PURPOSE
Adult T-cell leukemia/lymphoma (ATLL) is an aggressive disease caused by the human T-cell leukemia virus type 1. Real-world data of ATLL in Latin America are lacking.
PATIENTS AND METHODS
We analyzed patients with ATLL (acute, lymphomatous, chronic, and smoldering) encountered in 11 Latin American countries between 1995 and 2019. Treatment response was assessed according to the 2009 consensus report. Survival curves were estimated using the Kaplan-Meier method and log-rank test.
RESULTS
We identified 253 patients; 226 (lymphomatous: n = 122, acute: n = 73, chronic: n = 26, and smoldering: n = 5) had sufficient data for analysis (median age 57 years). Most patients with ATLL were from Peru (63%), Chile (17%), Argentina (8%), and Colombia (7%). Hypercalcemia was positively associated with acute type (57% v lymphomatous 27%, P = .014). The median survival times (months) were 4.3, 7.9, 21.1, and not reached for acute, lymphomatous, chronic, and smoldering forms, with 4-year survival rates of 8%, 22%, 40%, and 80%, respectively. First-line zidovudine (AZT)-interferon alfa (IFN) resulted in an overall response rate of 63% (complete response [CR] 24%) for acute. First-line chemotherapy yielded an overall response rate of 41% (CR 29%) for lymphomatous. CR rate was 42% for etoposide, cyclophosphamide, vincristine, doxorubicin, and prednisone versus 12% for cyclophosphamide, vincristine, doxorubicin, and prednisone–like regimen (P < .001). Progression-free survival at 1 year for acute type patients treated with AZT-IFN was 67%, whereas 2-year progression-free survival in lymphomatous type patients who achieved CR after chemotherapy was 77%.
CONCLUSION
This study confirms Latin American ATLL presents at a younger age and has a high incidence of lymphomatous type, low incidence of indolent subtypes, and worse survival rates as compared with Japanese patients. In aggressive ATLL, chemotherapy remains the preferred choice for lymphomatous favoring etoposide-based regimen (etoposide, cyclophosphamide, vincristine, doxorubicin, and prednisone), whereas AZT-IFN remains a good first-line option for acute subtype.
INTRODUCTION
Adult T-cell leukemia/lymphoma (ATLL) is a mature, peripheral T-cell neoplasm caused by the human T-cell leukemia virus type 1 (HTLV-1).1,2 HTLV-1 infects up to 10 million people worldwide and it is predominantly transmitted via breastfeeding, blood transfusion, sharing of needles, and sexual intercourse.3 The virus is endemic in southwestern Japan, the Caribbean Basin, South America, Western and Central Africa, and central Australia.3,4 In Latin America, the highest prevalence is found in the Dominican Republic, Brazil, and Peru.4,5
CONTEXT
Key Objective
Does adult T-cell leukemia/lymphoma (ATLL) present differently in Latin America than other parts of the world?
Knowledge Generated
Latin American ATLL overwhelmingly presents at a younger age and with aggressive disease, characterized by higher incidence of lymphomatous type, low incidence of indolent types, and low survival outcomes compared with Japanese patients.
Relevance
In aggressive ATLL, etoposide-based regimen (etoposide, cyclophosphamide, vincristine, doxorubicin, and prednisone) is a reasonable first-line choice over cyclophosphamide, vincristine, doxorubicin, and prednisone–like chemotherapy, whereas combination of zidovudine with interferon alfa-2b is a good option for nonbulky acute ATLL subtype.
ATLL is a generally incurable disease where CD4+ T cells carry clonally integrated copies of the HTLV-1 genome.6 The cumulative lifetime risk of developing ATLL is estimated as 4%-7% among HTLV-1 carriers, with most cases presenting during the sixth decade of life in Japan and during the fifth decade in the Hispanic and Afro-Caribbean population.5,7-9 Clinically, the disease is classified by the Shimoyama10 criteria into four subtypes: acute, lymphomatous, chronic, and smoldering. The acute and lymphomatous are the most common and are often grouped as aggressive ATLL. The chronic and smoldering have a less aggressive course and are grouped as indolent ATLL.5,7,10,11
ATLL carries a dismal prognosis despite modern intensive therapies, with shorter survival rates compared with other peripheral T-cell lymphomas.12 The treatment of ATLL remains challenging with no clearly established standard of care at the present time. To date, clinicoepidemiologic studies evaluating the incidence and management of ATLL in endemic and nonendemic areas for HTLV-1 infection in Latin America are lacking. Cognizant of this, the Grupo de Estudio Latinoamericano de Linfoproliferativos developed a clinical data registry of patients diagnosed with ATLL in Latin America. Herein, we describe the group's analysis and provide an in-depth insight into the epidemiology, clinical features, treatment, and disease outcome of patients with ATLL encountered collectively over the past 25 years in participating sites.
PATIENTS AND METHODS
Patients
We conducted a retrospective analysis of patients diagnosed with ATLL between January 1995 and December 2019. Twenty-four centers from 11 Latin American countries participated in the ATLL database query (Appendix Table A1). Patient demographics and clinical data were obtained from available medical records. Each Institutional Review Board approved this study.
Diagnosis, Disease Classification, and Risk Stratification
The diagnosis of ATLL was based on serologic evidence of HTLV-1 by enzyme-linked immunosorbent assay. Confirmation by reflex Western blot was performed in most cases, but not in some because of unavailability or high cost. In all cases, identification of clonal CD4+ CD7– CD25+/– T cells in peripheral blood and/or tissues was determined by histology, immunophenotyping, and gene rearrangement studies. Patient cases were classified according to the Shimoyama10 criteria. Two indexes were used for risk stratification in aggressive ATLL: the International Prognostic Index (IPI) and the Prognostic Index for Peripheral T-Cell Lymphoma (PIT).13,14
Therapy Approaches
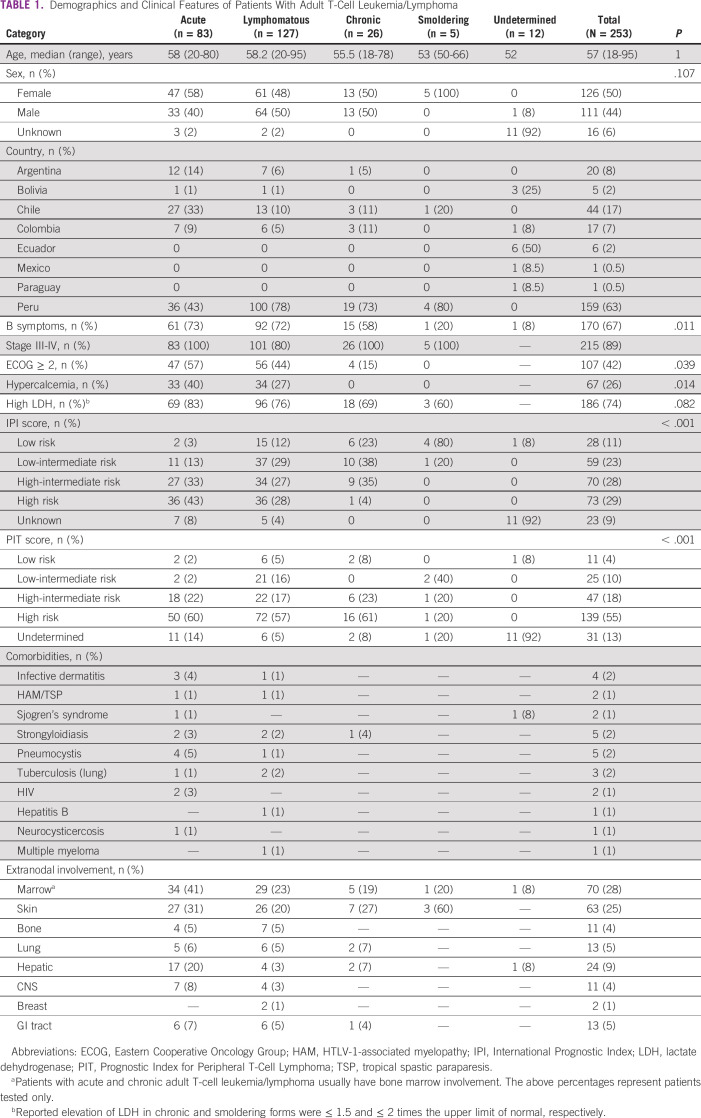
The therapy approaches investigated were classified into first-line therapy with (1) zidovudine (AZT)-interferon alfa (IFN) alone, (2) multiagent chemotherapy alone, (3) combined chemotherapy with AZT-IFN, and (4) single-agent chemotherapy and/or regional therapy (ie, topical therapy and phototherapy). The centers that used parenteral AZT-IFN had a comparable therapy protocol on the basis of previously published data.15 In general, the chemotherapy regimens used at any time included cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP)–like regimens; etoposide-based regimens (eg, etoposide, cyclophosphamide, vincristine, doxorubicin, and prednisone [CHOEP] and EPOCH), platinum-based regimens, other non–platinum-based regimens, single-agent chemotherapy, and allogeneic transplant (allogeneic hematopoietic stem-cell transplant [allo-HSCT]). Regimens were selected by the treating physician on the basis of ATLL subtype and according to local institutional practices (Table 1).
