PURPOSE
For unresectable gallbladder cancer (GBC), gemcitabine and platinum is standard combination; however, outcome is poor. We conducted this study to find feasibility of modified flourouracil, oxaliplatin, and irinotecan in this group.
MATERIALS AND METHODS
We conducted a prospective, phase II single-arm pilot study. Inclusion criteria were histologically proven GBC and Eastern Cooperative Oncology Group 0-1. Primary end points were overall response rates and overall survival. The following treatment was given: oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, and irinotecan 150 mg/m2, all once on day 1, fluorouracil 2,400 mg/m2 continuous intra-venous infusion over 46 hours repeated every 2 weeks, and maximum 12 doses, with primary granulocyte colony-stimulating factor prophylaxis.
RESULTS
Between February 2019 and July 2020, 29 patients with unresectable GBC were enrolled. The median age was 52 years, and 18 were females. The Eastern Cooperative Oncology Group was 0 in 4. Five had bilirubin > normal, and 15 each had high serum alkaline phosphatase and carbohydrate antigen 19-9. Twenty-five patients had stage IV disease, and remaining unresectable locally advanced disease. A median of 8.5 cycles was given, and 11 completed treatment. Nine stopped chemotherapy because of progression, and one because of toxicity, and treatment is ongoing in three. Twenty-two required dose reduction. A treatment delay of 1-2 weeks was seen in 25 patients. Best response was complete response 1, partial response 13 (overall response rate 48.2%), and stable disease 9. Four patients with metastatic disease underwent R0 resection. As on cutoff date, nine are surviving (three without disease). Eighteen died of PD, and in two, cause was unknown. There was no toxic death. The median overall survival and progression-free survival were 309 and 252 days, respectively. Twenty-three patients experienced grade III or IV toxicity, and common were diarrhea (13), vomiting (12), and anemia (7).
CONCLUSION
First-line modified flourouracil, oxaliplatin, and irinotecan is feasible in unresectable GBC with encouraging responses. Toxicities are higher but manageable. Higher response rates make this an option to explore in borderline resectable cases.
INTRODUCTION
Gallbladder cancer (GBC) is one of the common cancers among north and north-eastern Indian women (along Gangetic belt), and this is the commonest biliary tract cancer (BTC) in this part. In Delhi, GBC incidence is 11.8/100,000 population and this is the third commonest among females.1 In India, each year nearly 26,000 new GBCs are diagnosed.2 The only curative therapy is surgery, and only a small subset (about 10%-20% of patients) is operable. Even with established chemotherapy combinations, the median survival is between 9.0 and 11.5 months. Gemcitabine and platinum (oxaliplatin or cisplatin) is the common chemotherapy protocol used in unresectable or metastatic disease.3,4 In a direct comparison, equivalence of GemCis to that of mGemOx could not be established. mGemOx has shown an increased median survival of 9.0 months compared with 8.3 months in GemCis. This was not statistically significant, and moreover, study was not powered to address the issue of superiority.5
CONTEXT
Key Objective
We studied modified flourouracil, oxaliplatin, and irinotecan as first line in unresectable gallbladder cancer (GBC). This has not been studied or reported so far.
Knowledge Generated
Modified flourouracil, oxaliplatin, and irinotecan has higher response rates and predictable and manageable toxicities. This has potential to be a new therapeutic option for patients with nonresectable or metastatic GBC.
Relevance
This study suggests that new combination being used in advanced nonresectable GBC may provide higher response rates. Furthermore, this also generates new hypothesis: (1) whether this combination should be compared with standard gem-platinum compound and (2) whether this should be explored in borderline resectable cases as neoadjuvant therapy.
