PURPOSE
Nasopharyngeal carcinoma (NPC), a malignant neoplasm of the epithelium covering the nasopharynx, is a rare disease in most parts of the world. Epstein-Barr virus (EBV), the most potent oncogenic virus, coupled with environmental and genetic factors has been identified to play a role in the development of NPC. An array of methods for detecting the virus do exist, from serologic detection of antibodies to DNA amplification. There is paucity of local data on the status of EBV infection in relation to NPC within the region, and this study attempts to shed more light on the subject.
METHODS
This was a retrospective cross-sectional laboratory-based study on histologically confirmed, archived tissues from July 2015 to June 2019. Immunohistochemistry expression of latent membrane protein-1 (LMP-1) was used to detect EBV infection in the tissues.
RESULTS
A total of 71 cases were enrolled in this study. The mean age was 47.87 years ± 16.84 years with a male-to-female ratio of 1.5:1. There was a unimodal distribution of EBV detection, with the peak (26.8%) at 36-45 years. About 45.1% of the 71 samples tested positive for LMP-1, all of which were nonkeratinizing carcinoma. Nonkeratinizing carcinoma was the most common histopathologic subtype (n = 67; 94.4%), with the majority (38 of 67; 56.7%) being undifferentiated and 29 of 67 (43.3%) differentiated. Keratinizing and basaloid subtypes had two cases each, representing 2.8%.
CONCLUSION
A significant proportion of NPC, particularly nonkeratinizing histologic subtype, seems to show LMP-1 positivity by immunohistochemistry, which may be adopted in resource-constrained settings to detect EBV infection in these tissue biopsies.
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is a malignant neoplasm of the epithelium covering the nasopharynx and is the most common tumor affecting this anatomic area. In early stages, it has a tendency to localize to the lateral wall of the nasopharynx, particularly the fossa of Rosenmuller.1-3
CONTEXT
Key Objective
To determine a difference in expression of Epstein-Barr virus (EBV) infection among the different histologic types of nasopharyngeal carcinoma (NPC).
Knowledge Generated
There is a unimodal peak in age distribution among patients with NPC and EBV, latent membrane protein-1 detection, and only nondifferentiated keratinizing carcinoma, the most prevalent histologic type, tested positive for EBV.
Relevance
With the prospects of immunotherapy for NPC, adoption of routine EBV testing, even with immunohistochemistry in resource-constrained settings, would be an added advantage in identifying those who would benefit from this modality of treatment.
A rare disease in most parts of the world, it has a high incidence in regions such as Southeast Asia (Thailand and Philippines) and North Africa (Algeria and Morocco) with the highest incidence in Hong Kong.1,4 In Tanzania, it accounts for 14.2% of head and neck cancers at Muhimbili National Hospital.5
WHO has classified NPC into three distinct entities on the basis of the histologic findings. They are nonkeratinizing carcinoma, keratinizing squamous cell carcinoma, and basaloid squamous cell carcinoma. This is a modification from the traditional numerical classification of WHO I, II, and III, which has been replaced.1,2,6 Of the three variants, keratinizing carcinoma is of a greater proportion in the low-incidence areas in comparison with the higher-incidence areas.1,7,8
Genetic and environmental factors together with Epstein-Barr virus (EBV) infection play the greatest role in the development of NPC.9,10 EBV is detected in 100% of nonkeratinizing NPC1 regardless of geographical region, with low levels detected in keratinizing and basaloid in high endemic areas in comparison with higher levels in low-incidence areas for NPC. The association of EBV with NPC was postulated because of the presence of elevated IgG and IgA antibody titers to the viral capsid antigen, which correlated with the tumor burden, remission, and recurrence.11
EBV is a DNA virus of the Herpesviridae family and Gammaherpesviridae subfamily. It exhibits tropism for epithelial and lymphoid tissues because of the cellular expression of its receptor, which is also C3d receptor for the complement system. EBV has been linked to conditions such as infectious mononucleosis, oral hairy leucoplakia, and malignancies such as Burkitt's lymphoma, B-cell lymphomas, and gastric carcinomas.12 Virtually all human beings become infected with EBV at some point in their lifetime. For those in developing countries and lower socioeconomic status, infection is acquired in early childhood and remains subclinical but for those in the higher socioeconomic status, it is acquired in adolescence, mostly presenting with infectious mononucleosis.11 Transmission is primarily through saliva.11,13
