PURPOSE:
The COVID-19 pandemic has posed significant challenges in the care of patients with cancer, including how to manage outpatients who are COVID-positive but do not require hospitalization. We explored the use of a remote patient monitoring (RPM) program to care for such outpatients.
METHODS:
Consecutive patients who were tested for COVID-19 because of symptom onset but were clinically stable were offered enrollment into a pilot RPM program. Patients were provided equipment for vital sign measurements and a computer tablet to enter results three times per day. The results were monitored centrally by clinical staff. The goal was to closely monitor patients and escalate care as warranted.
RESULTS:
Between March and June of 2020, 29 patients were approached and 26 were enrolled. The mean age was 57 years old (range, 30-88), 14 were women, and patients remained in the program for an average of 16 days (range, 2-63). Twenty-four patients (83%) were on active anticancer therapy. During that time period, only one patient was admitted to the hospital for worsening respiratory symptoms. The percentage of days during which at least one set of data and all three sets of data were entered was 97.2% and 65.7%, respectively. There was no association between the demographic factors of age, sex, or the reason for being monitored with the level of engagement (P > .05).
CONCLUSION:
In this pilot study, patients with cancer were readily enrolled in a remote home monitoring program. Monitoring was feasible, and there was a high rate of engagement with the program. The role of RPM should be further tested as the COVID pandemic continues.
INTRODUCTION
The global pandemic caused by the SARS-CoV-2 and the infectious illness, COVID-19, has profoundly disrupted the diagnosis and care of patients with cancer. Patients with cancer who contract COVID-19 infection have higher mortality rates than the general population.1-3 How to triage and manage patients with cancer who might have COVID-19 is an urgent question in outpatient clinical care.
Patients with COVID-19 infection may be asymptomatic or have mild, moderate, or severe symptoms.4-6 Symptoms develop on average 4 days after a positive polymerase chain reaction test, with a range of 3-7 days.6 In the general population, patients with mild symptoms do not need additional evaluations or interventions. However, some of those patients may decompensate with more moderate to severe symptoms, usually 1 week after initial symptom onset.7 In patients with risk factors for severe disease, Gandhi et al7 recommended that they undergo close monitoring for clinical progression with a low threshold for additional evaluations.
During the pandemic, questions have arisen related to the unique risks that COVID-19 poses to patients with cancer and the logistics of how to safely continue care in the outpatient clinical and infusion settings. In March 2020, our cancer center clinical leadership team developed outpatient screening methods and established a COVID testing clinic to identify patients who may be COVID-positive and to distinguish that from other causes of fever or respiratory symptoms. For patients who were COVID-positive but stable, the clinical question arose on how to best manage their care.
The US Department of Health and Human Services has defined remote patient monitoring (RPM) as “the use of connected electronic tools to record personal health and medical data in one location for review by a provider in another location, usually at a different time.”8 Recognizing the increased impact and potential for increased morbidity and mortality posed by the novel SARS-CoV-2 virus to our cancer patient population, our institution implemented an RPM program for patients who were either persons under investigation (PUIs) or COVID-19–positive with mild or no symptoms.
METHODS
Patients
Symptom screening by phone and at the entrance to our cancer center were based on Centers for Disease Control guidance (fever, respiratory symptoms, new loss of smell and taste, and exposure history). Patients on active cancer therapy with such symptoms were escorted to a COVID testing clinic separated from the rest of the cancer center. Appropriate patients were evaluated by a nurse and advanced practice provider (APP) wearing appropriate personal protective equipment. Patients were assessed and categorized as having mild symptoms if they were hemodynamically stable, with an oxygen saturation of 94% or greater. Patients with more severe illness were escorted to the hospital’s Emergency Department.
