PURPOSE
Despite the successes achieved in chronic myeloid leukemia (CML) with tyrosine kinase inhibitor (TKI) therapy, resistance remains an obstacle. The most common mechanism of resistance is the acquisition of a point mutation in the BCR-ABL kinase domain. Few studies have reported African patients with CML in regard to such mutations. We here report the types of BCR-ABL mutations in Ethiopian imatinib-resistant patients with CML and their outcome.
PATIENTS AND METHODS
Patients with CML with a diagnosis of imatinib resistance who were tested for BCR-ABL mutation between 2014 and September 2019 were included.
RESULTS
A total of 962 cases of CML on imatinib therapy were reviewed and 164 cases of failure were found. Of these, only 31 cases (19%) had mutation analysis performed. Most cases (94%) were secondary failures. At the time of CML diagnosis, the median age was 33 years and the majority presented with features of advanced-phase disease. Of the 31 patients, 22 mutations were found (65%). The types of mutations detected were as follows: non–P-loop mutations 36% (11), P-loop mutations 13% (four), and alternatively spliced BCR-ABL variants 23% (seven). The splice variant frequently detected was BCR-ABL35INS (20%). Twenty-six of the 31 patients (84%) were switched to second-line TKIs, whereas in four patients (13%), imatinib dose escalation was done. Overall, the outcome revealed that 16 patients (52%) were alive with complete hematologic response, whereas 12 patients (39%) had died. All patients who expressed BCR-ABL135INS were treated with second-line TKIs, and two of them (33%) had died because of disease progression.
CONCLUSION
In Ethiopia, CML affects the young and point mutations were frequently detected in imatinib-resistant patients. BCR-ABL1 35INS was also prevalent and associated with disease progression.
INTRODUCTION
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm marked by the increased proliferation of a granulocytic cell line that retains the ability to differentiate. Greater than 95% of patients with CML have cytogenetic evidence of the Philadelphia (Ph) chromosome,1,2 a reciprocal translocation between the long arms of chromosome 22 at the BCR gene and chromosome 9 at the ABL gene (t[9;22]).3 As a result, a BCR-ABL fusion gene is created that generates a chimeric protein with constitutively active tyrosine kinase activity.1
CONTEXT
Key Objective
Imatinib has revolutionized the treatment of chronic myeloid leukemia (CML) in chronic phase. However, the emergence of resistance mutations within the Abl kinase domain remains an obstacle to cure in some patients. Findings from several studies in different populations indicate a wide variability in the frequency and type of resistance mutations. The aim of this study was to identify the resistance mutations among imatinib-resistant Ethiopian patients with CML.
Knowledge Generated
The analysis demonstrated younger age and more advanced disease at presentation compared with North America and European countries. The splice variant BCR-ABL135INS was frequently detected (20%) and appears associated with disease progression.
Relevance
These findings could suggest a different disease biology of CML in younger patients and BCR-ABL135INS may play a role in disease progression and affect outcomes.
Tyrosine kinase inhibitor (TKI) therapy has revolutionized the treatment of CML, giving patients with chronic-phase (CP) disease a near-normal age-adjusted lifespan.4 For patients with CP-CML, the first-generation TKI imatinib gives a complete cytogenetic remission (CCyR) in roughly 60%-70% of cases and a major molecular response (MMR) in 50%-60% of cases, with overall survivals (OSs) of > 90%. The second-generation TKIs (dasatinib and nilotinib) are significantly more potent than imatinib and thus give higher MMR rates (70%-80%). However, the OSs of patients with CP-CML treated with imatinib or the second-line agents are similar. In Ethiopia, imatinib has become the first-line treatment for CML since 2004 through a partnership program between Novartis and The Max Foundation to provide free imatinib for lower-income countries and patient outcomes have since improved immensely.5 Over the past 5 years, second- and third-generation TKIs became available as well by donation through The Max Foundation from different drug manufacturers for imatinib-resistant patients.
