PURPOSE:
The COVID-19 pandemic has posed significant pressures on healthcare systems, raising concern that related care delays will result in excess cancer-related deaths. Because data regarding the impact on patients with breast cancer are urgently needed, we aimed to provide a preliminary estimate of the impact of COVID-19 on time to treatment initiation (TTI) for patients newly diagnosed with breast cancer cared for at a large academic center.
METHODS:
We conducted a retrospective study of patients with newly diagnosed early-stage breast cancer between January 1, 2020, and May 15, 2020, a time period during which care was affected by COVID-19, and an unaffected cohort diagnosed between January 1, 2018 and May 15, 2018. Outcomes included patient volume, TTI, and initial treatment modality. Adjusted TTI was compared using multivariable linear regression.
RESULTS:
Three hundred sixty-six patients were included. There was an 18.8% decrease in patient volume in 2020 (n = 164) versus 2018 (n = 202). There was no association between time of diagnosis (pre-COVID-19 or during COVID-19) and adjusted TTI (P = .926). There were fewer in situ diagnoses in the 2020 cohort (P = .040). There was increased use of preoperative systemic therapy in 2020 (43.9% overall, 20.7% chemotherapy, and 23.2% hormonal therapy) versus 2018 (16.4% overall, 12.4% chemotherapy, and 4.0% hormonal therapy) (P < .001).
CONCLUSION:
TTI was maintained among patients diagnosed and treated for breast cancer during the COVID-19 pandemic at a single large academic center. There was a decrease in patient volume, specifically in patients with in situ disease and a shift in initial therapy toward the use of preoperative hormonal therapy.
INTRODUCTION
Even before COVID-19, time from breast cancer diagnosis to treatment initiation had increased by approximately 10 days in the last decade.1 This potentially puts the 275,000 women who are diagnosed with breast cancer in the United States every year at risk of inferior outcomes.2 Delays in treatment of breast cancer have been linked with increased mortality, such that each 60-day delay in surgery was shown to be associated with a 26% increased risk of death because of breast cancer among patients with early-stage invasive breast cancer.3-5 This finding helped drive development of time-based quality metric and guideline recommendations for receipt of chemotherapy and radiation following early-stage breast cancer diagnosis.6,7
The COVID-19 pandemic has posed significant pressures on healthcare system resources and treatment decisions at the provider and patient level. This has led to growing concern that delays in diagnosis and care will result in thousands of excess cancer deaths over the next several years.8-12 As a result, several organizations have published consensus guidelines for management of breast cancer during the pandemic, aiming to preserve hospital resources for patients being treated for COVID-19 by deferring breast cancer treatments without significantly compromising the outcomes and quality of care for individuals with breast cancer.8
Data regarding the impact of the pandemic on volume of patient with breast cancer, time to treatment initiation (TTI), stage at presentation, and treatment sequence are urgently needed. However, these are not readily available from national databases without substantial delay. To meet this need, we aimed to evaluate the impact of COVID-19 on patients receiving care for newly diagnosed breast cancer at a large academic center via collection of current patient-level data. We hypothesized that TTI would be longer in the 2020 COVID-19 era compared with our 2018 comparator cohort.
METHODS
Setting
Penn Medicine is a six-hospital health system headquartered in Philadelphia, caring for more than 17,000 patients with new adult cancer every year.13 The Hospital of the University of Pennsylvania (HUP) is the primary academic medical center and includes the main campus and two additional outpatient satellite sites.