Laboratory tests, including a complete blood count, a rapid flu swab, and a nasopharynx swab for a polymerase chain reaction test using the Abbott RealTime SARS-CoV-2 on the m2000 system or dry swab using the COVID Abbott ID NOW for rapid testing (Abbott Park, IL), were performed. PUIs or COVID-positive patients with mild symptoms were offered home monitoring. These patients continued in outpatient cancer care management, receiving nonimmunosuppressive systemic cancer therapy per physician discretion (eg, oral targeted therapy, trastuzumab/pertuzumab), blood product transfusions, and intravenous hydration in our COVID clinic. This sequestered management continued until the patient had two negative COVID tests at least 24 hours apart, at which time the patient could return to the usual infusion and provider clinics.
Intervention
The Locus Health platform was originally developed to perform remote home monitoring of infants discharged from the Neonatal Intensive Care Unit, where it was found to be safe and effective.9,10 In our pilot program, patients with cancer were offered free enrollment in the RPM program if they had mild symptoms and were stable. They were educated on the program parameters and provided with an automatic blood pressure cuff, oral thermometer, finger pulse oximeter, and a configured Apple iPad to enter vital results. The iPad configuration could be in either English or Spanish. Patients were asked to enter vital measurements of temperature, blood pressure, heart rate, and pulse oximetry three times a day. Since we did not know at that time the natural history of acute COVID-19 infection, we proposed three measurements per day. They also answered two questions: “Do you feel better, worse, or the same as yesterday?” and “Are you experiencing shortness of breath at this time?” We generated these patient-reported outcome questions as part of this pilot study.
A password-protected patient dashboard was available to clinicians at the cancer center. APPs monitored the patient dashboard 12 hours a day, 7 days a week. Patients were instructed that if they had worsening symptoms at night to call the on-call provider or come into the Emergency Department. Abnormal values were pulse oximetry of 93% or less, temperatures more than 100.0° F, a systolic blood pressure < 100 or a diastolic blood pressure < 60 mmHg, or heart rates more than 100 beats per minute. If a patient entered an abnormal value or answered “yes” to the question regarding feeling shortness of breath, a warning would prompt them to call their provider’s office, and that value would be highlighted on the clinical dashboard and alert the APP team. The patient would be contacted, and the APP and treating physician would make the clinical determination on the appropriate next step. This communication and clinical recommendation was documented in the electronic medical record (EMR).
Patients who were PUIs remained in the RPM program until their tests returned negative and they were no longer symptomatic. Patients who had tested positive for COVID-19 remained on RPM until they were no longer symptomatic and had two negative COVID tests done at least 24 hours apart.
Statistical Methods
Descriptive statistics were used to present demographic information, including age, sex, and primary cancer diagnosis, as well as duration of RPM and outcomes. Patient engagement was defined as the compliance with vital measurement and data entry three times per day by the patients on RPM. We measured the number of days patients entered at least one set of data (engagement 1×) during their enrollment period and the number of days when they entered all three sets of expected data (engagement 3×) during their enrollment. We explored associations of engagement and the demographic variables of age, sex, reason for RPM, and duration of enrollment using Pearson correlation coefficient (PCC), two-sample t test, Wilcoxon rank-sum test, analysis of variance, Kruskal-Wallis test, and Fisher’s Exact test, wherever is appropriate. SAS software Version 9.4 (SAS Inc) was used for statistical analysis. P values < .05 were considered statistically significant.
Institutional Review Board
The institutional review board reviewed and approved this retrospective study of patients who were offered enrollment in the RPM program.
RESULTS
Twenty-nine consecutive patients were offered RPM, and a total of 26 (93%) were enrolled between March and June 2020. Two patients declined to enroll because of perceived complexity and stress of completing measurement tasks, and one patient lacked a sufficient home internet connection. Early in the program, participants were primarily patients under investigation (PUIs), when COVID-19 test results could take 7-10 days to return, whose results later were negative (n = 11, 42%). After a rapid testing process was instituted with the Abbott ID NOW test, with the results returned within 30 minutes, patients offered RPM were those who had tested positive (n = 12, 46%) or were negative but had a high clinical suspicion given symptoms and history, including close contact with a COVID-positive patient (n = 3, 12%); these were included given the possible false negative rate from rapid COVID-19 testing.
