PURPOSE
There are sparse data on the outcome of patients with locally advanced breast cancer (LABC). This report is on the prognostic factors and long-term outcome from Cancer Institute, Chennai.
METHODS
This is an analysis of untreated patients with LABC (stages IIIA-C) who were treated from January 2006 to December 2013.
RESULTS
Of the 4,577 patients with breast cancer who were treated, 2,137 patients (47%) with LABC were included for analysis. The median follow-up was 75 months (range, 1-170 months), and 2.3% (n = 49) were lost to follow-up at 5 years. The initial treatment was neoadjuvant concurrent chemoradiation (NACR) (77%), neoadjuvant chemotherapy (15%), or others (8%). Patients with triple-negative breast cancer had a pathologic complete response (PCR) of 41%. The 10-year overall survival was for stage IIIA (65.1%), stage IIIB (41.2%), and stage IIIC (26.7%). Recurrence of cancer was observed in 27% of patients (local 13% and distant 87%). Multivariate analysis showed that patients with a tumor size > 10 cm (hazard ratio [HR], 2.19; 95% CI, 1.62 to 2.98; P = .001), hormone receptor negativity (HR, 1.45; 95% CI, 1.22 to 1.72; P = .001), treatment modality (neoadjuvant chemotherapy, HR, 0.56; 95% CI, 0.43 to 0.73; P = .001), lack of PCR (HR, 2.36; 95% CI, 1.85 to 3.02; P = .001), and the presence of lymphovascular invasion (HR, 1.97; 95% CI, 1.60 to 2.44; P = .001) had decreased overall survival.
CONCLUSION
NACR was feasible in inoperable LABC and gave satisfactory long-term survival. PCR was significantly higher in patients with triple-negative breast cancer. The tumor size > 10 cm was significantly associated with inferior survival. However, this report acknowledges the limitations inherent in experience of management of LABC from a single center.
INTRODUCTION
Breast cancer is the most common cancer in Indian women with an age-adjusted incidence rate of 25.8 per 1,00,000 women.1 The incidence has increased by 40% between 1990 and 2016.2 The cause for the increase is multifactorial and is due to cancer awareness, delayed marriage, late age at first childbirth, lower parity, dietary factors, higher socioeconomic status, increasing life expectancy, and improved health care facilities.3 Women in developing countries often present at an advanced stage where initial surgery is not feasible. The reason for this delayed presentation is due to the stigma associated with a diagnosis of cancer, belief in the inevitability of death, guilt, and fear of transmission.4 There are few randomized controlled trials in locally advanced breast cancer (LABC), which is inoperable at presentation. The standard treatment for LABC has been preoperative chemotherapy followed by reassessment for surgery. This was initially developed in the West to allow surgeons to perform breast-conserving surgery. However, the extent and size of tumors do not allow this to be performed in India as a routine. Therefore, an approach using concurrent chemoradiation initially followed by surgery was developed at Cancer Institute, Chennai, to treat inoperable LABC. A previous report showed that patients with LABC (n = 1,117) who were treated with neoadjuvant concurrent chemoradiation (NACR) had downstaging of tumor (45%) and nodal (57.5%). Furthermore, patients who achieved pathologic complete response (PCR) had better survival, and the 15-year disease-free survival (DFS) for the entire cohort was 58%.5 Several phase II trials have shown that NACR with drugs such as capecitabine,6 paclitaxel,7 paclitaxel and vinorelbine,8 fluorouracil and vinorelbine9 are feasible and effective. Combination chemotherapy (fluorouracil, mitoxantrone, and cyclophosphamide) has also been combined with radiation in phase III trials in the adjuvant setting.10,11 In contrast to head and neck cancer and rectal cancer, NACR has not been developed as a standard of treatment in LABC. This is in part due to concerns in toxicity and cosmesis following NACR. Very few centers globally have evaluated this approach. We report on the analysis of a large cohort of patients with LABC treated at a single center evaluating prognostic factors and outcomes.
CONTEXT
Key Objective
To assess the real-world outcome and prognostic factors of patients with locally advanced breast cancer.
Knowledge Generated
Patients with triple-negative breast cancer had high pathologic complete response with neoadjuvant concurrent chemoradiation (NACR). Patients with a tumor size > 10 cm had inferior survival and warrant consideration for upstaging to T4. Patients with hormone receptor negativity, lack of pathologic complete response, and the presence of lymphovascular invasion and those treated with NACR had decreased survival.
