PURPOSE
Patients with cancer are at increased risk for unfavorable outcomes from COVID-19. Knowledge about the outcome determinants of severe acute respiratory syndrome coronavirus 2 infection in this population is essential for risk stratification and definition of appropriate management. Our objective was to evaluate prognostic factors for all-cause mortality in patients diagnosed with both cancer and COVID-19.
METHODS
All consecutive patients with cancer hospitalized at our institution with COVID-19 were included. Electronic medical records were reviewed for clinical and laboratory characteristics potentially associated with outcomes.
RESULTS
Five hundred seventy-six consecutive patients with cancer and COVID-19 were included in the present study. An overall in-hospital mortality rate of 49.3% was demonstrated. Clinical factors associated with increased risk of death because of COVID-19 were age over 65 years, Eastern Cooperative Oncology Group performance status > 0 zero, best supportive care, primary lung cancer, and the presence of lung metastases. Laboratory findings associated with a higher risk of unfavorable outcomes were neutrophilia, lymphopenia, and elevated levels of D-dimer, creatinine, C-reactive protein, or AST.
CONCLUSION
A high mortality rate in patients with cancer who were diagnosed with COVID-19 was demonstrated in the present study, emphasizing the need for close surveillance in this group of patients, especially in those with unfavorable prognostic characteristics.
INTRODUCTION
From a local epidemic of respiratory disease initially described in the Chinese province of Wuhan, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the resulting COVID-19 emerged as a pandemic that has transformed health care delivery around the globe.1 Early reports already indicated that the risk of morbidity and mortality from COVID-19 was not uniform across infected patients but was rather dependent on patients' characteristics such as age and comorbidities.2 Thus, concerns about the risk of unfavorable outcomes in patients with cancer, because of immunosuppression from the disease and antineoplastic therapies,3 were raised by initial studies reporting higher mortality rates in this population.4
CONTEXT
Key Objective
To evaluate the mortality rates of patients with cancer hospitalized with COVID-19 in Brazil and the factors driving their outcomes.
Knowledge Generated
In this cohort of 576 patients with cancer and COVID-19, an overall in-hospital mortality rate of 49.3% was demonstrated. Among the clinical factors associated with increased risk of death were age, Eastern Cooperative Oncology Group performance status greater than zero, best supportive care alone, primary lung cancer, and the presence of lung metastases. Patients with signs of renal and hepatic dysfunction and laboratory alterations in inflammatory parameters at the time of COVID-19 diagnosis were also at increased risk of death. Mortality was higher for patients with metastatic disease than for those with no evidence of disease, although no significant interaction was demonstrated between recent cancer treatment and outcomes.
Relevance
This is the largest Latin American cohort to assess the characteristics, determinants of mortality, and clinical outcomes of hospitalized patients with cancer diagnosed with COVID-19. Our data emphasize the need for close surveillance in this group of patients, and provide local evidence on determinants of unfavorable outcomes.
Clinicians involved in cancer treatment are faced with potentially competing risks of complications from cancer progression and from COVID-19. Data regarding the interaction between these conditions, critical to inform clinical practice, are still scarce and, often, conflicting.5-7
Brazil is one of the most affected countries, currently accumulating more than eight million confirmed cases and more than 200,000 deaths.8 Thus, greater knowledge of local data regarding the COVID-19 outbreak is of utmost importance for the management of patients with cancer.9 Therefore, the present study evaluates the clinical characteristics, outcomes, and determinants of mortality in a high-risk group of patients with cancer who were infected with SARS-Cov-2.
METHODS
Study Design and Participants
Our study sample included all consecutive patients with cancer hospitalized with COVID-19 infection in the Instituto do Cancer do Estado de São Paulo—Brazil, from March 31, 2020, until September 2, 2020. We reviewed the electronic medical records of all patients. The majority of patients had confirmed COVID-19 on the basis of a reverse transcriptase-polymerase chain reaction from nasopharyngeal swab or positive serology for SARS-Cov-2. A minority of patients with a positive epidemiology, clinical presentation, and radiological alterations highly suggestive of SARS-Cov-2 infection were also included in the analysis to respect the inclusion methodology of all consecutive patients, thus minimizing the risk of selection bias. All patients had a confirmed diagnosis of invasive hematologic or solid malignancy.
COVID-19 management followed Instituto do Cancer do Estado de São Paulo's Infection Control Committee Guidelines, in accordance with University of Sao Paulo Medical School's Infectious Disease Department protocols. Management guidelines were regularly updated as new evidence became available. During the study period, patients were tested for SARS-Cov-2 infection mainly if they presented symptoms with clinical deterioration. Patients who had light symptoms, not requiring hospitalization, were not systematically tested because of the limited number of tests available at the time. Those patients were treated in the outpatient care setting and received proper orientation regarding isolation and symptom management. A few asymptomatic patients were diagnosed because of COVID-19 screening before surgical procedures.