TABLE 1.
Demographics and Clinical Features of Patients With Adult T-Cell Leukemia/Lymphoma
Response Criteria
Therapy responses were assessed according to the 2009 consensus report for ATLL.16 The Cheson criteria was used to assess response through computerized tomography imaging.17 Complete response (CR) required a decrease in the absolute lymphocyte count to < 4 × 109/L with < 5% of flower cells remaining along normalization of all nodal and extranodal lesions (including bone marrow); partial response (PR) was defined as ≥ 50% disease reduction; and progressive disease (PD) as ≥ 50% increase in the count of flower cells and absolute lymphocyte count > 4 × 109/L at any time, and/or ≥ 50% increase in size of any nodal and/or extranodal lesions. Stable disease (SD) was defined as a failure to attain CR or PR in the absence of PD. Responses (CR, PR, and SD) should persist for at least 4 weeks to be classified as such.
Statistical Analysis
Demographics, clinical features, and therapies received were summarized using descriptive statistics. The primary study outcomes were treatment response, progression-free survival (PFS), and overall survival (OS). Event-free patients were censored at the date of last clinical follow-up. Alive patients were censored at last follow-up in clinic or by telephone. Survival estimates were calculated by the Kaplan-Meier method and compared using the log-rank test. The Clopper-Pearson method was used to estimate the two-sided 95% CI in response rates. Statistical analysis was performed using IBM SPSS Statistics version 23.
RESULTS
Epidemiologic and Clinical Features
A total of 253 patients with ATLL were identified between January 1995 and December 2019. Demographic and clinical features of patients with ATLL are shown in Figure 1 and Table 1.
FIG 1.
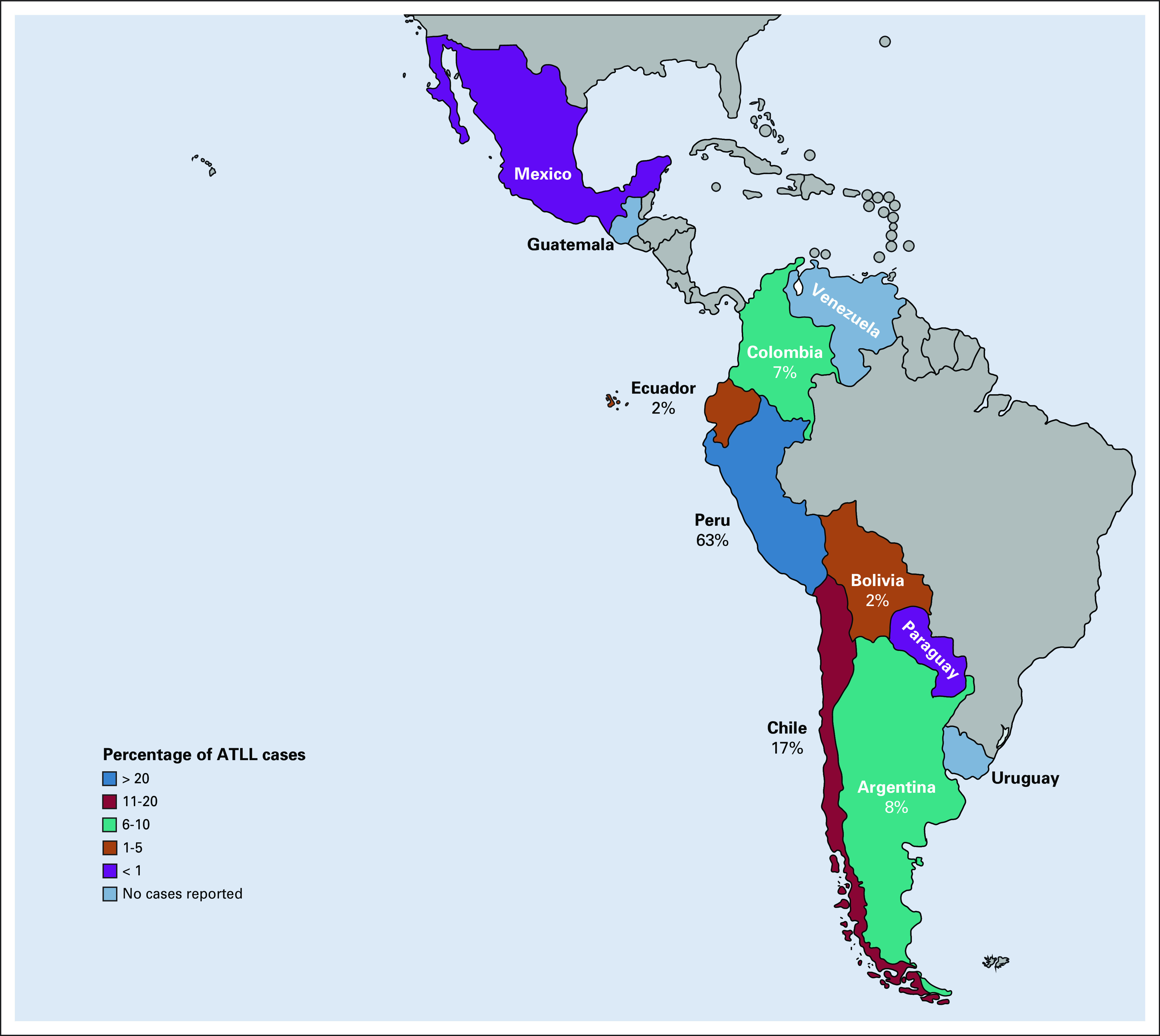
Geographical distribution map of patients with ATLL encountered in Latin America in Grupo de Estudio Latinoamericano de Linfoproliferativos cohort (N = 253). ATLL, adult T-cell leukemia/lymphoma.