Data Source and Study Population
2020 Cohort.
Eligible patients had a new histologic diagnosis of invasive breast cancer, ductal carcinoma in situ (DCIS), or lobular carcinoma in situ (LCIS) between January 1, 2020, and May 15, 2020, and were treated at HUP or one of its satellite sites. Inclusion start date was chosen based on the estimated TTI of 2 months, and treatment courses were expected to extend several months. Thus, an early threshold maximized capture of all patients whose evaluation and treatment overlapped with a pandemic-affected period, late February and early March 2020. Patients who did not continue their care at HUP after initial consultation were excluded. Patients were identified by electronic medical record report of new patient visits to an oncology specialist with diagnosis of breast cancer at HUP and satellite sites between January 1, 2020, and May 15, 2020. Data integrity was verified by manual chart review of systematically sampled patients, with every fifth patient reviewed. Review indicated that all eligible patients had new patient visits with a breast surgical specialty and subsequently, patients without a breast surgery visit were excluded. These data were merged with prospectively collected log of patients with new histologic diagnosis of invasive breast cancer between April 1, 2020, and May 15, 2020, maintained by pathology. This log was a response by operational leadership to track patients whose treatment might have been affected by the COVID-19 pandemic. By merging pathology-identified new diagnoses of breast cancer with our report of new patient visits with breast surgery, we ensured that all patients with an early breast cancer diagnosis were included in the study period. Manual chart review was then conducted by two overlapping reviewers. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Pennsylvania. In March 2020, as part of disease team operational response to COVID-19, a standardized electronic medical record element, here referred to as the Covid delay questionnaire, was added to visit notes in which the clinician or a retrospective reviewer denoted any type of care delay attributable to the COVID-19 pandemic. The COVID delay questionnaire enabled analysis of sequencing of initial treatments, including patients who were started on preoperative hormonal therapy to delay surgery during the COVID-19 pandemic.
2018 Cohort.
This cohort served as a comparison cohort to the primary 2020 cohort and included patients identified from the Penn Medicine Cancer Registry, a system maintained by professional certified tumor registrars that abide by state and national standards for submission to the Pennsylvania State Cancer Registry and Commission on Cancer National Cancer Database (NCDB). The registry is organized by hospital site, with any hospital with a primary role in the diagnosis or treatment of a tumor reporting. There is approximately a 10-month lag period for complete reporting. Thus, to receive complete data and account for potential seasonality, the comparator time period was established as January 1, 2018-May 15, 2018, the most recent year for which data were available for a comparable time period. Eligible patients had new diagnoses of invasive breast cancer, DCIS, or LCIS diagnosed between January 1, 2018, and May 15, 2018, at HUP and two satellite sites. Registry data were confirmed by systematic sampling for manual chart review.
Outcomes
The primary outcome was TTI, defined as time in days from diagnosis to the start of a patient’s initial treatment. Date of diagnosis was defined as date of histologic diagnosis (core biopsy, punch biopsy, and fine needle aspiration of breast or lymph node) in the 2020 cohort. Date of diagnosis for the 2018 cohort is a registrar collected field; investigators’ manual chart review confirmed that this date corresponded to the date of histologic diagnosis. Initial treatment was defined as the recorded date when a patient received their first step in treatment (surgery, chemotherapy, hormonal therapy, or radiation therapy). This was collected by manual chart review for the 2020 cohort and registrar collected in the 2018 cohort, which was subsequently systematically confirmed by manual chart review.
Exploratory outcomes available only for the 2020 cohort included type of initial presentation (mammography versus self-palpation) and more detailed descriptors of initial therapy, including surgery type with or without reconstruction and use of human epidermal growth factor receptor 2 (HER2)–targeted agents.
Independent Variables
The exposure was year of diagnosis (pre-COVID-19 or during COVID-19). Covariates included clinical stage at diagnosis (American Joint Committee on Cancer 8th edition), breast cancer subtype for invasive cancers, age at diagnosis, race, and histology.
Statistical Analyses
Adjusted TTI was compared between the exposed and unexposed cohorts using multivariable linear regression with robust standard errors. We tested for an association between exposure and TTI using a Wald test. We evaluated for differences in patient characteristics between the exposed and unexposed cohorts using chi-squared tests. We conducted secondary analyses to determine whether changes in TTI pre-COVID-19 and during COVID-19 differed by race by including interaction terms between the exposure and race variable in our model. A significance level of two-sided P value < .05 was set a priori. All analyses were performed using Stata/IC 15.1 for Windows (StataCorp, College Station, TX). This data collection and analysis were determined to be quality improvement and quality assurance related by operational leaders and were exempt from Institutional Review Board review.
