Abstract
Radiation therapy to anatomic regions, including the head and neck, chest wall, and extremities, can produce radiation-induced fibrosis (RIF). To elucidate the cellular and molecular mechanism(s) involved in RIF, female C57BL/6J mice were irradiated to the right flank to 35 Gy in single fraction using 6 Mv electrons. Radiation fibrosis was detected by day 14, was increased by day 28, and confirmed by Masson’s trichrome histological staining for collagen. Biopsied tissue at day 14 showed an increase in expression of fibrosis-related genes including transforming growth factor-β (TGF-β) and collagens 1–6. A single adipose-derived stem cell (ASC) injection on day 28 at the irradiated site decreased by day 40: epithelial thickness, collagen deposition, and significantly improved limb excursion compared with irradiated controls. Noncontact transwell coculture of ASCs above a monolayer of irradiated human foreskin fibroblasts downregulated fibrosis-related genes TGF-β, connective tissue growth factor, interleukin-1, NF-kB, tumor necrosis factor, and collagens 1–6. Hepatocyte growth factor (HGF) secreted by ASCs was identified as a novel mechanism by which ASCs exert antifibrotic effects by downregulating fibrotic gene expression in irradiated cells and recruiting bone marrow cells to the irradiated site. In conclusion, these data indicate a mechanistic role of HGF secreted by ASCs in reducing RIF.
Keywords: Adipose-derived stem cells, Radiation, Fibrosis, Skin, Transforming growth factor, Hepatocyte growth factor
INTRODUCTION
Clinical radiation therapy can induce late changes in the skin and muscle, which are directly dependent upon radiation total dose, fraction size, and volume treated. Radiation-induced fibrosis (RIF) can result in progressive functional and anatomic impairment, including decreased tissue elasticity, limited mobility, muscle atrophy, telangiectasias, xerosis, delayed wound healing, and alopecia [1]. The functional loss of hair follicles and sebaceous glands can be accompanied by altered skin pigmentation and necrosis [2,3].
The molecular mechanism of radiation fibrosis (RF) is poorly defined but involves progressive proliferation and differentiation of fibroblasts to myofibroblasts, which deposit fibrous matrix [3]. There can also be interstitial and microvascular deposition of components of extracellular matrix. Proliferation of myofibroblasts is driven by profibrotic cytokines, including transforming growth factor-β1 (TGF-β1) and connective tissue growth factor (CTGF). TGF-β1 is a 25 kDa polypeptide with multiple functions [3]. TGF-β1 binds to its receptor and triggers a transcriptional response that facilitates proliferation and differentiation of fibroblasts to myofibroblasts [4]. Hypoxia and production of reactive oxygen species and reactive nitrogen species by fibrotic tissues also contribute to induction and persistence of fibrosis [5].
There has been a recently renewed interest in stem cell therapies, especially adipose derived, for the treatment of RF. Clinical protocols have included injection of mesenchymal stem cells (MSCs) and endothelial cells [6]. Recent results indicate that enriched adipose-derived stem cells (ASCs), as well as, autologous fat can be used to treat patients, who require reconstructive surgery including that associated with RF [7]. ASCs reside within the adipose tissue (AT), which is a highly specialized connective tissue that contributes to both mechanical and metabolic stability [8,9]. ASCs have been found to display the immune phenotype of CD34+CD90+CD29+CD45–CD31– and are cells of mesenchymal origin [10,11]. ASCs exhibit trilineage differentiation capacity for the bone, cartilage, and fat [12]. Harvest of ASCs for clinical applications revealed an approximately 500-fold higher yield of cells, and harvesting by lipo-aspiration from subcutaneous AT sites is practically more feasible in clinical settings compared with the relatively low abundance and difficulty of harvest of bone marrow-derived MSCs [13]. The interaction of ASCs with both innate and adaptive immune response cells, the capacity of ASCs to downregulate T cell proliferation across HLA genotypes, and the low immunogenicity of ASCs themselves suggests that ASCs could be adapted for use in cell therapy of RF. We investigated whether autologous fat-derived ASCs ameliorated RF in vivo. Furthermore, we also defined the noncell contact mechanism by which ASCs ameliorate irradiation fibrosis using a transwell coculture system in vitro.
MATERIALS AND METHODS
Mice
Mice C57BL/6, C57BL/6 green fluorescent protein+/luciferase+ (GFP+/Luc+), and C57BL/6 Smad–/– were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International-approved facility. They were treated according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Irradiation
Mice were irradiated as previously published [14]. Legs to be irradiated were shaved. Approximately 5 minutes before irradiation, each mouse was anesthetized by injecting 1.25 mg/kg of Nembutal (Lundbeck, Copenhagen, Denmark). A 6-MeV electron beam from a Varian 23EX linear accelerator (Varian Medical Systems, Inc., Palo Alto, CA) was used to generate β-irradiation burns. Irradiation was conducted using a 25 × 25-cm applicator, a dosage rate of 1,000 MU/minute, and a source-to-mouse skin surface distance of 100 cm. During irradiation, a 3-cm-thick bolus was placed to prevent the deep penetration of radiations. The setup is such that only the shaved upper right rear leg of each mouse was exposed to an irradiation field of 2 × 2 cm. All monitor units were calculated by incorporating the appropriate applicator factor and cutout factors such that the doses delivered to mouse skin was 35 Gy.