Data were collected on electronic case report forms (RedCap), including demographic characteristics, comorbidities, Eastern Cooperative Oncology Group performance status (ECOG PS), oncologic history, COVID-19 symptoms, laboratory tests, treatment received, and outcomes. The symptoms included in this analysis were those reported by the patients and described in the medical records by the attending physicians at the time of hospital admission. Best supportive care (BSC) was defined in this study as the group of patients who, before SARS-Cov-2 infection, were receiving supportive treatments alone, without systemic anticancer therapy.
The study's primary objective was to evaluate prognostic factors for all-cause mortality in patients diagnosed with both cancer and COVID-19. The Local Ethics Committee approved this study (CAAE 31688420.7.0000.0068). Signed informed consent was applied for patients who could be contacted.
Statistical Analysis
Descriptive statistics were used to summarize characteristics and outcomes from the study population. Continuous variables were presented using median and range, whereas categorical variables were presented using absolute and relative numbers.
Mortality was evaluated as the number of deaths during the hospitalization with COVID-19 in relation to the total number of cancer patients with confirmed SARS-Cov-2 infection.
Factors associated with COVID-19 mortality were evaluated using logistic regression. The following variables were evaluated in the univariate logistic regression: age, ECOG PS, diabetes, hypertension, cardiopathy, smoking history, chronic use of corticosteroids, chronic use of angiotensin II receptor blocker or angiotensin-converting enzyme inhibitors, primary tumor site, oncologic treatment status, lung metastases, chemotherapy or surgery 30 days before COVID-19 diagnosis, COVID-19 symptoms (dyspnea, ageusia, and anosmia), use of therapies directed to COVID-19, and laboratory tests (neutrophilia, lymphopenia, D-dimer, creatinine, C-reactive protein, AST, and ALT).
Variables were selected for inclusion in multivariate analysis if they presented a P < .05 in the univariate logistic regression. Two multivariate models were performed, one with clinical and demographic factors and the other with laboratory tests at the time of COVID-19 diagnosis.
No imputation method was used for missing data. Statistical analyses were performed using Stata software, version 15.1 (StataCorp, TX). P values were considered statistically significant when < .05.
RESULTS
Clinical and Demographic Characteristics
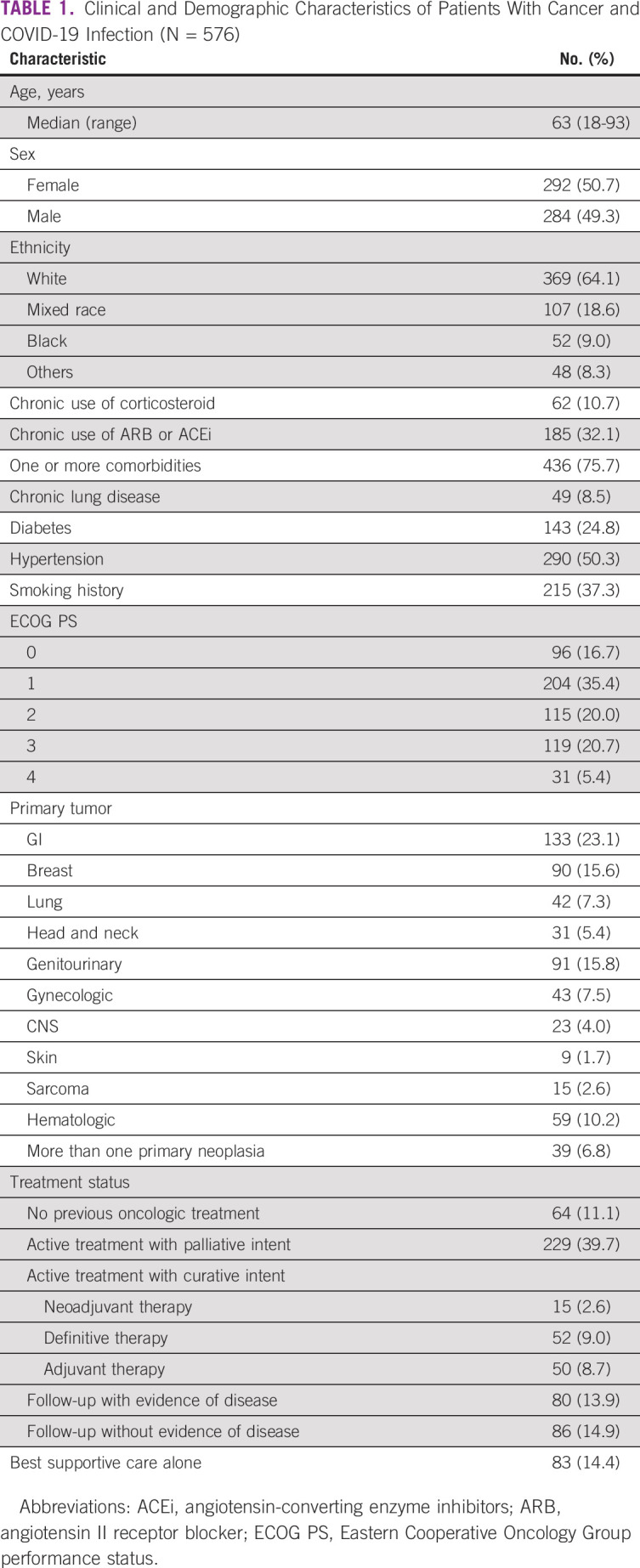
A total of 576 consecutive patients with cancer and confirmed COVID-19 were included in the study. The mean age was 63 years (range 18-93 years). Most patients (75.7%) had at least one comorbidity; 290 (50.3%) had hypertension, 143 (24.8%) had diabetes, and 215 (37.3%) had a smoking history.