The majority (n = 211; 83%) had aggressive ATLL (lymphomatous: n = 127, 50%; acute: n = 83, 33%), and 31 (12%) had indolent ATLL (chronic: n = 26, 10%; smoldering: n = 5, 2%). Twelve cases (5%) were unclassifiable because of lack of laboratory or imaging information. The median age at diagnosis was 57 years, with a female predominance in acute (n = 47; 58%) and smoldering (n = 5; 100%) subtypes. Most patients with ATLL were from Peru (n = 159; 63%), Chile (n = 44; 17%), Argentina (n = 20; 8%), and Colombia (n = 17; 7%) (Fig 1). During the ATLL data query, participating centers from Guatemala, Uruguay, and Venezuela did not report ATLL cases during the past 10-15 years.
Hypercalcemia was associated with acute ATLL (40% v lymphomatous 27%, P = .014). The PIT score yielded a higher risk classification than the IPI score (high-risk PIT [n = 139, 55%] v high-risk IPI [n = 73, 29%], respectively; P < .001). Coinfections were seen in 7% (n = 17) of cases with strongyloidiasis (n = 5) and Pneumocystis jirovecii pneumonia (n = 5) as the most commonly observed.
Disease Outcome
Two hundred twenty-six patients (lymphomatous: n = 122, acute: n = 73, and chronic and smoldering: n = 31) had sufficient data for analysis. With a median follow-up of 12 months (range 1 month to 15 years), the median OS times were 4.3 months, 7.9 months, 21.1 months, and not reached for acute, lymphomatous, chronic, and smoldering ATLL (P < .001), with 4-year survival rates of 8%, 22%, 40%, and 80%, respectively (Fig 2).
FIG 2.
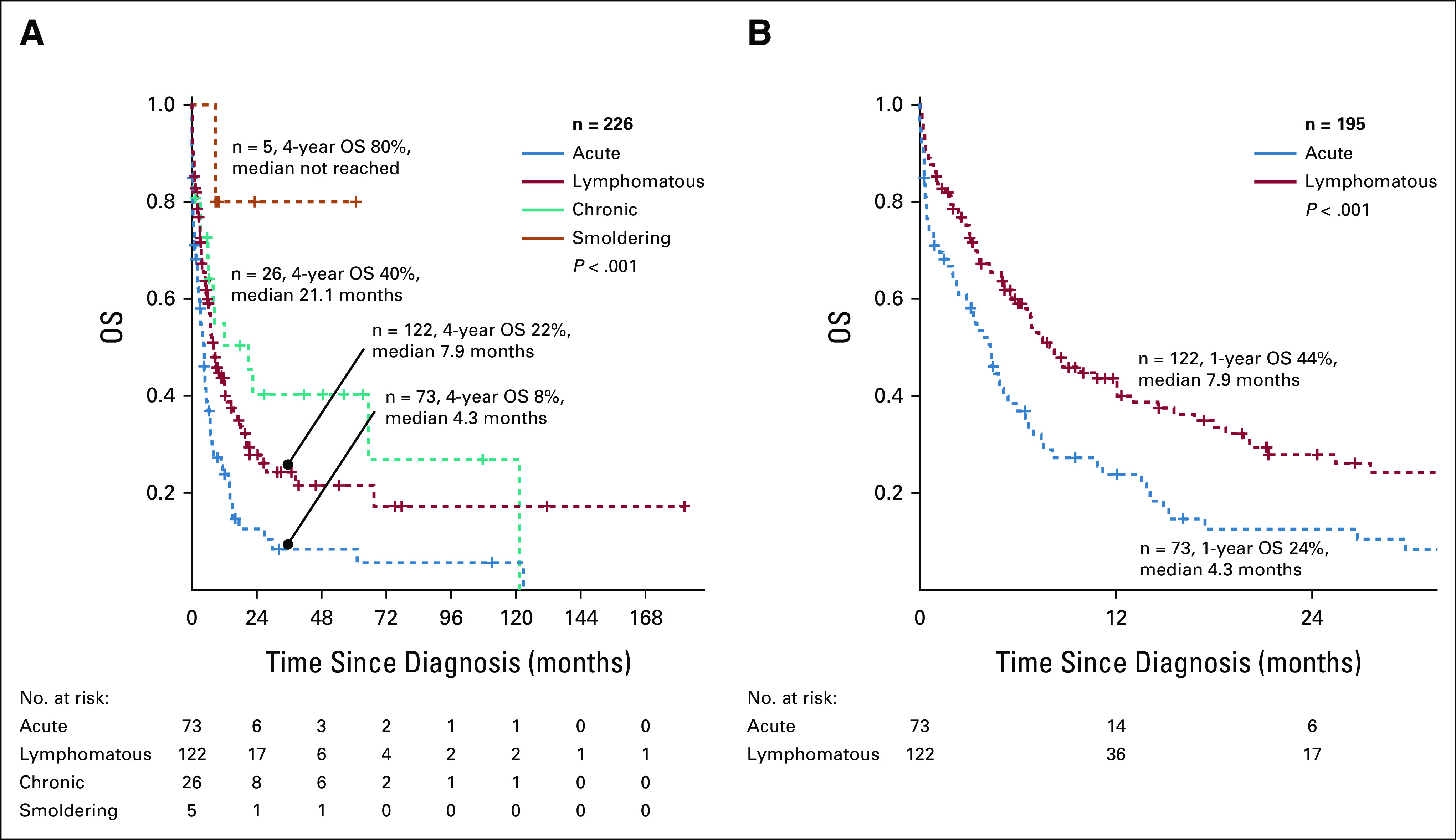
OS of patients with ATLL according to clinical subtype: (A) all ATLL and (B) aggressive ATLL. ATLL, adult T-cell leukemia/lymphoma; OS, overall survival.
Therapy Approaches and Responses
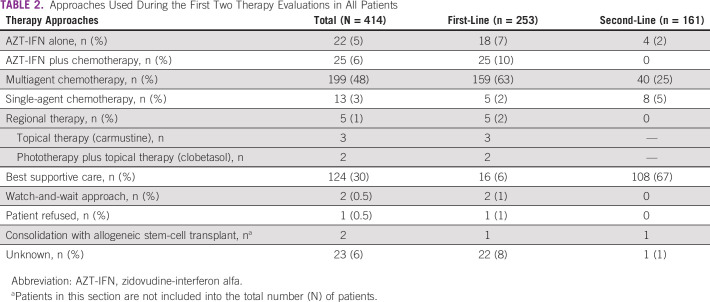
The therapy approaches used during the first two lines of therapy are summarized in Table 2. The median number of treatment regimens administered in all patients was 1 (range 1-3). Sixteen (6%) patients did not receive first-line treatment and had best supportive care because of very poor performance status led by a rapid disease progression. No biologic agents were used in our cohort mainly because of lack of access to these agents. Data on patients managed for CNS involvement are presented in Appendix Table A2.
TABLE 2.
