PURPOSE
There are deficient data on prevalence of germline mutations in breast cancer susceptibility genes 1 and 2 (BRCA1/BRCA2) in Indian patients with ovarian cancer who are not selected by clinical features.
METHODS
This prospective, cross-sectional, noninterventional study in nine Indian centers included patients with newly diagnosed or relapsed epithelial ovarian, primary peritoneal, or fallopian tube cancer. The primary objective was to assess the prevalence of BRCA1/BRCA2 mutations, and the secondary objective was to correlate BRCA1/BRCA2 status with clinicopathologic characteristics. Mutation testing was performed by a standard next-generation sequencing assay.
RESULTS
Between March 2018 and December 2018, 239 patients with a median age of 53.0 (range, 23.0-86.0 years) years were included, of whom 203 (84.9%) had newly diagnosed disease, 36 (15.1%) had family history of ovarian or breast cancer, and 159 (66.5%) had serous subtype of epithelial ovarian cancer. Germline pathogenic or likely pathogenic mutations in BRCA1 and BRCA2 were detected in 37 (15.5%; 95% CI, 11.1 to 20.7) and 14 (5.9%; 95% CI, 3.2 to 9.6) patients, respectively, whereas variants of uncertain significance in these genes were seen in four (1.7%; 95% CI, 0.5 to 4.2) and six (2.5%; 95% CI, 0.9 to 5.4) patients, respectively. The prevalence of pathogenic or likely pathogenic BRCA mutations in patients with serous versus nonserous tumors, with versus without relevant family history, and ≤ 50 years versus > 50 years, were 40 of 159 (25.2%; 95% CI, 18.6 to 32.6) versus 11 of 80 (13.8%; 95% CI, 7.1 to 23.3; P = .0636), 20 of 36 (55.6%; 95% CI, 38.1 to 72.1) versus 41 of 203 (20.2%; 95% CI, 14.9 to 26.4; P < .0001), and 20 of 90 (22.2%; 95% CI, 14.1 to 32.2) versus 31 of 149 (20.8%; 95% CI, 14.6 to 28.2; P = .7956), respectively.
CONCLUSION
There is a high prevalence of pathogenic or likely pathogenic germline BRCA mutations in Indian patients with ovarian cancer.
INTRODUCTION
Ovarian cancer is one of the most common gynecologic cancers, with 313,959 new cases and 207,252 deaths reported worldwide in 2020.1 It accounted for more deaths than any other cancer of the female reproductive system.2 In the Indian context, ovarian cancer is the third leading cancer among women, after cervical and breast cancer.3
CONTEXT
Key Objective
What is the incidence of germline mutations in BRCA1 or BRCA2 genes in Indian patients with ovarian cancer who are not selected by clinical criteria like family history or age?
Knowledge Generated
In this multicenter Indian study involving nine tertiary centers and 239 patients, germline pathogenic or likely pathogenic mutations in BRCA1 and BRCA2 were detected in 15.5% and 5.9% of patients, respectively. The prevalence of these mutations in patients with nonserous tumors, without relevant family history, and with age > 50 years was 13.8%, 20.2%, and 20.8%, respectively. This suggests that selection of patients for germline testing by serous histology, suggestive family history, and young age is likely to miss BRCA1 and BRCA2 mutations in many patients.
Relevance
There is a high incidence of germline BRCA1 and BRCA2 mutations in Indian patients with epithelial ovarian cancer. Efforts should be made to make such testing widely available in India.
A majority of patients with ovarian cancer are diagnosed at an advanced stage, wherein the 5-year survival is < 30%.4-7 Family history of ovarian or breast cancer is one of the important predisposing factors, with first- and second-degree relatives having four-fold and two-fold higher risk of developing ovarian cancer, respectively.8-10 Inherited mutations in the key tumor suppressor genes, the breast cancer susceptibility gene 1 or 2 (BRCA1 or BRCA2), are prevalent in 3%-27% of patients with ovarian cancer who are not selected on the basis of clinical features like family history.11,12 By age 70 years, ovarian cancer risk is 40% in BRCA1 and 18% in BRCA2 mutation carriers.13 Germline mutations in BRCA genes also confer high risk for the development of fallopian tube carcinoma and primary papillary serous carcinoma of the peritoneum.14-16
Some studies have suggested that ovarian cancer patients with germline BRCA1/2 mutations (especially BRCA2) have improved prognosis compared with those lacking BRCA mutations.17-21 Thus, as recommended by numerous clinical guidelines, screening for BRCA mutations may help not only in developing patient management strategies but also in prognosticating patients with ovarian cancer.22-26 For example, the recent National Comprehensive Cancer Network guidelines (version 1, 2020) recommend genetic testing for BRCA1/2 mutations along with other panels of mutations like CDH1, PALB2, PTEN, and TP53 in patients with breast cancer, ovarian cancer, and pancreatic cancer on the basis of their family history, ethnicity, age, and tumor histology.27 Although a few regional studies have been reported,11,28,29 these have included patients with breast and/or ovarian cancer who were chosen because of clinical features like suggestive family history or young age, with pathogenic or likely pathogenic mutation being reported in 25.5%-30.1% of them. Hence, this multicenter Indian study was undertaken in patients with ovarian cancer not selected for any predisposing clinical features, who were evaluated for prevalence of germline BRCA mutations and its association with clinical and pathologic characteristics.