Looking at the poor prognosis, there is need for better drugs or drug combinations. Recently, three-drug combination of flourouracil, oxaliplatin, and irinotecan (FOLFIRINOX) has been used in ductal adenocarcinoma of pancreas. The combination, FOLFIRINOX, has shown improved survival in metastatic pancreatic adenocarcinoma compared with gemcitabine, and modified FOLFIRINOX (mFOLFIRINOX) has shown improved survival compared with gemcitabine in adjuvant setting.6,7 In metastatic pancreatic cancer, Conroy et al6 reported improvement in median overall survival (OS). Median OS and progression-free survival (PFS) were significantly higher in the FOLFIRINOX arm (11.1 and 6.4 months, hazard ratio = 0.57 [0.9-0.73], P < .0001) compared with gemcitabine arm, respectively (6.8 and 3.3 months). In the adjuvant study, the median OS reported was 54.4 months in mFOLFIRINOX group compared with 35 months in the gemcitabine group.7
To reduce the toxicity of FOLFIRINOX, various modifications were done such as using growth factors (granulocyte colony-stimulating factor) prophylaxis, omission of bolus fluorouracil (FU), and reducing the dose of irinotecan.7,8 The irinotecan dose used in the mFOLFIRINOX protocol by Conroy et al7 was 150 mg/m2. In mFOLFIRINOX protocol developed by Hemchandra et al,8 only bolus FU was omitted and there was no change in dose of irinotecan.
Study Rationale and Hypothesis
Although biology of GBC and pancreatic cancer is different, some embryologic similarities do exist between gallbladder and pancreatic cancer, and many pancreatic cancer protocols have been tried in GBC. Prognosis of unresectable GBC is poor with the median survival being < 10 months.3,5 mFOLFIRINOX has shown superior survival compared with gemcitabine in resected pancreatic adenocarcinoma (adjuvant setting).7 FOLFIRINOX has been used as a second-line therapy in BTC.9 This phase II pilot study is to test safety and efficacy of mFOLFIRINOX in unresectable or metastatic GBC.
MATERIALS AND METHODS
Study Design
This was a prospective, phase II single-arm study conducted at Department of Medical Oncology, All India Institute of Medical Sciences, New Delhi.
Study protocol was approved by institute ethics committee. Study was conducted in accordance with Declaration of Helsinki and good Clinical Practice Guidelines and registered at Clinical Trial Registry of India (CTRI/2019/02/017562 registered on August 2, 2019). All eligible patients were enrolled after obtaining written informed consent.
Study Subjects
Inclusion criteria are (1) histologically proven cases of adenocarcinoma of gallbladder, which are locally advanced unresectable or metastatic; (2) subjects having measurable disease; (3) Eastern Cooperative Oncology Group performance status ≤ 1; (4) age > 18 years; (5) adequate bone marrow functions reflected as Hb > 10 g/dL, total leucocytes count > 4,000/mm3 (absolute neutrophil count > 1,500/mm3), and platelets > 1,00,000/mm3; (6) serum creatinine < 1.8 mg%; (7) serum bilirubin ≤ 3 mg%; and (8) hepatic transaminases (serum glutamic-oxaloacetic transaminase [SGOT] and serum glutamic pyruvic transaminase [SGPT]) within three times of upper normal limit (up to five times in the case of diffuse liver involvement). Patients were allowed for recruitment if their recurrence was after a gap of 6 months since last adjuvant chemotherapy or radiotherapy. (9) Prior radical surgery was allowed if recurrence was unresectable.
Exclusion criteria included (1) women of reproductive age group not practicing contraception; (2) lactating and pregnant women; (3) patients who are on other investigational drugs; and (4) patients with uncontrolled concurrent diabetes mellitus, hypertension, or any other cardiac disorder.
Those who presented with hyperbilirubinemia were managed with biliary diversions and were enrolled if serum bilirubin returned to ≤ 3 mg/dL.