Main primary sites for solid tumors were GI (n = 133, 23.1%), breast (n = 90, 15.6%), genitourinary (n = 91, 15.8%), and lung (n = 43, 7.3%). Fifty-nine patients (10.2%) had a hematologic malignancy. Most patients (n = 490, 85.0%) had an active oncologic disease or had received active oncologic treatment in the past three months. Only 10 patients had received treatment with an immune checkpoint inhibitor. Eighty-three patients (14.4%) were receiving BSC alone. Table 1 summarizes clinical and demographic features from the study population.
TABLE 1.
Clinical and Demographic Characteristics of Patients With Cancer and COVID-19 Infection (N = 576)
SARS-Cov-2 Infection
Symptoms most frequently reported by patients were dyspnea (n = 391, 62.8%), cough (n = 255, 44.3%), and fever (n = 218, 37.8%). Few patients reported diarrhea (10.6%), anosmia (6.4%), or ageusia (9.9%). Most patients had a positive SARS-Cov-2 reverse transcriptase-polymerase chain reaction (n = 556, 96.5%). The other patients had clinical manifestations associated with COVID-19 plus a positive serology (n = 15, 2.6%) or tomographic findings consistent with COVID-19 (n = 5, 0.9%). Overall, 78.9% of the patients had a tomographic image suggestive of COVID-19 infection.
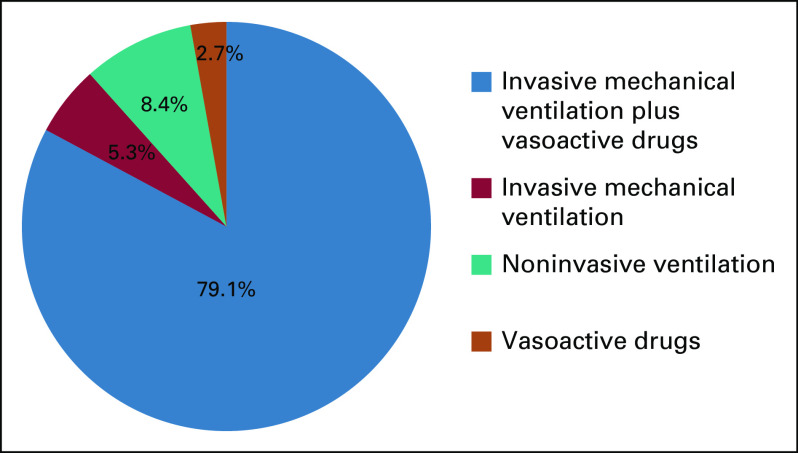
Seventy-six patients (13.2%) did not require hospitalization and were treated at home. On the other hand, 495 patients (85.9%) required hospitalization and 225 patients (39.1%) were hospitalized in an intensive care unit (ICU). Among patients who went to ICU, 190 (84.4%) received invasive mechanical ventilation and 184 (81.8%) received vasoactive drugs. Figure 1 summarizes the supportive therapy received by patients treated in the ICU.
FIG 1.
Supportive therapy received by patients with cancer hospitalized in an intensive care unit during COVID-19 infection (n = 225).
Considering COVID-19–specific treatment, 340 patients (59%) received a therapy that was considered directed to the COVID-19 infection. Azithromycin was given to 328 patients (56.9%), sometimes for the treatment of bacterial coinfections. Ivermectin and chloroquine were included in the therapeutic regimens used by 1.7% and 2.1% of patients, respectively. Most patients received anticoagulation, being either prophylactic (56.9%) or therapeutic (14.2%).