RESULTS
Population
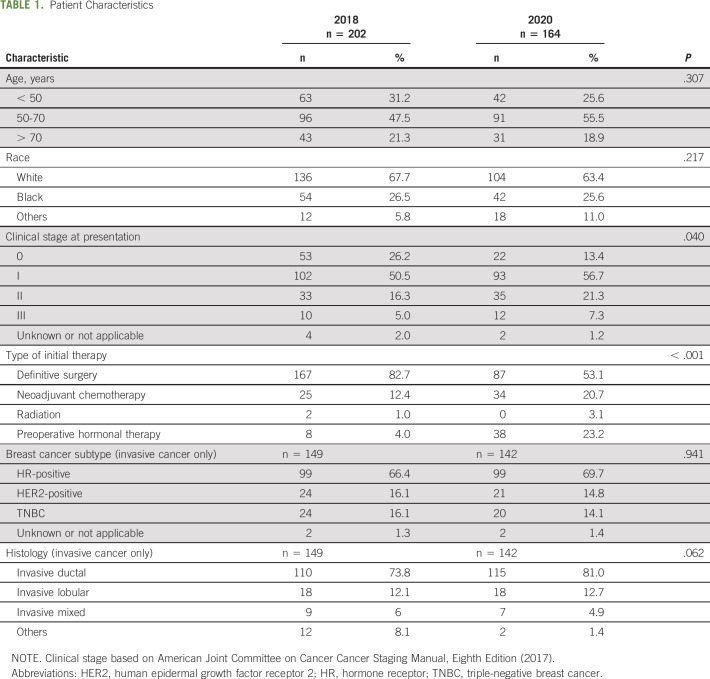
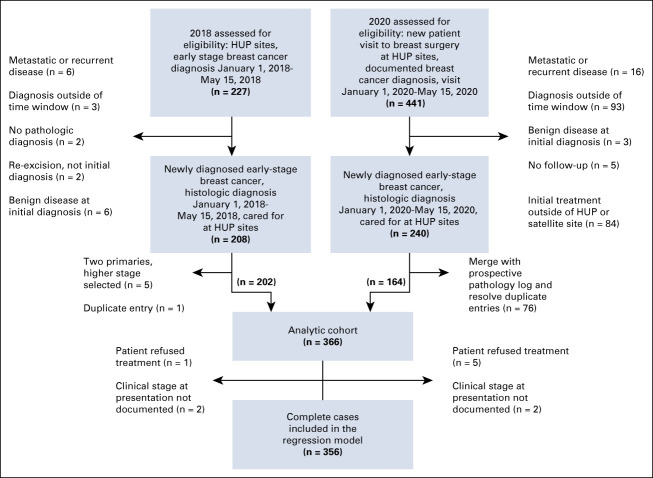
A total of 366 patients met our inclusion criteria. Patient volume of new breast cancer diagnosis decreased by 18.8% in 2020 (n = 164) compared with 2018 (n = 202). Patient characteristics were similar between the 2018 and 2020 cohorts: the majority of patients were between 50 and 70 years old and White race (Table 1). Breast cancer subtype (P = .941) and histology (P = .062) among invasive cancers were also similarly distributed between the two cohorts. Most patients with invasive cancer had hormone receptor–positive (HR+ HER2−) disease, representing 66.4% and 69.7% in the 2018 and 2020 cohorts, respectively (Table 1). Two patients missing clinical stage at presentation (0.5%) and six patients who refused treatment (1.6%) were excluded from the regression analysis (Appendix Fig A1, online only).
TABLE 1.
Patient Characteristics
Clinical stage at presentation differed between the 2018 and 2020 cohort (P = .040). Specifically, presentation with clinical stage 0 (DCIS) was more common in 2018 as compared with 2020 (n = 53, 26.2% v n = 22, 13.4%). Patients with clinical stage I and II disease at presentation were represented in lower proportion of newly diagnosed patients in the 2018 versus 2020 cohort; however, the number of patients diagnosed at each of these stages was similar pre-COVID-19 and during COVID-19 (Table 1). Additionally, the type of initial therapy differed between cohorts (P < .001), with fewer patients beginning with preoperative systemic therapy in 2018 as compared with 2020 (16.4% v 43.9%, respectively). This was largely driven by changes in preoperative hormonal therapy (4.0% in 2018 v 23.2% in 2020) rather than chemotherapy (12.4% chemotherapy in 2018 v 20.7% in 2020) (Table 1).