METHODS
The study protocol (ClinicalTrials.gov identifier: NCT03471572) was approved by the regulatory authorities and the ethics committees or institutional review boards of all the participating centers. The study was conducted in accordance with the Declaration of Helsinki, the International Council on Harmonization Good Clinical Practice guidelines, Good Pharmacoepidemiological Practice, and the applicable legislation(s) on noninterventional studies and/or observational studies.30,31 All patients provided written informed consent before their study participation.
Study Population
Women age 18 years or older with previously or newly diagnosed ovarian, primary peritoneal, or fallopian tube cancer were enrolled in the study. Patients were excluded if they failed to provide written informed consent or had any medical condition that, in the opinion of the investigator, would interfere with safe completion of the study, or were participating in any other clinical trial.
Study Design and End Points
This was a prospective, noninterventional, cross-sectional, multicenter study that enrolled patients between March 2018 and December 2018 at 9 centers across India (Appendix Table A1). Data pertaining to demographics, family history of breast and/or ovarian cancer, and medical and surgical history were collected from patients' available medical records and transcribed on to the electronic case report forms. Blood samples were collected, coded for confidentiality, stored at ambient temperature, and sent to a central laboratory at Bangalore, India, for germline mutation testing. DNA was extracted from blood using a QIAamp DNA mini kit (Qiagen, Germany), and next-generation sequencing (NGS) was performed on the extracted DNA using the TruSight cancer sequencing panel (Illumina, San Diego, CA), covering 94 high-risk genes associated with cancer predisposition. The list of genes is the same as that previously reported.28 From 50 ng of input genomic DNA of each patient, NGS libraries were prepared and hybridized to a custom pool of oligonucleotides, targeting genomic regions as previously described, followed by paired end sequencing of up to 150 base pair read lengths.28 The mutations were classified as pathogenic or likely pathogenic or variants of uncertain significance (VUS) as per International Agency for Research on Cancer classification.32 At the devolution visit, the investigator informed patients about their BRCA mutation test results and appropriate genetic counseling was provided as per the local standard of care. The primary objective was to determine the proportion of patients with germline pathogenic or likely pathogenic BRCA1 and BRCA2 mutations and variants of uncertain significance. We also assessed the association of BRCA1 and BRCA2 mutation with family history of breast and/or ovarian cancer and histopathologic type of ovarian cancer. The study did not aim to provide any new or interventional drug to the patients.