The estimated global incidence of CML is 0.6-2.0 cases per 100,000 individuals per year.6 Little is known about the incidence of CML in Africa, including in Ethiopia, with one report in 2017 estimating 0.4 cases per 100,000 populations per year.7 The difference in incidences might be because of geographic and/or ethnic variations.6 Unfortunately, most CML cases occur in areas of the world without access to the technology for the diagnosis and monitoring of CML. In 2018, among the estimated 503,000 prevalent cases of CML globally, 53% were estimated to be living in resource-limited settings.8 According to CML patient registry at Tikur Anbessa Specialized Hospital, the only center in Ethiopia treating CML, more than 1,800 patients with CML were diagnosed so far. Despite its relatively low incidence, CML will become a very prevalent oncologic diagnosis in the future because of transformed survival by TKI therapy. Thus, it should be expected that emerging resistance or progression is likely to increase.4
In the developed world, the majority of cases are diagnosed in CP, with a median age at diagnosis of approximately 65 years.4 In low-income countries, the diagnosis of CML is made more often in advanced-phase disease, with a median age at diagnosis of approximately 39 years.9 In Ethiopia, the median age is reported as 36 years, with more than one-third (37%) in the age group of adolescents and young adults (AYAs; 15- to 29-year-old), and the majority were high risk based on European Treatment and Outcome Study and Sokal prognostic scoring system (79% and 56%, respectively).10 Little is known about the reason for these differences in the natural history of the disease, whether it is because of the pyramid of ages of the African people or unknown environmental and/or genetic factors.11 Luckily, survival for CP-CML in industrialized countries is similar to that in low-income countries.12
Despite the success achieved with imatinib, development of resistance is an ongoing obstacle. The most commonly described mechanism associated with resistance is the point mutations in the BCR-ABL1 gene, although other rarer mechanisms occur (eg, BCR-ABL gene amplification and clonal evolution and activation of alternative splicing of BCR-ABL transcripts). Findings from several studies indicate that BCR-ABL point mutations are detected with a frequency ranging from 12% to 63% in imatinib-resistant patients with CML. Although more than 90 different mutations have been described, two third of mutated cases involve mutations of amino acids in T315, Y253, E255, M351, G250, F359, and H396.13-19 Emergence of new mutations during imatinib treatment predicts loss of CCyR, shorter progression-free survival, shorter time to BC progression, and shorter OS.16 Moreover, certain mutations may direct the pick of the TKI for salvage therapy.20,21
The types and frequencies of BCR-ABL mutations reported in different population studies have shown wide variability. However, there are very few data regarding such mutations in African patients and none reported from Ethiopia. The aim of this study was to describe the BCR-ABL mutations among Ethiopian patients with CML resistant to imatinib therapy and to report on their treatment outcomes.
PATIENTS AND METHODS
We conducted a retrospective descriptive analysis of data obtained from medical records of imatinib-resistant patients with CML at Tikur Anbessa Specialized Hospital in Addis Ababa, Ethiopia. All patients who were tested for mutation analysis in view of suboptimal response or loss of response to first-line imatinib treatment between January 2014 and September 2019 were included. Imatinib failure was defined as loss of CCyR, MMR, or complete hematologic remission (CHR; secondary resistance), or failure to achieve a CHR by 3 months (primary resistance).20,21 Mutation screening test by Sanger method was carried out at a private pathology laboratory in India on peripheral blood samples.22
We used SPSS version 20.0 for data entry and analysis. Descriptive statistics was applied to summarize sociodemographic, clinical, test-related and treatment-related findings. Measures of central tendency, measures of dispersion, frequency distributions, and tables were used to depict the major findings of the study.
RESULTS
Sociodemographic Characteristics
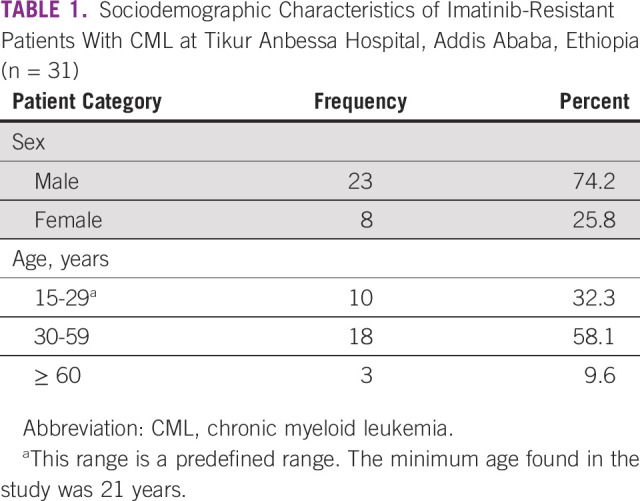
A total of 962 cases of CML on imatinib were reviewed and 164 cases of CML with imatinib failure were found. Of the 164 imatinib-resistant patients with CML, only 31 patients (19%) were identified to have tested for mutation. This is because mutation screening test is not available in Ethiopia and only very few patients can afford to have it done abroad. The median age at the time of CML diagnosis was 33 years with interquartile range (IQR) of 23 years, and the male-to-female ratio was found to be 2.9:1 (Table 1).