Approaches Used During the First Two Therapy Evaluations in All Patients
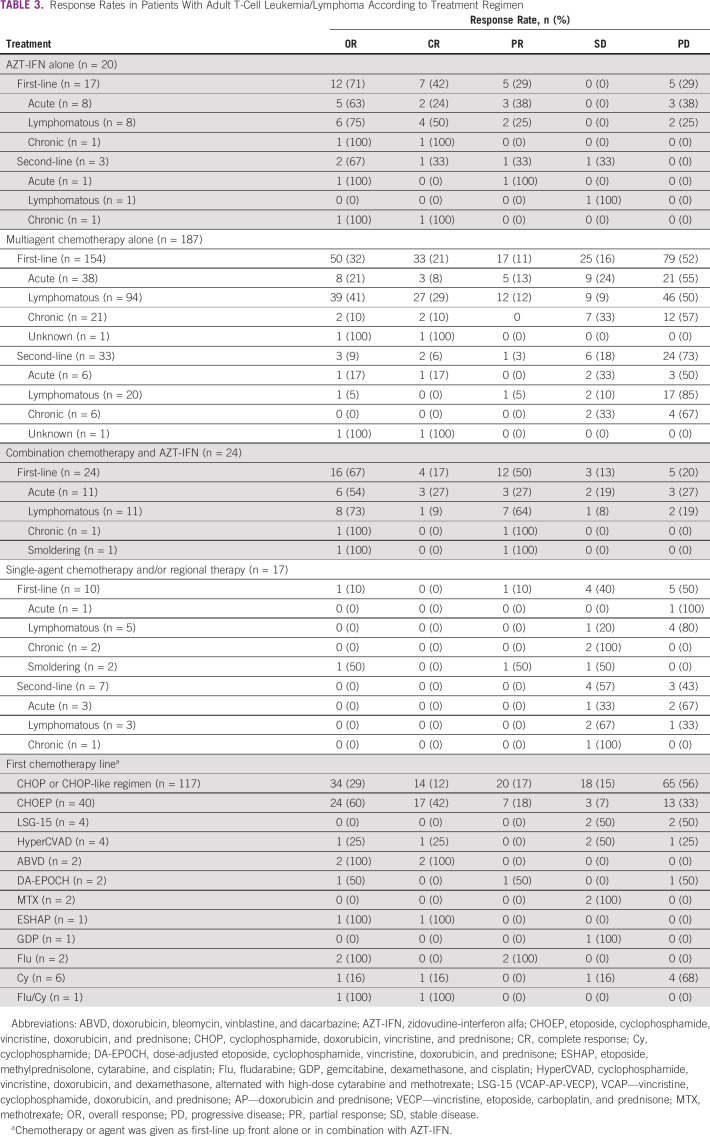
AZT-IFN–Based Regimen
Table 3 summarizes the response rates in patients with ATLL according to treatment regimen. Of the evaluable patients, 44 received either AZT-IFN alone (n = 20) or in combination with multiagent chemotherapy (n = 24). First-line AZT-IFN alone (n = 17) resulted in an overall response rate (ORR) of 71% and CR of 42% (acute: n = 8, ORR 63%, CR 24%; lymphomatous: n = 8, ORR 75%, CR 50%; and smoldering: n = 1, CR 100%). First-line combination of AZT-IFN with multiagent chemotherapy (either concurrent or sequentially) resulted in an ORR of 67% and a CR of 17% (acute: n = 11, ORR 54%, CR 27%; lymphomatous: n = 11, ORR 73%, CR 9%; chronic: n = 1, PR 100%; and smoldering: n = 1, PR 100%). Second-line AZT-IFN alone (n = 3) yielded an ORR of 67% and a CR of 33% (one acute PR, one chronic CR, and one lymphomatous SD).
TABLE 3.
Response Rates in Patients With Adult T-Cell Leukemia/Lymphoma According to Treatment Regimen
Chemotherapy-Based Regimen
A total of 187 patients received multiagent chemotherapy either as first line (n = 154) or as second line (n = 33). First-line chemotherapy resulted in an ORR of 32% and a CR of 21% (acute: n = 38, ORR 21%, CR 8%; lymphomatous: ORR 41%, CR 29%; chronic: n = 21, ORR 10%, CR 10%; and one with unknown ATLL subtype had CR). Second-line chemotherapy resulted in an ORR of 9% and a CR of 6% (acute: n = 6, ORR 17%, CR 17%; lymphomatous: n = 20, ORR 5%, PR 5%; chronic: n = 6, ORR 0%; and one with unknown ATLL subtype had CR). The most commonly used first-line regimens were CHOP or CHOP-like regimen (n = 117, 59%) and CHOEP regimens (n = 40, 20%) with an ORR of 29% (CR 12%) and 60% (CR 42%), respectively (P < .001) (Table 3). LSG-15–like regimen was used in four patients with an ORR of 0% (SD, n = 2, 50%) and hyperCVAD in four patients with one patient achieving CR (SD, n = 2, 50%).
Comparing Therapy Regimens
There were no differences in PFS in patients with acute or lymphomatous ATLL receiving either first-line AZT-IFN alone, chemotherapy alone, or combination therapy (Appendix Fig A1). However, in patients with aggressive ATLL who achieved CR, first-line AZT-IFN (alone or in combination with chemotherapy) yielded a better PFS compared with chemotherapy alone in acute ATLL (AZT-IFN–based: n = 5, median 30.4 months, 1-year PFS 67% v chemotherapy-based: n = 3, median 2.8 months, 1-year PFS 0%). Chemotherapy resulted in improved PFS in patients with lymphomatous ATLL compared with AZT-IFN–based approach (AZT-IFN–based: n = 4, median 17.7 months, 2-year PFS 37% v chemotherapy-based: n = 26, median 67.1 months, 2-year PFS 77%) (Appendix Fig A1). However, these differences were not statistically significant.