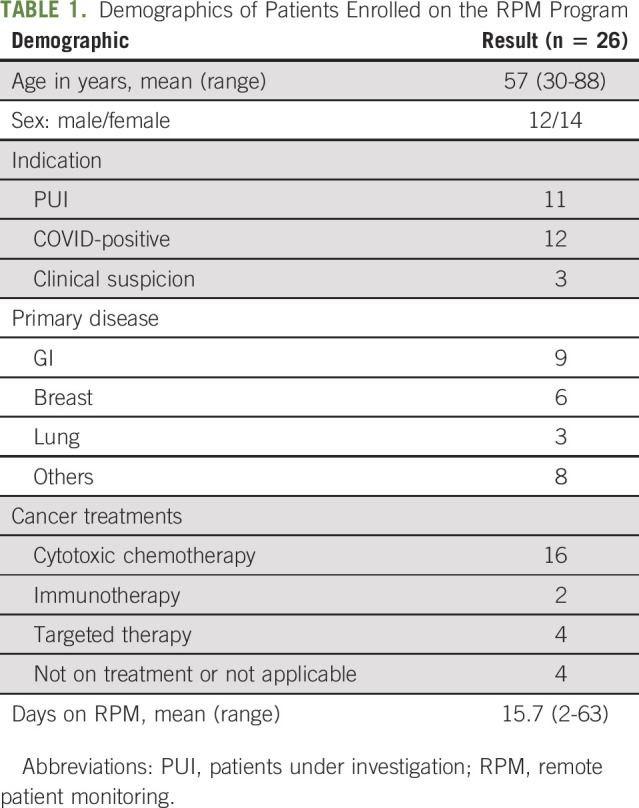
The mean age of the patients was 57 years, with a range from 30 to 88 years old. A majority were female. The most frequent diagnoses were cancers of the GI tract, breast, and lungs. Two patients had sickle cell disease. The majority of patients were on active anticancer treatment (n = 22, 85%), including cytotoxic chemotherapy (n = 16, 62%), immunotherapy (n = 2, 8%), and targeted therapies (n = 4, 15%). Four patients were not on anticancer treatment (15%), including the two patients with sickle cell disease, one with newly diagnosed lymphoma who had not yet started treatment and the other patient with chronic lymphocytic leukemia who was in remission. In most cases, anticancer treatment was held until two negative COVID tests were returned (n = 20, 91%). Two patients who were PUIs remained on systemic targeted therapy while awaiting test results. Patient demographics are included in Table 1.
TABLE 1.
Demographics of Patients Enrolled on the RPM Program
Patients remained on home monitoring for an average of 15.7 days (range, 2-63 days). One patient tested positive by nasal swab multiple times for more than 7 weeks and was on monitoring for 63 days (or an additional 14 days after her last positive test). Two patients used the Spanish language version of the RPM program. Figure 1 shows the clinical trending dashboard on of individual patient that providers monitored centrally at our cancer center.
FIG 1.