Relevance
Neoadjuvant concurrent chemoradiation is a treatment option in inoperable locally advanced breast cancer in the context of a clinical trial.
METHODS
Patients
This is an analysis of previously untreated patients with LABC who presented to a Cancer Institute (WIA), Chennai, from January 2006 to December 2013. These patients were enrolled in the breast cancer cohort of the Indian Council of Medical Research patterns of care and survival study for breast, cervix, and head and neck cancers.12,13 The study was conducted according to ICH-GCP guidelines. The study was approved by the Institutional Ethics Committee of Cancer Institute (WIA), Chennai. Patient information including clinical, demographic, histopathology, treatment, and follow-up details were collected from the individual case records. The inclusion criteria were patients with untreated invasive breast cancer with stages IIIA-C. The exclusion criteria were patients with bilateral breast cancer, synchronous double primary, and sarcoma or lymphoma of the breast. The staging investigations were chest x-ray, ultrasound of abdomen or pelvis, bone scan, and a contralateral breast mammogram. In patients with LABC at diagnosis, those staged as cT3N1 alone were considered operable. Trucut biopsy was performed before treatment, and immunohistochemistry (IHC) for expression of estrogen and progesterone receptors was performed routinely. Grading of tumors was performed according to the Nottingham grading system.14 IHC for expression of human epidermal growth factor receptor 2 (HER2) was performed routinely from 2007. All tumors whose score on IHC for HER2 was 3+ were taken as positive.15 Fluorescence in situ hybridization was not performed routinely when the score for HER2 on IHC was 2+. Molecular subtyping on the basis of IHC into luminal A and luminal B was not possible as Ki 67 was not performed.
Treatment
All patients underwent treatment after discussion in a multidisciplinary breast tumor board. The treatment approach was predominantly NACR or neoadjuvant chemotherapy (NAC) on the basis of physician judgment. The choice was largely determined by the size of the tumor, ability to achieve primary skin closure, axillary nodal status, the patient’s ability to tolerate combined chemoradiation, and whether surgery was feasible. In some patients, neoadjuvant hormonal therapy was given because of poor performance status. The majority of patients received six cycles of combination chemotherapy with either cyclophosphamide methotrexate fluorouracil or anthracycline-containing regimens. Radiation was delivered either preoperatively in combination with chemotherapy or postoperatively. The neoadjuvant radiation schedule was an external beam radiation of 40 Gy given at 200 cGy per fraction per day for 5 days a week. In selected patients, who had positive margins after surgery following NACR, further radiation was administered. The adjuvant radiation schedule was 46 Gy given at 200 cGy per fraction per day for 5 days a week. Preoperative radiation was given to the involved breast, axilla, supraclavicular lymph nodes with or without internal mammary region. Adjuvant radiation was given to the involved breast or chest wall, supraclavicular nodes, with or without internal mammary region. The response was assessed clinically by physical examination before starting chemotherapy and after every two cycles. Complete response (CR) was no palpable tumor, partial response (PR) was decrease in tumor size, stable disease (SD) was no response, and progressive disease (PD) was an increase in tumor size. The surgery was either modified radical mastectomy or breast conservation surgery. PCR was defined as no invasive residual disease in the breast and axillary nodes with or without ductal carcinoma in situ. Premenopausal women received 5 years of adjuvant tamoxifen, and postmenopausal women received 5 years of adjuvant letrozole. Patients who remained premenopausal after chemotherapy received ovarian suppression (oophorectomy or radiocastration). During the period under study, patients whose tumors were HER2-positive did not receive trastuzumab routinely. This was due to inability to afford the innovator drug trastuzumab (Roche, Basel, Switzerland) and biosimilars were unavailable.
Statistical Analysis
DFS was calculated from the date of diagnosis to date of recurrence or death. Overall survival (OS) was calculated from the date of diagnosis to the date of death because of any cause. Descriptive statistics were used to analyze the baseline characteristics. Survival was estimated using the Kaplan-Meier method.10 Univariate and multivariate analyses were performed by Cox proportional hazards model.9 Statistical analyses were performed using STATA version 11, and a two-sided P value < .05 was considered significant.
