duration between initial diagnosis and adv/met stage and the duration of adv/met disease before start of study treatment are presented in Table 1 for the collective study population and individual tumor types. Across tumor types, the median time from initial diagnosis to adv/met stage ranged from 0 months (patients with IFS, n = 29) to 36.5 months (salivary gland cancer, n = 19).
TABLE 1.
Duration Between Initial Diagnosis and Locally Advanced or Metastatic Stage and Between Advanced or Metastatic Disease and Initiation of Larotrectinib
Because adv/met disease may be present but not immediately detectable at initial diagnosis, we also determined the proportion of patients for whom adv/met disease was detected within 2 months of initial diagnosis, with the rationale that a very short duration may be indicative of delays in diagnostics (eg, from imaging), rather than being true progression to a more advanced stage of disease. These patients were considered to be representative of a population likely to have had adv/met disease at diagnosis. Although nearly half (74 of 153; 48%) of all patients met those criteria, sample sizes were too small for several specific tumor types represented to draw meaningful conclusions. Among tumor types represented by six or more patients, the criteria were met by 75% (6 of 8) of patients with colon cancer, 79% (23 of 29) IFS, 85% (11 of 13) lung, 33% (2 of 6) melanoma, 5% (1 of 19) salivary gland, 41% (14 of 34) soft-tissue sarcoma, and 38% (10 of 26) thyroid cancers (Data Supplement).
Subgroup Analysis: Patient Characteristics
To further examine the impact of clinical course of patients on clinical outcomes, we conducted two subgroup analyses: (1) by prior lines of therapy (0, 1-2, or ≥ 3) and (2) by duration of adv/met disease before larotrectinib imitation (short, medium, or long).
In the first subgroup analysis, cohorts by line of therapy included 36, 84, and 44 patients with 0, 1-2, and ≥ 3 prior lines of therapy, respectively. At baseline, patient characteristics were generally balanced between cohorts who had 0 versus 1-2 prior lines of therapy (Data Supplement). The cohort of patients receiving 3 or more lines of previous therapy was older, with more female patients and slightly worse Eastern Cooperative Oncology Group performance status. Gene fusions involving NTRK3 were more frequent in patients with 0 prior lines of therapy, compared with NTRK1, which was more common in patients with 1-2 or ≥ 3 prior lines of therapy.
For the second subgroup analysis by duration of pre-larotrectinib adv/met disease, each cohort included 51 patients. More than half of patients with the shortest duration were children, with the median age of 12.4 years, where those with medium and long durations of adv/met disease were older (median age, 38.0 and 52.0 years, respectively; Data Supplement). Gene fusions involving NTRK3 were also more frequent in the short-duration group, compared with NTRK1, which was more common in the medium and long-duration groups.
Subgroup Analysis: Efficacy Analysis by Line of Therapy
The median time from initial diagnosis to locally adv/met stage was 0.9, 1.2, and 9.4 months for patients receiving 0, 1-2, and ≥ 3 prior lines of therapy, respectively; the median time from adv/met disease to larotrectinib initiation was 1.5, 5.8, and 29.0 months, respectively.
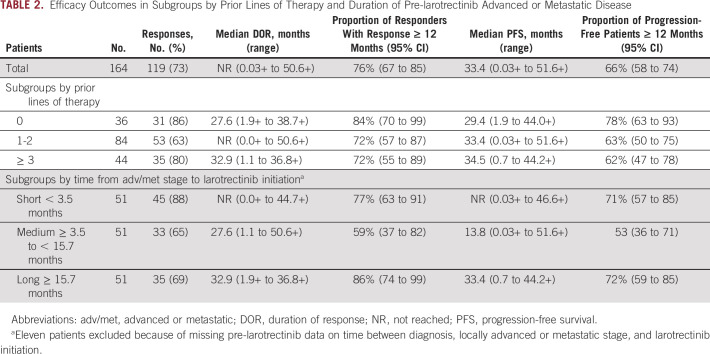
ORR by line of therapy was 86%, 63%, and 80% for 0, 1-2, and ≥ 3 prior lines of therapy, with the median DOR of 27.6, not reached (NR), and 32.9 months, respectively; the proportion of responders with DOR ≥ 12 months was 84%, 72%, and 72%, respectively (Table 2; Fig 1A). The median PFS was 29.4, 33.4, and 34.5 months, respectively; the proportion of patients with PFS ≥ 12 months was 78%, 63%, and 62%, respectively (Table 2; Fig 1B). The median follow-up time for DOR was 14.7, 12.0, and 16.6 months, respectively; for PFS, the median follow-up was 16.3, 13.7, and 19.3 months, respectively.