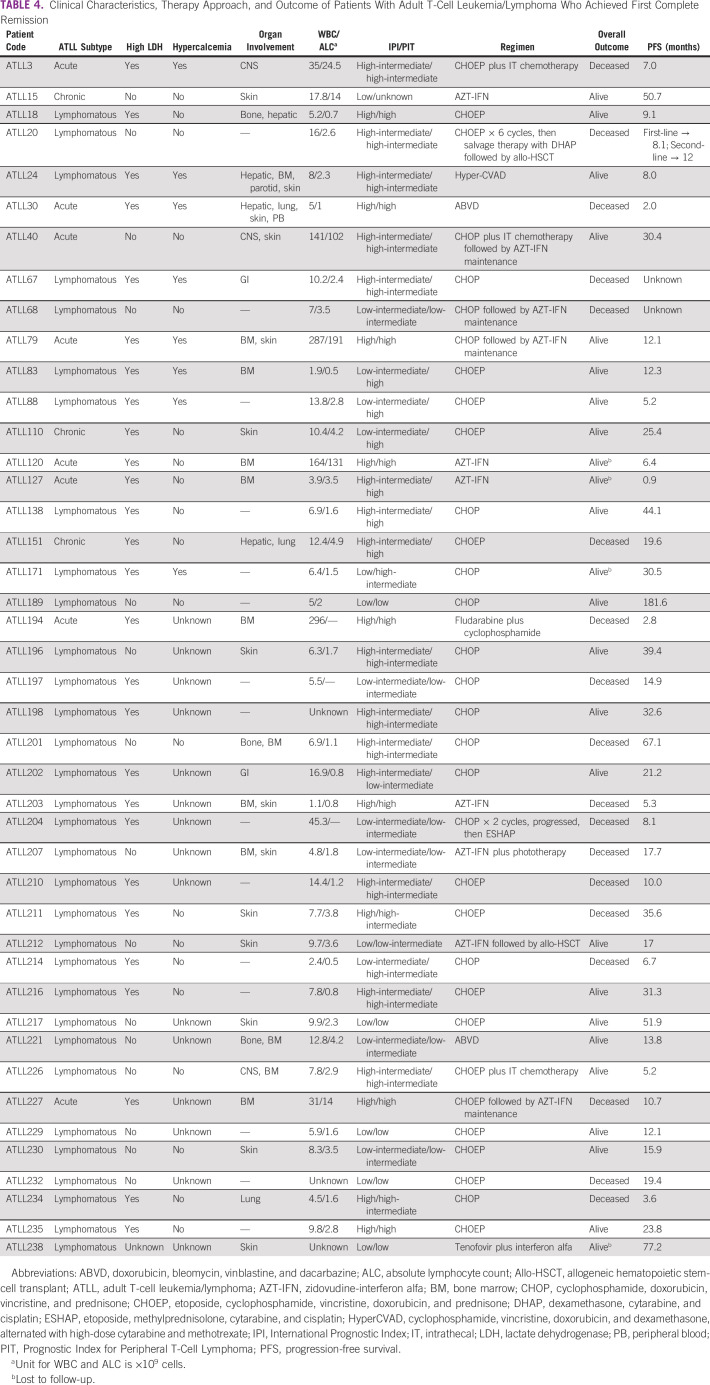
Table 4 summarizes the therapy approaches and outcomes of patients with ATLL who achieved first complete remission.
TABLE 4.
Clinical Characteristics, Therapy Approach, and Outcome of Patients With Adult T-Cell Leukemia/Lymphoma Who Achieved First Complete Remission
DISCUSSION
To our knowledge, this study is the first international, multi-institutional study evaluating the epidemiology, diagnosis, therapy approaches, and outcomes of patients with HTLV-1–related ATLL encountered in different Latin American countries over the past 25 years. In this study, Latin American patients with ATLL were predominantly women and were diagnosed at a younger age (median 57 years, range 18-95), which is in line with previous Latin American and US reports describing that Hispanic and Afro-Caribbean patients are diagnosed approximately a decade younger than Japanese patients.5,7,18-22
HTLV-1 is commonly found in indigenous peoples of the Pacific Islands (Australia and Melanesia) and the Americas.23-27 In Latin America, there are more than 400 indigenous groups, ranging from 45 to 50 million individuals.28-30 The 2020 International Work Group for Indigenous Affairs reported Bolivia as the country housing the largest indigenous population in Latin America (48% of the population), followed by Guatemala (43.8%), Mexico (21.5%), Chile (12.8%), and Peru (12.5%).28 In countries such as Venezuela, Paraguay, Brazil, El Salvador, and Uruguay, indigenous people comprise < 3% of the population.28-30 Besides Brazil, HTLV-1 infection is predominantly presented in indigenous people living in the Andes of Peru (Quechua and Aymara), Chile (Mapuche and Rapa Nui), and Colombia (Paez); in the Peruvian Amazon (Shipibo-Konibo); and in the Chaco region (Qulla and Puná of northwestern Argentina).3,26,27 The prevalence of HTLV-1 infection in countries of Central America and Mexico is poorly known; however, it is estimated to be low on the basis of few studies performed in blood donors.25-27,31
In our study, ATLL cases were primarily encountered in Peru, encompassing 63% of the studied patients. Chile, Argentina, and Colombia also reported numerous cases, most of them in indigenous people living in areas close to the mountains and where the virus has been reported as endemic. Participating centers from Bolivia, Ecuador, Mexico, and Paraguay reported very few cases, whereas centers from Venezuela, Uruguay, and Guatemala did not report any cases during the past 2 decades. Possible explanations to our findings are as follows: (1) some participating centers in this study are located far from known HTLV-1 endemic areas, which might have yielded to underreporting the number of ATLL cases; however, all participating centers are referral cancer centers in their countries, with ATLL as a common motive for referral; and (2) limited resources and lack of standardized protocols for HTLV testing in Latin America (countries particularly known for having large indigenous population such as Bolivia, Ecuador, and Mexico had screening rates of HTLV-1/2 in blood donors of 0%, 8%, and 0%, respectively, during 2016-2017).31 Although our findings seem to correlate with what is known regarding the geographical distribution of HTLV-1 infection in Latin America, there is an unmet need to better understanding the current incidence and prevalence of HTLV-1 infection in this region of the world. Brazil, a known highly endemic country for HTLV-1 infection, was not included in this study because of its recent incorporation to the Grupo de Estudio Latinoamericano de Linfoproliferativos; nonetheless, data of ATLL in Brazil are well-established, whereas these same data are lacking in other Latin American countries.32-35
In our cohort, lymphomatous (50%) ATLL was most commonly encountered compared with acute (33%) ATLL. This finding correlates with what has been previously described in other Latin American and US studies5,20,36 but is dissimilar to Japanese and some other US cohorts where acute ATLL was the most common subtype.7,21,22 We acknowledge that our patients were classified using archived records, therefore representing a potential risk for biases. However, there was a distinction between the acute and lymphomatous groups in our cohort, including higher rates of hypercalcemia and poorer outcomes in patients with acute ATLL. The 1-year OS rates of 24% for acute ATLL compared with 44% for lymphomatous ATLL (P < .001) are consistent with the natural history of ATLL (Fig 2B). A relative poorer survival has been reported in Hispanic and Afro-Caribbean patients as opposed to Japanese patients.5,7,20-22,36 Although limited health care access in Latin American countries could ultimately influence patient outcome, our clinical findings suggest that Latin American patients with ATLL present with more aggressive clinical course than Japanese patients. Furthermore, a recent study reported that North American ATLL (mostly composed of patients of Caribbean descent) has a distinct genomic landscape with a high frequency of prognostic epigenetic mutations affecting p53 function and is consequently associated to chemorefractoriness.37 This could potentially explain the differences in aggressiveness seen in American and Caribbean ATLL populations compared with Japanese population, despite sharing the same HTLV-1 serotype.