RESULTS
Patients
A total of 4,577 patients with newly diagnosed breast cancer were treated in our hospital from January 2006 to December 2013. The clinical stage at presentation was stages I (n = 90; 1.97%), II (n = 1,796, 39.24%), III (n = 2,137, 46.69%), IV (n = 535, 11.69%), and unknown (n = 19, 0.42%). Only patients with LABC (n = 2,137, 46.69%) have been included in this analysis. The median follow-up of patients is 75 months (range, 1-170 months), and 2.3% (n = 49) were lost to follow-up at 5 years. However, the data from the patients who were lost to follow-up were included for analysis of outcome. The data of all patients were frozen on December 31, 2018, for analysis. The median age at diagnosis was 50 years, and 99% were women. A co-morbid illness was present in 41%, with the most common being hypertension (23%) followed by diabetes mellitus (21%). The majority had inoperable LABC at presentation (70%) with a median tumor size of 6.5 cm (range, 2-25 cm). The most common histology was infiltrating ductal carcinoma (94%), and grade 3 was the differentiation in the majority (71%). In the biopsy from the primary tumor, expression of estrogen receptor, progesterone receptor, and HER2 (3+) was 55%, 45%, and 25%, respectively. Twelve percentage of tumors did not express any of the above receptors (triple-negative) (Table 1).
TABLE 1.
Clinical Characteristics and Tumor Profile (N = 2,137)

Treatment
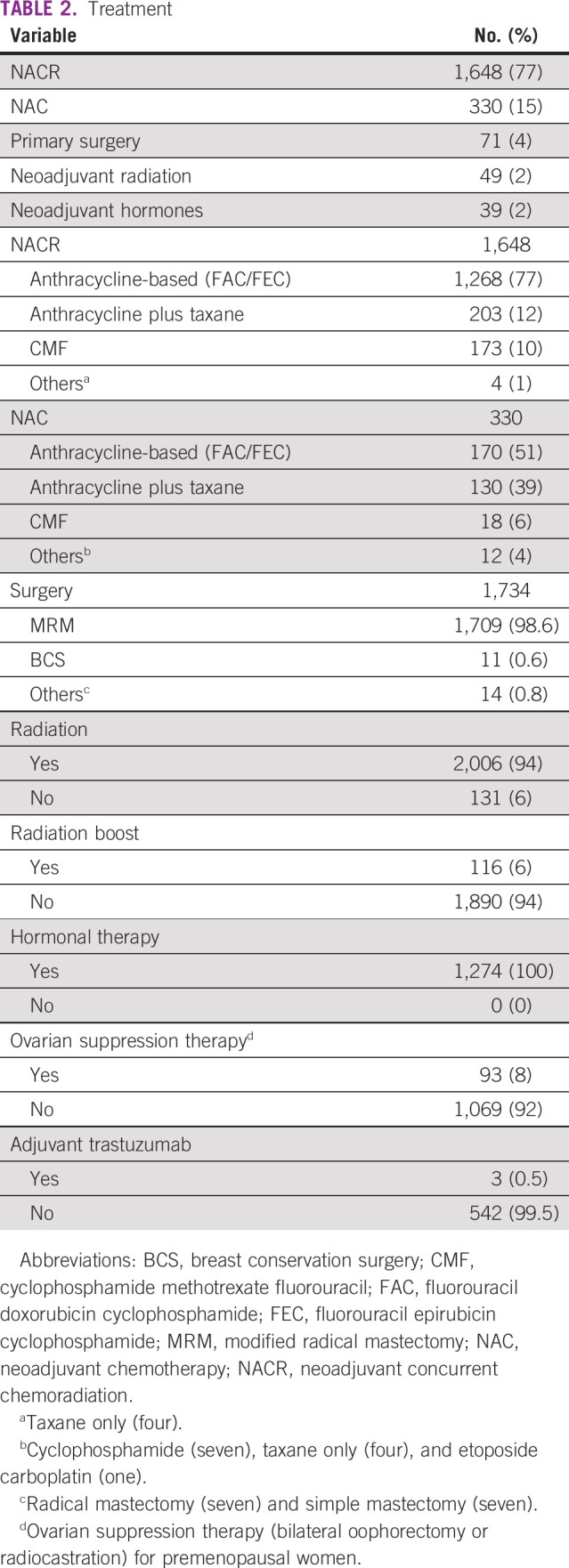
Seventy-one (4%) patients with LABC underwent initial surgery. Among them, the majority had operable LABC (cT3N1) (n = 47, 66%). The adjuvant treatment in these patients was based on histology and expression of receptors. Accordingly, patients were treated with chemotherapy (60%), radiation (48%), and hormones (68%), and 8% did not receive any adjuvant treatment. The reasons for not receiving chemotherapy were advanced age, significant co-morbid illness, poor general condition, and patient’s unwillingness for chemotherapy.