Measurements of Fibrosis-Induced Leg Contracture, Histological Evaluation of Skin Damage and Collagen Deposition
The extent of the leg movement was measured using a protractor and represented as degree of motion [14]. For histological evaluation, tissue samples were fixed in 10% formalin, embedded in paraffin, and cut into 5-μm sections. Relative collagen levels were assed using Masson’s trichrome staining. Slides were examined by a pathologist for signs of fibrosis. Skin sections were evaluated for signs of skin damage via hematoxylin and eosin stains [14].
Generation of Bone Marrow Chimeric Mice
C57BL/6 mouse and GFP+ Luc+ marrow chimeric mice were prepared with 10 Gy total-body irradiation; followed by i.v injection of 1 × 106 GFP+ Luc+ marrow cells 24 hours later [15]. Chimerism was documented by bioluminescence imaging as reported [15].
Live Cell Imaging In Situ of Cells at Injection Sites or in the Bone Marrow
All animals treated with GFP+ Luc+ cells, across all groups, were evaluated at 7 day intervals with in vivo fluorescence optical imaging to evaluate persistence, distribution, and relative signal of donor cells using a Xenogen IVIS Imaging System 200 Series (PerkinElmer, MA, USA) [15].
Derivation of Tissue Fibrosis Cell Lines From Explanted Fibrotic Tissue
Mice either treated with 106 ASCs or saline at day 1 following 35 Gy (hind limb) skin irradiation were euthanized, and irradiated skin was surgically collected in phosphate-buffered saline (PBS). The skin tissue was washed twice in PBS and minced into small pieces. Minced tissue was digested with 200 U/ml collagenase (CLS type I, Worthington Biochemical Corp., Lakewood, NJ) and 2% w/v bovine serum albumin (BSA; Sigma, MO, USA) in a shaking water bath for 60 minutes at 37°C and 150 rpm. Digested tissue was passed through a 70-μm cell strainer and centrifuged for 10 minutes at 200 relative centrifugal force (RCF) at room temperature. Pelleted cells were resuspended in Dulbecco’s modified Eagle’s medium (DMEM) 10% fetal bovine serum (FBS) and incubated at 37°C with 5% CO2, grown for 8–10 days, and then were imaged by fluorescence microscopy.
Isolation of Human and Mouse ASCs
ASCs were isolated as explained [11,16]. Briefly, human subcutaneous white AT (sWAT) samples were harvested from donors undergoing routine abdominoplasty at the Department of Plastic Surgery, University of Pittsburgh. The tissue collection methods were approved by the Institute Review Board. All sWAT samples were obtained from the lower abdomen. None of the donors had medical record of history of diabetes, liver, renal, or other metabolic diseases. AT biopsies after surgery procedures were transferred to lab in sterile container before sterile processing in a laminar flow sterile work bench class II. Mice were euthanized after anesthesia. Subcutaneous fat was surgically removed and transferred to the tissue culture lab for processing. Tissue was rinsed three times with PBS (Sigma) followed by removal of fibrous material and blood vessels by dissection.
The tissue was cut into pieces (~1 to 2 mg) and digested in digestion buffer (PBS) containing 200 U/ml collagenase (CLS type I) and 2% w/v BSA (Sigma) under stirring for 60 minutes at 37°C and 450 rpm, 1 mg AT/3 ml digestion buffer. The dispersed tissue was centrifuged for 10 minutes at 200 RCF at room temperature. The floating adipocytes were aspirated and the sedimented stromal-vascular fraction (SVF) was suspended in erythrocyte lysis buffer (0.155 M NH4CI, 5.7 mM K2HPO4, 0.1 mM EDTA, pH 7.3) and incubated for 10 minutes at room temperature [11,16].
To remove tissue debris, the cell suspension was filtered through a nylon mesh (pore size 100 μm, BD). After another centrifugation step (10 minutes at 200 RCF), the pelleted SVF was suspended in DMEM/F12 (Sigma) supplemented with 10% FBS (Sigma) and filtered through a 40 μm mesh to remove residual cell aggregates. SVF cells were inoculated into cell culture flasks at a density of 30,000/cm2. The attached cell population at 1 week postculture was referred to as the ASC fraction, which was used for further studies. The ASC cell population contains an enriched population of adipose stem/progenitor cells [11].