Three types of latency infections have been identified with EBV depending on the viral products expressed. For NPC, it is type 2 latency infection with the expression of EBV nuclear antigen-1, latent membrane protein-1 (LMP-1), latent membrane protein-2, and EBV early RNA.14 These products are what are assayed to detect presence or absence of EBV infection. Detection methods include in situ hybridization (ISH), polymerase chain reaction, and immunohistochemistry (IHC). Serologic assays are becoming a commonplace in testing for EBV but they have their drawbacks such as a wide variability and lack of specificity with false positives seen in autoimmune disorders unrelated to EBV.15
LMP-1 regulates a number of signaling pathways in the pathogenesis of NPC, mainly through the three functional domains of its C terminal activating regions 1, 2, and 3.16,17 Through the NF-κB signaling pathway, LMP-1 regulates cell proliferation, apoptosis, transformation, metastasis, and invasion ultimately causing immortalization through the p65 subunit. LMP-1 plays a crucial role in the chemokine ligand 5–mediated cancer angiogenesis by activating the phosphoinositide 3-kinase protein kinase B (PI3K AKT) and hypoxia-inducible factor 1α signaling pathways. The Janus tyrosine kinase and signal transducer and transcription activator pathway activated by LMP-1 mediates expression of programmed cell death protein ligand 1 aiding the cancer cells escape the body's immune surveillance.17 LMP-1 makes more cells susceptible to the virus by secretion of matrix metalloproteases, which facilitate the degradation of the extracellular matrix. The stability of p53, a tumor suppressor gene, is interfered with by LMP-1, such that there is a lack of induced cell apoptosis in the cell cycle. LMP-1 also mimics the CD40 causing an overexpression of cancer stem cell markers leading to high metastatic features in NPC.18
METHODS
This was a retrospective, cross-sectional, laboratory-based study conducted at Muhimbili National Hospital, Tanzania. The study population was nasopharyngeal biopsies submitted to the histopathology unit to confirm diagnosis of NPC.
Inclusion Criteria
All histologically confirmed NPC biopsies submitted to the histopathology department from July 2015 to June 2019 were included.
Exclusion Criteria
Crushed tissues, unavailable tissue blocks, and cases lacking patient information were excluded.
Histopathologic Evaluation and Microscopy
This was done as previously described.19 Briefly, review of the diagnoses was done by the first author (V.E.S.) and a qualified, senior anatomical pathologist (A.R.M.) on hematoxylin and eosin sections and classification using the WHO system. EBV status was determined using immunohistochemical expression of LMP-1. Photomicrography was performed by the first author (V.E.S.) and the senior histopathologist (A.R.M.) using an Olympus (CX31RBSF Model) light microscope equipped with a digital camera (Olympus Corporation, Tokyo, Japan) as previously described.19,20
IHC Staining
This was done according to methods previously described.20 The paraffin-embedded tissue blocks were cut into 3 μm–thick sequential sections; the slides were dried, deparaffinized in xylene, and rehydrated through graded alcohol; tissue sections were circled with a hydrophobic pen (Dako pen); and endogenous peroxide activity was blocked for 15 minutes using peroxidase blocking solution (Dako ready-to-use reagent) and antigen retrieved by pressure cooking for 10 minutes in citrate buffer (pH = 6). Slides were allowed to cool using tap water for another 10 minutes and then rinsed with wash buffer (phosphate-buffered saline [PBS]) for 5 minutes. Sections were incubated with mouse antibody anti-EBV-LMP (CS1-4) prediluted by Medaysis for 30 minutes, washed with wash buffer for 5 minutes and thereafter incubated with a universal Horseradish peroxidase for 30 minutes and washed with PBS twice each for 3 minutes. Sections were then incubated with 3,3′diaminobenzidine (Dako DAB) for 10 minutes followed by rinsing in water for 2 minutes then counterstaining with hematoxylin for 17 dips and bluing for 5 minutes. Sections were dehydrated in the ascending grades of alcohol (ethanol 70%, ethanol 80%, ethanol 95%, and ethanol 100%) and then cleared in two changes of xylene for 5 minutes in each and covered using mounting medium by using Sakura Tissue Tek coverslipper. All these procedures were performed in the humidity chamber to make sure slides are not drying in between the steps. Both negative and positive controls were run alongside the tests.