Intervention
mFOLFIRINOX regimen comprised oxaliplatin 85 mg/m2 intravenous (IV) infusion over 2 hours, leucovorin 400 mg/m2 IV infusion in over 90 minutes concurrently with irinotecan 150 mg/m2 IV infusion over 90 minutes on day 1, and FU 2,400 mg/m2 continuous IV infusion over 46 hours. Cycles were repeated every 2 weeks until disease progression, intolerable toxicity, or 6 months, whichever was earlier. All patients received primary colony stimulating growth factor prophylaxis on days 4, 6, and 8 with each cycle.
Assessments
After detailed history and physical examination, following investigations were performed: complete blood counts, renal functions, liver functions, and tumor markers (carcinoembryonic antigen and carbohydrate antigen 19-9); ECG; chest x-ray; and contrast-enhanced computed tomography of chest, abdomen, and pelvis (in some cases, additionally, FDG-18 positron emission tomography-computed tomography scan was also performed).
Any patient who received at least one dose of chemotherapy was evaluated for toxicity, safety, and survival. Clinical response was assessed before every cycle, and radiologic response was assessed after every four cycles. Response was assessed using RECIST 1.1 criteria.
Toxicity was assessed by Common Terminology Criteria for Adverse Events version 4.0. Patients were allowed to go for curative resection postchemotherapy in the case of good response.
Outcomes
There were two co-primary end points: overall response rates (ORRs) (sum of complete response [CR] and partial response [PR]) and OS, defined as time from date of enrollment to date of death or last seen alive. Secondary end points were toxicity and complete resection rates.
Study period
Study accrual period was between February 2019 and July 2020. Data analysis cutoff date was November 30, 2020.
Sample Size Calculation
This was a phase II feasibility pilot study. A formal sample size was not calculated. Initially, we planned for accrual of 20 patients, which was later amended to include up to 30 patients.
Statistical Analysis
All statistical computations were performed using IBM-SPSS 16.0 software. Descriptive analysis summarized the baseline characteristics. OS and PFS were analyzed using Kaplan-Meier method. OS was calculated from date of enrollment to date of death or last seen alive. Median survival was calculated and reported with 95% CI. It was an intent-to-treat analysis.
RESULTS
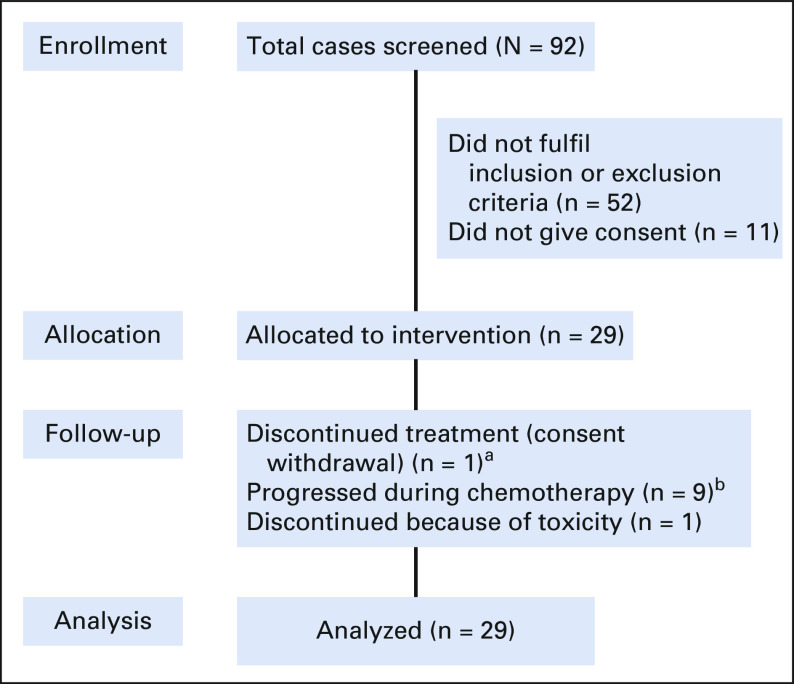