Culture of ASCs
Upon reaching 70% confluence in T175 flask (Thermo Fisher Scientific, MA, USA), ASCs were washed with PBS (Thermo Fisher Scientific) and trypsinized using 3 ml 0.5% trypsin-EDTA ×1 (Sigma). Trypsin was neutralized using 7 ml DMEM/F12, 10% FBS medium plus 50 μg/ml Gentamicin (Invitrogen, CA, USA) and removed by centrifugation at 300 RCF for 5 minutes, and the cells were again seeded at a density of 5,000–7,000 cells per centimeter square in DMEM/F12 plus 10% FBS and maintained at 37°C with 5% CO2. ASC was passaged by seeding at 5,000–7,000 cells per centimeter square, medium was changed every third day, and the cells were grown to 70% confluence before splitting. ASCs cultivated to passage 4 were used in this study.
Cultivation of Human Foreskin Fibroblasts
Human foreskin fibroblasts (HFFs) were isolated and cultivated using methods described [17]. Briefly, 30,000 cells per centimeter square were seeded in six well plates in DMEM (Sigma) supplemented with 10% fetal calf serum (FCS).
Derivation of Mouse Bone Marrow Stromal Cell Lines
The method for derivation of bone marrow stromal cell lines has been previously published [18]. Briefly, the contents of adult mouse femurs and tibias were flushed through a 17-gauge needle into 25 cm2 flasks (Corning, Corning, NY) and maintained in a high-humidity incubator with 7% CO-2, in Fisher’s medium supplemented with 15% heat-inactivated FCS with penicillin and streptomycin. Cultures were supplemented with 10–5 M hydrocortisone sodium hemisuccinate. Fresh corticosteroid was added weekly according to published methods. Nonadherent cells were removed weekly and total cells counted and then plated in 0.8% methylcellulose-containing medium supplemented with growth factors for hematopoiesis according to published methods [19]. Adherent cells from long-term bone marrow cultures at 20 weeks were transferred by trypsinization to plastic petri dishes and grown in DMEM supplemented with 10% FCS, penicillin, and streptomycin [19]. Cell lines were expanded conservatively and then cloned in Poisson distribution methods according to published procedures [18].
Transwell Coculture Experiments
Transwell system (0.4 pm pore size, polyester membrane; Corning) was used. HFFs or mouse bone marrow stromal cells were cultured in the bottom surface of a 9 cm2 culture dishes. Above the adherent layer in the transwell human or mouse, ASCs were cultured in the upper transwell basket. Briefly, HFF or bone marrow stromal cells were grown to confluence and were irradiated using 10 Gy radiation dose. Twenty-four hours postirradiations, 3 × 105 ASCs were added to the upper transwell basket. Coculture was maintained for 48 hours, and culture supernatant was collected for estimation of cytokines and growth factors using Luminex assay. Cells were lysed, and total RNA was isolated using TRIzol (Sigma) reagent following manufacturer’s protocol. cDNA was synthesized, and expression of profibrotic gene battery (TGF-β1, CTGF, NF-κB, interleukin-1 [IL-1], and collagens 1–6 [Col1–Col6]) was analyzed using gene-specific TaqMan primers employing quantitative real-time polymerase chain reaction (PCR).
Real-Time PCR
RNA was extracted from mouse skin or from explanted and cultured cells using the TRIzol reagent following manufacturer’s instruction. RNA was reverse transcribed using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, CA, USA), according to manufacturer’s protocol. Using cDNA as a template and gene-specific primers (Thermo Fisher), expression of TGF-01 (Mm01178820_m1), CTGF (Mm01192933_g1), tumor necrosis factor [TNF] (Mm00443258_ m1), NF-kB (Mm00476361_m1), IL-1 (Mm00439620_m1), Col1a1 (Mm00801666_g1), Col2a1 (Mm01309565_m1), Col3a1 (Mm01254476_m1), Col4a1 (Mm01210125_m1), Col5a1 (Mm00489342_ m1), Col6a1 (Mm00487160_m1), and hepatocyte growth factor (HGF; Hs00300159_m1) was quantified by quantitative real-time PCR using the Eppendorf Realplex2 Mastercycler (Hamburg, Germany). Data for each gene transcript were normalized by calculating the difference (ΔCt) from the Ct-housekeeping and Ct-target genes. The relative increase or decrease in expression was calculated by comparing the reference gene with target gene calculated by comparing the reference gene with the target gene (ΔΔCt) and using the formula for relative expression (=2ΔΔCt).
Protein Measurements by Luminex Assay
The concentration of HGF and other proteins in conditioned medium or in cell pellets was analyzed by the multiplex Luminex assay from R&D Systems (MN, USA) according to manufacture protocol. Cell culture supernatant samples were centrifuged for 4 minutes at 16,000g immediately prior to use or dilution. Samples were diluted two-fold by adding 75 μl supernatant to 75 μl of Calibrator Diluent mix (Kit). Add 50 μl sample or standard (Kit) to 96 well plate according to assay scheme and incubate for 2 hours at room temperature after adding 50 μl Microparticle Cocktail (Kit). Each well was washed three times with 100 μl wash buffer (Kit). Add 50 μl diluted biotin antibody cocktail to each well and incubate for 1 hour with shaking at 800 rpm. Wash three times with wash buffer. Add 50 μl diluted Streptavidin-PE to each well and incubate for 30 minutes with shaking at 800 rpm. Wash again three times with wash buffer. Readings were taken using Luminex 100/200 analyzer (Luminex Inc., Austin,TX.) and protein concentration reported.