IHC Evaluation
The slides were then reviewed under a light microscope. Brown granular cytoplasmic and membrane staining was interpreted as positive for EBV LMP-1, whereas bluish staining of the cytoplasm and membrane was interpreted as negative for EBV LMP-1. A positive control included a tissue known to have EBV infection, whereas for negative controls the test antibody was omitted and replaced by PBS.19
Furthermore, an internal negative control included parts of the section (stroma) that did not stain to the antibody.
Data Analysis
Data analysis was performed using Statistical Package for Social Sciences SPSS version 26. Results were presented in cross tabulations and figures. Association of EBV with age and sex as well as histologic classification of NPC was analyzed using Fisher's exact t test and a P value of < .05 was considered significant.
Ethical Approval
Ethical clearance was sought from the Research and Publication Committee of the School of Medicine and from the Senate Research and Publications Committee of the Muhimbili University of Health and Allied Sciences. Administrative permission to conduct the study and waiver of consent, to be allowed access to the tissue bank, were granted from Muhimbili National Hospital as this was a retrospective study.
RESULTS
General Findings
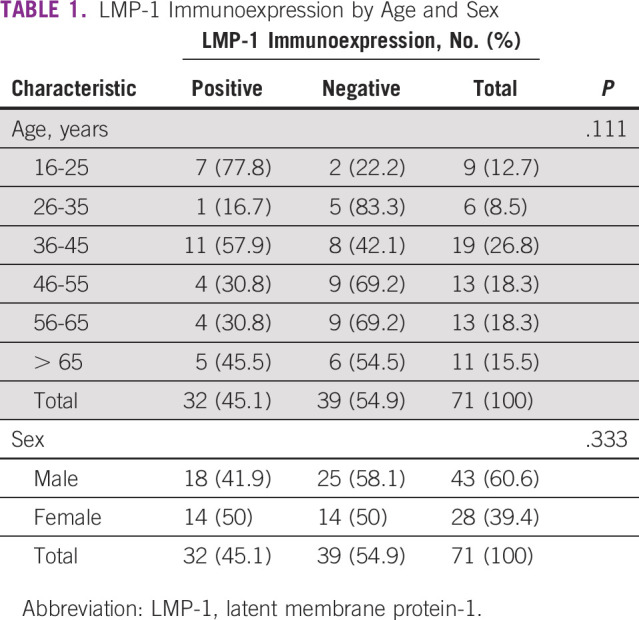
A total of 71 cases were enrolled to this study. The patients' age ranged from 16 to 82 years with a mean age of 47.87 years ± 16.84 years. The most frequent (26.8%) age group was 36-45 years, reflecting a unimodal peak. There were 43 males enrolled, with a male-to-female ratio of 1.5:1. Thirty-two (45.1%) of the 71 samples tested positive for LMP-1. There is a unimodal peak in EBV tissue immunoexpression across the age groups, with the highest positivity rate seen among the 36-45 years group (26.8%) and the lowest being among the 26- to 35-year-olds (8.5%) although this was not statistically significant (P value of .111; Table 1).
TABLE 1.