TABLE 1.
Sociodemographic Characteristics of Imatinib-Resistant Patients With CML at Tikur Anbessa Hospital, Addis Ababa, Ethiopia (n = 31)
Clinical Characteristics and Laboratory Abnormalities
Thirty of 31 patients had their CML diagnosis year documented, which ranged from 2007 to 2019. The median duration of illness before CML diagnosis was 5.0 months (IQR, 4.3 months). The majority (28 patients, 90.0%) presented in CP-CML, whereas the remaining (three patients, 9.7%) presented in accelerated phase (AP) CML.
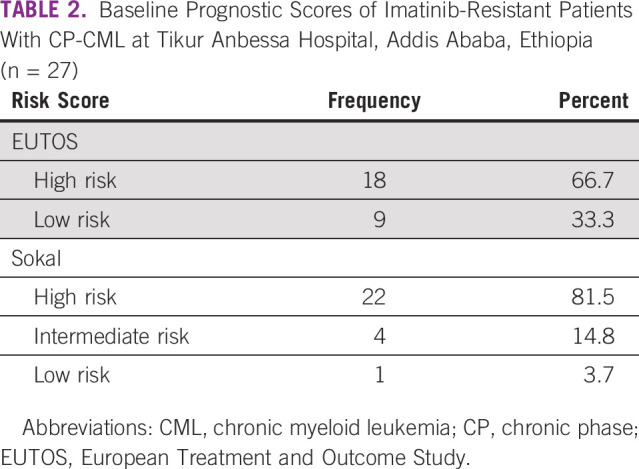
All patients had splenomegaly with mean spleen size of 15.3 cm below the left costal margin (overall range: 3-26 cm). Among the 28 patients with CP-CML, baseline prognostic scores were recorded for 27 patients, and it revealed that 22 patients (81.5%) had Sokal high-risk disease (Table 2). Comorbidity was identified in seven patients (23.3%): HIV infection in three, HBV infection in two, hyperthyroidism in one, and major depressive disorder in one.
TABLE 2.
Baseline Prognostic Scores of Imatinib-Resistant Patients With CP-CML at Tikur Anbessa Hospital, Addis Ababa, Ethiopia (n = 27)
Analysis of baseline hematologic profile was done for 30 patients. Leukocytosis and anemia were the commonly identified abnormalities. All the patients had leukocytosis with mean WBC count of 380,618/µL (range: 75,000-723,730/µL). Almost all patients (96.7%) had anemia (< 13 g/dL for men; < 12 g/dL for women). None of the patients had thrombocytopenia; however, seven patients (23.3%) were found to have thrombocytosis. The median basophil and blast percentage in the peripheral blood was found to be 6.5% (IQR, 8.0%) and 5.0% (IQR, 3%), respectively.
Imatinib Initiation and BCR-ABL Mutations
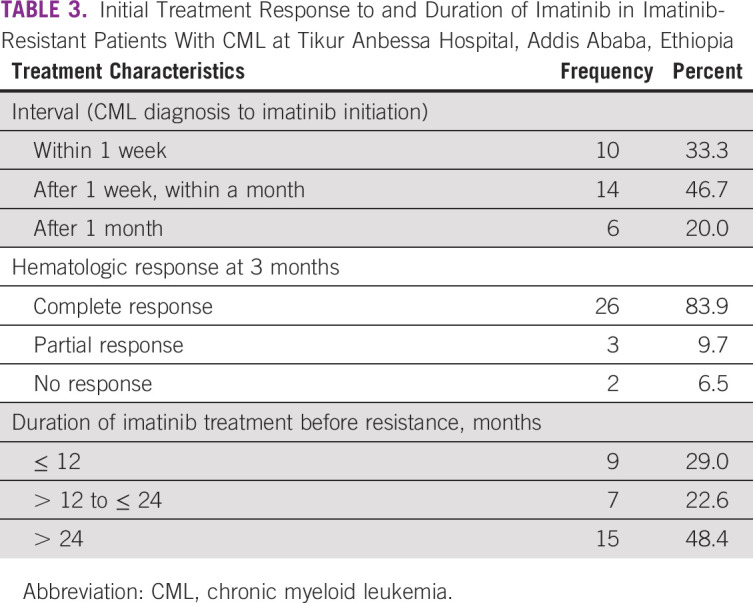
All the 31 patients were initially started on imatinib treatment, after a median time of 26 days from CML diagnosis date (IQR, 15 days). The dose of imatinib was 400 mg/day for CP patients and 600-800 mg/day for AP patients. Hematologic response was assessed 3 months after the initiation of imatinib, and 26 patients (84%) were found to have achieved CHR. BCR-ABL1 transcript level at 3 months was checked only for two patients, one with partial hematologic response and another with no hematologic response, and their levels were 24% international scale (IS) and 43% IS, respectively.