Western Blotting
Western blotting was performed according to published methods [11]. c-Met antibody (D1C2 XP, Cell Signaling, MA, USA) was used.
Statistical Analyses
Data are reported as the mean ± SEM. We assessed subject variability of the measured outcome. Student’s t test and analysis of variance test was performed where applicable using GraphPad Prism software. Error bars are represented as the mean ±SEM.
RESULTS
Quantitation of Parameters of Radiation-Induced Hind Limb Fibrosis in C57BL/6 Mice
We first used a mouse model of hind limb RF. We delivered 35 Gy to the hind limbs of adult female C57BL/6 mice with field size, electron beam dosimetry, and technique, as reported previously [14]. We quantitated fibrosis on explanted fixed tissues by histology and measured hind limb range of motion prior to tissue explant at serial time points out to 30 days after 35 Gy. Constriction of the range of motion and soft tissue histologic fibrosis at day 30 post irradiation is shown in Figure 1A, 1B, respectively. Day 30 was selected for histological analyses based on the strongest decrease in hind limb range of motion observed at this time point.
Figure 1.
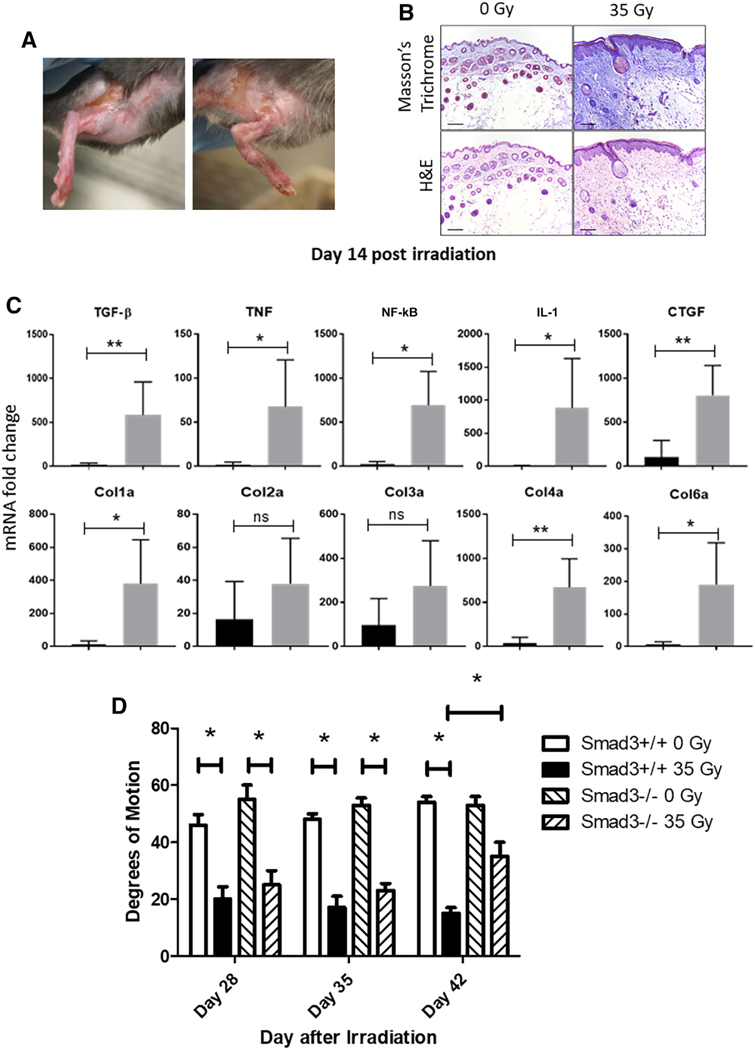
Irradiation-induced hind limb fibrosis in C57BL/6 mice. (A): Fibrotic lesions were observed at day 24 post 35 Gy irradiation, demonstrating a successful establishment of radiation-induced fibrosis model for investigating adipose-derived stem cells-based cell therapy. (B): Deposition of Col using Masson’s trichrome, and skin damage was assessed at day 24 employing histopathologic staining. Scale bar = 100 μm in all images. (C): Profibrotic gene mRNA expression was analyzed by quantitative real-time polymerase chain reaction from irradiated skin tissue at day 14 postirradiation. Expression was normalized using glyceraldehyde-3-phosphate dehydrogenase as a house keeping gene (n = 5 for each time point). (D): Smad3–/– and wild-type mice were irradiated to 35 Gy to one hind limb. Limb excursion was measured by protractor and plotted as degree of motion at each of three time points (n = 5). *, p < .05; **, p < .01. Abbreviations: Col, collagen; CTGF, connective tissue growth factor; IL-1, interleukin-1; TGF-β, transforming growth factor-β; TNF, tumor necrosis factor.