The initial treatment of other patients with inoperable LABC was NACR (77%), NAC (15%), or others (8%) (Appendix Table A1). The most common chemotherapy regimens administered during NACR were either anthracycline-based (77%) or anthracycline and taxane (12%), which was based on individual physician judgment. The most common chemotherapy regimen administered as NAC was either anthracycline-based (51%) or anthracycline- and taxane-based regimen (39%). In those patients who were given concurrent chemoradiation, the median dose of radiation was 40 Gy (Gray) with five fractions/week. Overall, internal mammary nodes were irradiated in 76% of patients.
Modified radical mastectomy was the most common surgery (98%) followed by breast conservation in 1%. The median time to surgery in patients treated with either NAC or NACR was 4 months (range, 2-20 months) and 6 months (range, 3-27 months), respectively. The median number of chemotherapy cycles before surgery in the NAC or NACR patients was three cycles (range, 1- 6) and six cycles (range, 1-6), respectively. All hormone-positive patients (100%) received adjuvant hormonal therapy, and 8% of premenopausal women proceeded to ovarian suppression therapy (bilateral oophorectomy or radiocastration). Among the HER2-positive subset, three patients (0.5%) received adjuvant trastuzumab (Table 2). Two patients completed 1 year of adjuvant trastuzumab, one patient had symptomatic heart failure after the second dose of trastuzumab, and further trastuzumab was withheld.
TABLE 2.
Treatment
Outcome
The clinical response after NACR was CR, PR, SD, PD, and unknown in 15.4%, 81.9%, 0.2%, 1.8%, and 0.5%, respectively. The clinical response after NAC was CR, PR, SD, PD, and unknown in 7.9%, 88.2%, 1.2%, 2.4%, and 0.3%, respectively. PR was any decrease in size tumor size by clinical examination, which is a limitation. Patients who received NACR had a higher PCR (25%) as compared with those who received NAC (18%), which was statistically significant (P = .019) (Table 3).
TABLE 3.
PCR

The 5-year OS (Fig 1) and DFS (Fig 2) of the entire cohort were 65% and 60%, respectively. The 5-year DFS for stages IIIA, IIIB, and IIIC was 72%, 49%, and 29%, respectively. The 5-year OS for stages IIIA, IIIB, and IIIC was 76%, 54%, and 39%, respectively (Appendix Table A2; Fig 3). The 5-year DFS and OS of patients who received NACR were 59.1% and 64%, respectively. The 5-year DFS and OS of patients who received NAC were 69.3% and 75%, respectively (Appendix Table A2). Patients with a tumor size > 10 cm at diagnosis had a significantly worse survival (hazard ratio [HR], 2.04; 95% CI, 1.52 to 2.73; P = .001) (Fig 4). Patients with triple-negative breast cancer (TNBC) had a 10-year DFS of 48.9% (Appendix Table A2; Fig 5). Patients who received NACR had a significantly worse DFS (HR, 0.62; 95% CI, 0.49 to 0.79; P = .001) (Fig 6) and OS (HR, 0.64; 95% CI, 0.52 to 0.79; P = .001) (Appendix Fig A1) as compared with those who received NAC.
FIG 1.
OS of all patients. Kaplan-Meier curve showing the OS of 2,137 patients. Numbers within parentheses are events. OS, overall survival.
FIG 2.
DFS of all patients. Kaplan-Meier curve showing the DFS of 2,137 patients. Numbers within parentheses are events. DFS, disease-free survival.
FIG 3.
DFS according to stage. Kaplan-Meier curve showing the stagewise DFS. Numbers within parentheses are events. DFS, disease-free survival.
FIG 4.
DFS and tumor size. Kaplan-Meier curve showing the DFS according to the tumor size. Numbers within parentheses are events. DFS, disease-free survival.
FIG 5.
DFS of patients with TNBC. Kaplan-Meier curve showing the DFS of patients with TNBC. Numbers within parentheses are events. DFS, disease-free survival; TNBC, triple-negative breast cancer.
FIG 6.
DFS comparing NACR and NAC. Kaplan-Meier curve comparing DFS of patients treated with NAC and neoadjuvant concurrent chemoradiation. Numbers within parentheses are events. DFS, disease-free survival; NAC, neoadjuvant chemotherapy; NACR, XXX.