The median duration of imatinib treatment before resistance was found to be 24 months (IQR, 45 months; range, 3-106 months; Table 3).
TABLE 3.
Initial Treatment Response to and Duration of Imatinib in Imatinib-Resistant Patients With CML at Tikur Anbessa Hospital, Addis Ababa, Ethiopia
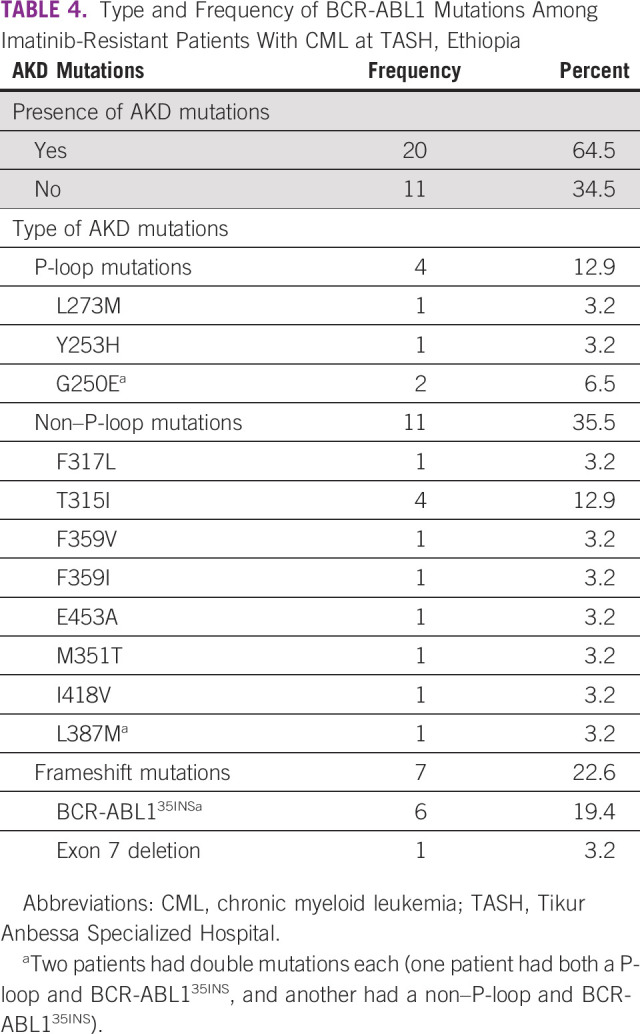
Of the 31 patients with imatinib resistance, two patients (6.5%) were primary failures and 29 patients (93.5%) were secondary failures. BCR-ABL mutations were found in 20 patients (64.5%).
The types of mutations detected in these 20 patients were non–P-loop mutations in 11 patients (35.5%), P-loop mutations in four patients (12.9%), and alternatively spliced BCR-ABL variants in seven patients (22.6%). Among the non–P-loop mutations, T315I was detected in four patients (12.9%) and F317L, F359V, F359I, E453A, M351T, I418V, and L387M were detected in one patient each (3.2% each). Among the P-loop mutations, G250E was detected in two patients (6.5%), whereas L273M and Y253H were detected in one patient each (3.2% each).
Regarding the types of the alternatively spliced BCR-ABL variants, six patients (19.4%) harbored intron-derived insertion or truncation mutation (p.C475fs*11; c.1423_1424ins35),14 whereas one patient (3.2%) had 185bp deletion (p.R362fs*20/c.1086_1270del185) involving exon 7 of the BCR-ABL kinase domain. Two of these patients had double mutations: one patient had both a P-loop (G250E) and BCR-ABL135INS, whereas the second patient had non–P-loop (L387M) and BCR-ABL135INS (Table 4).
TABLE 4.
Type and Frequency of BCR-ABL1 Mutations Among Imatinib-Resistant Patients With CML at TASH, Ethiopia
Response to Change in TKI Therapy and Overall Outcome
As seen in Table 5, a total of 26 patients (83.9%) were switched to second-line TKIs, whereas in four of the patients (12.9%) dose escalation of imatinib was done. One patient with T315I mutation was treated with salvage therapy because ponatinib was not available at the time.