Outcomes
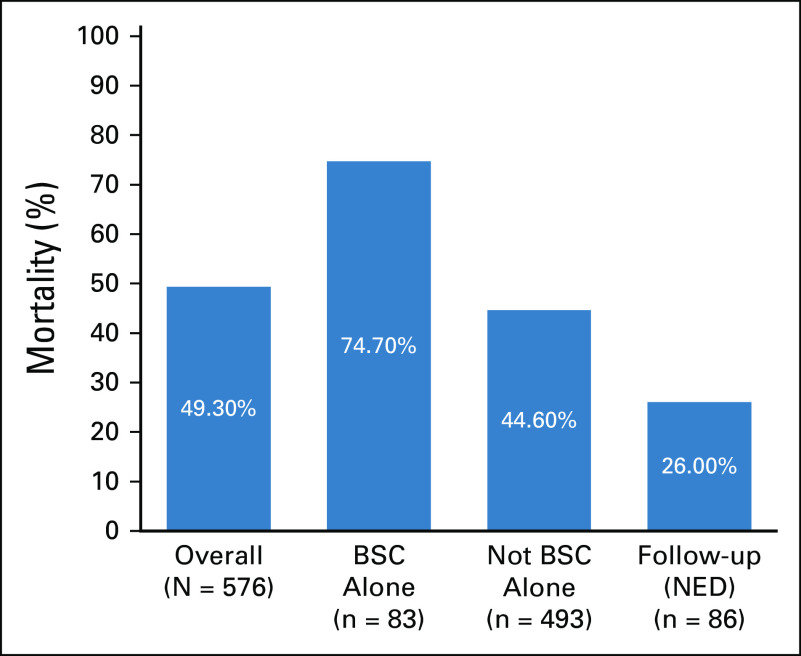
In the overall study population, 284 individuals with cancer (49.3%) died after diagnosis of COVID-19. The mortality rate was higher (74.7%) among patients with cancer receiving BSC alone before COVID-19. When BSC patients were excluded, the mortality rate declined to 44.6% (n = 220/493). In the cohort of patients who died and were not receiving BSC alone (n = 220), 46 patients (19.5%) did not go to an ICU. This decision took into consideration the cancer prognosis, other comorbidities, and performance status. Our center did not experience shortage of ICU beds.
Among the 86 patients who were in follow-up with no evidence of disease, the mortality rate was 26%. Figure 2 shows a graphic representation of the mortality because of COVID-19.
FIG 2.
Graphical representation of mortality rates of patients with cancer and COVID-19 infection. BSC, best supportive care; NED, no evidence of disease.
Mortality rates among patients diagnosed with COVID-19 between March and May and between June and September were 53.7% and 46.1%, respectively (odds ratio [OR], 0.73; P = .072; 95% CI, 0.52 to 1.02).
Prognostic Factors
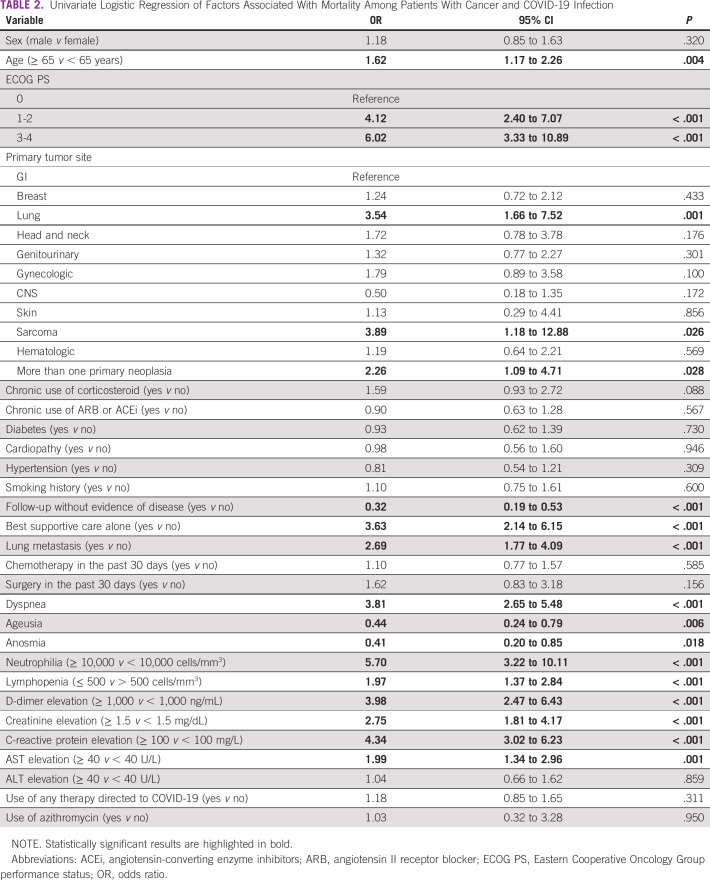
Univariate logistic regression showed that age above 65 years (OR, 1.62; P = .004) and ECOG PS (1-2 v 0: OR, 4.12; P < .001; 3-4 v 0: OR, 6.02; P < .001) were associated with higher mortality. No association was seen between mortality and other comorbidities besides cancer, although 75.7% of the patients included had one or more comorbidities.
Regarding the oncologic history, mortality because of COVID-19 infection was higher among patients with lung cancer (OR, 3.54; P = .001), sarcoma (OR, 3.89; P = .026), and more than one primary neoplasia (OR, 2.26; P = .028). Moreover, patients who presented with lung metastasis had a risk of death 2.69 times higher than those without it (P < .001).