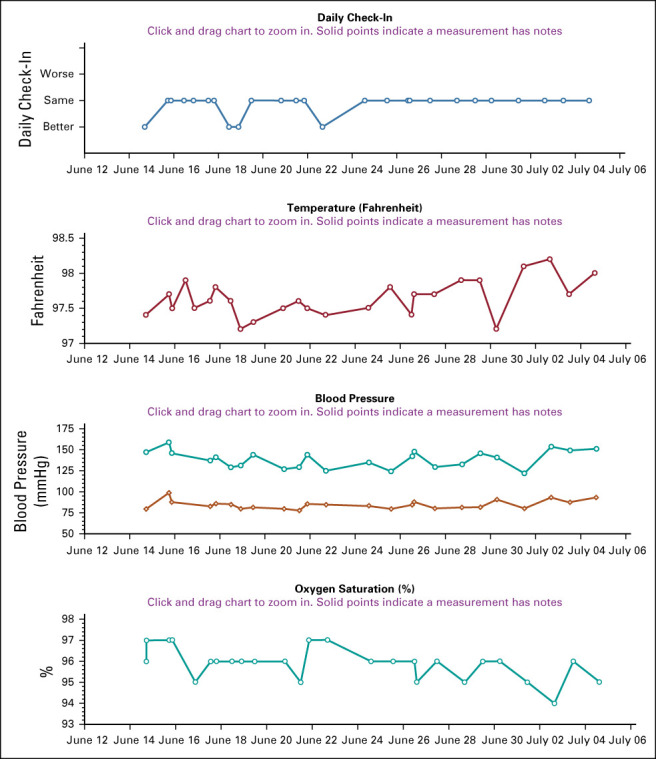
Example of dashboard trending report on participant in RPM program. RPM, remote patient monitoring.
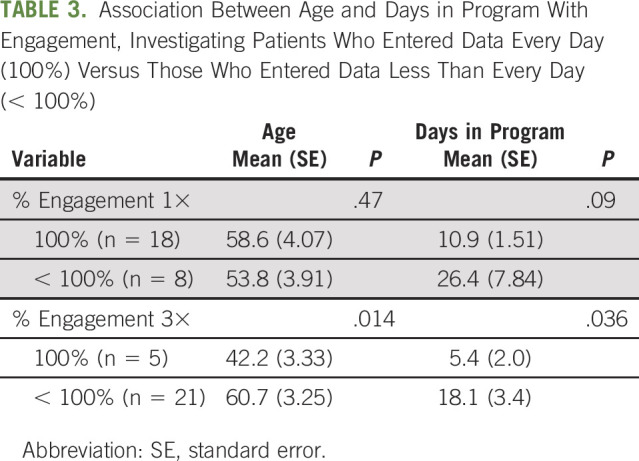
There was a high compliance, or engagement rate, by patients enrolled in RPM program. For all patients, the percentage of days during which at least one set of data was entered was 97.2% (range, 80%-100%). The percentage of days during which data were entered all three times was 65.7% (range, 0%-100%). There was no association between the demographic factors of age, gender, reason for monitoring, or days in the program with the level of engagement (Table 2). When we compared patients who entered data all three times each day for every day they were in the program (n = 5) with those who did this for fewer than all the days enrolled (n = 21), we did find a relationship with age (42.2 v 60.7 years; P = .01) and total days in the program (5.4 v 18.1 days; P = .04) (Table 3).
TABLE 2.
Patient Variables and Association With Engagement in Remote Monitoring Program During Enrollment
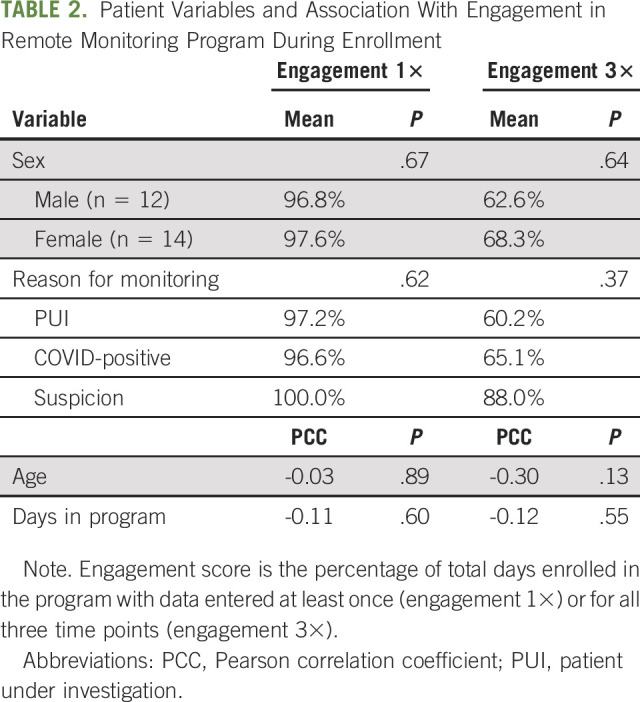
TABLE 3.