There was a relapse of breast cancer in 27% of patients with distant in 87% and locoregional in 13% (Appendix Table A3). The locoregional recurrences were similar between the NACR (3.6%) and NAC (3.3%), but the distant recurrences were higher for NACR (25.9%) than NAC (17.9) (Appendix Table A4). On follow-up, second primary cancers (SPCs) were observed in 2% of patients, with the most common being breast followed by ovary or fallopian tube cancer (Appendix Table A3).
Prognostic Factors
Univariate analysis (Appendix Table A5) showed that tumor size, stage, hormonal status, treatment modality, type of chemotherapy, PCR, and lymphovascular invasion (LVI) had a significant correlation with DFS and OS. Multivariate analysis (Table 4) showed that patients with a tumor size > 10 cm (HR, 2.19; CI, 1.62 to 2.98; P = .001), hormone receptor negativity (HR, 1.45; CI, 1.22 to 1.72; P = .001), treatment modality (NAC, HR, 0.56; CI, 0.43 to 0.73; P = .001), lack of PCR (HR, 2.36; CI, 1.85 to 3.02; P = .001), and the presence of LVI (HR, 1.97; CI, 1.60 to 2.44; P = .001) had decreased OS.
TABLE 4.
Multivariate Analysis

DISCUSSION
This report describes the clinical details and outcome of one of the largest series of patients with LABC from a single center in India. The management of breast cancer globally is continuously evolving. The adoption of breast screening programs, particularly in the West, has allowed the detection of cancer at earlier stages of the disease.16 The primary management has been surgery, and adjuvant therapy has been tailored to the molecular attributes of the tumor. This has led to a gradual decline in mortality and improvement in OS. In developing countries, such as India, because of advanced disease at presentation, different strategies have been adopted. In general, at most centers in India, for LABC, preoperative chemotherapy has been the preferred option before definitive surgery.17 At our center, concurrent chemoradiation has evolved to address the problem of managing really large noninflammatory breast tumors.5 In part, this NACR approach was pursued to take advantage of the synergistic effect of chemotherapy and radiation. Concurrent chemoradiation has been used in management of carcinoma of head and neck, rectum, and cervix. However, in breast cancer, although theoretically, this has remained less explored, it offers an advantage.
LABC forms a significant proportion of breast cancer in developing countries. For the duration of this study, it formed 46% of all patients treated at our center. Although this report is from a consecutive series of patients treated at our center, it is not a prospective trial evaluating the role of concurrent chemoradiation in LABC. Nevertheless, the results capture a real-world scenario in this part of the world. The overall outcomes of this difficult group of patients with adequate follow-up were reasonable with more than 50% alive and disease-free at 10 years. The outcomes decreased with increasing stages such as IIIA, which were superior compared with stage IIIC. These results are comparable with those reported from New York where chemoradiation was administered to patients with LABC (Appendix Table A5).18 Similarly, the outcomes are also comparable with those centers where the initial approach was only preoperative chemotherapy. These data suggest an apparent advantage for NAC as compared with NACR with respect to DFS and OS. However, it must be noted that the number of patients who received NAC were less and patients with larger tumors (stage IIIB and IIIC) received predominantly NACR (Appendix Table A1). Besides this, patients with TNBC who have a poor prognosis also had a 10-year OS of more than 50%. During this period, in India, the cost of trastuzumab was so prohibitive that it was not possible to treat all patients whose tumors had overexpression of HER2. Currently, with the availability of generic versions of trastuzumab, which have been proven to be equivalent in efficacy, it is possible to treat patients.19 Interestingly, the survival of these patients was also similar to those who had hormone-sensitive and HER2-negative tumors.
The size of the tumor is an important prognostic factor, and by definition, for LABC, all have tumors equal to or more than 5 cm in size. In this report, we were able to identify a group of patients whose tumors were more than 10 cm (9%) and these patients had a significantly inferior outcome. Thus, it may be prudent to consider including patients with a tumor size > 10 cm in T4 stage rather than T3. Furthermore, patients with very large tumors had no distant metastases. This is corroborated by a report in 819,647 women with invasive breast cancer, which showed that the correlation between tumor size and risk of distant metastasis was not linear.20 Of course, the detection of metastasis could be improved by additional investigations. It was shown recently that positron emission tomography computed tomography as compared with chest x-ray or ultrasound of abdomen or pelvis upstaged extra-axillary regional lymph nodes by 18% and distant metastatic sites by 28%.21
A PCR is a good indicator of response to therapy and does correlate with outcome. In this report, patients who received NACR had a 6% higher PCR rate as compared with those treated with NAC. This could be because patients who received NACR received more number of chemotherapy cycles before surgery. Despite this, patients who received NAC had a better survival as compared with NACR. This is possibly because the majority of patients with stage IIIA or operable tumors were offered NAC, whereas the rest with more advanced disease had NACR (Appendix Table A1). Interestingly, patients with triple-negative LABC had a high PCR (41%) without the addition of platinum-based chemotherapy. The cohort of 545 patients with HER2-positive tumors had a PCR rate of 18% with either NACR or NAC. Patients with HER2-positive LABC treated with dual anti-HER2 therapy (trastuzumab and pertuzumab) in combination with chemotherapy can have a PCR of up to 45%.22 These results clearly indicate that chemoradiation does lead to robust pathologic responses comparable with chemotherapy alone.