Importantly, patients receiving BSC alone for their cancer treatment had a higher risk of death because of COVID-19 infection (OR, 3.63; P < .001). On the other hand, patients who were in follow-up without evidence of disease had better outcomes (OR, 0.32; P < .001).
Symptoms related to COVID-19 infection were also associated with outcomes. Patients who reported anosmia (OR, 0.44; P = .006) and ageusia (OR, 0.41; P = .018) had lower mortality. Otherwise, patients with dyspnea had a higher risk of death (OR, 3.81; P < .001).
Finally, alterations in six laboratory markers at the time of COVID-19 diagnosis were associated with a higher mortality: neutrophilia > 10,000 cells/mm3 (OR, 5.70; P < .001), lymphopenia lower than 500 cells/mm3 (OR, 1.97; P < .001), D-dimer > 1,000 ng/mL (OR, 3.98; P < .001), creatinine > 1.5 mg/dL (OR, 2.75; P < .001), C-reactive protein > 100 mg/L (OR, 4.34; P < .001), and AST > 40 U/L (OR, 1.99; P = .001). The use of commonly used therapies directed to COVID-19 did not seem to affect mortality. Table 2 shows the results of univariate logistic regression.
TABLE 2.
Univariate Logistic Regression of Factors Associated With Mortality Among Patients With Cancer and COVID-19 Infection
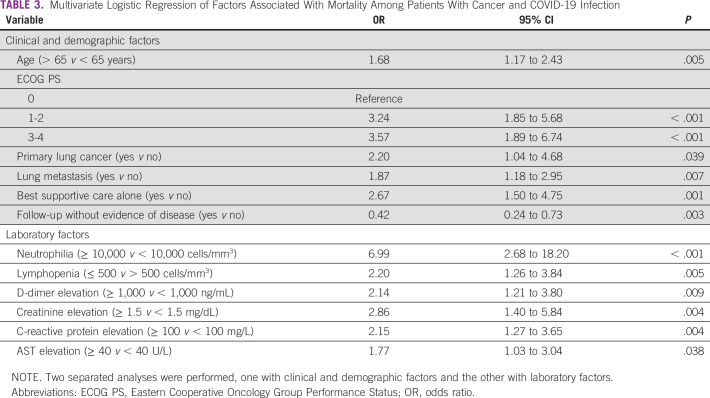
Variables included in the multivariate model of clinical and demographic factors were age, EGOG PS, primary lung cancer, BSC alone, and follow-up without evidence of disease. The multivariate model showed that age older than 65 years, ECOG PS, primary lung cancer, lung metastases, and BSC alone remained associated with a higher risk of death. Follow-up without evidence of disease continued to be a favorable prognostic factor. Additionally, neutrophilia, lymphopenia, D-dimer elevation, R-reactive protein elevation, creatinine elevation, and AST elevation remained all independently associated with mortality in the multivariate model for laboratory factors. The results of the multivariate analyses are detailed in Table 3.
TABLE 3.
Multivariate Logistic Regression of Factors Associated With Mortality Among Patients With Cancer and COVID-19 Infection
DISCUSSION
In this retrospective, single-institute cohort, including 576 consecutive patients with cancer and COVID-19, an overall in-hospital mortality rate of 49.3% was demonstrated. This rate was significantly higher in patients in BSC and lower in those without evidence of disease. Clinical factors significantly associated with increased risk of death because of COVID-19 were age over 65 years, ECOG PS > 0, BSC, primary lung cancer, and the presence of lung metastases. In addition, laboratory findings associated with a higher risk of unfavorable outcomes were neutrophilia, lymphopenia, and elevated levels of D-dimer, creatinine, C-reactive protein, and AST.