Association Between Age and Days in Program With Engagement, Investigating Patients Who Entered Data Every Day (100%) Versus Those Who Entered Data Less Than Every Day (< 100%)
Of the 26 patients, only one patient was admitted to the hospital for worsening symptoms. The patient was a PUI, on monitoring for 3 days, and admitted for progressive cough and fever. During his admission, he tested negative twice for COVID-19 and recovered with systemic antibiotics for a presumed bacterial upper respiratory infection. Another patient had worsening fevers but on evaluation was found to have cellulitis and was admitted for intravenous antibiotics. A third patient was admitted for bleeding at his ostomy site. Five patients reported feeling short of breath at some point during monitoring, and eight patients reported feeling worse than the day before.
A total of 23 patients (88%) were monitored at home and did not require hospital admission. Five patients developed worsening respiratory status (defined as one oxygen saturation measure below 93%) while on home monitoring, but on evaluation remained hemodynamically stable and were not sent to the emergency room nor admitted and later fully recovered.
One patient was a 38-year-old Hispanic woman with pancreatic adenocarcinoma on neoadjuvant chemotherapy. She was in her third cycle of treatment when she reported that her babysitter tested positive for COVID-19. She had a restaging computed tomography scan that demonstrated incidental patchy ground glass opacities in a peripheral distribution suspicious for COVID-19, along with a decrease in size of the pancreatic head mass. The patient was brought in for COVID-19 testing, and an initial test was negative. At the time of her first visit, she reported chills without fever and a slight cough. She agreed to enroll in the home monitoring program, and further chemotherapy was temporarily held. While being monitored, she became more symptomatic with body aches and headache in addition to chills. Repeat COVID testing was positive. She was hemodynamically stable and thus continued on home monitoring. Throughout the monitoring, her symptoms remained mostly mild, but she did experience periodic episodes of shortness of breath and hypoxia with activity. The APP monitoring team spoke with the patient frequently during the time frame she was entering abnormal values. After 2 weeks of quarantine, she had two consecutive negative COVID-19 tests and was able to proceed with definitive surgery.
Another patient was 44-year-old with newly diagnosed stage IV marginal zone lymphoma. He was scheduled to begin treatment and was tested for COVID-19 in accordance with guidelines from the American Society of Hematology.13 He was asymptomatic but was found to be positive. His only known exposure was a co-worker’s daughter who had tested positive 3 days before. The patients’ oncologic treatment was initially deferred, and the patient was placed on home quarantine with home monitoring. The patient was brought in for retesting 14 and 21 days later and remained persistently positive. Treatment with rituximab was started. He eventually tested negative 10 weeks after his initial positive test.
DISCUSSION
As the body of knowledge on the SARS-CoV-2 and the COVID-19 infection has grown since January 2020, a common observation is that most patients infected with the virus will have no or only mild symptoms. It was unknown whether this would apply equally to patients with cancer as the general population. However, it was apparent early in the pandemic that patients with cancer who develop severe symptoms have a higher mortality rate.1-3 This raises the hypothesis that close supervision of patients with cancer to allow prompt intervention when severe COVID-19 symptoms develop may improve outcomes.
As testing became more widely available in the United States and elsewhere, more patients with cancer were diagnosed with COVID-19. Recommendations from the American Society of Hematology suggested that all lymphoma and leukemia adult patients should be tested before admission for induction or consolidation chemotherapy.11 ASCO recommended testing all patients with newly diagnosed solid tumor before their first chemotherapy treatment.12 Thus, medical oncologists and hematologists will be facing the dilemma of what to do with a stable, newly diagnosed COVID-positive patient in their clinic. Our study highlights the feasibility of RPM in this patient population.