TABLE 5.
Treatment Type and Overall Outcome Among Imatinib-Resistant Patients With CML at Tikur Anbessa Hospital, Addis Ababa, Ethiopia
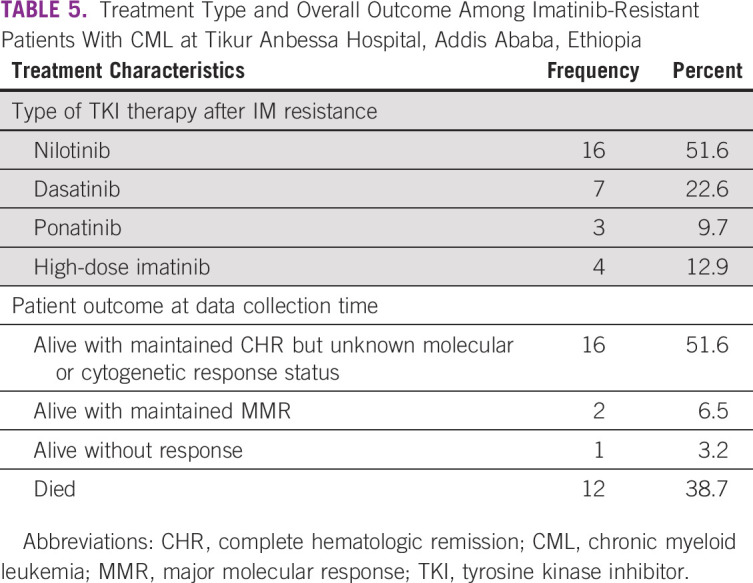
Assessment of response in CML to TKI therapy is by hematologic parameters and molecular test (BCR-ABL result). But due to financial reason, only very few patients can afford the molecular test. Overall, the results revealed that 16 patients (51.6%) were alive with maintained CHR response but unknown molecular or cytogenetic response, whereas 12 patients (38.7%) had died, and the cause of death in all patients was related to disease progression (Table 5).
With regard to outcomes on the basis of mutation type, 15 (75.0%) of the 20 patients with mutations were alive with CHR (but unknown molecular or cytogenetic response), whereas seven (63.6%) of the 11 patients without detectable mutations had died. Among patients with mutations, 75% of patients with P-loop mutations and T315I mutations were alive with CHR, whereas only four of the seven patients (57.1%) with splice variants were alive at the time of data collection.
Among the 12 patients (38.7%) who had died, mutation was not detected in seven patients (58.3%), whereas five patients (41.7%) had mutation. The types of mutations in these patients were P-loop mutation (one patient), T315I mutation (one patient), alternatively spliced BCR-ABL variants (two patients; BCR-ABL135INS and exon 7 deletion in each patient), and double mutation with non–P-loop and BCR-ABL135INS (one patient).
The three HIV-positive patients were on first-line HAART treatment with undetectable viral load, and all were alive with a median follow-up of 10.3 months. Mutations were detected in two of them (F359I and M351T), whereas one patient had no mutation. They were treated with dasatinib (n = 2) and nilotinib (n = 1).
Analysis of Patients With Alternatively Spliced BCR-ABL Variants
The median age of our patients with splice variants was 33 years (range, 24-54 years) with male-to-female ratio of 6:1. With regard to response to change in TKI therapy among these seven patients, four patients with BCR-ABL135INS were treated with nilotinib and two of them were alive with CHR but the other two had died because of disease progression. Two of the patients with BCR-ABL135INS were treated with dasatinib as second-line therapy and both were alive with maintained CHR. The patient with exon 7 deletion was treated with dose escalation of imatinib to 800 mg but died because of disease progression.
DISCUSSION
In this study, we have analyzed patients with CML who had suboptimal response or loss of response according to the European LeukemiaNet and National Comprehensive Cancer Network recommendations to first-line imatinib treatment over the past 5 years and had documented mutation analysis results.20,21 Hence, we found only 31 patients; of whom, two patients (6%) were primary resistance cases and 29 patients (94%) were secondary resistance cases. We found several features in our cohort that were different than in cases from developed countries, but also some similar features. Our patients were young (median age 33 years) and despite a short period of disease symptoms (median 5 months), more than 80% presented with high-risk Sokal score. A large percentage of cases (49%) had point mutations, and 23% had splice variants.