LMP-1 Immunoexpression by Age and Sex
Histopathologic and Immunohistologic Results
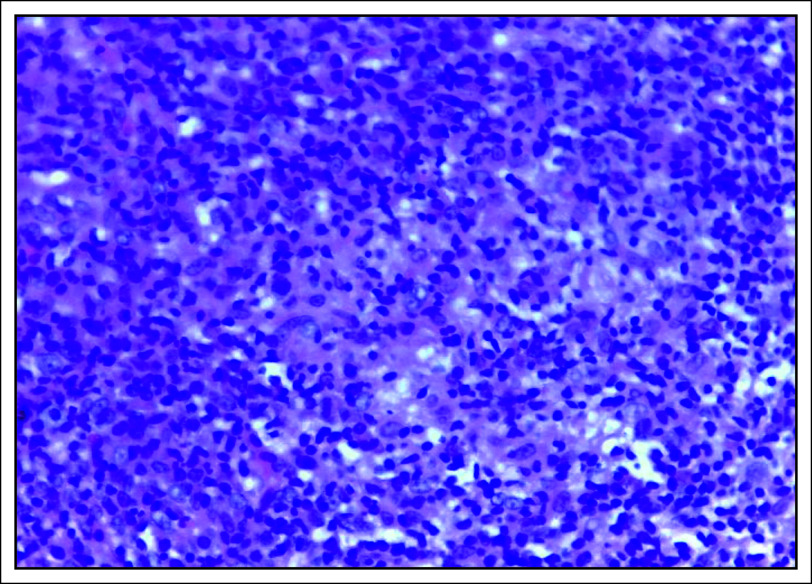
Nonkeratinizing carcinoma (Fig 1) was the most common (67 of 71; 94.4%) histopathologic subtype, majority (38 of 67; 56.7%) of which were undifferentiated and 29 of 67 (43.3%) differentiated, although this difference was not statistically significant (P value = .277). Furthermore, keratinizing squamous cell carcinoma and basaloid type each had two cases, representing 2.8%, respectively.
FIG 1.
Hematoxylin and eosin–stained photomicrograph showing nonkeratinizing undifferentiated carcinoma, ×40 magnification.
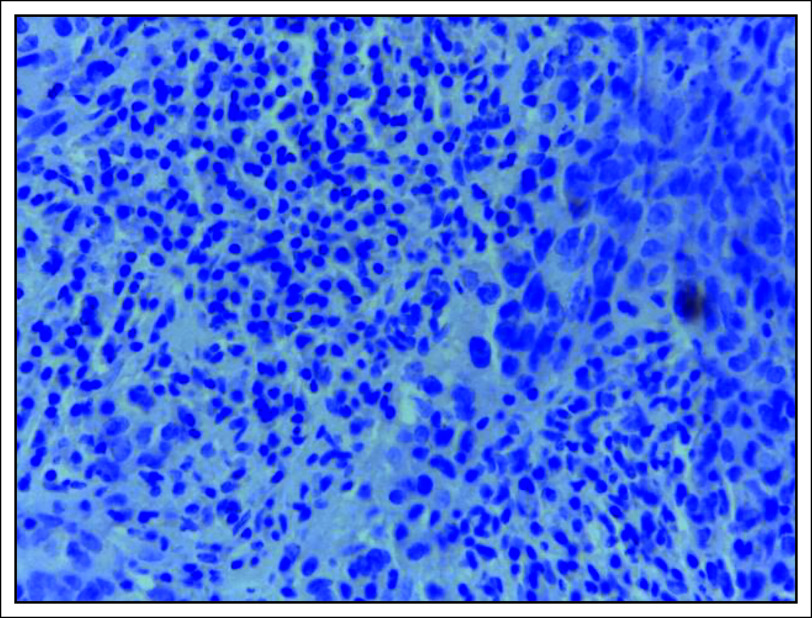
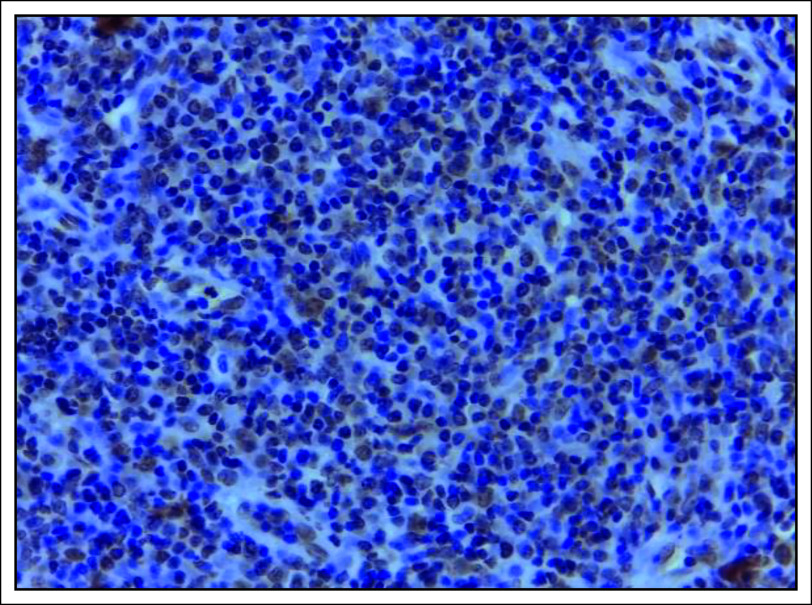
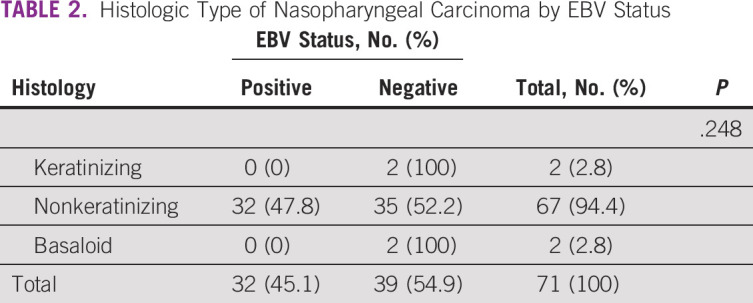
A significant number (32 of 71 [45.1%]) of the tissues tested positive for LMP-1 antibody by IHC and all these were nonkeratinizing carcinoma (Figs 2 and 3). On the contrary, keratinizing and basaloid carcinomas all tested negative for LMP-1, although this difference in EBV immunoreactivity across the three histologic types of NPC was not statistically significant, with a P value of .248 (Table 2). Moreover, majority (41 of 71 [58.1%]) of the males tested negative for LMP-1, whereas the females showed equal distribution of the same, although this difference was also not statistically significant, with a P value of .333 (Table 1).
FIG 2.
LMP-1 immunohistochemistry photomicrograph showing absence of staining of the nuclei and cytoplasm, which is defined as negative for LMP-1 immunoexpression, ×40 magnification. LMP-1, latent membrane protein-1.
FIG 3.
LMP-1 immunohistochemistry photomicrograph showing diffuse staining of the nuclei and cytoplasm, which is defined as positive for LMP-1 immunoexpression, ×40 magnification. LMP-1, latent membrane protein-1.
TABLE 2.