We next analyzed the levels of expression of fibrosis-related genes, TGF-β1, CTGF, TNF, NF-κB, IL-1, and Col1–Col6 in surgically excised irradiated mouse skin at day 14 postirradiation using quantitative real-time PCR. The results showed significant upregulation in expression of profibrotic genes, TGF-β1, CTGF, TNF, NF-κB, IL-1, Col1, Col4, and Col6 as compared with nonirradiated control mice skin tissue by day 14 postirradiation (Fig. 1C).
The above results confirm the prior studies showing increase in levels of TGF-β1 during the development of RF in mice [3, 20, 21]. To further confirm the role of TGF-β1 in the mouse model of RF, we tested Smad3 knock out (Smad3–/–) mice. Smad3–/– mice have been reported to have an absent canonical TGF-β1 signaling pathway [22, 23]. After limb irradiation, there was a decrease in limb excursion in Smad3–/– mice during the early time points postirradiation compared with that in wild-type mice (Fig. 1D). By day 42 after irradiation, Smad3–/– mice showed significant improvement in limb excursion compared with wild-type mice. The initial decrease in limb excursion observed in Smad3–/– mice was most likely due to edema, which is observed postirradiation [24]. Smad3–/– mice showed no detectable RF in tissues examined out to 100 days in later stages [22, 23]. These results confirm the absence of RF in Smad3–/– mice [22, 23].
Injection of ASCs Into Irradiated Tissue Mitigates RF
To evaluate the effect of local injection of ASCs in the irradiated mouse limb, we injected 106 ASCs into the triceps muscles of each mouse hind limb. We used GFP+ Luc+ mice, as donors of the ASC cells, and injected them at the irradiated site of C57BL/6 wild-type mice at day 28 postirradiation. The 28-day time point was the time of early fibrosis characterized by a significant decrease in the limb excursion. Control mice received saline injection.
A single ASCs injection significantly improved limb excursion measured by degree of motion compared with the irradiated then saline injected group (Fig. 2A). There was visible improvement in skin texture in ASCs-injected mice. Histological analyses of formalin-fixed skin sectioned stained with H&E stain (Fig. 2B) revealed that in saline-injected irradiated mice, the epidermis was severely hyperplastic with formation of deep rete pegs and presence of parakeratotic crusts on the epithelial surface. Masson’s trichrome staining of the underlying dermis showed a denser connective tissue (Fig. 2D, 2E) compared with control nonirradiated mice skin (Fig. 2C). In contrast, epithelial thickness (Fig. 2D, 2E) and collagen density (Fig. 2F, 2G) in ASCs-treated mice was improved and comparable to nonirradiated control mouse skin, respectively (Fig. 2B, 2C).
Figure 2.
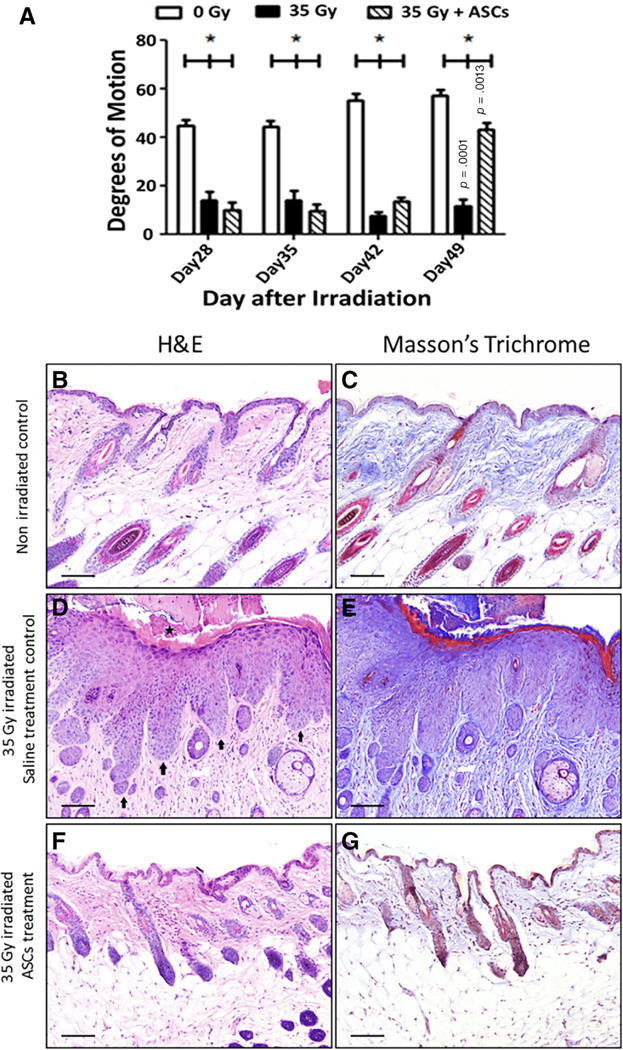
Local ASCs injection mitigates irradiation-induced hind limb fibrosis. (A): C57bl/6 mice were irradiated to 35 Gy and injected locally at day 28 postirradiation with 106 ASCs isolated from GFP+ Luc+ mice. Irradiated leg movement was measured by protractor as degrees of motion and plotted (n = 8). (B–G): Deposition of collagen was measured using Masson’s trichrome staining and H&E staining at day 50 postirradiation. In the control irradiated skin (D), the epidermis was severely hyperplastic with formation of deep rete pegs (arrows). Parakeratotic crusts were present on the epithelial surface (star). Trichrome staining of the underlying dermis revealed denser connective tissue (E) compared with the control unirradiated animal (C). The ASCs-treated irradiated animal (F, G) was similar to the non-irradiated control (B, C) with similar epithelial thickness and collagen density appearance in both the H&E-stained and trichrome-stained sections. Scale bar = 100 μm in all images. Abbreviation: ASC, adipose-derived stem cell.