Statistical Analysis
On the basis of an estimated BRCA1/2 prevalence rate of 15.8% in the study population, a sample size of 228 was calculated with a precision of 5%.33 With an assumed dropout proportion of 10%, a total of 240 patients were planned to be included in this study. Statistical analyses were performed using Statistical Analysis Software (version 9.4). The BRCA1/2 mutation–positive status was summarized in terms of frequency (n) and percentages along with corresponding binomial exact 95% CI using Clopper Pearson method. For analysis of secondary end points, chi-square test was used to test the differences in BRCA 1/2 mutation status between subgroups defined by histopathologic type and family history of breast and/or ovarian cancer at a significance level of 0.05.
RESULTS
Clinical and Pathologic Characteristics
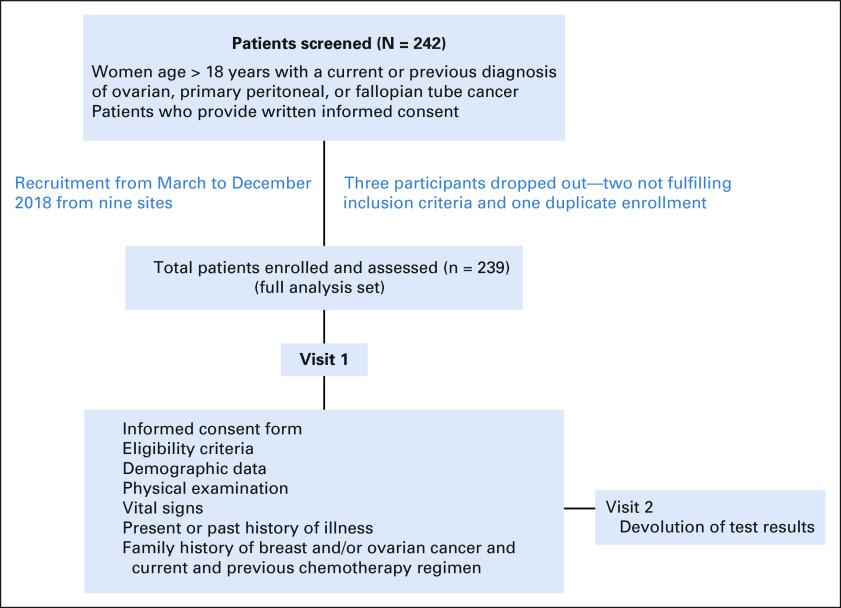
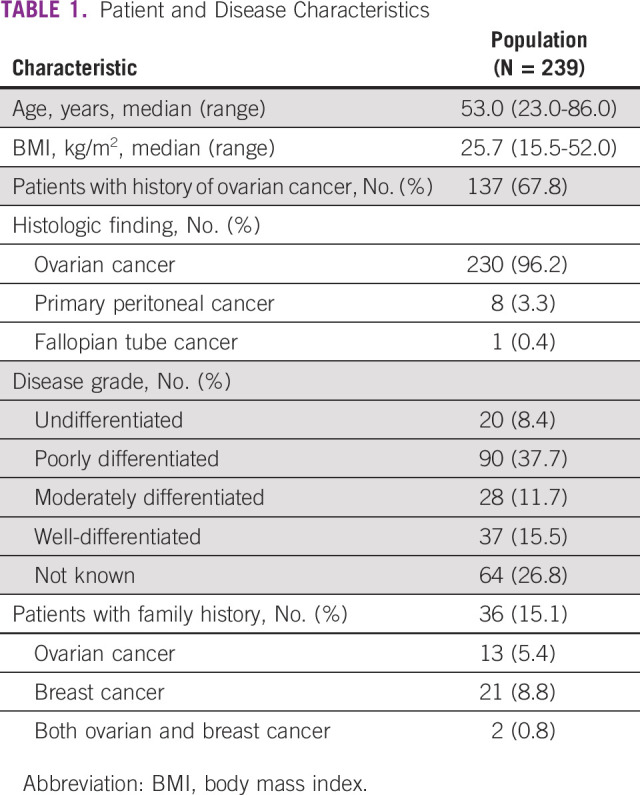
Between March and December 2018, 242 female patients with epithelial ovarian or primary peritoneal cancer were enrolled in the study, of whom three patients had to be excluded from the analysis, two because of not fulfilling the eligibility criteria and one being a duplicate enrollment. Table 1 shows the demographic characteristics of the patients, and Appendix Figure A1 shows the study flow. The median age of patients was 53.0 (range, 23.0-86.0 years) years; 203 (84.9%) patients had newly diagnosed disease. A majority of patients (230, 96.2%) had ovarian cancer followed by primary peritoneal cancer (8, 3.3%) and fallopian tube cancer (1, 0.4%). Most patients (203, 84.9%) did not have a family history of ovarian or breast cancer.
TABLE 1.
Patient and Disease Characteristics
Prevalence of BRCA1 and BRCA2 Mutations
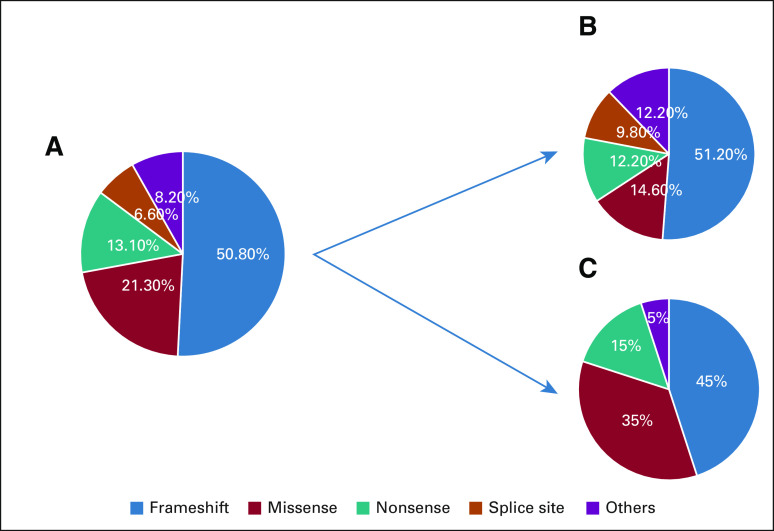
Of the analyzed 239 patients, pathogenic or likely pathogenic BRCA1/2 mutations were detected in 51 (21.3%; 95% CI, 16.3 to 27.1) patients, 37 (15.5%; 95% CI, 11.1 to 20.7) with BRCA1 mutations and 14 (5.9%; 95% CI, 3.2 to 9.6) with BRCA2 mutations. None of the patients had mutations in both genes. Variants of uncertain significance (VUS) were detected in 10 (4.2%; 95% CI, 2.0 to 7.6) patients, four (1.7%; 95% CI, 0.5 to 4.2) in BRCA1 and six (2.5%; 95% CI, 0.9 to 5.4) in BRCA2. Among the 61 patients with BRCA1/2 or VUS mutations, the numbers of patients (percentage) with frameshift mutation, missense mutation, nonsense mutation, splice site mutation, and other mutations were 31 (50.8%), 13 (21.3%), eight (13.1%), four (6.6%), and five (8.2%), respectively (Fig 1).