Time to Treatment Initiation
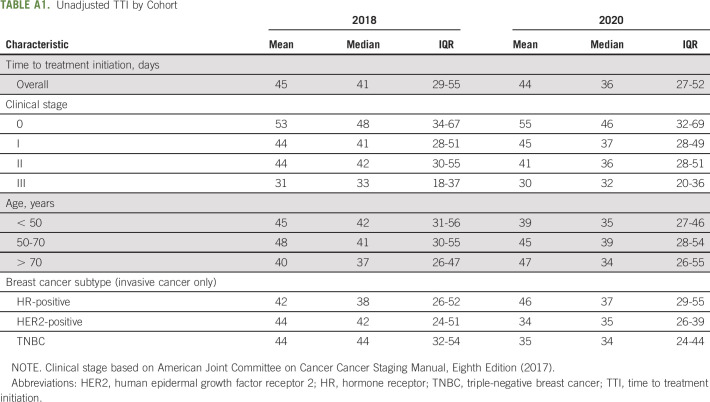
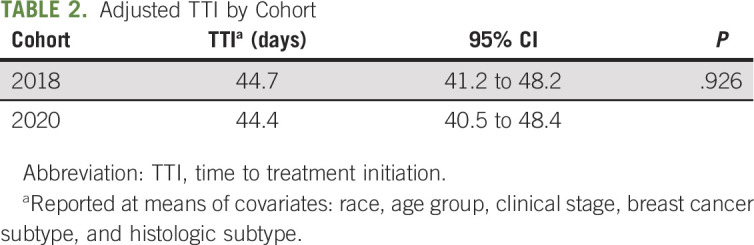
The mean unadjusted TTI in 2018 was 45 days (median 41, IQR 29-55) versus 44 days (median 36, IQR 27-52) in 2020 (Appendix Table A1, online only). After adjusting for race, age, clinical stage, breast cancer subtype, and histologic subtype, there was no association between time of diagnosis (pre-COVID-19 or during COVID-19) and TTI (P = .926) (Table 2). This was true regardless of a patient’s race for interaction between exposure and race in our secondary analysis. However, across cohorts, patients who are Black were treated 16 days slower as compared with those who are White (15.7; 95% CI, 6.9 to 24.6; P = .001). Given that unadjusted TTI was shorter than that estimated at time of inclusion criteria determination, sensitivity analyses were conducted with only patients diagnosed after February 1, 2020, and March 1, 2020. In both cases, unadjusted mean TTI was less than the analyzed 2020 cohort such that conclusions of this study were unchanged.
TABLE 2.
Adjusted TTI by Cohort
2020 Cohort Exploratory Outcomes
The mean time from presentation to histologic diagnosis was 38 days (median 23, IQR 13-48), from histologic diagnosis to initial appointment 17 days (median 14, IQR 8-21), and from initial appointment to initial treatment 27 days (median 22, IQR 15-33). 52.4% of patients presented because of screening mammogram, followed by self-palpated mass (41.8%), and others (5.8%) (Table 3).
TABLE 3.