Only 12% of patients in the NACR received taxane; however, there was no difference in survival between those who received anthracycline only and those who received anthracycline and taxane. Interestingly, the locoregional recurrence was similar in patients both receiving preoperative and postoperative radiation; however, the distant metastases were more in NACR. The long-term complications of chemoradiation such as cosmesis and SPC are a concern.23 In this report, second malignancies (2%) were predominantly breast cancer followed by ovary or fallopian tube cancer. There were no hematologic malignancies. The prevalence of SPC was less as compared with the National Cancer Institute SEER where early-stage breast cancers had the SPC of 7% at 10 years, 14% at 15 years, and 20% at 20 years.24 Furthermore, prospective evaluation of survivors for cardiac function has not been performed, which is desirable particularly for those with left-sided tumors.
Patients were not screened routinely for variants in genes that predispose to breast cancer. However, independent of this report, 16% of patients (n = 91) had pathogenic mutations in BRCA1 or BRCA2.25 With the availability of next-generation sequencing, this was more sensitive in detecting variants.26
The multivariate analysis identified several independent prognostic factors that correlated with the outcome in this group of patients. Although some factors such as the size of the tumor, the expression of ER/PR, and PCR are well-known, the expression of HER2 was not significant. Interestingly, LVI was an independent factor for outcome. A recent report showed that LVI was an independent prognostic factor and had a better correlation with survival than PCR.27 Patients whose tumors were positive for HER2 (2+) by IHC did not undergo further examination by fluorescence in situ hybridization primarily because of financial constraint and inability to treat them with trastuzumab. The local and systemic toxicities following NACR were not adequately captured for any inferences to be made.
The initial treatment for LABC is still an open question, and the role of chemoradiation has not been proven. This report provides the basis for further evaluation of this approach in LABC. Increasingly, an NAC approach is being recommended particularly in TNBC.28 The optimal regimen that can be safely combined with breast radiation has to be defined. In our experience, if the doxorubicin cyclophosphamide (AC)/paclitaxel is the preferred chemotherapy approach, then paclitaxel can be safely combined with radiation initially followed by AC subsequently before surgery.29 A similar study evaluated NAC with AC initially followed by concurrent chemoradiation with twice weekly paclitaxel in LABC.30 The outcome of other studies in patients with LABC is mentioned in Appendix Table A6. Currently, a randomized trial is being conducted in our institute comparing chemoradiation and chemotherapy in the initial management of LABC. Concurrent chemoradiation for LABC is a therapeutic option that can be considered in a clinical trial setting.
In conclusion, this study has the largest sample size of patients with LABC worldwide. LABC is the most common presentation in India, which is often inoperable. Patients with a tumor size > 10 cm had an unfavorable outcome irrespective of treatment and warrant a consideration for upstaging to T4 in American Joint Committee on Cancer. Patients with TNBC had high PCR. Stage, hormonal status, treatment modality, type of chemotherapy, PCR, and LVI had a significant correlation with survival. We await the results of the randomized controlled trial comparing NACR with NAC in patients with LABC from our Institute.
ACKNOWLEDGMENT
We would like to thank all the faculty of the Department of Epidemiology, Tumor Registry, and Biostatistics for the data collection.
APPENDIX
FIG A1.
OS comparing NACR and NAC. Kaplan-Meier curve comparing OS of patients treated with NAC and NACR. Numbers within parentheses are events. HR, hazard ratio; NAC, neoadjuvant chemotherapy; NACR, neoadjuvant concurrent chemoradiation; OS, overall survival.
TABLE A1.