FIG 1.
(A) Distribution of pathogenic or likely pathogenic and VUS BRCA1/2 mutations per mutation type (n = 61). (B) BRCA1 (n = 41). (C) BRCA2 (n = 20). VUS, variant of uncertain significance.
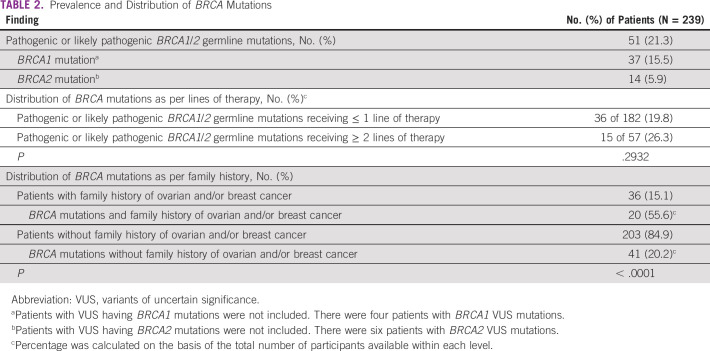
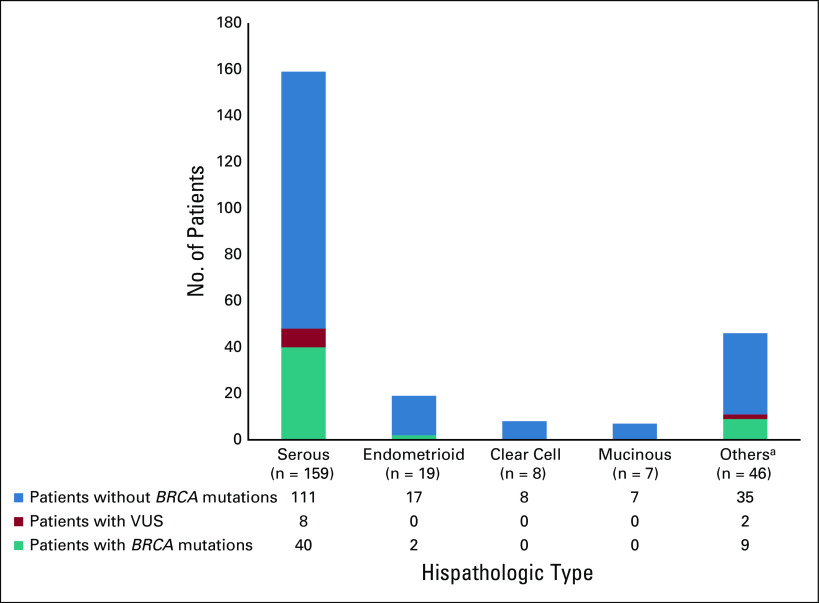
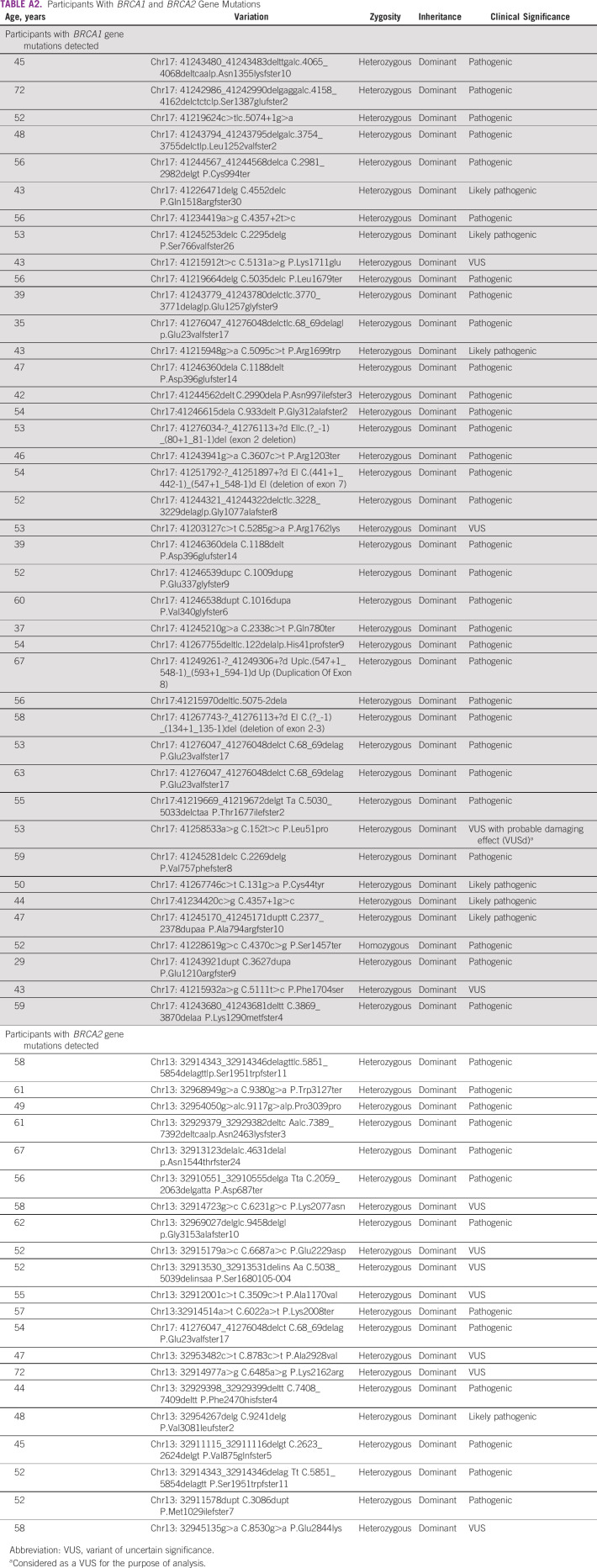
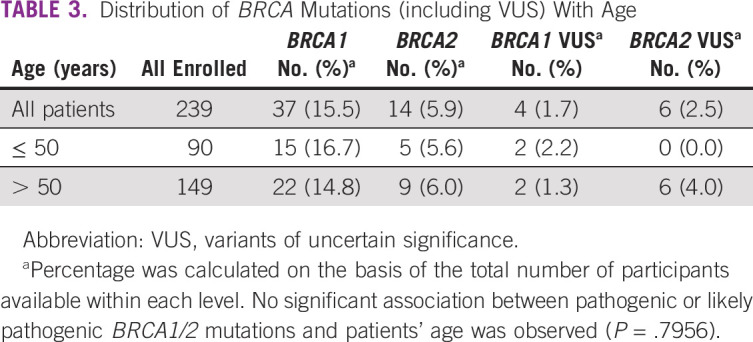
Table 2 presents the prevalence and distribution of BRCA mutations according to the number of lines of systemic therapy and family history. Of the 182 (76.2%) patients who had received ≤ 1 line of treatment, 36 (19.8%; 95% CI, 14.3 to 26.3) had germline pathogenic or likely pathogenic mutation compared with 15 of 57 (26.3%; 95% CI, 15.5 to 39.7) patients who had received ≥ 2 lines of treatment. Of the 36 patients with family history of breast and/or ovarian cancer, 20 (55.6%; 95% CI, 38.1 to 72.1) had pathogenic or likely pathogenic BRCA1/2 mutations compared with 41 of 203 (20.2%; 95% CI, 14.9 to 26.4) patients with no family history (P < .0001). There was a trend toward higher prevalence of pathogenic or likely pathogenic BRCA1/2 mutations in patients with serous subtype of ovarian cancer (40 of 159, 25.2%; 95% CI, 18.6 to 32.6) compared with patients with nonserous subtypes (11 of 80, 13.8%; 95% CI, 7.1 to 23.3; Fig 2). Among patients with known endometrioid, clear cell, or mucinous histology, two of 34 (5.9%; 95% CI, 0.7 to 19.7) had germline BRCA1/2 mutations, including, notably, none of the 15 patients with clear cell or mucinous histology. There was no statistically significant difference in the association of BRCA mutations with tumor histology (P = .0636). There was no significant association of pathogenic or likely pathogenic BRCA1/2 mutations with patients' age, with prevalence being 20 of 90 (22.2%; 95% CI, 14.1 to 32.2) in patients ≤ 50 years compared with 31 of 149 (20.8%; 95% CI, 14.6 to 28.2) in patients > 50 years (P = .7956; Table 3). The variations for the detected mutations in BRCA 1/2 are reported in Appendix Table A2.
TABLE 2.
Prevalence and Distribution of BRCA Mutations
FIG 2.