In this pilot investigation using RPM in stable PUIs and COVID-positive patients in a community cancer center, we found that most patients were willing to participate in the home monitoring program, with only three of 29 patients not enrolling. We also found a high degree of engagement, with patients entering at least one set of data 97% of the days on RPM and entering data all three times in a day 66% of the time. This high degree of patient participation in remote monitoring was likely in part due to the high degree of patient and public awareness of the danger posed by an active COVID-19 infection, but may also reflect ease of use and comfort level with the program.
The majority of patients in the RPM program did not need referral to the emergency room or an urgent care center—something that patients are very reluctant to do during the COVID pandemic. None of these patients required hospital admission for COVID-19 infection complications. It is important to note we did not prove that RPM improved clinical outcomes. Instead, we showed that RPM was feasible in this patient population. The findings in this pilot study should be tested in larger patient populations to further define the role of RPM in the COVID era, especially if we will be confronting this pandemic for the foreseeable future.
Since this pilot study during the first wave of COVID-19 infections in the spring of 2020, our RPM program has continued with no substantive changes. With the increasing cases being diagnosed in our region during the second wave in the fall and winter of 2020, we are actively monitoring more than a dozen patients per day in this program.
We identified several potential limitations with the home monitoring program. One was language interpretation. Second, although most patients reported great ease with the use of the equipment and remained compliant with entering values, there may be selected patients who are not familiar with the technology and/or cannot operate the equipment on their own, especially older patients. That said, we did not see an association between age and engagement, with older patients completing the RPM tasks as well as younger patients. One patient did not participate because of the lack of a home internet connection.
One additional drawback of the program is that it did not interface with the EMR system, although the platform could have been configured to do so. Given the need to rapidly deploy this program as the COVID pandemic hit our region, we did not want to wait for the time it would take for full EMR integration. We did develop a separate monitoring note template for documenting the program and patient contact within our EMR. Since this pilot program was completed we have developed this EMR interface.
A key challenge for initiating and maintaining an RPM program is cost. However, recent action by the Centers for Medicare & Medicaid Services improves RPM reimbursement for established patients.13
With the advances in telemedicine brought on by COVID-19 and recent CMS announcements that may allow billing for such services, additional applications of RPM could be investigated in the care of patients with cancer, including, for example, the management of outpatients with low-risk febrile neutropenia, patients with leukemia during their consolidation treatment, and for tracking vital signs, weight, and calorie counts during chemoradiation for patients with head and neck, esophageal, and anal cancer. The COVID-19 pandemic has greatly accelerated the use of telemedicine in terms of clinic evaluations and patient care. We found that remote home monitoring was feasible in managing oncology patients during the COVID-19 pandemic. Further investigations are warranted into using this tool for the duration of the pandemic and in other aspects of cancer care.
Nancy Addison
Employment: Locus Health
Timothy L. Cannon
Consulting or Advisory Role: Loxo, Navican
Other Relationship: Navican/Intermountain Healthcare
William B. Ershler
Stock and Other Ownership Interests: Global Blood Therapeutics
Consulting or Advisory Role: Pharmacosmos, Novartis, Global Blood Therapeutics
Speakers' Bureau: Pharmacosmos, Novartis, Global Blood Therapeutics
Danielle Shafer
Research Funding: EMD Serono
Sekwon Jang
Consulting or Advisory Role: Bristol Myers Squibb, EMD Serono, Novartis, Sanofi, Sun Biopharma, Genentech
Kirby Farrell
Employment: Locus Health
Leadership: Locus Health
Stock and Other Ownership Interests: Locus Health
Travel, Accommodations, Expenses: Locus Health
John F. Deeken
Research Funding: Merck, Loxo Oncology
Patents, Royalties, Other Intellectual Property: UpToDate
No other potential conflicts of interest were reported.