The COVID-19 pandemic profoundly affected health care systems around the world, including centers dedicated to cancer care.5 Early concerns about the risk of unfavorable outcomes because of COVID-19 in patients with cancer emerged from the rationale of systemic immunosuppressive state caused by the underlying malignancy and anticancer treatments.3,10 Indeed, early reports suggested that patients with cancer and COVID-19 had a higher risk of negative outcomes.11 Given the necessity of stratifying patients with cancer according to the risk of unfavorable outcomes, as well as expanding our understanding of the interaction between SARS-Cov-2 virus and both cancer and antineoplastic therapies, the search for determinants of severe COVID-19 in patients with cancer became of paramount importance. Among the clinical factors identified as predictors of increased risk of death in our study, increased age and performance status were also systematically reported in other similar surveys, including the cohorts of Gustave Roussy Hospital,12 COVID-19 and Cancer Consortium (CCC19), and a multi-institution registry including centers across the United States, Canada, and Spain13 and also in the Italian cohort from the Veneto region.14 In our cohort, a BSC treatment intent defined before SARS-Cov-2 infection was independently associated with an increased risk of death compared with patients under active cancer treatment and those in follow-up. Perhaps this should not come as a surprise, considering the short expected survival for this group anyway. Several cohorts evaluating patients with cancer and COVID-19 have not reported the proportion of included patients who were on BSC,13,15 which might have influenced the reported mortality rates, since these patients appear to be at higher risk of unfavorable outcomes. Thus, the outcomes of patients in BSC should be systematically reported both combined and separately from the overall population, since the absence of this subgroup analysis may lead to misinterpretation (and possibly overestimation) of overall mortality data of patients with cancer. A primary lung cancer and the presence of lung metastases were independently associated with an increased risk of death in our cohort, a mortality determinant also reported in another Brazilian cohort.9 As the lung is one of the target organs more commonly affected by COVID-19, the lung involvement by neoplasia could be associated with higher rates and severity of respiratory complications, possibly as a result of worse baseline lung function.16
Another point of interest was the possible impact that cancer therapies could have on the outcomes of patients with COVID-19, since these treatments commonly lead to immunologic modulation including suppression3 or stimulation.17 The outcomes of SARS-Cov-2 infection appear to be largely determined by the inflammatory response triggered by the virus.18 In our cohort, recent use of chemotherapy was not associated with an increased risk of death, as was also demonstrated by the large cohorts from CCC1913 and UK Coronavirus Cancer Monitoring Project.15 However, in a recent meta-analysis that included data from 16 studies, patients who received chemotherapy within the past 30 days before COVID-19 diagnosis had an increased risk of death, even after adjusting for confounding variables (age, sex, cancer type, cancer treatment subtype, duration of cancer diagnosis, smoking status, obesity, performance status, presence of metastasis, and comorbidities),19 cautioning about the need for attentive care for patients undergoing cytotoxic chemotherapy because of a possible increased risk of unfavorable outcomes.
Laboratory data have also been explored as easily accessible predictors of COVID-19 outcomes in patients with cancer. Among the baseline biologic determinants of overall survival in our cohort, lymphopenia, elevated inflammatory markers (eg, C-reactive protein), and elevated D-dimer have also been reported in other studies in patients with12,20 and without cancer.21,22 Noteworthily, in sepsis, neutrophils are commonly hyperactivated with delayed apoptosis disorder, along with the depletion and exhaustion of CD4 and CD8 T lymphocytes as a result of apoptosis,23 which are frequent events in patients with severe COVID-19.24 Thus, increases in neutrophil count and lymphocyte depletion, identified as predictors of a higher risk of death in our cohort, may occur as a result of excessive inflammation and immune suppression in sepsis triggered by SARS-CoV-2 infection and have already been correlated with severity and mortality in patients with COVID-19.22 In our study, elevated liver enzymes and creatinine, previously associated with worse outcomes in patients without cancer in other studies,21 were also independently related to higher mortality rate in patients with cancer. Taken together, clinical and laboratory data associated with a higher risk of unfavorable outcomes in patients with cancer and COVID-19 are important tools for stratifying patients into risk groups, thus allowing appropriate preventive measures and early identification of patients under higher risk for severe disease.
The general population of patients hospitalized because of COVID-19 has an estimated mortality that varies between 21% and 38%.25,26 Considering the Brazilian population, a recent large cohort with more than 250,000 hospitalized patients showed a mortality rate of 38%. In comparison with these numbers, hospitalized patients with cancer and COVID-19 appear to have an even higher risk of death.27 Different studies using variable mortality definitions and including different patient populations reported markedly heterogeneous mortality rates in patients with cancer hospitalized because of COVID-19, ranging from 7% to 55%.28,29 A meta-analysis evaluating in-hospital mortality in patients with cancer and COVID-19 including 13 studies (2,922 patients) that analyzed data from predominantly hospitalized patients demonstrated a pooled 30-day mortality rate of 30% (95% CI, 25% to 35%).27
The heterogeneity of in-hospital mortality rates between different studies heightens the discussion about what drives the increased mortality in patients with cancer and COVID-19 and whether this is related to underlying patient characteristics, cancer type, or cancer therapies.27 The population included in our study included predominantly sick, hospitalized patients and was enriched with high-risk characteristics, such as age (median age 63 years), concomitant comorbidities (75.7%), compromised performance status (46% with ECOG PS ≥ 2), and active disease and/or active oncologic treatment (85%), which might have contributed to the high rate of in-hospital mortality. The severity of COVID-19 in our population is also illustrated by the high ICU admission rate (39.1%), markedly higher than that reported in other cohorts (7% and 14% in the UK Coronavirus Cancer Monitoring Project15 and CCC1913 cohorts, respectively). Additionally, caution should be taken when comparing mortality rates reported by different studies, since some of them jointly analyze the mixture of inpatient and outpatient populations. As patients with cancer who are treated as outpatients have much lower mortality than those admitted to the hospital with COVID-19,27,30 failure to separate patients according to the location of treatment can underestimate the mortality reported for hospitalized patients.