Between February 2019 and July 2020, a total of 92 patients of unresectable GBC were screened. Twenty-nine were enrolled into this study (Fig 1). All patients were seen, evaluated in a multidisciplinary tumor clinic, and deemed unresectable. Baseline parameters are shown in Table 1. The median age was 52 years (range, 31-65 years). Of 29 patients, 18 were females. Pain was the commonest symptom, and all patients reported it, which was followed by weight loss and vomiting seen in 7 (24%) and 6 (21%) patients, respectively. Four patients had type II diabetes, four had hypertension, and seven had hypothyroidism as associated co-morbidity. Two had family history of unknown GI cancer in first-degree relative. Twenty-five patients had an Eastern Cooperative Oncology Group performance status score of I. Baseline blood parameters are shown in Table 1. Positron emission tomography-computed tomography scan was available in 20 patients at baseline. Twenty-five patients had clinical stage IV, and four had unresectable locally advanced unresectable stage at presentations. A median of 8.5 cycles was given (range, 1-12), and 11 completed 12 cycles. Chemotherapy was discontinued in nine patients because of disease progression (this includes four patients who initially had PR or stable disease and progressed later on) and in one because of toxicity, and another withdrew consent after five cycles and did not continue chemotherapy after that. Three patients are still receiving chemotherapy at last follow-up. Three patients were lost to follow-up, and one had early death. Reason for discontinuation of therapy is shown in Table 2. Varying degrees of dose reduction were required in 22 patients (25% dose reduction in 19). A dose delay of 1-2 weeks was seen in 25 patients. ORRs observed were CR in 1, PR in 13, stable disease in 9, and PD in 5, and in one patient, response could not be evaluated. Four of these patients who initially had unresectable metastatic disease showed good radiologic response (CR or PR) and were deemed fit to undergo surgery. All these four had R0 resection after six cycles (three completed remaining cycles after surgery). The results are shown in Table 3. At the data cutoff date, nine patients are alive; among them, three are disease-free.
FIG 1.
Study participation and follow-up (patient disposition diagram). aConsent withdrawal after five cycles. bThis includes patients who initially had documented stable disease or partial response and then progressed.
TABLE 1.
Baseline Characteristics
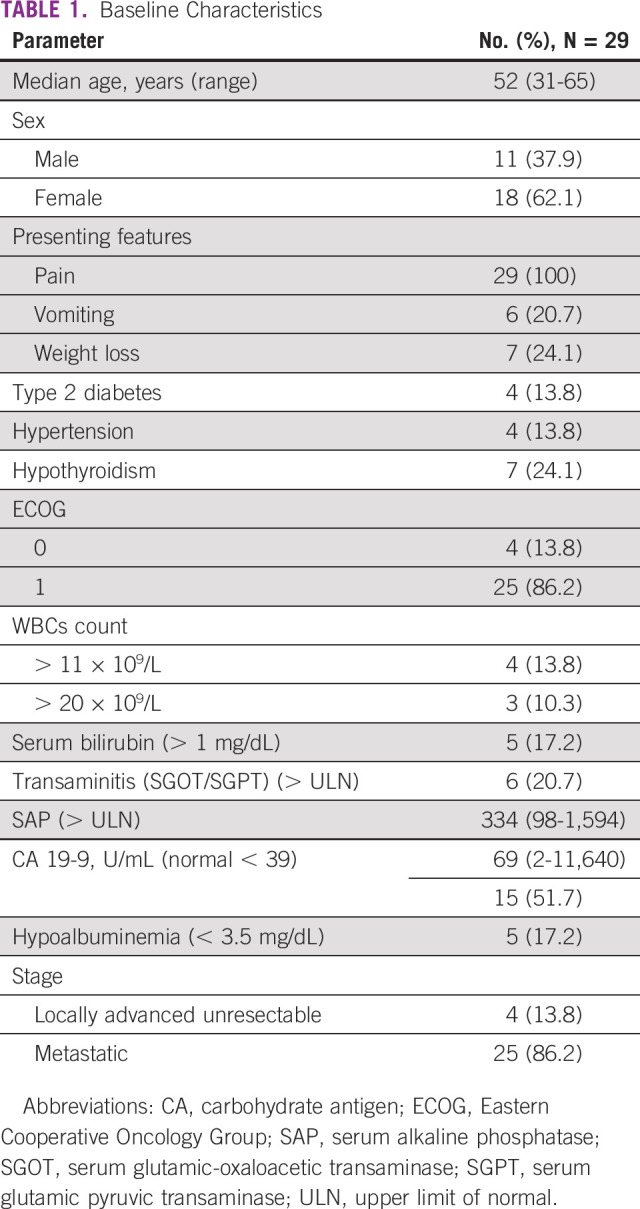
TABLE 2.