Exploratory Outcomes in the 2020 Cohort
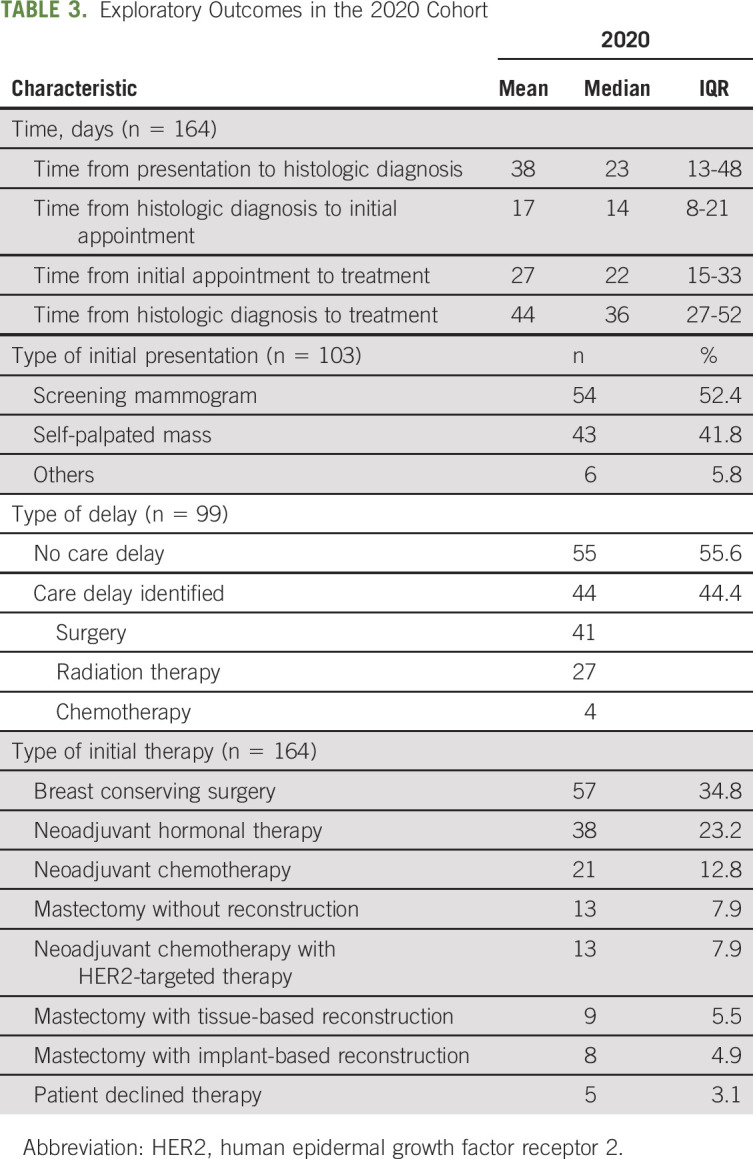
Among the 2020 cohort, initial therapy in order of frequency included breast conserving surgery (34.8%), neoadjuvant hormonal therapy (23.2%), neoadjuvant chemotherapy (12.8%), mastectomy without reconstruction (7.9%), neoadjuvant chemotherapy with HER2–targeted therapy (7.9%), mastectomy with tissue-based reconstruction (5.5%), and mastectomy with implant-based reconstruction (4.9%). Of the patient group that received neoadjuvant hormonal therapy (38 patients), 11 remained on hormonal therapy at time of analysis and the mean duration of hormonal therapy was 67 days (median 61, IQR 41-96). Additionally, review of the 13 patients who underwent mastectomy without reconstruction indicated that four of the 13 preferred to have reconstruction, two of whom were counseled against reconstruction because of clinical factors, and thus, only two planned for delayed reconstruction presumably because of COVID-19–related circumstances.
Of the 99 patients with completed COVID-19 delay questionnaires, 55 (55.6%) patients had no care delay and 44 (44.4%) had delay in care, of which surgery (n = 41) and radiation therapy (n = 27) were the most frequent. Among patients identified on questionnaires as having a delay in surgery because of COVID-19, 70.7% (n = 29) were started on preoperative hormonal therapy.
DISCUSSION
Patients with newly diagnosed early-stage breast cancer treated at our institution during the COVID-19 pandemic did not wait longer to start therapy than those treated in previous years. Although similar numbers of patients were diagnosed with invasive breast cancer during this period as compared with previous years, fewer patients were diagnosed with noninvasive disease during COVID-19. We also found that during COVID-19, choice of initial therapy for newly diagnosed HR+ patients shifted from surgery toward hormonal therapy. Collectively, these findings suggest that, at our institution, treatment delays for patients with newly diagnosed early-stage breast cancer were avoided by increasing the use of preoperative hormonal therapy along with multidisciplinary mitigation strategies.