To our knowledge, this is the largest Latin American cohort to assess the characteristics, determinants of mortality, and clinical outcomes of hospitalized patients with cancer who were diagnosed with COVID-19. Among the limitations of our study, the retrospective nature and the accrual in a single public institution may limit the generalization of our findings. Finally, restricted numbers of specific malignancy types limit our ability to accurately address the influences of individual cancer types and systemic therapeutic regimens on clinical outcomes.
In conclusion, delivering care for patients with cancer during the COVID-19 pandemic has become a major challenge for the cancer care community. Our data demonstrate a high mortality rate in patients with cancer who were diagnosed with COVID-19, emphasizing the need for close surveillance in this group of patients, and provide evidence on determinants of unfavorable outcomes in hospitalized patients with cancer.
Guilherme Nader Marta
Travel, Accommodations, Expenses: Bayer Schering Pharma, Roche
Renata Colombo Bonadio
Research Funding: Novartis, AstraZeneca
Expert Testimony: Ache
Travel, Accommodations, Expenses: Roche, AstraZeneca
Driele Peixoto Bittencourt
Honoraria: Novo Nordisk, EMS, GE Healthcare, Novartis
Consulting or Advisory Role: Bayer
Camilla Oliveira Hoff
Leadership: Rede D'Or
Stock and Other Ownership Interests: Oncostar
Honoraria: Bayer, Exelixis, Lilly, United Medical
Consulting or Advisory Role: Bayer, Exelixis, United Medical, Lilly
Research Funding: Exelixis, Lilly
Juliana Pereira
Consulting or Advisory Role: AstraZeneca
Vanderson Rocha
Honoraria: Takeda, Novartis, Roche
Consulting or Advisory Role: Takeda, Agios, Zodiac Pharma, Novartis, AbbVie
Speakers' Bureau: Bristol Myers Squibb, Takeda, Amgen, Agios, Pfizer, Janssen
Paulo M. Hoff
Leadership: Oncologia D'Or
Stock and Other Ownership Interests: OncoStar Oncology Clinics
Honoraria: Bayer Health, United Medical
Consulting or Advisory Role: United Health Group, Bayer, Lilly, Exelixis
Research Funding: Bayer, MSD Oncology, Novartis, Exelixis, Roche/Genentech, AstraZeneca/MedImmune, Lilly
No other potential conflicts of interest were reported.
Footnotes
G.N.M. and R.C.B. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Guilherme Nader Marta, Renata Colombo Bonadio, Odeli Nicole Encinas Sejas, Maria Cecilia Mathias Machado, Camila Motta Venchiarutti Moniz, Edson Abdala, Maria Del Pilar Estevez-Diz, Paulo M. Hoff
Financial support: Paulo M. Hoff
Administrative support: Paulo M. Hoff
Provision of study materials or patients: Odeli Nicole Encinas Sejas, Ulysses Ribeiro Jr, Vanderson Rocha
Collection and assembly of data: Guilherme Nader Marta, Renata Colombo Bonadio, Odeli Nicole Encinas Sejas, Gabriel Watarai, Maria Cecilia Mathias Machado, Lorena Teixeira Frasson, Camila Motta Venchiarutti Moniz, Raquel Keiko de Luca Ito, Camilla Oliveira Hoff, Veruska Menegatti Anastacio, Ulysses Ribeiro Jr, Juliana Pereira, Edson Abdala, Maria Del Pilar Estevez-Diz, Paulo M. Hoff
Data analysis and interpretation: Guilherme Nader Marta, Renata Colombo Bonadio, Odeli Nicole Encinas Sejas, Maria Cecilia Mathias Machado, Camila Motta Venchiarutti Moniz, Driele Peixoto, Ulysses Ribeiro Jr, Juliana Pereira, Vanderson Rocha, Edson Abdala, Maria Del Pilar Estevez-Diz, Paulo M. Hoff
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Guilherme Nader Marta
Travel, Accommodations, Expenses: Bayer Schering Pharma, Roche
Renata Colombo Bonadio
Research Funding: Novartis, AstraZeneca
Expert Testimony: Ache
Travel, Accommodations, Expenses: Roche, AstraZeneca
Driele Peixoto Bittencourt
Honoraria: Novo Nordisk, EMS, GE Healthcare, Novartis
Consulting or Advisory Role: Bayer
Camilla Oliveira Hoff
Leadership: Rede D'Or
Stock and Other Ownership Interests: Oncostar
Honoraria: Bayer, Exelixis, Lilly, United Medical
Consulting or Advisory Role: Bayer, Exelixis, United Medical, Lilly
Research Funding: Exelixis, Lilly
Juliana Pereira
Consulting or Advisory Role: AstraZeneca
Vanderson Rocha
Honoraria: Takeda, Novartis, Roche
Consulting or Advisory Role: Takeda, Agios, Zodiac Pharma, Novartis, AbbVie
Speakers' Bureau: Bristol Myers Squibb, Takeda, Amgen, Agios, Pfizer, Janssen
Paulo M. Hoff
Leadership: Oncologia D'Or
Stock and Other Ownership Interests: OncoStar Oncology Clinics
Honoraria: Bayer Health, United Medical
Consulting or Advisory Role: United Health Group, Bayer, Lilly, Exelixis
Research Funding: Bayer, MSD Oncology, Novartis, Exelixis, Roche/Genentech, AstraZeneca/MedImmune, Lilly
No other potential conflicts of interest were reported.