Reasons for Discontinuation of Chemotherapy
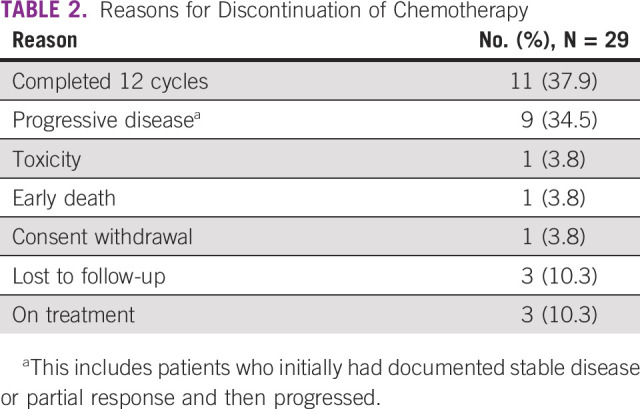
TABLE 3.
Results
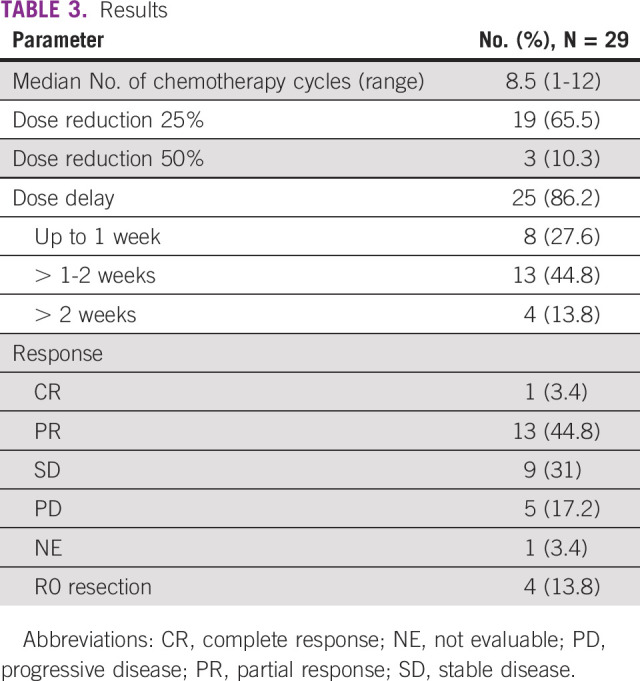
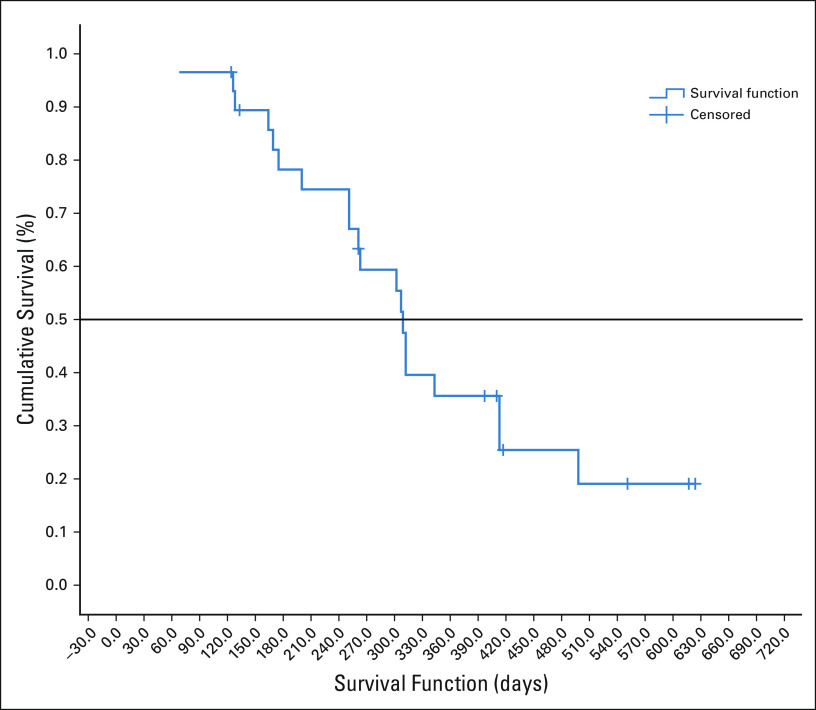
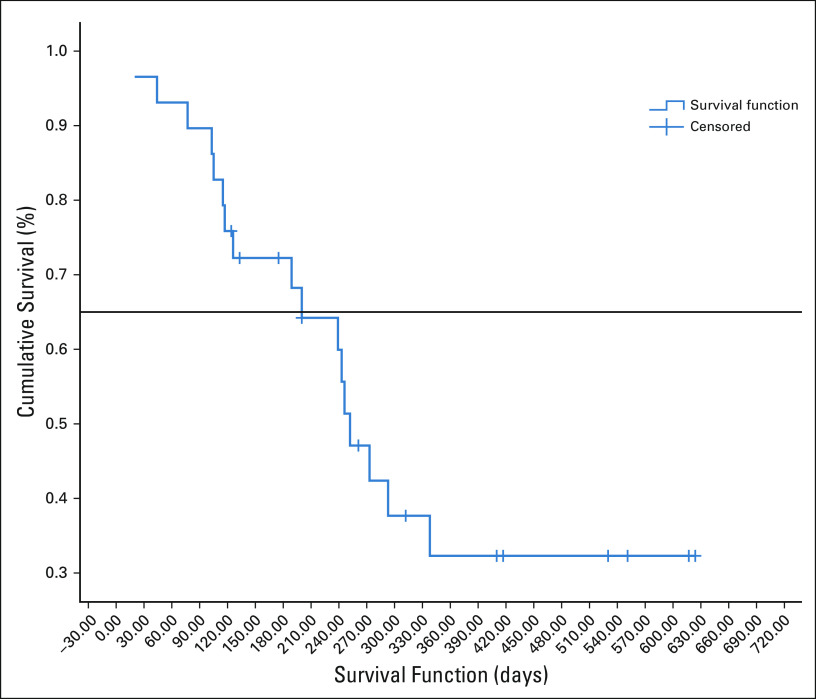
The median follow-up of surviving patients is 410 days. The median OS was 309 days ([10.3 months]; 95% CI, 296.92 to 321.07) as shown in Figure 2. The median OS of patients who had either CR or PR was 413 days ([13.76 months]; 95% CI, 266.59 to 559.40). The median PFS was 252 days ([8.4 months]; 95% CI, 207.96 to 296.03), which is shown in Figure 3. Eighteen died because of progressive disease, and two patients died at home and in them, cause of death could not be established.
FIG 2.
Overall survival in days.
FIG 3.
Progression-free survival in days.