TABLE 2.
Efficacy Outcomes in Subgroups by Prior Lines of Therapy and Duration of Pre-larotrectinib Advanced or Metastatic Disease
FIG 1.
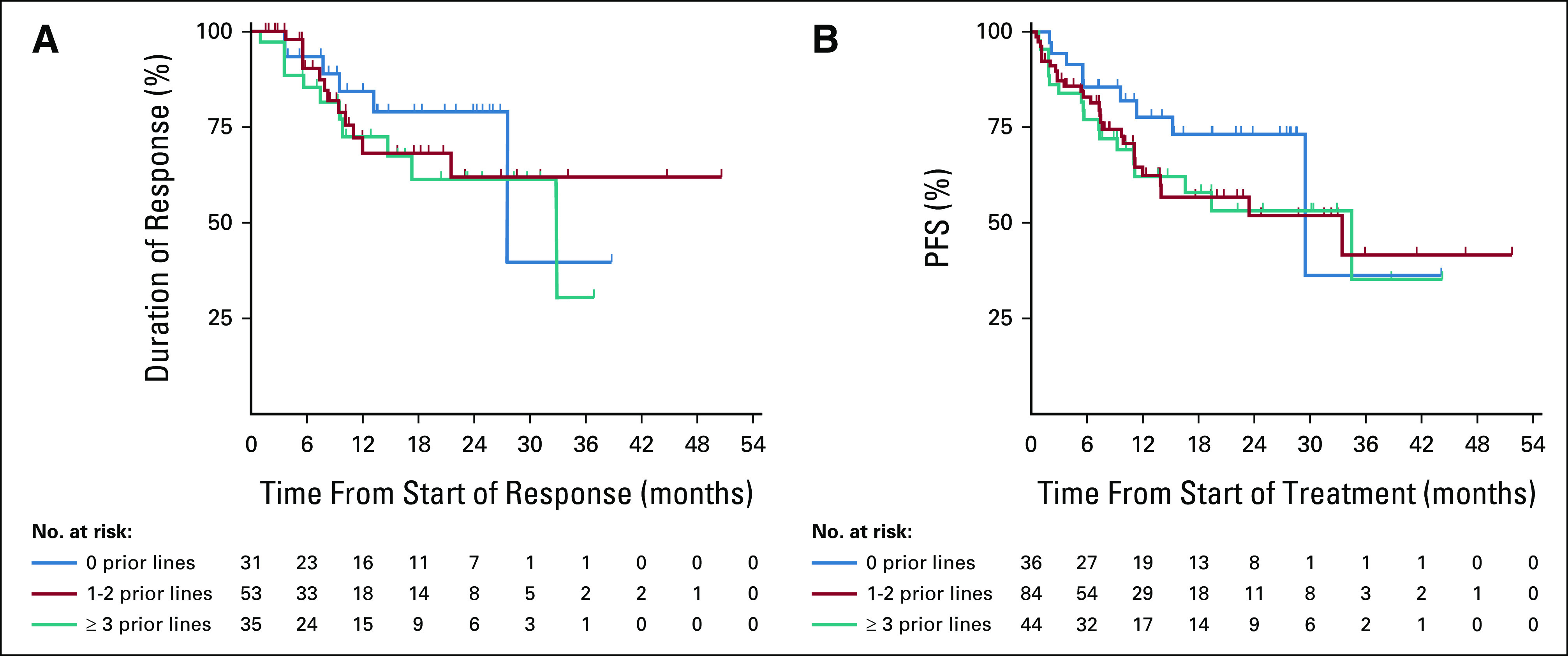
Kaplan-Meier plots of DOR and PFS according to prior lines of therapy (0, 1-2, or ≥ 3): (A) DOR in patients with confirmed responses and (B) PFS. Tick marks indicate censored patients. DOR, duration of response; PFS, progression-free survival.
Subgroup Analysis: Efficacy Analysis by Duration of Adv/Met Disease Before Larotrectinib
For subgroups by duration of pre-larotrectinib adv/met disease, the median time from adv/met diagnosis to larotrectinib initiation was 1.3, 7.3, and 37.8 months for the short, medium, and long subgroups, respectively.