Injected ASCs Persist at the Site of Radiation Injury
We next determined the fate of transplanted GFP+ Luc+ ASCs at the site of irradiation and performed in situ imaging of transplanted cells at the injected sites. The result showed the presence of luciferase signal in the irradiated leg injected with GFP+ Luc+ ASCs at day 77 postinjection, whereas the contralateral nonirradiated leg also injected with GFP+ Luc+ ASCs showed no signal (Fig. 3A). We further confirmed the persistence of GFP+ Luc+ ASCs in explanted skin tissue. At day 80 post cell injection, we prepared single cell suspensions and quantitated GFP+ cells. No GFP positive cells were observed in cell suspension explanted from nonirradiated but GFP+ Luc+ ASCs injected legs, whereas an average 10.4% of the cells explanted from irradiated legs were positive for GFP signal (Fig. 3B). These results indicate a persistence of irradiated ASCs injected at the irradiated sites.
Figure 3.
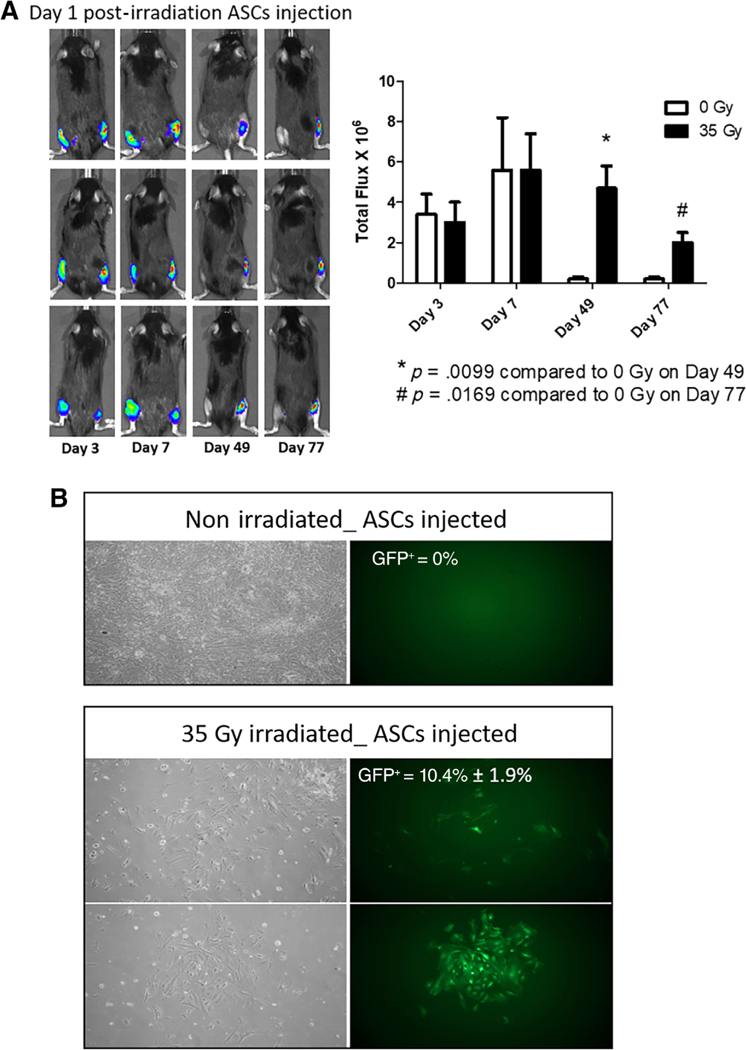
Injected ASCs persist at the site of 35 Gy irradiation. (A): Retention at day 1of injected GFP+ Luc+ ASCs at the right hind limb-irradiated site and continuous persistence out to 77 days postirradiation. In contrast, the unirradiated left hind limb showed less retention of the same number of injected cells at days 49 and 77. (B): Single-cell suspensions from explanted irradiated skin were cultured at day 77 and GFP+ cells were counted per 100 cells (image magnification ×10). *, p < .05; **, p < .01. Abbreviation: ASC, adipose-derived stem cell.