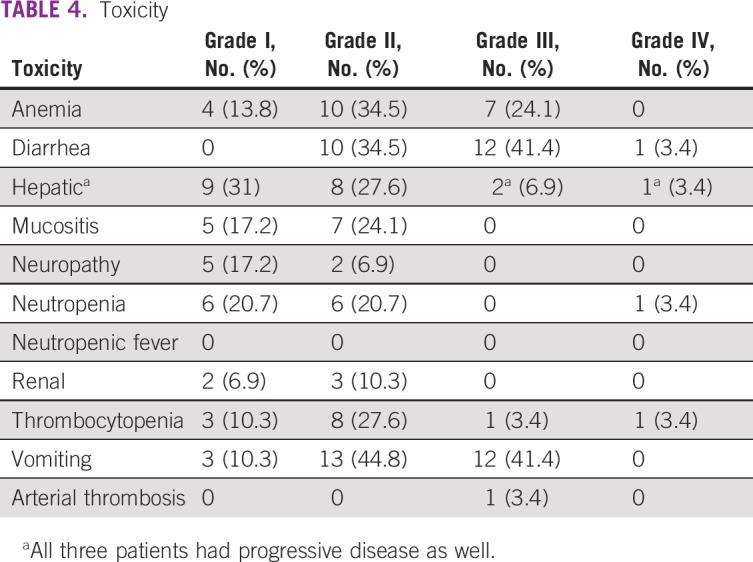
Adverse Events
Twenty-three (79.3%) of 29 patients had one or another grade III or IV toxicity; however, there was no toxic death. This includes three patients who had anemia only, and one patient with progressive disease had grade IV liver toxicity. Common grade III and IV toxicities observed were diarrhea in 13 patients (45%), vomiting in 12 (41%), anemia in 7 (24%), and hepatic in 3 (all three had progressive disease at the same time). One patient developed arterial thrombosis. No patient developed febrile neutropenia. Toxicity data are shown in Table 4.
TABLE 4.
Toxicity
DISCUSSION
Three-drug combination of FOLFIRINOX and its modification (mFOLFIRINOX), where bolus FU was done away with and irinotecan dose was reduced, have shown to improve response rates and survival in pancreatic adenocarcinoma.6-8 There are limited publications using FOLFIRINOX or mFOLFIRINOX in gall bladder cancer or BTC. There is an ongoing phase II/III study for unresectable or metastatic BTC, the results of which are yet unknown.10
To our knowledge, the study reported here is probably the first prospective study using mFOLFIRINOX in unresectable GBC as a first-line therapy. An ORR of 48.3% and a total disease control rate of 79% are much higher than previous reports, where the ORR was about 25%-26%.3-5 Achieving a response is important because those who showed objective response had a median OS of 413 days (13.76 months). As expected, side effects were common in this protocol. Varying degrees of dose reductions (76%) and 79% of patients experiencing one or another grade III or IV toxicity are of concern and warrant adequate precautions. Even after understanding that four of these patients had either anemia or liver toxicity only (in the background of progressive disease), the incidence of cumulative grade III or IV toxicity was 65%. A significant number of grade III and IV toxicities have been reported by other investigators as well. Even in ABC-02 study using GemCis protocol, total incidence of grade III or IV toxicities was reported in about 70% of patients.4 Using mFOLFIRINOX protocol, Conroy et al7 reported incidence of grade III and IV toxicity to be around 75%. Recently reported study by Belkouz et al9 has reported somewhat similar toxicity. In another recent report using FOLFIRINOX as second-line therapy in 30 patients, overall grade III or IV neutropenia was seen in 50%. In the same report, dose reductions of 80%-85% were also reported. The ORR was just 10%.9 In a report from China, 15 patients with BTC were given mFOLFIRINOX and a third of patients had grade III or IV toxicity.11 Both these studies have included all subtypes of patients with BTC unlike only GBC in the current study.
Possible reasons for increased toxicity could be because of deranged liver functions or lower albumin. With the use of primary granulocyte colony-stimulating factor prophylaxis, there were no neutropenic fever and no toxic death. This is reassuring. Although the median OS is only 10.3 months (309 days), this is higher than our previous two studies.4,5 The higher response rates and the fact that four of the patients with metastatic liver disease could undergo R0 resection give suggestion that this combination may be explored as neoadjuvant therapy in borderline resectable GBC as well.