The median age is comparable with prior reports from Ethiopia,10 other African countries, and Asia of patients with CML,9 but is lower than reports from Western countries.4 The association of young age with more aggressive disease is counterintuitive but in keeping with analyses from developed countries that compare AYAs (they represent 8%-13% of the study patient population) with adults and elders with newly diagnosed CP-CML.23-25 In the German CML IV study group, AYAs had inferior response rates with early molecular response at 3 months but no differences in cytogenetic and molecular remissions or progression rates. In comparison, other studies suggest that AYAs have significantly lower cytogenetic and molecular responses and higher probability of transformation compared with older adults.23-25 These findings could suggest a different disease biology of CML in younger patients, with more aggressive disease characteristics, lower response rates, and relatively higher probability of early disease progression.11,23-25
Since only few of the patients with imatinib resistance had mutation analyses performed, there is a certain degree of uncertainty as to the estimate of resistance mutations. However, we found BCR-ABL point mutations in 15 patients (49%), which is similar to reports from Egypt (57%),26 China (58%),27 and Italy (43%)28 but showed higher frequency compared with the frequency reported in study populations from United States (36%),29 India (30%),30 Nigeria (25%),31 Australia (19%),32 and United Kingdom (12%).33 Similarly, the spectrum of types of point mutations was similar to other reports, as was the response to secondary TKIs used once resistance occurred. Indeed, only two of the 13 patients (15%) had died (one of them was a patient with T315I mutation who couldn't be initiated on ponatinib).
Alternatively spliced BCR-ABL variants were detected in seven patients (23%), two of them (29.5%) had additional point mutations. The BCR-ABL splice variant frequently detected from our cohort was BCR-ABL35INS (six patients, 19.4%), whereas one patient (3.2%) had exon 7 deletion of the BCR-ABL kinase domain. Among patients with the alternatively spliced variant, five were in the CP and two in the AP during initial presentation. Our finding of high frequency of BCR-ABL35INS among patients with imatinib resistance is consistent with a large screening studies of such mutations by Berman et al34 but quite higher than a report from Laudadio et al (1.7%).35 The median age of our patients with splice variant was 33 years (range, 24-54 years), which is consistent with case reports and clinical studies, which also reported a lower median age.34-38
BCR-ABL35INS is an alternatively spliced BCR-ABL mRNA with a 35-bp insertion derived from intron 8 and positioned between the junction of ABL exons 8 and 9, resulting in a frameshift leading to the addition of 10 residues and truncation of 653 residues because of early termination.14,39 This premature termination induces a conformational change of BCR-ABL protein, which hinders TKI to bind BCR-ABL, such as BCR-ABL1 T315I mutation39 and also enhances genomic instability.34 Alternative splicing in BCR-ABL, including BCR-ABL35INS and exon 7 deletion, has been implicated as the mechanism of TKI resistance,14,30,34,37-42 although the contribution of this mutation to TKI resistance is inconsistent.35,36,43,44
In our cohort, the splicing variants were associated with imatinib resistance and disease progression. Thus, of the seven patients with alternatively spliced BCR-ABL who had begun imatinib as first-line therapy, one patient (14%) had no response and six patients (86%) had a response but then progressed while on imatinib after a median duration of 27.5 months (range, 8-94). These patients were treated with nilotinib (n = 4), dasatinib (n = 2), and high-dose imatinib (n = 1). Despite change of therapy, three of the seven patients (43%), who were treated with nilotinib (n = 2) and high-dose imatinib (n = 1), had died because of disease progression. The two patients who were treated with dasatinib were alive with CHR. Dasatinib has been reported to overcome this resistance in previous case reports.37,38 These findings suggest that these mutations may play a role in disease progression and affect outcomes; therefore, a prompt change of treatment is essential.
Our study had several limitations. In Ethiopia, the structural and socioeconomic factors limit access to disease monitoring, making the measurement of short- and long-term response rates difficult. For example, > 80% of our patients with imatinib resistance were not tested for BCR-ABL mutation because of financial limitation; thus, these findings might not be representative of our entire CML population. Moreover, socioeconomic and cultural factors may play into the high male-to-female ratio of our cohort.
In conclusion, our study results show that almost half of imatinib-resistant Ethiopian patients with CML had point mutations. The splice variant BCR-ABL135INS was also a common finding in our patients and it was associated with disease progression and mortality. The use of dasatinib in BCR-ABL35INS allows for a better therapeutic response, although suggested by few patients. We suggest that large number of Ethiopian patients with CML with imatinib resistance need to be assessed to fully characterize features of BCR-ABL mutations, including its actual frequency, its contribution to TKI resistance, and identifying the alternative TKI.