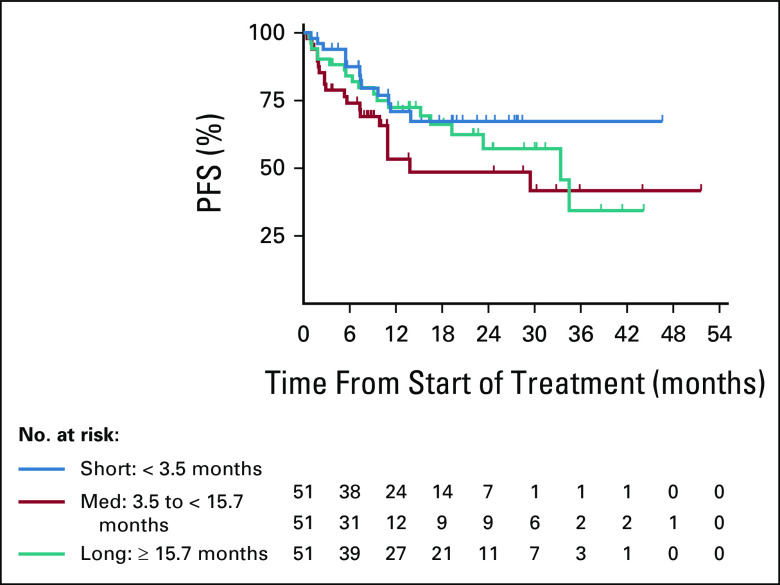
ORR was 88%, 65%, and 69% for patients who had short, medium, and long durations of adv/met disease, with the median DOR of NR, 27.6, and 32.9 months, respectively; the proportion of responders with DOR ≥ 12 months was 77%, 59%, and 86%, respectively (Table 2). The median PFS was NR, 13.8, and 33.4 months, respectively; the proportion of patients with PFS ≥ 12 months was 71%, 53%, and 72%, respectively (Table 2; Fig 2). The median follow-up time for DOR was 13.6, 9.2, and 20.4 months, respectively; for PFS, the median follow-up was 15.3, 10.9, and 21.9 months, respectively.
FIG 2.
Kaplan-Meier plot of PFS according to time from adv/met stage to larotrectinib initiation. Subgroups were defined as short (< 3.5 months), medium (3.5 to < 15.7 months), and long (≥ 15.7 months) durations between adv/met stage and larotrectinib initiation. Tick marks indicate censored patients. adv/met, advanced or metastatic; PFS, progression-free survival.
Most children with IFS were observed to have adv/met disease at diagnosis (Data Supplement) and previously shown to have among the most sensitive histologic types to larotrectinib.9 IFS has been associated with generally good prognosis and high sensitivity to systemic therapy, although a subset of patients have recurrent disease that can result in amputation and poor quality of life.17 Pediatric cancers in general have been associated with longer survival rates than adult cancers and could influence the results of an integrated analysis.18 Furthermore, as a means to avoid disfiguring surgery or limb amputation, the larotrectinib pediatric phase I/II trial allowed patients with IFS to enroll without a requirement for progression after previous therapy or lack of standard of care, thus increasing the proportion of patients with IFS who had no prior lines of therapy. Therefore, to eliminate the possibility that findings from our analyses were driven primarily by the IFS or pediatric populations, we conducted sensitivity analyses, using the same subgroups while excluding patients with IFS or excluding pediatric patients. Exclusion of pediatric patients from the analysis showed ORRs ranging from 53% to 76%, with DORs of 32.9 months to NR. Exclusion of patients with IFS from the analyses showed ORRs ranging from 52% to 83%, with DORs of 27.6 months to NR (Data Supplement). These findings demonstrate consistent clinical benefit in subsets of adult patients only or in non-IFS patients, supporting the robustness of the data (Data Supplement).
DISCUSSION
Larotrectinib is a highly selective TRK inhibitor with rapid, potent, and durable antitumor activity in both children and adults with solid tumors that harbor NTRK gene fusions. Primary data from clinical trials have validated TRK fusions as highly effective targets to larotrectinib's tumor-agnostic profile. Drilon et al10 first presented pooled efficacy from the initial 55 patients from the three larotrectinib trials, reporting an ORR of 75% by independent review and 80% by investigator assessment. Median DOR and PFS had not been reached at a median follow-up of 9.4 months.10 Hong et al9 followed this study with an expanded analysis of 159 enrolled patients, reporting an ORR of 79% by investigator assessment, and median DOR of 35.2 months after a follow-up of 12.9 months. The median PFS was 28.3 months after a median follow-up of 11.1 months.9 The present analysis was designed to better understand whether the natural course of TRK fusion cancer influences larotrectinib efficacy outcomes. Across all histologic types, we observed a short duration between initial diagnosis and locally adv/met stages of disease. This rapid progression to more aggressive stages of disease supports earlier observations that the presence of NTRK gene fusion events does not generally have a positive prognostic value.15,16 To further explore whether differences in progression dynamics could influence outcomes from larotrectinib treatment, we conducted two separate subgroup analyses: (1) by prior line of therapy and (2) by duration of adv/met disease before larotrectinib initiation. Although, as expected, the subgroup categorizations yielded some differences in patient demographics and baseline characteristics, treatment with larotrectinib led to high ORR and sustained response durations across all lines of therapy and irrespective of the duration of time to develop adv/met disease.