There is concern that the COVID-19 pandemic will lead to care delays and poor outcomes for patients with cancer. Current understanding of the impact of the COVID-19 pandemic on patients with breast cancer is limited and subject to significant reporting delays with typical state and national reporting outlets.
Consistent with our hypothesis, there was an 18.8% decrease in number of patients diagnosed with breast cancer between January and May 2020 compared with historical data from 2018, suggesting that fewer patients were seeking breast cancer care or breast cancer preventive services, such as screening mammography, during the pandemic period. This reduction in caseload was largely driven by DCIS, presumably because of suspension of screening mammography in late March and April at our institution. A higher proportion of breast cancer was detected by self-palpation in 2020 compared with historical cohorts.14 This finding has important implications in that missing in situ diagnoses over this time period may lead to a bolus of in situ cases over the next few months as screening mammography is resumed and possibly even a shift toward higher clinical stage I disease.
Reassuringly, there was no difference in TTI among patients diagnosed in 2020 compared with 2018. This may be due to care delay mitigation efforts undertaken at the institution during the pandemic, which included increased frequency of multidisciplinary tumor board from one to three times weekly to facilitate presentation and triage of new cases (99 of the 164 patients in the 2020 cohort), tracking of these presented cases, and increasing use of upfront hormonal therapy for newly diagnosed early-stage patients with HR+, HER2− disease. In addition, several other care-delivery interventions might have also helped prevent treatment delays. There was increased adoption of every 3- or 2-week dosing schedules, where appropriate, for patients being treated with systemic therapy for both early- and late-stage breast cancer to avoid weekly schedules and decrease patient and provider volumes in the clinic. Our institution also substantially increased home administration of luteinizing hormone releasing hormone agonist for patients with breast cancer during the study pandemic period.15 Although clinicians and operational reviewers cited care delays among 44.4% of patients in our COVID-19 tracking cohort, most of this reflected a delay in surgery that is not captured in the TTI end point mainly because of the substitution of preoperative hormonal therapy. Before COVID-19, many of these patients would have gone directly to definitive surgery without preoperative hormonal therapy and/or would have received neoadjuvant chemotherapy.
Although our data suggest that TTI at our institution did not worsen during COVID-19, it is also notable that both the 2018 and 2020 cohorts have a higher TTI than the national median of 24 days for breast cancer reported in studies using the National Cancer Database.2 Quality improvement efforts to address this difference have been underway at our sites, and it is possible that part of these gains might have been negatively affected by COVID-19. It is known that increased time to surgery and time to chemotherapy are associated with lower overall and disease-specific survival, with studies showing delayed time to chemotherapy being particularly detrimental to patients with triple-negative breast cancer (TNBC).3,5
Additionally, we acknowledge that treatment of breast cancer has evolved rapidly since 2018, with a shift toward neoadjuvant systemic therapy for TNBC and HER2+ breast cancer. Thus, the high proportion of patients receiving neoadjuvant systemic chemotherapy and targeted therapy in 2020 is expected and unlikely attributable to pandemic-related factors in the TNBC and HER2+ groups. However, the increased use of hormonal therapy before surgery in the HR+ group does represent a practice shift at this institution, noting that the duration of hormonal therapy of mean 67 days would be better described as temporizing hormonal therapy as opposed to neoadjuvant hormonal therapy with intent of downstaging, which is typically administered for a duration of 3-9 months.16-20 This practice shift toward preoperative hormonal therapy is consistent with delivery of quality care and concordant with national working group recommendations for the treatment of hormone receptor breast cancer during COVID-19,8 which cited studies of tamoxifen with or without surgery that demonstrated no difference in survival within the first 3 years, suggesting that short-term deferment of surgery with hormonal therapy should not adversely affect breast cancer–specific survival.21-23 However, the preoperative use of hormonal therapy confounds interpretation of gene expression profiles typically conducted on the surgical specimen. Thus, the preoperative use of hormonal therapy resulted in a downstream practice shift in that gene expression profiles such as Oncotype DX or MammaPrint were ordered on the diagnostic breast core biopsy (rather than on their surgically excised breast tumor) at time of diagnosis.