Jerald Radich
Consulting or Advisory Role: Novartis, Bfs, Amgen, Takeda Science Foundation, Bio-Rad
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Fisihatsion Tadesse, Abdulaziz Abubeker, Amha Gebremedhin
Administrative support: Fisihatsion Tadesse, Getahun Asres
Provision of study materials or patients: Fisihatsion Tadesse, Abdulaziz Abubeker, Amha Gebremedhin
Collection and assembly of data: Fisihatsion Tadesse, Getahun Asres
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jerald Radich
Consulting or Advisory Role: Novartis, Bfs, Amgen, Takeda Science Foundation, Bio-Rad
No other potential conflicts of interest were reported.
REFERENCES
- 1.Rowley JD: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 243(5405):290-293, 1973 [DOI] [PubMed] [Google Scholar]
- 2.Torgerson SR, Haddad RY, Atallah E: Chronic myelogenous leukaemia for primary care physicians. Dis Mon 58:168-176, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Faderl S Talpaz M Estrov Z, et al. : The biology of chronic myeloid leukaemia. N Engl J Med 341:164-172, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Huang X, Cortes J, Kantarjian H: Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer 118:3123-3127, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novartis and The Max Foundation transform pioneering cancer access program for people in lower-income countries. https://www.novartis.com/news/media-releases/novartis-and-max-foundation-transform-pioneering-cancer-access-program-people
- 6.Rohrbacher M, Hasford J: Epidemiology of chronic myeloid leukaemia (CML). Best Pract Res Clin Haematol 22:295-302, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Tadwalkar S: The global incidence and prevalence of chronic myeloid leukemia over the next ten years (2017-2027). J Blood Disord Transfus 8:2155-2164, 2017 [Google Scholar]
- 8.Tadwalkar S, Hughes M: Global impact of tyrosine kinase inhibitors on chronic myeloid leukemia epidemiology over the next ten years. JCO Glob Oncol 4:107s, 2018. (suppl 2) [Google Scholar]
- 9.Mendizabal AM, Garcia-Gonzalez P, Levine PH: Regional variations in age at diagnosis and overall survival among patients with chronic myeloid leukemia from low and middle income countries. Cancer Epidemiol 37:247-254, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Mulu Fentie A Tadesse F Engidawork E, et al. : Prevalence and determinants of non-adherence to Imatinib in the first 3-months treatment among newly diagnosed Ethiopian’s with chronic myeloid leukemia. PLoS One 14:e0213557, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malhotra H, Radich J, Garcia-Gonzalez P: Meeting the needs of CML patients in resource-poor countries. Hematology Am Soc Hematol Educ Program 2019:433-442, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umeh CA Garcia-Gonzalez P Tremblay D, et al. : The survival of patients enrolled in a global direct-to-patient cancer medicine donation program: The Glivec International Patient Assistance Program (GIPAP). EClinicalMedicine 19:100257, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apperley JF: Part I: Mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol 8:1018-1029, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Ma W Kantarjian H Yeh C-H, et al. : BCR-ABL truncation due to premature translation termination as a mechanism of resistance to kinase inhibitors. Acta Haematol 121:27-31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soverini S Colarossi S Gnani A, et al. : Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia positive patients: By the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res 12:7374-7379, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Soverini S Branford S Nicolini FE, et al. : Implications of BCR-ABL1 kinase domain-mediated resistance in chronic myeloid leukemia. Leuk Res 38:10-20, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Soverini S Hochhaus A Nicolini FE, et al. : BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: Recommendations from an expert panel on behalf of European LeukemiaNet. Blood 118:1208-1215, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Weisberg E Manley PW Cowan-Jacob SW, et al. : Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia. Nat Rev Cancer 7:345-356, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Branford S, Melo JV, Hughes TP: Selecting optimal second-line tyrosine kinase inhibitor therapy for chronic myeloid leukemia patients after imatinib failure: Does the BCR-ABL mutation status really matter? Blood 114:5426-5435, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Hochhaus A Baccarani M Silver RT, et al. : European Leukemia Net 2020 recommendations for treating chronic myeloid leukemia. Leukemia 34:966-984, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radich JP Deininger M Abboud CN, et al. : Chronic myeloid leukemia, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 16:1108-1135, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Moore FR, Yang F, Press RD: Detection of BCR-ABL1 kinase domain mutations causing imatinib resistance in chronic myelogenous leukemia. Methods