The medical literature has a limited number of studies that compare the clinical course of patients with NTRK gene fusions versus NTRK wild-type patients. Nonclinical studies have shown that constitutive activation of TRK fusion proteins drives pro-oncogenic transcription in tumors that harbor the NTRK gene fusion, whereas transforming effects of nonfusion NTRK mutations (ie, overexpression and somatic mutations) are less well-established.1 In a retrospective study of adult patients with genomically profiled solid tumors from the Flatiron Health-Foundation Medicine-Genomic Database, Bazhenova et al15 presented findings that co-occurrence of oncogenic alterations in ALK, BRAF, ERBB2, EGFR, ROS1, and KRAS was infrequently found in patients with NTRK gene fusions, suggesting that the NTRK gene fusion may be a primary oncogenic driver in TRK fusion–positive tumors. Importantly, a trend was also observed toward shorter OS in patients with TRK fusion cancer, compared with a NTRK wild-type matched cohort (12.5 v 16.5 months; P = .648).15 This finding is consistent with our assessment that NTRK gene fusions are not prognostic for favorable outcomes.
In another analysis, Rosen et al16 presented a comparison of patients with NTRK gene fusions versus NTRK wild-type enrolled at MSKCC from April 2015 to August 2018. Among 76 patients with TRK fusion cancer, 51 (67%) had developed advanced or recurrent disease and had an ORR of 46.7% with first-line, non-TRK inhibitor therapy, and median PFS of 9.1 months.16 Our analysis showed that patients receiving first-line larotrectinib had an ORR of 86% and median PFS of 29.4 months. In the Rosen et al. study, patients with advanced or recurrent disease who received a TRK inhibitor (any line of therapy) had an ORR of 67.6%, which was closely aligned with expectations for TRK inhibitor therapy.16 Immuno-oncology regimens given to 12 of these patients resulted in only one response, suggesting that TRK inhibitors are more appropriately suited for solid tumors that harbor NTRK gene fusions. ORR of these patients receiving chemotherapy was similar to that of TRK inhibitor therapies (62.5% v 67.6%); however, neither data on prior therapies, which could influence response rates, nor safety findings were presented in that analysis, thus limiting comparisons of risk-benefit from either therapy.16 Larotrectinib is associated with a favorable tolerability profile9,10; our analysis showed no new or unexpected safety signals, consistent with earlier reports (data not shown). Although the MSKCC patients and our patient population cannot be directly compared, the results reported by Rosen et al continue to highlight the efficacy advantage of larotrectinib over other types of therapies.
Our analysis included 29 patients with IFS whose median time from diagnosis to adv/met stage was 0 months, and the median duration of adv/met disease before larotrectinib was 3.3 months. In the expanded larotrectinib analysis by Hong et al,9 patients with IFS achieved an ORR of 96%. Given that the population of patients with IFS both comprised a sizable proportion (20%) of the full analysis population and that most already had locally adv/met disease at diagnosis, we wanted to ensure that our observations were not driven primarily by patients with IFS. Exclusion of patients with IFS from the analysis showed consistent findings with the full study population, demonstrating that favorable efficacy outcomes were observed across all subgroups, even when discounting one of the most sensitive patient populations in our analysis. In a separate analysis, the exclusion of the pediatric population (who had a 92% ORR by investigator assessment9) did not alter the efficacy findings compared with the full study population.
Limitations to our analysis include the small sample sizes of individual tumor types, which precludes drawing conclusions on the basis of histologic types. In addition, these analyses did not account for the types of prior therapies and their impact on disease progression before larotrectinib, nor was disease progression measured by standardized time intervals before study inclusion.