Limitations of this study include the small sample size and the single-institution cohort design. However, these findings represent important early data to assess the impact of COVID-19 on breast cancer care until larger registry data become available. Additionally, the use of a comparison cohort from 2018 cancer registry data limits our ability to control for secular trends when comparing COVID-19 versus pre-COVID-19 TTI and patient volume. However, because secular trends suggest an increasing TTI over time, this would likely bias our findings away from, rather than toward, the null. Additionally, the primary outcome of TTI relies on the patient receiving treatment for their breast cancer. Thus, patients who refused care potentially because of COVID-19 would not have met our inclusion criteria. However, in the 2020 cohort, only five patients refused treatment and five patients had no follow-up, which is unlikely to have a significant impact on the results (Appendix Fig A1).
In summary, our findings demonstrate that patients treated for breast cancer did not experience higher rates of treatment delays during COVID-19. This may be due to the adoption of consensus guidelines and mitigation strategies surrounding treatment of patients with breast cancer during the pandemic. Because these strategies were evidence-based, this also suggests that timely, high-quality care continued to be delivered during this period. Although this is reassuring, the decrease in number of in situ diagnoses and short follow-up period mean that ongoing monitoring is needed to determine the downstream effects of the pandemic, particularly with respect to overall survival, which are likely to be seen for years to come.
ACKNOWLEDGMENT
Dr Peter Gabriel, Abigail Doucette, Drs Kevin Fox, Jennifer Matro, Susan Domchek, Angela Bradbury, Angela DeMichele, Haley Knollman, Payal Shah, Julia Tchou, David Anderson, Jami Rothman, Gary Freedman, Neil Taunk, Emily Conant, Susan Weinstein, Anupma Nayak, and David Mankoff.
Appendix
FIG A1.
Patient flow diagram.
TABLE A1.
Unadjusted TTI by Cohort
Lawrence N. Shulman
Research Funding: Celgene
Ira J. Bleiweiss
Honoraria: Bard Biopsy
Speakers' Bureau: Bard Biopsy, Philips Digital Imaging
Rachel C. Jankowitz
Honoraria: Eisai
Consulting or Advisory Role: Merck
No other potential conflicts of interest were reported.
Footnotes
R.C.J. and A.I.L. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Kathryn Hawrot, Lawrence N. Shulman, Rachel C. Jankowitz, Amy I. Laughlin
Administrative support: Lawrence N. Shulman, Elizabeth J. Wilkie, Amy I. Laughlin
Provision of study materials or patients: Kathryn Hawrot, Ira J. Bleiweiss
Collection and assembly of data: Kathryn Hawrot, Ira J. Bleiweiss, Elizabeth J. Wilkie, Rachel C. Jankowitz, Amy I. Laughlin
Data analysis and interpretation: Lawrence N. Shulman, Ira J. Bleiweiss, Zachary A. K. Frosch, Rachel C. Jankowitz, Amy I. Laughlin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Time to Treatment Initiation for Breast Cancer During the 2020 COVID-19 Pandemic
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Lawrence N. Shulman
Research Funding: Celgene
Ira J. Bleiweiss
Honoraria: Bard Biopsy
Speakers' Bureau: Bard Biopsy, Philips Digital Imaging
Rachel C. Jankowitz
Honoraria: Eisai
Consulting or Advisory Role: Merck
No other potential conflicts of interest were reported.