In this study, patients with acute ATLL who received first-line AZT-IFN either alone (n = 16) or in combination with chemotherapy (n = 22) had a tendency toward higher ORR (AZT-IFN alone: ORR 63% [CR 24%]; AZT-IFN combination: ORR 54% [CR 27%]) as compared with those treated with first-line multiagent chemotherapy (n = 132; ORR 21% [CR 8%]). This finding is in line with previous meta-analysis where patients with aggressive ATLL had an ORR of 66% and a CR of 35%.38 Importantly, patients with acute ATLL who achieved CR after AZT-IFN had a longer PFS as compared with those who achieved CR after chemotherapy alone (30.4 months v 2.8 months, respectively) as only three of 38 acute type patients achieved CR after chemotherapy (Appendix Fig A1C). Previous studies have reported this positive outcome, suggesting that AZT-IFN is a good first-line option to attempt for patients with leukemic ATLL.5,38 Although not formally studied in this cohort, a common disease feature we saw in responders to AZT-IFN was lymph node size of ≤ 3 cm in patients with acute ATLL and mass ≤ 3.5 cm, suggesting that this therapy may only be effective in ATLL presenting with nonbulky disease. Despite the long-term responses seen with AZT-IFN, most patients will eventually experience disease progression, which confirms this therapy is suppressive but not curative, reason why patients in clinical remission should be evaluated for stem-cell transplantation or remain on maintenance therapy indefinitely, if deemed unfit for transplantation.5,15,38
In lymphomatous ATLL, although response rates were low in patients treated with first-line multiagent chemotherapy (ORR 41% and CR 29%), superior outcomes were observed in those who achieved a CR compared with first-line AZT-IFN (67.1 months v 17.7 months, respectively). However, this was not statistically significant because of the low number of patients treated (Appendix Fig A1D). This finding provides further evidence that chemotherapy should remain the standard first-line treatment of this subtype, as was observed in previous studies.5,16,38 In this study, CHOEP regimen (n = 40) yielded higher response rates compared with CHOP or CHOP-like regimen (n = 117) (ORR 60%, CR 42% v ORR 29%, CR 12%, respectively; P < .001). Only four patients received the modified intensive regimen vincristine, cyclophosphamide, doxorubicin, and prednisone-doxorubicin and prednisone-vincristine, etoposide, carboplatin, and prednisone (known as LSG-15 regimen, vincristine, cyclophosphamide, doxorubicin, and prednisone-doxorubicin, ranimustine and prednisone-vincristine, etoposide, carboplatin, and prednisone), of whom two had SD and the other two PD. A previous study found no difference in outcomes between the above regimens, but this could have been related to the small sample size in the study.5 Therefore, it would be reasonable to use CHOEP, or a similar regimen, in patients with aggressive ATLL who are good candidates for high-dose chemotherapy and allo-HSCT. In our study, only two patients with lymphomatous ATLL underwent allo-HSCT, one as second line after achieving CR with dexamethasone, cytarabine, and cisplatin (PFS 12 months, died from relapsed disease) and the other after achieving CR with first-line AZT-IFN (remains disease-free, PFS 17 months).
Finally, this study not only provides a comprehensive assessment of this rare and generally incurable disease but also offers a better understanding on the limitations the different participating institutions face during the initial and subsequent evaluation of patients with ATLL in Latin America. We identified disparities among countries in the following: (1) sophisticated imaging such as positron emission tomography and magnetic resonance imaging almost universally lacked in public or governmental institutions; (2) biologic agents (eg, interferon alfa-2b, mogamulizumab, and alemtuzumab) were not readily available for the management of ATLL in most Latin American countries, and if available, the cost was borne by the patient, which makes its use almost impossible; and (3) there was limited access to clinical trials for ATLL in Latin American countries (only two countries had mogamulizumab as a therapy option under a clinical trial in the past 25 years).
In conclusion, ATLL continues to carry a dismal outcome with conventional therapies, urging the development of novel approaches. Our study found that in Latin America, patients present at a younger age, with female predominance, high incidence of lymphomatous type, low incidence of indolent types, and worse survival rates as compared with previously reported Japanese patients. AZT-IFN produced durable responses in patients with acute ATLL who achieved CR as compared with chemotherapy alone. On the other hand, chemotherapy responses, especially after CHOEP, were more durable in lymphomatous type patients who achieved CR than the acute type. Our findings suggest that in the management of aggressive ATLL, chemotherapy remains the preferred first-line choice for lymphomatous type favoring the etoposide-based regimen CHOEP, whereas AZT-IFN is a good option to attempt for acute type up front, with consideration of allo-HSCT in those patients who achieve a good response to therapy. Further characterization of Latin American ATLL through genomic analysis is planned.
Appendix
FIG A1.
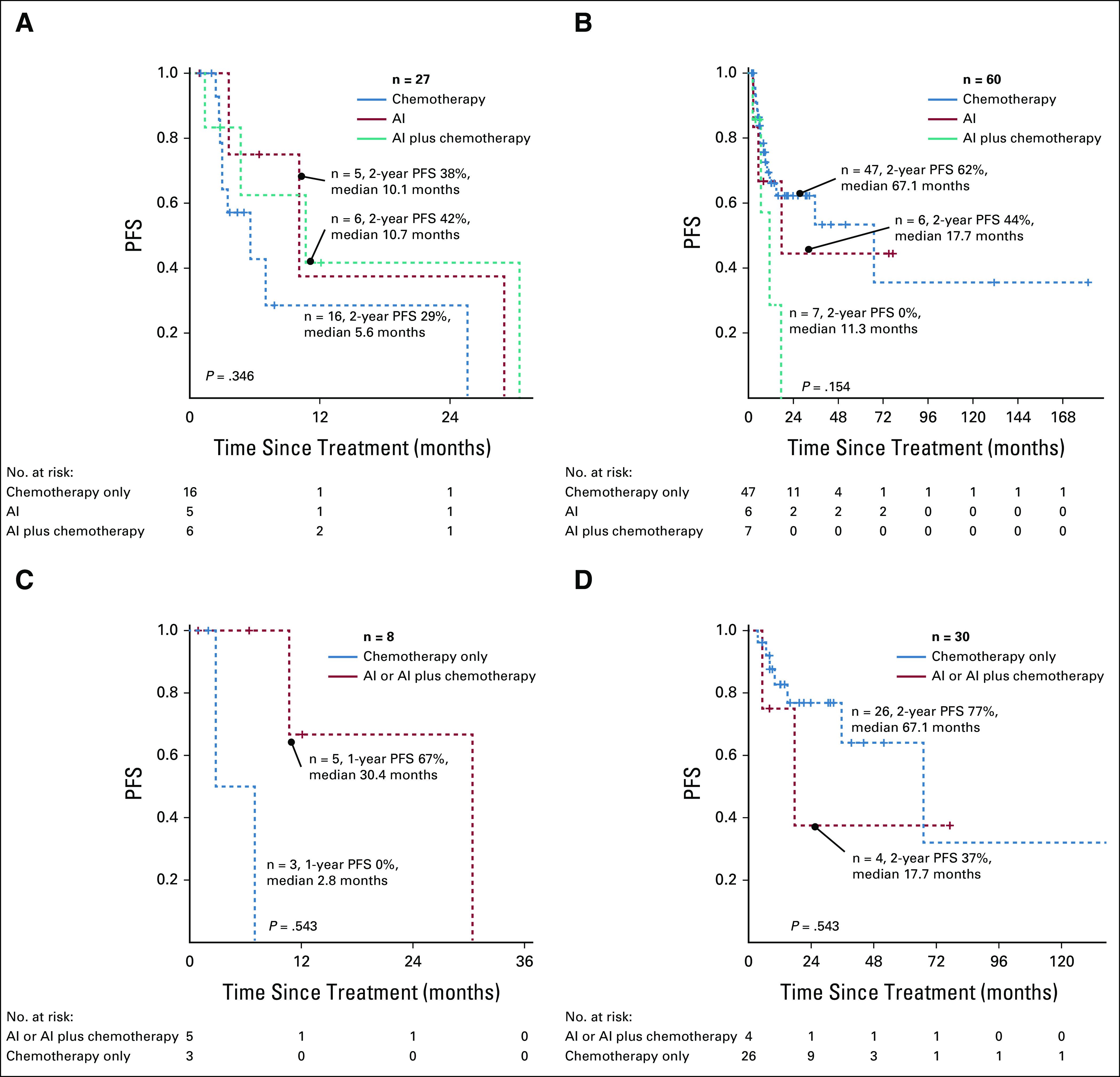
PFS of patients with aggressive ATLL after first-line therapy according to clinical subtype and treatment approach: (A) acute ATLL, (B) lymphomatous ATLL, (C) acute ATLL after CR, and (D) lymphomatous ATLL after CR. AI, AZT (zidovudine) with Interferon alpha 2b; ATLL, adult T-cell leukemia/lymphoma; CR, complete response; PFS, progression-free survival.