Mol Biol 999:25-39, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Kalmanti L Saussele S Lauseker M, et al. : Schweizerische Arbeitsgemeinschaft für Klinische Krebsforschung: Younger patients with chronic myeloid leukemia do well in spite of poor prognostic indicators: Results from the randomized CML study IV. Ann Hematol 93:71-80, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pemmaraju N Kantarjian H Shan J, et al. : Analysis of outcomes in adolescents and young adults with chronic myelogenous leukemia treated with upfront tyrosine kinase inhibitor therapy. Haematologica 97:1029-1035, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castagnetti F Gugliotta G Baccarani M, et al. : Differences among young adults, adults and elderly chronic myeloid leukemia patients. Ann Oncol 26:185-192, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Elnahass YH Mahmoud HK Ali FT, et al. : Abl kinase domain mutations in imatinib-treated Egyptian patients with chronic myeloid leukemia. J Leuk 1:106, 2013 [Google Scholar]
- 27.Qin Y Chen S Jiang B, et al. : Characteristics of BCR-ABL kinase domain point mutations in Chinese imatinib-resistant chronic myeloid leukemia patients. Ann Hematol 90:47-52, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Soverini S Colarossi S Gnani A, et al. : Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: By the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res 12:7374-7379, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Jabbour E Kantarjian H Jones D, et al. : Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia 20:1767-1773, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Patkar N Ghodke K Joshi S, et al. : Characteristics of BCR-ABL kinase domain mutations in chronic myeloid leukemia from India: Not just missense mutations but insertions and deletions are also associated with TKI resistance. Leuk Lymphoma 57:2653-2660, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Oyekunle A Bolarinwa R Owojuyigbe T, et al. : H396R, F359V and E255K mutations of the Abl kinase domain in imatinib-resistant Nigerian patients with chronic myeloid leukemia. Afr J Haematol Oncol 1:79-83, 2010 [Google Scholar]
- 32.Branford S Rudzki Z Walsh S, et al. : Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtu-ally always accompanied by clinical resistance, and mutations in the ATPphosphate-binding loop (P-loop) are associated with a poor prognosis. Blood 102:276-283, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Khorashad JS de Lavallade H Apperley JF, et al. : Finding of kinase domain mutations in patients with chronic phase chronicmyeloid leukemia responding to imatinib may identify those at high risk of disease progression. J Clin Oncol 26:4806-4813, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berman E Jhanwar S Hedvat C, et al. : Resistance to imatinib in patients with chronic myelogenous leukemia and the splice variant BCR-ABL1(35INS). Leuk Res 49:108-112, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laudadio J Deininger MWN Mauro M, et al. : An intron-derived insertion/truncation mutation in the BCR-ABL kinase domain in chronic myeloid leukemia patients undergoing kinase inhibitor therapy. J Mol Diagn 10:177-180, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishida T Miyazaki K Okina S, et al. : The clinical outcomes of chronic myeloid leukemia patients harboring alternatively spliced BCR-ABL variants. Hematology 24:49-51, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Park SH Chi HS Kwon MR, et al. : Cys475Tyrfs*11 in the BCR/ABL kinase domain in CML. Ann Lab Med 32:452-454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu SC, Tang JL, Li CC: Dasatinib in chronic myelogenous leukemia. N Engl J Med 355:1062-1063, 2006 [PubMed] [Google Scholar]
- 39.Lee T-S Ma W Zhang X, et al. : BCR-ABL alternative splicing as a common mechanism for imatinib resistance: Evidence for molecular dynamic simulations. Mol Cancer Ther 7:3834-3831, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Yuda J Miyamoto T Odawara J, et al. : Persistent detection of alternatively spliced BCR-ABL variant results in a failure to achieve deep molecular response. Cancer Sci 108:2204-2212, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherbenou DW Hantschel O Turaga L, et al. : Characterization of BCR-ABL deletion mutants from patients with chronic myeloid leukemia. Leukemia 22:1184-1190, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Mahadeo KM, Cole PD: Successful treatment using omacetaxine for a patient with CML and BCR-ABL1 35INS. Blood 115:3852, 2010 [DOI] [PubMed] [Google Scholar]
- 43.O’Hare T Zabriskie MS Eide C, et al. : The BCR-ABL35INS insertion/truncation mutant is kinase-inactive and does not contribute to resistance in chronic myeloid leukemia. Blood 118:5250-5254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaillard J-B Arnould C Bravo S, et al. : Exon 7 deletion in the bcr-abl gene is frequent in chronic myeloid leukemia patients and is not correlated with resistance against imatinib. Mol Cancer Ther 9:3083-3089, 2010 [DOI] [PubMed] [Google Scholar]