SUPPORT
This intervention and retrospective study was made possible by the generous support of the Mather Family whose philanthropic gift made it possible.
AUTHOR CONTRIBUTIONS
Conception and design: Mary Steimer, Jessica Leabo, David Heyer, Nancy Bowles, Kirby Farrell, John F. Deeken
Provision of study materials or patients: Timothy L. Cannon, Raymund Cuevo, William B. Ershler, Danielle Shafer, Sekwon Jang, Angela Pennisi, John F. Deeken
Collection and assembly of data: Mary Steimer, Jessica Leabo, Nancy Addison, John F. Deeken
Data analysis and interpretation: Hongkun Wang, David Heyer, Timothy L. Cannon, Raymund Cuevo, William B. Ershler, Danielle Shafer, Sekwon Jang, Angela Pennisi, Amjaad Al-Hussain, John F. Deeken
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Remote Home Monitoring of Patients With Cancer During the COVID Pandemic: A Pilot Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Nancy Addison
Employment: Locus Health
Timothy L. Cannon
Consulting or Advisory Role: Loxo, Navican
Other Relationship: Navican/Intermountain Healthcare
William B. Ershler
Stock and Other Ownership Interests: Global Blood Therapeutics
Consulting or Advisory Role: Pharmacosmos, Novartis, Global Blood Therapeutics
Speakers' Bureau: Pharmacosmos, Novartis, Global Blood Therapeutics
Danielle Shafer
Research Funding: EMD Serono
Sekwon Jang
Consulting or Advisory Role: Bristol Myers Squibb, EMD Serono, Novartis, Sanofi, Sun Biopharma, Genentech
Kirby Farrell
Employment: Locus Health
Leadership: Locus Health
Stock and Other Ownership Interests: Locus Health
Travel, Accommodations, Expenses: Locus Health
John F. Deeken
Research Funding: Merck, Loxo Oncology
Patents, Royalties, Other Intellectual Property: UpToDate
No other potential conflicts of interest were reported.
REFERENCES
- 1.Dai M, Liu D, Lie M, et al. : Patients with cancer appear more vulnerable to SARS-CoV-2: A multicenter study during the COVID-19 outbreak. Cancer Discov 10:783-791, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyashita H, Mikami T, Chopra N, et al. : Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol 31:1088-1089, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuderer NM, Choueiri TK, Shah DP, et al. : Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 395:1907-1918, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, McGoogan JM: Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323:1239-1242, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Gudbjartsson DF, Helgason A, Jonsson H, et al. : Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med 382:2302-2315, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakurai A, Sasaki T, Kato S, et al. : Natural history of asymptomatic SARS-CoV-2 infection. N Engl J Med 383:885-886, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi RT, Lynch JB, Del Rio C: Mild or moderate Covid-19. N Engl J Med 383:1757-1766, 2020 [DOI] [PubMed] [Google Scholar]
- 8. https://www.healthit.gov/topic/health-it-initiatives/telemedicine-and-telehealth
- 9.Vergales BD, Murray PD, Miller SE, et al. : Safety and efficacy of a home NG monitoring program for premature infants. Pediatr 146:389-390, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Vergales J, Peregoy L, Zalewski J, et al. : Use of a digital monitoring platform to improve outcomes in infants with a single ventricle. Pediatr 146:609, 2020 [DOI] [PubMed] [Google Scholar]
- 11.American Society of Hematology : COVID-19 Resources. www.hematology.org [Google Scholar]
- 12.American Society of Clinical Oncology : A Guide to Cancer Care Delivery During the COVID-10 Pandemic. www.asco.org.care-individuals-cancer-during-covid-19 [Google Scholar]
- 13.Centers for Medicare and Medicaid Services (CMS), HHS : https://www.federalregister.gov/documents/2019/11/15/2019-24086/medicare-program-cy-2020-revisions-to-payment-policies-under-the-physician-fee-schedule-and-other