In conclusion, the short-time interval from first diagnosis to development of locally adv/met stage suggests that the presence of NTRK gene fusions in tumors is unlikely to be a prognostic marker of a generally favorable clinical disease course. Larotrectinib treatment resulted in high ORR and sustained responses, regardless of prior lines of therapy and across different durations of pre-larotrectinib adv/met disease, supporting its strong clinical activity independent of the disease course before larotrectinib initiation. Importantly, on the basis of consistent clinical benefit across all subgroups, larotrectinib favorably altered the course of disease in patients with TRK fusion cancer. Even patients with rapid progression to advanced disease had much longer PFS on larotrectinib, compared with the previous disease course, thus providing further evidence that larotrectinib alters time to progression.
ACKNOWLEDGMENT
We would like to thank Ingrid Koo, PhD, for the editorial assistance.
Carsten Bokemeyer
Honoraria: Merck KGaA, Sanofi, Roche, Bayer, Bristol Myers Squibb, AstraZeneca, Merck Sharp & Dohme, Berlin Chemie
Consulting or Advisory Role: Lilly/ImClone, Merck Serono, Sanofi, Bayer Schering Pharma, Merck Sharp & Dohme, GSO, AOK Health Insurance, Novartis
Research Funding: AbbVie, ADC Therapeutics, Agile Therapeutics, Alexion Pharmaceuticals, Amgen, Apellis Pharmaceuticals, Astellas Pharma, AstraZeneca, Bayer, BerGenBio, Blueprint Medicines, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eisai, Gilead Sciences, Glycotope GmbH, GlaxoSmithKline, Incyte, IO Biotech, Isofol Medical, Janssen-Cilag, Karyopharm Therapeutics, Lilly, Millennium, MSD, Nektar, Novartis, Rafael Pharmaceuticals, Roche, Springworks Therapeutics, Taiho Pharmaceutical
Travel, Accommodations, Expenses: Merck Serono, Sanofi, Pfizer, Bristol Myers Squibb
Gilles Vassal
Consulting or Advisory Role: Bayer, Roche/Genentech, AstraZeneca, Bristol Myers Squibb, Lilly, Ipsen, Novartis
Travel, Accommodations, Expenses: Bristol Myers Squibb, Roche
Antoine Italiano
Honoraria: Bayer, Daiichi Sankyo, Lilly, Epizyme, Novartis, Roche, Ipsen
Consulting or Advisory Role: Roche, Daiichi Sankyo, Immune Design, Epizyme, Bayer, Lilly
Research Funding: Roche, Bayer, AstraZeneca/MedImmune, PharmaMar, MSD Oncology, Merck Serono
Patents, Royalties, Other Intellectual Property: BMS
Esther De La Cuesta
Employment: Bayer
Leadership: Bayer
Stock and Other Ownership Interests: Bayer
Florian Hiemeyer
Employment: Bayer
Stock and Other Ownership Interests: Bayer
Travel, Accommodations, Expenses: Bayer
Marc Fellous
Employment: Bayer
Marisca Marian
Employment: Bayer
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the Annual Meeting of the German, Austrian and Swiss Associations of Hematology and Medical Oncology (DGHO), October 9-October 11, 2020, Abstract 288.
SUPPORT
Supported by Bayer Pharmaceuticals.