Stage and Treatment
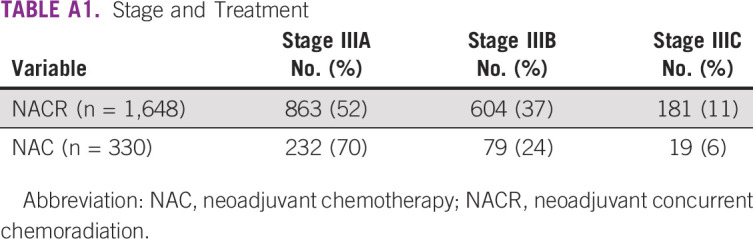
TABLE A2.
Survival of All Patients

TABLE A3.
Recurrence and Second Primary

TABLE A4.
Pattern of Recurrence

TABLE A5.
Univariate Analysis
TABLE A6.
Studies in Patients With LABC
PRIOR PRESENTATION
Presented as an oral paper in Indian Cancer Congress 2017, November 10, 2017, Bengaluru, India.
AUTHOR CONTRIBUTIONS
Conception and design: Manikandan Dhanushkodi, Viswanathan Shanta, Velusamy Sridevi, Ranganathan Rama, Ganesarajah Selvaluxmy, Trivadi S. Ganesan
Administrative support: Rajaraman Swaminathan
Collection and assembly of data: Manikandan Dhanushkodi, Ranganathan Rama, Rajaraman Swaminathan, Trivadi S. Ganesan
Data analysis and interpretation: Manikandan Dhanushkodi, Viswanathan Shanta, Ranganathan Rama, Rajaraman Swaminathan, Ganesarajah Selvaluxmy, Trivadi S. Ganesan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Malvia S Bagadi SA Dubey US, et al. : Epidemiology of breast cancer in Indian women. Asia Pac J Clin Oncol 13:289-295, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Dhillon PK Mathur P Nandakumar A, et al. : The burden of cancers and their variations across the states of India: The Global Burden of Disease Study 1990–2016. Lancet Oncol 19:1289-1306, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manoharan N Nair O Shukla NK, et al. : Descriptive epidemiology of female breast cancer in Delhi, India. Asian Pac J Cancer Prev 18:1015-1018, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyblade L Stockton M Travasso S, et al. : A qualitative exploration of cervical and breast cancer stigma in Karnataka, India. BMC Womens Health 17:58, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shanta V Swaminathan R Rama R, et al. : Retrospective analysis of locally advanced noninflammatory breast cancer from Chennai, South India, 1990-1999. Int J Radiat Oncol Biol Phys 70:51-58, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Gauide MFD Amorim G Arcuri RA, et al. : A phase II study of second-line neoadjuvant chemotherapy with capecitabine and radiation therapy for anthracycline-resistant locally advanced breast cancer. Am J Clin Oncol 30:78-81, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Chakravarthy AB Kelley MC McLaren B, et al. : Neoadjuvant concurrent paclitaxel and radiation in stage II/III breast cancer. Clin Cancer Res 12:1570-1576, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Kao J Conzen SD Jaskowiak NT, et al. : Concomitant radiation therapy and paclitaxel for unresectable locally advanced breast cancer: Results from two consecutive phase I/II trials. Int J Radiat Oncol Biol Phys 61:1045-1053, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Bollet MA Sigal-Zafrani B Gambotti L, et al. : Pathological response to preoperative concurrent chemo-radiotherapy for breast cancer: Results of a phase II study. Eur J Cancer 42:2286-2295, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Toledano A Azria D Garaud P, et al. : Phase III trial of concurrent or sequential adjuvant chemoradiotherapy after conservative surgery for early-stage breast cancer: Final results of the ARCOSEIN trial. J Clin Oncol 25:405-410, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Rouëssé J de la Lande B Bertheault-Cvitkovic F, et al. : A phase III randomized trial comparing adjuvant concomitant chemoradiotherapy versus standard adjuvant chemotherapy followed by radiotherapy in operable node-positive breast cancer: Final results. Int J Radiat Oncol Biol Phys 64:1072-1080, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Nandakumar A Kishor Rath G Chandra Kataki A, et al. : Concurrent chemoradiation for cancer of the cervix: Results of a multi-institutional study from the setting of a developing country (India). J Glob Oncol 1:11-22, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nandakumar A, Nandakumar A: Survival in head and neck cancers—Results of a multi- institution study. Asian Pac J Cancer Prev 17:1745-1754, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Elston CW, Ellis IO: Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 19:403-410, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Guideline summary: American Society of Clinical Oncology/College of American Pathologists Guideline recommendations for human epidermal growth factor receptor HER2 testing in breast cancer. JCO Oncol Pract 3:48-50, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson HD Fu R Cantor A, et al. : Effectiveness of breast cancer screening: Systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med 164:244-255, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Gogia A Raina V Deo SVS, et