ASCs in Coculture Downregulate Levels of Profibrotic Genes Expression in Transwell-Separated Irradiated Cells
We next investigated the role of secreted factors from ASCs in altering expression of genes associated with RF in irradiated cells. Transwell cocultures were setup using irradiated HFF as target cell population in the lower compartment and human ASCs in the upper transwell compartment (Fig. 4A). Irradiation of HFF using 10 Gy dose resulted in upregulation of fibrosis-inducing genes expression (Fig. 4B). The addition of ASCs for 48 hours in the upper layer above a Millipore filter down-regulated fibrosis-related genes in irradiated HFF (Fig. 4B). There was no detectable effect of ASCs on transcript levels in nonirradiated HFF cells. There was upregulation in expression of fibrosis-inducing genes in the HFF cells. We observed an increase in TGF-βi, Col1, CTGF, TNF, NF-κB, IL-1, and Col3 expression in HFF after irradiation. The addition of ASCs to the transwell above the membrane downregulated fibrosis-related genes. The significant downregulation was detected with TGF-β1 and Col1 expression.
Figure 4.
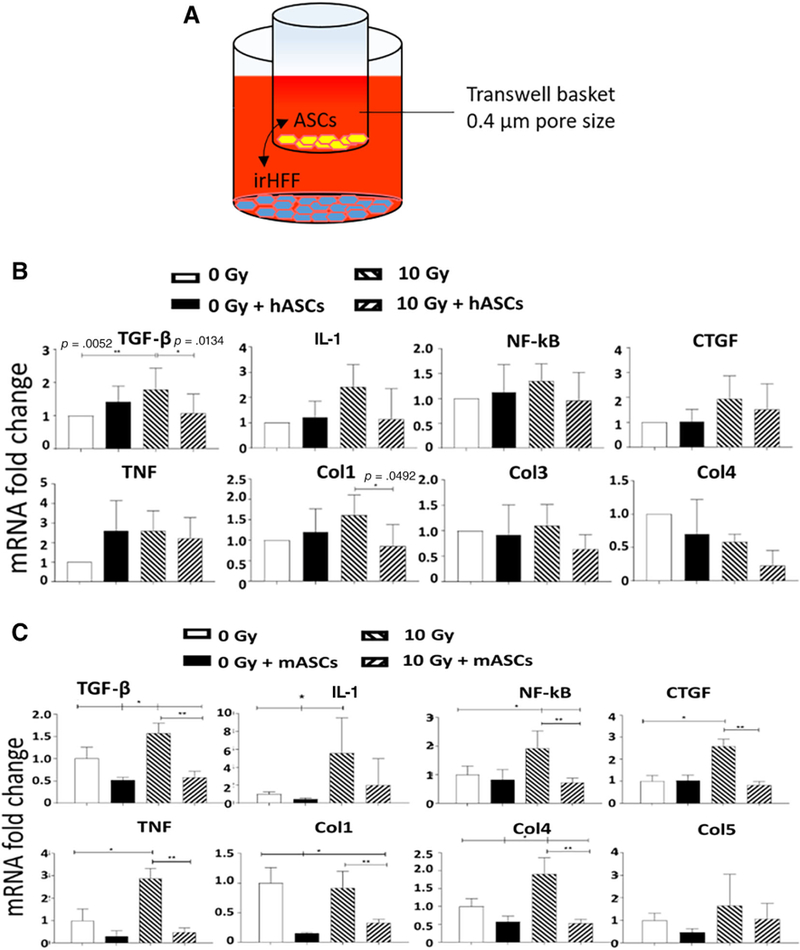
ASCs in transwell culture downregulate profibrotic gene expression in irradiated fibroblasts. (A): Schematic of transwell coculture. (B) Human ASCs were cocultured with nonirradiated or 10 Gy irHFF and tested at 48 hours post coculture. Fibrosis-related gene transcripts for TNF, NF-κB, IL-1, CTGF, TGF-β, Col1, Col4, and Col5 were measured by real-time polymerase chain reaction (RT-PCR) in the HFF cells. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene (n = 3). (C): Mouse bone marrow stromal cells were irradiated to 10 Gy and cocultured in transwells below mouse ASCs for 48 hours. mRNA expression of profibrosis genes TNF, NF-κB, IL-1, CTGF, TGF-β, Col1, Col4, and Col5 was measured by RT-PCR (n = 3). GAPDH was used as housekeeping gene. *, p < .05; **, p < .01. Abbreviations: ASC, adipose-derived stem cell; Col, collagen; CTGF, connective tissue growth factor; hASC, human adipose-derived stem cell; IL-1, interleukin-1; irHFF, irradiated human forskin fibroblast; TGF-β, transforming growth factor-β; TNF, tumor necrosis factor.
We next extended the transwell data to a study of ASC effects on irradiated bone marrow stromal cells. Mouse ASCs were placed on the upper level of the cultures. There was a significant upregulation of TGF-β, CTGF, TNF, NF-κB, IL-1, and Col4 in irradiated mouse stromal cells (Fig. 4C). After transwell coculture with mouse ASCs, a significant downregulation in the expression of TGF-β, Col1, CTGF, TNF, NF-κB, and Col4 genes in irradiated mouse stromal cells was observed (Fig. 4C). The results indicate the presence of an in vitro detectable paracrine-mediated effect of ASCs.