TABLE A1.
Latin American Hospitals With Expertise in Cancer Services Participating in Adult T-Cell Leukemia/Lymphoma Database Query
TABLE A2.
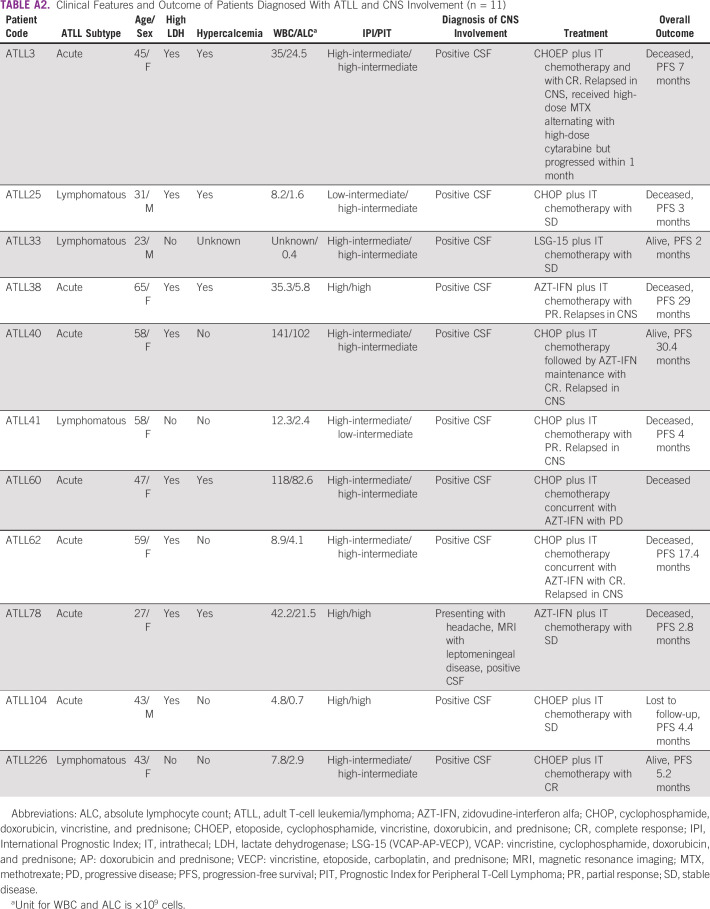
Clinical Features and Outcome of Patients Diagnosed With ATLL and CNS Involvement (n = 11)
Denisse A. Castro
Consulting or Advisory Role: Johnson & Johnson del Perú S.A.
Camila Peña
Honoraria: Janssen, Bristol Myers Squibb/Medarex
Consulting or Advisory Role: Janssen
Travel, Accommodations, Expenses: Tecnofarma
Victoria Otero
Employment: AstraZeneca
Eloisa Riva
Honoraria: Sanofi
Travel, Accommodations, Expenses: Roemmers
Maria A. Torres-Viera
Speakers' Bureau: Takeda
Jorge J. Castillo
Consulting or Advisory Role: Janssen, Roche/Genentech, Beigene, AbbVie/Pharmacyclics
Research Funding: Pharmacyclics, AbbVie, Janssen, BeiGene, TG Therapeutics
Juan Carlos Ramos
Research Funding: miRagen
Luis Villela
Consulting or Advisory Role: Jazz Pharmaceuticals, Roche-Syntex, AstraZeneca LATAM
Speakers' Bureau: Amgen Mexico, AbbVie
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the ASH Annual Meeting, virtual, December 2020.
L.M., D.J.E., D.A.C., C.P., and H.I. contributed equally to this work as first authors.
AUTHOR CONTRIBUTIONS
Conception and design: Luis Malpica, Daniel J. Enriquez, Camila Peña, Laura Aguirre-Martinez, Jorge J. Castillo, Brady E. Beltran
Administrative support: Milagros Altamirano
Provision of study materials or patients: Denisse A. Castro, Camila Peña, Lorena Fiad, Victoria Otero, Mirna Biglione, Gustavo Sandival-Ampuero, Ursula Aviles-Perez, Laura Aguirre-Martinez, Luciana Guanchiale, Pablo Soto, Jose L. Viñuela, Maria E. Cabrera, Eloisa Riva, Andrea Noboa, Myrna Candelaria, Fabiola Valvert, Maria A. Torres-Viera
Collection and assembly of data: Luis Malpica, Daniel J. Enriquez, Denisse A. Castro, Camila Peña, Henry Idrobo, Lorena Fiad, Maria Prates, Victoria Otero, Mirna Biglione, Milagros Altamirano, Gustavo Sandival-Ampuero, Ursula Aviles-Perez, Kelly Meza, Laura Aguirre-Martinez, Nancy Cristaldo, Juan L. Maradei, Luciana Guanchiale, Pablo Soto, Jose L. Viñuela, Maria E. Cabrera, Sally Rose Paredes, Marcos Di Stefano, Andrea Noboa, Juan A. Choque, Myrna Candelaria, Fabiola Valvert, Maria A. Torres-Viera
Data analysis and interpretation: Luis Malpica, Daniel J. Enriquez, Denisse A. Castro, Henry Idrobo, Jose L. Viñuela, Eloisa Riva, Alana Von Glasenapp, Jorge J. Castillo, Juan Carlos Ramos, Luis Villela, Brady E. Beltran
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Denisse A. Castro
Consulting or Advisory Role: Johnson & Johnson del Perú S.A.
Camila Peña
Honoraria: Janssen, Bristol Myers Squibb/Medarex
Consulting or Advisory Role: Janssen
Travel, Accommodations, Expenses: Tecnofarma
Victoria Otero
Employment: AstraZeneca
Eloisa Riva
Honoraria: Sanofi
Travel, Accommodations, Expenses: Roemmers
Maria A. Torres-Viera
Speakers' Bureau: Takeda
Jorge J. Castillo
Consulting or Advisory Role: Janssen, Roche/Genentech, Beigene, AbbVie/Pharmacyclics
Research Funding: Pharmacyclics, AbbVie, Janssen, BeiGene, TG Therapeutics
Juan Carlos Ramos
Research Funding: miRagen
Luis Villela
Consulting or Advisory Role: Jazz Pharmaceuticals, Roche-Syntex, AstraZeneca LATAM
Speakers' Bureau: Amgen Mexico, AbbVie
No other potential conflicts of interest were reported.