Association of histopathologic type and BRCA1/2 mutation status. aOther histopathologic subtypes include seromucinous, undifferentiated or other epithelial subtypes, or unclassified variants. VUS, variant of uncertain significance.
TABLE 3.
Distribution of BRCA Mutations (including VUS) With Age
DISCUSSION
Our analysis in this multicenter cohort of Indian patients with ovarian or primary peritoneal cancer suggests a high prevalence (21.3%) of germline pathogenic or likely pathogenic mutations in BRCA1 or BRCA2 genes with an additional 4.2% having variants of uncertain significance in these genes. To our knowledge, this is the first such report in a cohort from India that is not selected by clinical features like family history and provides important, clinically actionable information of relevance to patients and physicians.
Of note, our analysis suggests that, although the prevalence of BRCA1/2 mutations was higher in patients with family history of breast and/or ovarian cancer, a considerable minority of patients without such history (20.2%) also harbored the mutations. This suggests that the absence of family history is not adequate as a screening strategy for germline testing. The prevalence of these mutations was higher in patients with serous histology, and no patient with known clear cell or mucinous tumors had a pathogenic mutation, suggesting that histologic subtype may be used to triage patients for testing. Age was not associated with the prevalence of these mutations and should not be incorporated in clinical decision making to test for germline predisposition. The commonest pathogenic mutation reported in this data set was the 187delAG in BRCA1 (c.68_69delAG in four patients, Appendix Table A2), which is a frameshift, loss of function, and founder mutation in the Ashkenazi Jewish population and has also been reported in previous Indian studies with variable frequency.28,29 These patients were not of Ashkenazi Jewish descent and did not belong to any single geographical region in India. Among patients with a BRCA2 mutation, the commonest mutation was c.5851_5854del, which was reported in two unrelated patients and, to our knowledge, has not been reported in previous Indian studies.