al. : Neoadjuvant chemotherapy in locally advanced invasive lobular carcinoma: A limited institutional experience. South Asian J Cancer 7:64-65, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams S Chakravarthy AB Donach M, et al. : Preoperative concurrent paclitaxel-radiation in locally advanced breast cancer: Pathologic response correlates with five-year overall survival. Breast Cancer Res Treat 124:723-732, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rugo HS Barve A Waller CF, et al. : Effect of a proposed trastuzumab biosimilar compared with trastuzumab on overall response rate in patients with ERBB2 (HER2)-positive metastatic breast cancer: A randomized clinical trial. JAMA 317:37-47, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Sopik V, Narod SA: The relationship between tumour size, nodal status and distant metastases: On the origins of breast cancer. Breast Cancer Res Treat 170:647-656, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yararbas U Avci NC Yeniay L, et al. : The value of 18F-FDG PET/CT imaging in breast cancer staging. Bosn J Basic Med Sci 18:72-79, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gianni L Pienkowski T Im YH, et al. : Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13:25-32, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Matuschek C Nestle-Kraemling C Haussmann J, et al. : Long-term cosmetic outcome after preoperative radio-/chemotherapy in locally advanced breast cancer patients. Strahlenther Onkol 195:615-628, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Li D Weng S Zhong C, et al. : Risk of second primary cancers among long-term survivors of breast cancer. Front Oncol 9:1426, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soumittra N Meenakumari B Parija T, et al. : Molecular genetics analysis of hereditary breast and ovarian cancer patients in India. Hered Cancer Clin Pract 7:13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajkumar T Meenakumari B Mani S, et al. : Targeted resequencing of 30 genes improves the detection of deleterious mutations in South Indian women with breast and/or ovarian cancers. Asian Pac J Cancer Prev 16:5211-5217, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Ryu YJ Kang SJ Cho JS, et al. : Lymphovascular invasion can be better than pathologic complete response to predict prognosis in breast cancer treated with neoadjuvant chemotherapy. Medicine (Baltimore) 97:e11647, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia LY Hu QL Zhang J, et al. : Survival outcomes of neoadjuvant versus adjuvant chemotherapy in triple-negative breast cancer: A meta-analysis of 36,480 cases. World J Surg Oncol 18:129, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyer P Radhakrishnan V Balasubramanian A, et al. : Study of pathological complete response rate with neoadjuvant concurrent chemoradiation with paclitaxel in locally advanced breast cancer. Indian J Cancer 57:428, 2020 [DOI] [PubMed] [Google Scholar]
- 30.Kunheri B Kottarathil V Makuny D, et al. : Neoadjuvant chemotherapy followed by neoadjuvant concurrent chemoradiation for locally advanced breast cancer: A feasibility study and 10-year follow-up results. Indian J Gynecol Oncol 14:60, 2016 [Google Scholar]
- 31.Nair N Shet T Parmar V, et al. : Breast cancer in a tertiary cancer center in India—An audit, with outcome analysis. Indian J Cancer 55:16-22, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Raina V Kunjahari M Shukla NK, et al. : Outcome of combined modality treatment including neoadjuvant chemotherapy of 128 cases of locally advanced breast cancer: Data from a tertiary cancer center in northern India. Indian J Cancer 48:80, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Chakrabarti A Dasgupta P Haldar D, et al. : Assessment of prognostic factors for locally advanced breast cancer. J Indian Med Assoc 110:284-286, 2012 [PubMed] [Google Scholar]
- 34.Koca E Kuzan TY Dizdar O, et al. : Outcomes of locally advanced breast cancer patients with ≥ 10 positive axillary lymph nodes. Med Oncol 30:615, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Brenner B Siris N Rakowsky E, et al. : Prediction of outcome in locally advanced breast cancer by post-chemotherapy nodal status and baseline serum tumour markers. Br J Cancer 87:1404-1410, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cance WG Carey LA Calvo BF, et al. : Long-term outcome of neoadjuvant therapy for locally advanced breast carcinoma: Effective clinical downstaging allows breast preservation and predicts outstanding local control and survival. Ann Surg 236:295-302, 2002. discussion 302-303 [DOI] [PMC free article] [PubMed] [Google Scholar]