In conclusion, this study suggests that mFOLFIRINOX is safe and feasible in unresectable GBC. This has potential of improving response rates and possibly survival. Furthermore, phase III studies are needed to compare this with gemcitabine and platinum and also to explore its utility as neoadjuvant therapy in borderline resectable cases.
ACKNOWLEDGMENT
We thank All India Institute of Medical Sciences, New Delhi, India, for providing drugs for study.
SUPPORT
This was an investigator-initiated trial. This research did not receive any specific grant from funding agency in public, commercial, or not-for-profit organization. However, drugs were provided by the All India Institute of Medical Sciences, New Delhi, India.
CLINICAL TRIAL INFORMATION
CTRI/2019/02/017562 (registered on: August 2, 2019). http://ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=29670&EncHid=83508.86155&modid=1&compid=19%27,%2729670det%27.
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/GO.20.00657.
AUTHOR CONTRIBUTIONS
Conception and design: Atul Sharma, Sunil Kumar, Sandeep Bhoriwal, Sujoy Pal, Rakesh Kumar
Provision of study materials or patients: Sunil Kumar, Sandeep Bhoriwal, Sujoy Pal, Priyanshu Choudhary
Collection and assembly of data: Atul Sharma, Raja Pramanik, Akash Kumar, Sunil Kumar, Sandeep Bhoriwal, Sanjay Thulkar, Nihar Ranjan Dash, Sujoy Pal, Priyanshu Choudhary, Satyajit Pawar, Rakesh Kumar, Gaurav Gupta
Data analysis and interpretation: Atul Sharma, Sushmita Pathy, Sandeep Bhoriwal, Sujoy Pal, Rakesh Kumar, Gaurav Gupta
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Indian Council of Medical Research : National Cancer Registry Program and Population Based Cancer Registry report 2012-2016. https://main.icmr.nic.in/sites/default/files/reports/NCRP_2020_2012_16.pdf
- 2.International Agency on Cancer Research : Globocan 2018, Indian data. https://gco.iarc.fr/today/data/factsheets/populations/356-india-fact-sheets.pdf
- 3.Sharma A Dwary AD Mohanti BK, et al. : Best supportive care compared with chemotherapy for unresectable gallbladder cancer: A randomized controlled study. J Clin Oncol 28:981-986, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Valle JW Wasan H Palmer DH, et al. : Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273-1281, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Sharma A Mohanti BK Chaudhary SP, et al. : Modified gemcitabine and oxaliplatin (mGemOx) or gemcitabine + cisplatin (GemCis) in unresectable gallbladder cancer (GBC): Results of a phase III randomized controlled trial. Eur J Cancer 123:162-170, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Conroy T Desseigne F Ychou M, et al. : FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817-1825, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Conroy T Hammel P Hebbar M, et al. : FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 379:2395-2406, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Hemchandra M Brutcher E Kauh J, et al. : Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas 42:1311-1315, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Belkouz A de Vos-Geelen J Mathôt RAA, et al. : Efficacy and safety of FOLFIRINOX as salvage treatment in advanced biliary tract cancer: An open-label, single arm, phase 2 trial. Br J Cancer 122:634-639, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phelip JM Edeline J Blanc JF, et al. : Modified FOLFIRINOX versus CisGem first-line chemotherapy for locally advanced non resectable or metastatic biliary tract cancer (AMEBICA)-PRODIGE 38: Study protocol for a randomized controlled multicenter phase II/III study. Dig Liver Dis 51:318-320, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Ye LF Ren C Bai L, et al. : Efficacy and safety of modified FOLFIRINOX as salvage therapy for patients with refractory advanced biliary tract cancer: A retrospective study. Investig New Drugs 39:836-845, 2021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/GO.20.00657.