AUTHOR CONTRIBUTIONS
Conception and design: Carsten Bokemeyer, Gilles Vassal, Antoine Italiano
Collection and assembly of data: Esther De La Cuesta, Florian Hiemeyer
Data analysis and interpretation: Carsten Bokemeyer, Gilles Vassal, Antoine Italiano, Esther De La Cuesta, Florian Hiemeyer, Marc Fellous, Marisca Marian
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Carsten Bokemeyer
Honoraria: Merck KGaA, Sanofi, Roche, Bayer, Bristol Myers Squibb, AstraZeneca, Merck Sharp & Dohme, Berlin Chemie
Consulting or Advisory Role: Lilly/ImClone, Merck Serono, Sanofi, Bayer Schering Pharma, Merck Sharp & Dohme, GSO, AOK Health Insurance, Novartis
Research Funding: AbbVie, ADC Therapeutics, Agile Therapeutics, Alexion Pharmaceuticals, Amgen, Apellis Pharmaceuticals, Astellas Pharma, AstraZeneca, Bayer, BerGenBio, Blueprint Medicines, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eisai, Gilead Sciences, Glycotope GmbH, GlaxoSmithKline, Incyte, IO Biotech, Isofol Medical, Janssen-Cilag, Karyopharm Therapeutics, Lilly, Millennium, MSD, Nektar, Novartis, Rafael Pharmaceuticals, Roche, Springworks Therapeutics, Taiho Pharmaceutical
Travel, Accommodations, Expenses: Merck Serono, Sanofi, Pfizer, Bristol Myers Squibb
Gilles Vassal
Consulting or Advisory Role: Bayer, Roche/Genentech, AstraZeneca, Bristol Myers Squibb, Lilly, Ipsen, Novartis
Travel, Accommodations, Expenses: Bristol Myers Squibb, Roche
Antoine Italiano
Honoraria: Bayer, Daiichi Sankyo, Lilly, Epizyme, Novartis, Roche, Ipsen
Consulting or Advisory Role: Roche, Daiichi Sankyo, Immune Design, Epizyme, Bayer, Lilly
Research Funding: Roche, Bayer, AstraZeneca/MedImmune, PharmaMar, MSD Oncology, Merck Serono
Patents, Royalties, Other Intellectual Property: BMS
Esther De La Cuesta
Employment: Bayer
Leadership: Bayer
Stock and Other Ownership Interests: Bayer
Florian Hiemeyer
Employment: Bayer
Stock and Other Ownership Interests: Bayer
Travel, Accommodations, Expenses: Bayer
Marc Fellous
Employment: Bayer
Marisca Marian
Employment: Bayer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Cocco E, Scaltriti M, Drilon A: NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol 15:731-747, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stransky N Cerami E Schalm S, et al. : The landscape of kinase fusions in cancer. Nat Comm 5:4846, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kheder ES, Hong DS: Emerging targeted therapy for tumors with NTRK fusion proteins. Clin Cancer Res 24:5807-5814, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Skálová A Vanecek T Sima R, et al. : Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: A hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 34:599-608, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Tognon C Knezevich SR Huntsman D, et al. : Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell 2:367-376, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y Yang L Kucherlapati M, et al. : A Pan-cancer compendium of genes deregulated by somatic genomic rearrangement across more than 1,400 cases. Cell Rep 24:515-527, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourgeois JM Knezevich SR Mathers JA, et al. : Molecular detection of the ETV6-NTRK3 gene fusion differentiates congenital fibrosarcoma from other childhood spindle cell tumors. Am J Surg Pathol 24:937-946, 2000 [DOI] [PubMed] [Google Scholar]
- 8.El Demellawy D Cundiff CA Nasr A, et al. : Congenital mesoblastic nephroma: A study of 19 cases using immunohistochemistry and ETV6-NTRK3 fusion gene rearrangement. Pathology 48:47-50, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Hong DS DuBois SG Kummar S, et al. : Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol 21:531-540, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drilon A Laetsch TW Kummar S, et al. : Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med 378:731-739, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drilon A Siena S Ou S-HI, et al. : Safety and antitumor activity of the multitargeted Pan-TRK, ROS1, and ALK inhibitor entrectinib: Combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov 7:400-409, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doebele RC Drilon A Paz-Ares L, et al. : Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol 21:271-282, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US FDA Press Release : FDA approves larotrectinib for solid tumors with NTRK gene fusions, 2018. https://www.fda.gov/drugs/fda-approves-larotrectinib-solid-tumors-ntrk-gene-fusions
- 14.Yoshino T Pentheroudakis G Mishima S, et al. : JSCO-ESMO-ASCO-JSMO-TOS: International expert consensus recommendations for tumour-agnostic treatments in patients with solid tumours with microsatellite instability or NTRK fusions. Ann Oncol 31:861-872, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Bazhenova L Jiao X Lokker A, et al. : Cancers with NTRK gene fusions: Molecular characteristics and prognosis. Clin Cancer Res 26, 2020. (suppl 1; abstr 9) [Google Scholar]
- 16.Rosen EY Goldman DA Hechtman JF, et al. : TRK fusions are enriched in cancers with uncommon histologies and the absence of canonical driver mutations. Clin Cancer Res 26:1624-1632, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orbach D Rey A Cecchetto G, et al. : Infantile fibrosarcoma: Management based on the European experience. J Clin Oncol 28:318-323, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Institute of Medicine (US) and National Research Council (US) National Cancer Policy Board : 2, The epidemiology of childhood cancer, in Hewitt M, Weiner S, Simone J. (eds): Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington, DC, National Academies Press (US), 2003. https://www.ncbi.nlm.nih.gov/books/NBK221740/ [PubMed] [Google Scholar]