REFERENCES
- 1.Yoshida M, Miyoshi I, Hinuma Y: Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci USA 79:2031-2035, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poiesz BJ Ruscetti FW Reitz MS, et al. : Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sézary T-cell leukaemia. Nature 294:268-271, 1981 [DOI] [PubMed] [Google Scholar]
- 3.Verdonck K, González E, Van Dooren S, Vandamme A-M, Vanham G, Gotuzzo E: Human T-lymphotropic virus 1: Recent knowledge about an ancient infection. Lancet Infect Dis 7:266-281, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Gessain A, Cassar O: Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol 3:388, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malpica L Pimentel A Reis IM, et al. : Epidemiology, clinical features, and outcome of HTLV-1-related ATLL in an area of prevalence in the United States. Blood Adv 2:607-620, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azran I, Schavinsky-Khrapunsky Y, Aboud M: Role of Tax protein in human T-cell leukemia virus type-I leukemogenicity. Retrovirology 1(1):20, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katsuya H Ishitsuka K Utsunomiya A, et al. : Treatment and survival among 1594 patients with ATL. Blood 126:2570-2577, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Satake M Yamada Y Atogami S, et al. : The incidence of adult T-cell leukemia/lymphoma among human T-lymphotropic virus type 1 carriers in Japan. Leuk Lymphoma 56:1806-1812, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Malpica Castillo LE Peña C Rojas C, et al. : Identifying a simple clinical prognostic model for aggressive adult T-cell leukemia/lymphoma in Latin American population and its validation: A large International Study of the Latin America Working Group for Lymphomas (GELL). Blood 134, 2019. (suppl 1; abstr 4045) [Google Scholar]
- 10.Shimoyama M: Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. Br J Haematol 79:428-437, 1991 [DOI] [PubMed] [Google Scholar]
- 11.Castillo LM, Dittus C: Adult T-cell leukemia/lymphoma, in Novel Therapeutics for Rare Lymphomas. Springer Nature Switzerland AG, Springer International Publishing, 2019, pp 137-164 [Google Scholar]
- 12.Vose J, Armitage J, Weisenburger D: International T-cell lymphoma project. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J Clin Oncol 26:4124-4130, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Gallamini A Stelitano C Calvi R, et al. : Peripheral T-cell lymphoma unspecified (PTCL-U): A new prognostic model from a retrospective multicentric clinical study. Blood 103:2474-2479, 2004 [DOI] [PubMed] [Google Scholar]
- 14.International Non-Hodgkin's Lymphoma Prognostic Factors Project : A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med 329:987-994, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Ramos JC Ruiz P Ratner L, et al. : IRF-4 and c-Rel expression in antiviral-resistant adult T-cell leukemia/lymphoma. Blood 109:3060-3068, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsukasaki K Hermine O Bazarbachi A, et al. : Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: A proposal from an international consensus meeting. J Clin Oncol 27:453-459, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Connor OA Pro B Illidge T, et al. : Phase 3 trial of brentuximab vedotin and CHP versus CHOP in the frontline treatment of patients (pts) with CD30+ mature T-cell lymphomas (MTCL). J Clin Oncol 32, 2014. (abstr TPS8612) [Google Scholar]
- 18.Oliveira PD, de Carvalho RF, Bittencourt AL: Adult T-cell leukemia/lymphoma in South and Central America and the Caribbean: Systematic search and review. Int J STD AIDS 28:217-228, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Enriquez DJ Haro JC Rioja P, et al. : New prognostic classification for adult T cell lymphoma/leukemia based on hypercalcemia and neutrophil-to-lymphocyte ratio. Blood 130, 2017. (suppl 1; abstr 4164) [Google Scholar]
- 20.Beltran BE, Cotacallapa E, Castillo JJ: Adult T-cell leukemia/lymphoma in Peru: A report of 120 cases. Blood 120, 2012(abstr 5110) [Google Scholar]
- 21.Zell M Assal A Derman O, et al. : Adult T-cell leukemia/lymphoma in the Caribbean cohort is a distinct clinical entity with dismal response to conventional chemotherapy. Oncotarget 7:51981-51990, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips AA Shapira I Willim RD, et al. : A critical analysis of prognostic factors in North American patients with human T-cell lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma. Cancer 116:3438-3446, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Balduin B Priore C Acosta C, et al. : Infección por virus linfotrópico de células T humanas (HTLV) en Uruguay: identificación de problemas. Anales de la Facultad de Medicina Universidad de la República Uruguay 4:41-51, 2017. http://www.anfamed.edu.uy/index.php/rev/article/view/286 [Google Scholar]
- 24.Mosquera-Herrera CE Aspiazu-Miranda EP de Waard JH, et al. : A high prevalence of human T-lymphotropic virus (HTLV 1/2) infection among Afro-descendants, Esmeraldas province, Ecuador—Need for the implementation of surveys and control programs. Infect Drug Resist 12:1969-1974, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palma P Barrientos JM Posadas MA, et al. : Prevalencia del virus linfotrópico de células T humanas (HTLV) I/II en donantes de sangre. Cienc Tecnol Salud 4:15-50, 2017 [Google Scholar]
- 26.Eusebio-Ponce E, Candel FJ, Anguita E: Human T-cell lymphotropic virus type 1 and associated diseases in Latin America. Trop Med Int Heal 24:934-953, 2019 [DOI] [PubMed] [Google Scholar]
- 27.Paiva A, Casseb J: Origin and prevalence of human T-lymphotropic virus type 1 (HTLV-1) and type 2 (HTLV-2) among indigenous populations in the Americas. Rev Inst Med Trop Sao Paulo 57:1-13, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathaniel Berger D Bulanin N García-Alix L, et al. : 2 IWGIA-the indigenous world-2020. www.NickPurserDesign.com
- 29.Congressional Research Service : Indigenous Peoples in Latin America: Statistical Information. Bethesda, MD, 2020. https://fas.org/sgp/crs/row/R46225.pdf [Google Scholar]
- 30.Cruz-Saco MA: Indigenous Communities and Social Inclusion in Latin America—United Nations. Connecticut, 2018. https://www.un.org/development/desa/family/wp-content/uploads/sites/23/2018/05/2-1.pdf [Google Scholar]
- 31.Organización Panamericana de la Salud : Suministro de sangre para transfusiones en los países de América Latina y el Caribe 2016-2017. 2020. https://iris.paho.org/handle/10665.2/52150
- 32.Ishak R, De Oliveira Guimarães Ishak M, Vallinoto ACR: The challenge of describing the epidemiology of HTLV in the Amazon region of Brazil. Retrovirology 17:4, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosadas C Puccioni‐Sohler M Oliveira ACP, et al. : Adult T‐cell leukaemia/lymphoma in Brazil: A rare disease or rarely diagnosed? Br J Haematol 188:e46-e49, 2020 [DOI] [PubMed] [Google Scholar]
- 34.Pereira FM de Almeida MdCC Santos FLN, et al. : Evidence of new endemic clusters of human T-cell leukemia virus (HTLV) infection in Bahia, Brazil. Front Microbiol 10:1002, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pombo De Oliveira MS Loureiro P Bittencourt A, et al. : Geographic diversity of adult T-cell leukemia/lymphoma in Brazil. Int J Cancer 83:291-298, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Beltran B Quiñones P Morales D, et al. : Different prognostic factors for survival in acute and lymphomatous adult T-cell leukemia/lymphoma. Leuk Res 35:334-339, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Shah UA Chung EY Giricz O, et al. : North American ATLL has a distinct mutational and transcriptional profile and responds to epigenetic therapies. Blood 132:1507-1518, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bazarbachi A Plumelle Y Carlos Ramos J, et al. : Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol 28:4177-4183, 2010 [DOI] [PubMed] [Google Scholar]