REFERENCES
- 1.Khorana AA, Tullio K, Elson P, et al. : Time to initial cancer treatment in the United States and association with survival over time: An observational study. PLoS One 14:e0213209, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Surveillance Epidemiology and End Results Program : Cancer Stat Facts: Female Breast Cancer Subtypes. 2020. https://seer.cancer.gov/statfacts/html/breast-subtypes.html [Google Scholar]
- 3.Bleicher RJ, Ruth K, Sigurdson ER, et al. : Time to surgery and breast cancer survival in the United States. JAMA Oncol 2:330, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mateo AM, Mazor AM, Obeid E, et al. : Time to surgery and the impact of delay in the non-neoadjuvant setting on triple-negative breast cancers and other phenotypes. Ann Surg Oncol 27:1679-1692, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, et al. : Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol 2:322, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American College of Surgeons : National Accreditation Program for Breast Centers Standards Manual. Chicago, IL, American College of Surgeons, 2018 [Google Scholar]
- 7.National Quality Forum : NQF #0559 C0559: Combination Chemotherapy Is Considered or Administered Within 4 Months (120 Days) of Diagnosis for Women Under 70 With AJCC T1c, or Stage II or III Hormone Receptor Negative Breast Cancer. 2007 [Google Scholar]
- 8.Dietz JR, Moran MS, Isakoff SJ, et al. : Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. The COVID-19 Pandemic Breast Cancer Consortium. Breast Cancer Res Treat 181:487-497, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spring LM, Specht MC, Jimenez RB, et al. : Case 22-2020: A 62-year-old woman with early breast cancer during the COVID-19 pandemic. N Engl J Med 383:262-272, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharpless NE: COVID-19 and cancer. Science 368:1290, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Marron JM, Joffe S, Jagsi R, et al. : Ethics and resource scarcity: ASCO recommendations for the oncology community during the COVID-19 pandemic. J Clin Oncol 38:2201-2205, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Mauri D, Kamposioras K, Tolia M, et al. : Summary of international recommendations in 23 languages for patients with cancer during the COVID-19 pandemic. Lancet Oncol 21:759-760, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penn Medicine : A Vision for the Future: Division of Hematology/Oncology Strategic Plan, 2018/2023. 2018. https://www.pennmedicine.org/departments-and-centers/department-of-medicine/divisions/hematology-and-oncology/about-us/a-vision-for-the-future [Google Scholar]
- 14.Haakinson DJ, Stucky CC, Dueck AC, et al. : A significant number of women present with palpable breast cancer even with a normal mammogram within 1 year. Am J Surg 200:712-718, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Laughlin AI, Begley M, Delaney T, et al. : Accelerating the delivery of cancer care at home during the COVID-19 pandemic. N Engl J Med Catal, 2020. 10.1056/CAT.20.0258 [DOI] [Google Scholar]
- 16.Eiermann W, Paepke S, Appfelstaedt J, et al. : Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann Oncol 12:1527-1532, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Smith IE, Dowsett M, Ebbs SR, et al. : Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: The immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 23:5108-5116, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Cataliotti L, Buzdar AU, Noguchi S, et al. : Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer. Cancer 106:2095-2103, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Semiglazov V, Kletsel A, Semiglazov V, et al. : Exemestane (E) vs tamoxifen (T) as neoadjuvant endocrine therapy for postmenopausal women with ER+ breast cancer (T2N1–2, T3N0–1, T4N0M0). J Clin Oncol 23, 2005. (suppl; abstr 530) [Google Scholar]
- 20.Ellis MJ, Coop A, Singh B, et al. : Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1– and/or ErbB-2–positive, estrogen receptor–positive primary breast cancer: Evidence from a phase III randomized trial. J Clin Oncol 19:3808-3816, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Mustacchi G, Milani S, Pluchinotta A, et al. : Tamoxifen or surgery plus tamoxifen as primary treatment for elderly patients with operable breast cancer: The G.R.E.T.A. Trial. Group for Research on Endocrine Therapy in the Elderly. Anticancer Res 14:2197-2200, 1994 [PubMed] [Google Scholar]
- 22.Mustacchi G, Ceccherini R, Milani S, et al. : Tamoxifen alone versus adjuvant tamoxifen for operable breast cancer of the elderly: Long-term results of the phase III randomized controlled multicenter GRETA trial. Ann Oncol 14:414-420, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Fennessy M, Bates T, MacRae K, et al. : Late follow-up of a randomized trial of surgery plus tamoxifen versus tamoxifen alone in women aged over 70 years with operable breast cancer. Br J Surg 91:699-704, 2004 [DOI] [PubMed] [Google Scholar]