Histologic Type of Nasopharyngeal Carcinoma by EBV Status
DISCUSSION
Sub-Saharan Africa is considered to be a low endemic group for this particular malignancy with expected bimodal peak in age incidence and a male predominance.1,2,7,21 In this study, there was a unimodal peak at the 36-45 years age group similar to other studies within the continent. Edris et al22 in Sudan found the same unimodal peak at 41-60 years, so did other studies in Nigeria and Kenya with a peak at 40-49 and 31-40 years, respectively.8,23 Mremi et al24 in Tanzania found a peak at the 31-50 years age group. This similarity might be suggestive of comparable pathogenetic mechanisms and risk factors. However, a more extensive study on NPC in the region should be carried out to establish whether there is a change in age distribution pattern. In this study, there were more males who tested positive for EBV, a finding that is replicated in a study from Finland with 74% positive cases being male. Edris et al in Sudan found 60% of men tested positive for EBV, whereas Abdalazez et al found an equal number of positive and negative cases among men. EBV detection rates were high amongst females with NPC, with positivity rates of 82.7%, 66.6%, 77% in Finland and the last two in Sudan respectively.22,25,26
In our current study, the prevalence of EBV infection in NPC tissue sections was found to be 45% using LMP-1 IHC, although this was not statistically significant, which could partly be because of the small sample size. This compares well with a previous study from Sudan that showed almost similar findings at 41%.25 However, among studies that used a similar EBV detection method, a previous report from Finland showed a prevalence of 62%,26 whereas two in Nigeria showed 77.3% and 86%,27,28 respectively. These studies were in low endemic regions for NPC but the type of antibody used and the detection system applied might partly account for the differences seen in the rates. On the contrary, a previous report from Ghana showed a low 25% detection rate despite using EBV DNA polymerase chain reaction, a method considered to be superior to IHC.12 LMP-1 is expressed in all NPCs but the cells express variable levels of the protein within the tumor foci and this might explain the low detection rate of EBV observed in this study.29 Despite EBV early RNA ISH being considered the gold standard1 for EBV detection, other methods such as IHC are now being adapted in low-resource settings.25,27,28 This is especially true considering that a number of studies have noted that there is no significant difference among the various tests in detection rates.4,30-32 However, Fanaian et al33 compared the ISH and IHC methods of EBV detection and found that automated ISH had a sensitivity and specificity of 94% and 69%, respectively, whereas IHC had a sensitivity of 44% and specificity of 93%.
With advancements in genomic research, there are biomarkers such as p53R2, fibronectin, Mac-2 binding protein, plasminogen activator inhibitor-1, ceruloplasmin, and serum amyloid A that are being identified to be key in early diagnosis, response to treatment, and prognosis of NPC.34,35 It has been shown that expression of LMP-1 and Cripto-1, a member of the epidermal growth factor and a modulator in embryogenesis and oncogenesis, is positively related. LMP-1 can therefore be used as biomarker in tumor progression and metastasis.36 There is also promising use of immunotherapy in NPC with LMP-1–specific autologous cytotoxic lymphocytes–targeted therapy for recurrent disease. A vaccine based on LMP-1 has also shown tumor growth and metastasis suppression in mouse models; however, human trials are yet to be done.37
The histopathologic classification of NPC has undergone several changes, with the current classification by WHO identifying three distinct types: nonkeratinizing, keratinizing, and basaloid carcinoma.1,2 Nonkeratinizing carcinoma was the most prevalent type of NPC in this study, at 94.4%, which seems to be similar to the global picture across all risk strata.1,2,6,8,23,24,38 This histologic type is further classified into undifferentiated and differentiated with the former accounting for majority of the cases at 36%-95%, but this classification has no clinical significance and there might be cases wherein the two coexist in one tumor.1,2,6,23,26 In our current study of the various histologic types, EBV was apparently only detected in nonkeratinizing carcinoma tissues, a finding that seems comparable with reports from elsewhere.
In conclusion, this current study determined the association of EBV infection in NPCs at Muhimbili National Hospital, Tanzania, using the LMP-1 IHC and found that apparently about half (45%) of the cases tested positive, reflecting almost one in every two patients, although this appears to be lower than in majority of previous reports from elsewhere.
The age and sex distribution of NPC appeared similar to other studies from Africa showing a unimodal peak, but this is contrary to previous studies that indicate a bimodal peak with a majority of those tested positive for EBV being males and younger than 45 years. The most common histologic type of NPC appeared to be nonkeratinizing carcinoma, and only this type seemed to be associated with LMP-1 tissue positivity.