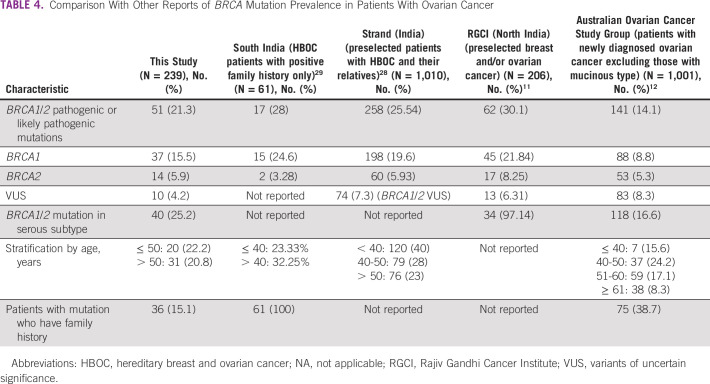
Table 4 summarizes selected previous reports from India and other countries, in patients with ovarian cancer.11,12,28,29 The prevalence of germline pathogenic or likely pathogenic BRCA 1/2 mutations in patients not selected by family history or histology ranged from 14.1% to 30.1%, and in previous reports selecting patients with relevant family history, it ranged from 38.7% to 100%. In previous reports of patients with serous histology, the prevalence of germline pathogenic or likely pathogenic BRCA 1/2 mutations ranged from 16.6% to 97.14%. Our results do not show any notable outliers compared with what has been reported by others. Another incidental finding in our study is that 36 of 182 (19.8%) patients who had received ≤ 1 line of treatment had germline pathogenic or likely pathogenic mutation, whereas 15 of 57 (26.3%) patients receiving ≥ 2 lines of treatment had germline pathogenic or likely pathogenic mutation. This finding needs to be evaluated further since ours is a cross-sectional study. It is possible that patients who survive long enough to receive multiple lines of treatment are enriched for BRCA mutations, which is known to confer sensitivity to repeated courses of chemotherapy, as has also been reported earlier.12
TABLE 4.
Comparison With Other Reports of BRCA Mutation Prevalence in Patients With Ovarian Cancer
The strength of our analysis is inclusion of patients from multiple centers in India, of all age in the adult group, of all histologic subtypes, and those with or without relevant family history. This makes the results of this study generalizable to the patient population with ovarian cancer seen in routine practice in India. These study results can be used by physicians to counsel patients about the need for germline testing. Germline testing was performed in a single laboratory with a proven record of quality control. BRCA mutation testing in ovarian cancer offers prognostic ability and therapeutic decision making from the perspective of patient. BRCA mutation status can guide the use of poly(ADP ribose) polymerase inhibitors, which are associated with favorable outcomes in patients with BRCA mutations. Patients with germline mutations act as a proband for further testing of first-degree relatives (cascade screening).34 In this context, the results of this study reinforce recently published Indian guidelines, which recommend genetic testing in all patients with ovarian cancer and discuss the potential therapeutic and familial impact and likely challenges in the Indian context.35
There are some limitations of our study. Although the study was designed to include patients not selected by clinical features, it is difficult to establish that the included set is representative of the entire ovarian cancer population without access to the clinical and pathologic characteristics of the latter. Moreover, because India is a diverse country with many regions having populations of distinct genetic background, it is not certain that our sample captures this diversity. One exclusion criterion in our study was participation in any other research study, which could have introduced a selection bias. However, this is unlikely to be an important consideration because there were no ongoing clinical trials for BRCA mutation–positive patients at the participating sites during the recruitment period. We are unable to correlate the prevalence of BRCA mutations with sensitivity to chemotherapy because this was beyond the scope of this study. From a technical standpoint, copy number variations were not evaluated in this study, either as part of the analytical pipeline of NGS data36 or by multiplex ligation-dependent probe amplification technique, which could have resulted in some underestimation of pathogenic alterations in BRCA1 and BRCA2, especially large genetic rearrangements. Despite these limitations, our results provide valuable information relevant to the scope and need for germline testing in patients with ovarian, fallopian tube, or primary peritoneal cancers in India.
In conclusion, our results, in a cohort of Indian patients with epithelial ovarian, primary peritoneal, or fallopian tube cancers, suggest a high prevalence of germline pathogenic or likely pathogenic BRCA1/2 mutations with no association with age. Evaluation of germline mutations in BRCA1 and BRCA2 should be considered in most patients with this disease.