REFERENCES
- 1.Zhu N Zhang D Wang W, et al. : A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727-733, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F Yu T Du R, et al. : Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395:1054-1062, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J-Y Duan X-F Wang L-P, et al. : Selective depletion of regulatory T cell subsets by docetaxel treatment in patients with nonsmall cell lung cancer. J Immunol Res 2014:286170, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landman A, Feetham L, Stuckey D: Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol 21:335-337, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrag D, Hershman DL, Basch E: Oncology practice during the COVID-19 pandemic. JAMA 323:2005-2006, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Giannakoulis VG, Papoutsi E, Siempos: Effect of cancer on clinical outcomes of patients with COVID-19: A meta-analysis of patient data. JCO Glob Oncol 6:799-808, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brar G Pinheiro LC Shusterman M, et al. : COVID-19 severity and outcomes in cancer patients: A matched cohort study. J Clin Oncol 38:3914-3924, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong E, Du H, Gardner L: An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 20:533-534, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Melo AC Thuler LCS Da Silva JL, et al. : Cancer inpatients with COVID-19: A report from the Brazilian National Cancer Institute. PLoS One 15:e0241261, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longbottom ER Torrance HDT Owen HC, et al. : Features of postoperative immune suppression are reversible with interferon gamma and independent of interleukin-6 pathways. Ann Surg 264:370-377, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Liang W Guan W Chen R, et al. : Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol 21:335-337, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albiges L Foulon S Bayle A, et al. : Determinants of the outcomes of cancer patients infected with SARS-CoV-2: Results from the Gustave Roussy cohort. Nat Cancer 1:965-975, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Kuderer NM Choueiri TK Shah DP, et al. : Clinical impact of COVID-19 on cancer patients (CCC19): A cohort study. Lancet 395:1907-1918, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rugge M, Zorzi M, Guzzinati S: SARS-CoV-2 infection in the Italian Veneto region: Adverse outcomes in cancer patients. Nat Cancer 1:784-788, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Lee LYW Cazier JB Angelis V, et al. : COVID-19 mortality in cancer patients on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 395:1919-1926, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peravali M Joshi I Ahn J, et al. : A systematic review and meta-analysis of clinical characteristics and outcomes in lung cancer patients with COVID-19. JTO Clin Res Rep 2:100141, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marin-Acevedo JA, Chirila RM, Dronca RS. Immune checkpoint inhibitor toxicities. Mayo Clin Proc 94:1321-1329, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med 383:2255-2273, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yekedüz E, Utkan G, Ürün Y. A systematic review and meta-analysis: The effect of active cancer treatment on severity of COVID-19. Eur J Cancer 141:92-104, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robilotti EV Babady NE Mead PA, et al. : Determinants of COVID-19 disease severity in cancer patients. Nat Med 26:1218-1223, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi S Qin M Shen B, et al. : Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 5:802-810, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao D Zhou F Luo L, et al. : Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: A retrospective cohort study. Lancet Haematol 7:e671-e678, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Der Poll T Van De Veerdonk FL Scicluna BP, et al. : The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol 17:407-420, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y Fu B Zheng X, et al. : Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev 7:998-1002, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson S Hirsch JS Narasimhan M, et al. : Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA 323:2052-2059, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karagiannidis C Mostert C Hentschker C, et al. : Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: An observational study. Lancet Respir Med 8:853-862, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desai A Gupta R Advani S, et al. : Mortality in hospitalized cancer patients and coronavirus disease 2019: A systematic review and meta‐analysis of cohort studies. Cancer 127:1459-1468, 2021 [DOI] [PubMed] [Google Scholar]
- 28.Cook G John Ashcroft A Pratt G, et al. : Real-world assessment of the clinical impact of symptomatic infection with severe acute respiratory syndrome coronavirus (COVID-19 disease) in patients with multiple myeloma receiving systemic anti-cancer therapy. Br J Haematol 190:e83-e86, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vuagnat P Frelaut M Ramtohul T, et al. : COVID-19 in breast cancer patients: A cohort at the Institut Curie Hospitals in the Paris area. Breast Cancer Res 22:55, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saini KS Tagliamento M Lambertini M, et al. : Mortality in cancer patients and coronavirus disease 2019: A systematic review and pooled analysis of 52 studies. Eur J Cancer 139:43-50, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]