With the prospects of immunotherapy for NPC, adoption of routine EBV testing would be an added advantage in identifying those who would benefit from this modality of treatment.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Provision of study materials or patients: Valerie E. Salano, Amos R. Mwakigonja
Collection and assembly of data: Valerie E. Salano, Amos R. Mwakigonja
Data analysis and interpretation: Valerie E. Salano, Amos R. Mwakigonja, Ashfaq Abdulshakoor, Aveline A. Kahinga
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Leon B John E Peter R, et al. (eds): World Health Organization Classification of Tumors Pathology & Genetics Head and Neck Tumors. Lyon, France, IARC Press, 2005, pp 81-97 [Google Scholar]
- 2.Stelow EB, Wenig BM: Update from the 4th edition of the World Health Organization classification of head and neck tumours: Nasopharynx. Head Neck Pathol 11:16-22, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yua F Lu Y K Tay J, et al. : Establishment of EBV latency in nasopharyngeal tumor epithelial cells by in vivo cell-mediated transfer infection. Otorhinolaryngol Neck Surg 3:1-7, 2018 [Google Scholar]
- 4.Lao TD, Anh NTH, Nguyen DH: Pattern of EBNA-1, EBNA-2, LMP-1 and LMP-2 in nasopharyngeal carcinoma in Vietnamese patients. International Conference on the Development of Biomedical Engineering in Vietnam, Ho Chi Minh City, Vietnam, June 2016 (pp 243-247)
- 5.Mwansasu C, Liyombo E, Moshi N: Pattern of head and neck cancers among patients attending Muhimbili National Hospital Tanzania. Tanzan J Health Res 17, 2015. 10.4314/thrb.v17i1 [DOI] [Google Scholar]
- 6.Umar B, Ahmed R: Nasopharyngeal carcinoma, an analysis of histological subtypes and their association with EBV, a study of 100 cases of Pakistani population. Asian J Med Sci 5:16-20, 2014 [Google Scholar]
- 7.Salehiniya H Mohammadian M Mohammadian-Hafshejani A, et al. : Nasopharngeal cancer in the world: Incidence, mortality and risk factors. World Cancer Res J 5:e1046, 2018 [Google Scholar]
- 8.Muchiri M: Demographic study of nasopharyngeal carcinoma in a hospital setting. East Afr Med J 2008;85:181-186 [DOI] [PubMed] [Google Scholar]
- 9.Flint PW Haughey BH Lund VJ, et al. : Head and Neck Surgery, in: Cummings Otolaryngology (ed 6). Saunders, Philadelphia, PA, 2015, pp 1420-1431 [Google Scholar]
- 10.Chamberlin M, McGrath J, Waskell L: EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopahrynx. Nature: 228:227-231, 1970 [DOI] [PubMed] [Google Scholar]
- 11.Raab-Traub N: Epstein–Barr virus in the pathogenesis of NPC. Semin Cancer Biol 12:431-441, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Asante DB Asmah RH Adjei AA, et al. : Detection of human papillomavirus genotypes and Epstein-Barr virus in nasopharyngeal carcinomas at the Korle-Bu Teaching Hospital, Ghana. Sci World J 2017:2721367, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jha HC Pei Y Robertson ES, et al. Epstein–Barr virus: Diseases linked to infection and transformation. Front Microbiol 2016;7:1-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borthakur P Kataki K Keppen C, et al. : Expression of Epstein Barr virus encoded EBNA1 and LMP1 oncoproteins in nasopharyngeal carcinomas from northeast India. Asian Pac J Cancer Prev 17:3411-3416, 2016 [PubMed] [Google Scholar]
- 15.Smatti MK Al-Sadeq DW Ali NH, et al. : Epstein–Barr virus epidemiology, serology, and genetic variability of LMP-1 oncogene among healthy population: An update. Front Oncol 8:211, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandes Q Merhi M Raza A, et al. : Role of Epstein–Barr virus in the pathogenesis of head and neck cancers and its potential as an immunotherapeutic target. Front Oncol 8:257, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo Y Liu Y Wang C, et al. : Signaling pathways of EBV-induced oncogenesis. Cancer Cell Int 21:93, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheerathodi MR, Meckes DG: The Epstein–Barr virus LMP1 interactome: Biological implications and therapeutic targets. Future Virol 13:863-887, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mremi A Yahaya J