ACKNOWLEDGMENT
The authors would like to thank AstraZeneca Pharma India Ltd, Bangalore, and Tech-Observer India Pvt Ltd, New Delhi, the Contract Research Organization for supervising the study and providing administrative support. Medical writing assistance for the development of this manuscript was provided by Ms Prajakta Nachane, M. Pharm, from Covance Scientific Services & Solutions Pvt Ltd in accordance with GPP3 guidelines (http://www.ismpp.org/gpp3).
Appendix
FIG A1.
Study flow.
TABLE A1.
List of Participating Centers
TABLE A2.
Participants With BRCA1 and BRCA2 Gene Mutations
Sudeep Gupta
Research Funding: Roche, Sanofi, Johnson & Johnson, Amgen, Celltrion, Oncostem Diagnostics, Novartis
Shyam Aggarwal
Stock and Other Ownership Interests: NATCO Pharma, Lupin Pharmaceuticals
Honoraria: Bristol Myers Squibb, Novartis, Pfizer
Consulting or Advisory Role: Boringer Inglehiem, Novartis, AstraZeneca, Roche
Speakers' Bureau: Novartis, AstraZeneca, Intas, Pfizer
Research Funding: Cipla, NATC
Devavrat Arya
Honoraria: Eisai, Roche India, AstraZeneca, Merck Serono, Intas, Mylan, Novartis, Boehringer Ingelheim, Pfizer, Fresenius Kabi
Consulting or Advisory Role: Mylan, Intas
Speakers' Bureau: Mercke, Eisai
Rohit Kodagali
Employment: AstraZeneca
Research Funding: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the ESMO Asia 2019 as oral presentation (abstract no. 226O).
SUPPORT
Supported by AstraZeneca Pharma India Ltd.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Sudeep Gupta, Senthil Rajappa, Suresh Advani, Shyam Aggarwal, Satya Dattatreya Palanki, Shekhar Patil, Rohit Kodagali
Administrative support: Senthil Rajappa
Provision of study materials or patients: Sudeep Gupta, Senthil Rajappa, Amit Agarwal, Shyam Aggarwal, Chanchal Goswami, Devavrat Arya
Collection and assembly of data: Sudeep Gupta, Senthil Rajappa, Suresh Advani, Amit Agarwal, Shyam Aggarwal, Chanchal Goswami, Satya Dattatreya Palanki, Devavrat Arya, Shekhar Patil, Rohit Kodagali
Data analysis and interpretation: Sudeep Gupta, Senthil Rajappa, Suresh Advani, Amit Agarwal, Satya Dattatreya Palanki, Rohit Kodagali
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Sudeep Gupta
Research Funding: Roche, Sanofi, Johnson & Johnson, Amgen, Celltrion, Oncostem Diagnostics, Novartis
Shyam Aggarwal
Stock and Other Ownership Interests: NATCO Pharma, Lupin Pharmaceuticals
Honoraria: Bristol Myers Squibb, Novartis, Pfizer
Consulting or Advisory Role: Boringer Inglehiem, Novartis, AstraZeneca, Roche
Speakers' Bureau: Novartis, AstraZeneca, Intas, Pfizer
Research Funding: Cipla, NATC
Devavrat Arya
Honoraria: Eisai, Roche India, AstraZeneca, Merck Serono, Intas, Mylan, Novartis, Boehringer Ingelheim, Pfizer, Fresenius Kabi
Consulting or Advisory Role: Mylan, Intas
Speakers' Bureau: Mercke, Eisai
Rohit Kodagali
Employment: AstraZeneca
Research Funding: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sung H Ferlay J Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Ovarian Cancer Statistics | How Common is Ovarian Cancer. https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html [Google Scholar]
- 3.National Cancer Registry Program : Annual Reports 2012-2014. National Centre for Disease Informatics and Research. Indian Council of medical Research. https://ncdirindia.org/ncrp/ALL_NCRP_REPORTS/PBCR_REPORT_2012_2014/ALL_CONTENT/Printed_Version.htm [Google Scholar]
- 4.Basu P De P Mandal S, et al. : Study of “patterns of care” of ovarian cancer patients in a specialized cancer institute in Kolkata, eastern India. Indian J Cancer 46:28-33, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Cancer Statistics Review, 1975-2014—SEER Statistics. SEER. https://seer.cancer.gov/archive/csr/1975_2014/ [Google Scholar]
- 6.Maheshwari A, Kumar N, Mahantshetty U: Gynecological cancers: A summary of published Indian data. South Asian J Cancer 5:112-120, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh M Prasad CP Singh TD, et al. : Cancer research in India: Challenges & opportunities. Indian J Med Res 148:362-365, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whittemore AS, Harris R, Itnyre J: Characteristics relating to ovarian cancer risk: Collaborative analysis of 12 US case-control studies. II. Invasive epithelial ovarian cancers in white women. Collaborative Ovarian Cancer Group. Am J Epidemiol 136:1184-1203, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Armstrong N Ryder S Forbes C, et al. : A systematic review of the international prevalence of BRCA mutation in breast cancer. Clin Epidemiol 11:543-561, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel C, Fischer C: Breast cancer risks and risk prediction models. Breast Care (Basel) 10:7-12, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta A Vasudevan S Sharma SK, et al. : Germline BRCA1 and BRCA2 deleterious mutations and variants of unknown clinical significance associated with breast/ovarian cancer: A report from North India. Cancer Manag Res 10:6505-6516, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alsop K Fereday S Meldrum C, et al. : BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: A report from the Australian Ovarian Cancer Study Group. J Clin Oncol 30:2654-2663, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S, Parmigiani G: Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol 25:1329-1333, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall JM Lee MK Newman B, et al. : Linkage of early-onset familial breast cancer to chromosome 17q21. Science 250:1684-1689, 1990 [DOI] [PubMed] [Google Scholar]
- 15.Paley PJ Swisher EM Garcia RL, et al. : Occult cancer of the fallopian tube in BRCA-1 germline mutation carriers at prophylactic oophorectomy: A case for recommending hysterectomy at surgical prophylaxis. Gynecol Oncol 80:176-180, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Girolimetti G Perrone AM Santini D, et al. : BRCA-associated ovarian cancer: From molecular genetics to risk management. Biomed Res Int 2014:787143, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baretta Z Mocellin S Goldin E, et al. : Effect of BRCA germline mutations on breast cancer prognosis: A systematic review and meta-analysis. Medicine (Baltimore) 95:e4975, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun C Li N Ding D, et al. : The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: A systematic review of the literature with a meta-analysis. PLoS One 9:e95285, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y-W: Association of BRCA1/2 mutations with ovarian cancer prognosis. Medicine (Baltimore) 97:e9380, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolton KL Chenevix-Trench G Goh C, et al. : Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA 307:382-390, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huszno J, Kołosza Z, Grzybowska E: BRCA1 mutation in breast cancer patients: Analysis of prognostic factors and survival. Oncol Lett 17:1986-1995, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.UK Genetic Testing Network : Developing Testing Criteria for Familial Breast and Ovarian Cancer: Incorporating NICE Guidelines. https://silo.tips/download/uk-genetic-testing-network [Google Scholar]
- 23.Balmaña J Díez O Castiglione M, et al. : BRCA in breast cancer: ESMO clinical recommendations. Ann Oncol 20:19-20, 2009. (suppl 4) [DOI] [PubMed] [Google Scholar]
- 24.Colombo N Sessa C du Bois A, et al. : ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann Oncol 30:672-705, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Gori S Barberis M Bella MA, et al. : Recommendations for the implementation of BRCA testing in ovarian cancer patients and their relatives. Crit Rev Oncol Hematol 140:67-72, 2019 [DOI] [PubMed] [Google Scholar]
- 26.Marth C Hubalek M Petru E, et al. : AGO Austria recommendations for genetic testing of patients with ovarian cancer. Wien Klin Wochenschr 127:652-654, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daly MB Pilarski R Yurgelun MB, et al. : NCCN guidelines insights: Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic, version 1.2020. J Natl Compr Canc Netw 18:380-391, 2020 [DOI] [PubMed] [Google Scholar]
- 28.Singh J Thota N Singh S, et al. : Screening of over 1000 Indian patients with breast and/or ovarian cancer with a multi-gene panel: Prevalence of BRCA1/2 and non-BRCA mutations. Breast Cancer Res Treat 170:189-196, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Vaidyanathan K Lakhotia S Ravishankar HM, et al. : BRCA1 and BRCA2 germline mutation analysis among Indian women from south India: Identification of four novel mutations and high-frequency occurrence of 185delAG mutation. J Biosci 34:415-422, 2009 [DOI] [PubMed] [Google Scholar]
- 30.World Medical Association : World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310:2191-2194, 2013 [DOI] [PubMed] [Google Scholar]
- 31.ICH E6 (R2) Good Clinical Practice. European Medicines Agency, 2018. https://www.ema.europa.eu/en/ich-e6-r2-good-clinical-practice [Google Scholar]
- 32.Plon SE Eccles DM Easton D, et al. : Sequence variant classification and reporting: Recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat 29:1282-1291, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liede A Malik IA Aziz Z, et al. : Contribution of BRCA1 and BRCA2 mutations to breast and ovarian cancer in Pakistan. Am J Hum Genet 71:595-606, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caswell-Jin JL Zimmer AD Stedden W, et al. : Cascade genetic testing of relatives for hereditary cancer risk: Results of an online initiative. J Natl Cancer Inst 111:95-98, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malhotra H Kowtal P Mehra N, et al. : Genetic counseling, testing, and management of HBOC in India: An expert consensus document from Indian Society of Medical and Pediatric Oncology. JCO Glob Oncol 6:991-1008, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt AY Hansen T Ahlborn LB, et al. : Next-generation sequencing-based detection of germline copy number variations in BRCA1/BRCA2: Validation of a one-step diagnostic workflow. J Mol Diagn 19:809-816, 2017 [DOI] [PubMed] [Google Scholar]