Abraham Z, et al. : The role of a minimum immunohistochemical antibody panel in confirming undifferentiated nasopharyngeal carcinoma: A cross-sectional study at the Muhimbili National Hospital, Dar-es-Salaam, Tanzania. Niger Med J 60:279, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaaya EE Castaños-Velez E Ekman M, et al. : AIDS and non AIDS-related malignant lymphoma in Tanzania. Afr Health Sci 6:69-75, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nasopharyngeal Carcinoma, 2014 Review of Cancer Medicines on the WHO List of Essential Medicines. Geneva, Switzerland, Union for International Cancer Control, 2014, pp 1-9 [Google Scholar]
- 22.Edris A Mohamed MA Mohamed NS, et al. : Molecular detection of Epstein-Barr virus in nasopharyngeal carcinoma among Sudanese population. Infect Agent Cancer 11:60, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yates S Iliyasu Y Ahmed S, et al. : Nasopharyngeal carcinoma at the Ahmadu Bello University Teaching Hospital, Zaria: A 22-year histopathological review (1992–2013). Arch Med Surg 3:24, 2018 [Google Scholar]
- 24.Mremi A: The Histopatholical Diagnosis of Nasopharyngeal Carcinoma at Muhimbili National Hospital. Muhimbili University of Health and Allied Sciences, Tanzania, 2015 [Google Scholar]
- 25.Adam A Abdullah N El Hassan L, et al. : Detection of Epstein-Barr Virus in Nasopharyngeal Carcinoma in Sudanese by in Situ Hybridization. J Cancer Ther 5:517-522, 2014 [Google Scholar]
- 26.Ruuskanen M Irjala H Minn H, et al. : Epstein-Barr virus and human papillomaviruses as favorable prognostic factors in nasopharyngeal carcinoma: A nationwide study in Finland. Head Neck 41:349-357, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yates SM Iliyasu Y Ahmed SA, et al. : Immunohistochemical expression of Epstein-Barr virus latent membrane protein-1 in nasopharyngeal carcinoma. Ann Trop Pathol 9:99-103, 2017 [Google Scholar]
- 28.Omoseebi O Akinde OR Obadofin OO, et al. : Association of Epstein–Barr virus (EBV) with malignancy of the nasopharynx in Lagos, Nigeria. Ann Trop Pathol 8:29-33, 2018 [Google Scholar]
- 29.Shair KHY, Reddy A, Cooper VS: New insights from elucidating the role of LMP1 in nasopharyngeal carcinoma. Cancers (Basel) 10:1-22, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi ZL Han XQ Hu J, et al. : Comparison of three methods for the detection of Epstein-Barr virus in Hodgkin’s lymphoma in paraffin-embedded tissues. Mol Med Rep 7:89-92, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Adam AAM Abdullah NE El Hassan LAM, et al. : Detection of Epstein-Barr virus in nasopharyngeal carcinoma in Sudanese by in situ hybridization. J Cancer Ther 5:517, 2014 [Google Scholar]
- 32.Kurniawan AN Kodariah R Elisabeth -M, et al. : Evaluation of EBV-LMPI as prognostic indicator of nasopharyngeal carcinoma in fndonesian patients. Med J Indonesia 11:81, 2002 [Google Scholar]
- 33.Fanaian NK Cohen C Waldrop S, et al. : Epstein-Barr virus (EBV)-encoded RNA: Automated in-situ hybridization (ISH) compared with manual ISH and immunohistochemistry for detection of EBV in pediatric lymphoproliferative disorders. Pediatr Dev Pathol 12:195-199, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Chen J Li S Xiao Y, et al. : p53R2 as a novel prognostic biomarker in nasopharyngeal carcinoma. BMC Cancer 17:846, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S-Q Pan S-M Liang S-X, et al. : Research status and prospects of biomarkers for nasopharyngeal carcinoma in the era of high-throughput omics (Review). Int J Oncol 58:9, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye Q Li J Wang X, et al. : In vivo and in vitro study of co-expression of LMP1 and Cripto-1 in nasopharyngeal carcinoma. Braz J Otorhinolaryngol 86:617-625, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teow S-Y, Yap H-Y, Peh S-C: Epstein-Barr virus as a promising immunotherapeutic target for nasopharyngeal carcinoma treatment. J Pathog 2017:1-10, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang ET, Adami HO: The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 15:1765-1777, 2006 [DOI] [PubMed] [Google Scholar]