HGF Secreted by ASCs Contributes to the Downregulation of TGF-β in Transwell-Separated Irradiated Human Fibroblasts
To determine whether a paracrine factor(s) released by ASCs-mediated transcription changes seen in irradiated fibroblasts, we tested HGF. The known antifibrotic role of HGF [25, 26] and its secretions by ASCs was a factor of interest [27]. We measured the production of HGF in transwell cocultured supernatant using Luminex assay, revealing a high concentration of HGF in ASC culture supernatant (2000 pg/ml), confirming the presence of HGF production by ASCs. We observed a significant increase in HGF production in transwell culture supernatant upon coculture of ASCs with irradiated HFF (Fig. 5A). We next added recombinant HGF to irradiated HFF in culture with no ASCs present. There was an HGF-dose-dependent downregulation of TGF-β gene expression in irradiated HFFs after addition of HGF in an increasing concentration (Fig. 5B). We further analyzed the HGF-specific effect on HFF and confirmed the presence of the HGF receptor (c-Met) on HFF cells (Fig. 5C).
Figure 5.
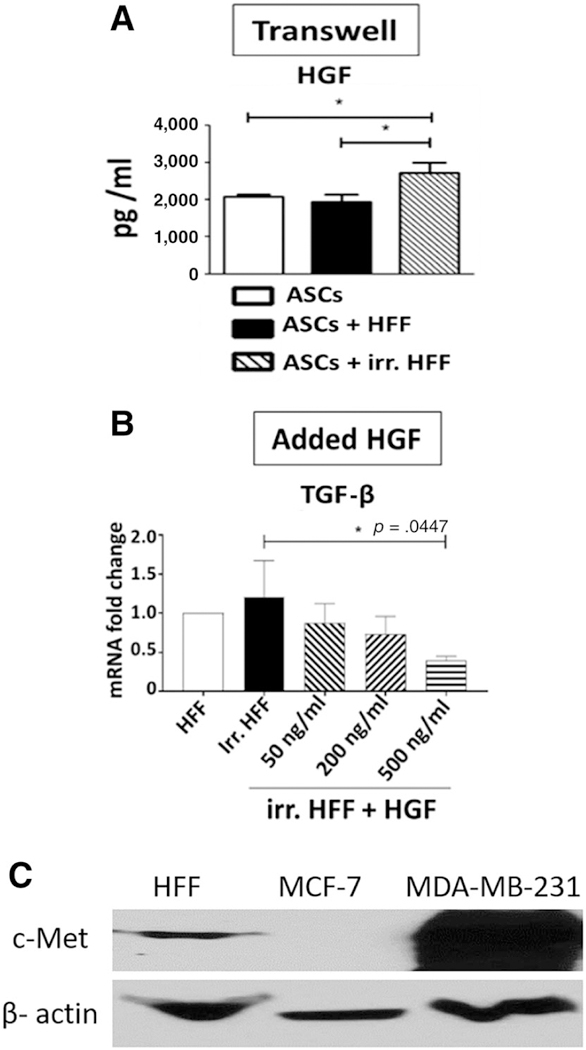
HGF downregulates TGF-β1 level in irradiated fibroblasts. (A): Secretion of HGF by ASCs in transwell coculture was measured by Luminex assay (n = 3). (B): The effect of recombinant HGF (50, 200, and 500 ng/ml) on expression of TGF-β mRNA in irr. HFF was analyzed by real-time polymerase chain reaction. Glyceraldehyde-3-phosphate dehydrogenase was used as a housekeeping gene. (C): HFF cell lysates were run on SDS-polyacrylamide gel electrophoresis, blotted on a nitrocellulose membrane and probed with c-Met antibody. Cell lysates from MCF-7 (negative control cells) and MDA-MB-231 (positive control cells) were used. β-actin was used as a loading control. *, p < .05. Abbreviations: ASC, adipose-derived stem cell; HFF, human forskin fibroblast; HGF, hepatocyte growth factor; irr. HFF, irradiated human forskin fibroblast; TGF-β, transforming growth factor-β.
ASCs Injection Into Irradiated Fibrotic Tissue Elicits Migration of Bone Marrow Cells to the Injection Site
HGF plays an important role in cell proliferation, migration, and morphogenesis [28]. Studies have shown a chemokine-like role of HGF in enhancing migration of bone marrow origin cells and promote tissue regeneration [29–31]. Our transwell data indicated a direct role of HGF produced by ASCs in downregulating fibrosis-related genes expression in irradiated damaged cells. We hypothesized that in addition to a direct role, HGF produced by ASCs may have been contributing an indirect role by promoting migration of bone marrow cells to the irradiated injury site to contribute in damage repair in vivo. To test this hypothesis, we generated GFP+ Luc+ bone marrow chimeric mice. At 4 weeks after total body irradiation and bone marrow transplantation, a leg was irradiated with 35 Gy and ASCs or saline was injected. At day 21 postirradiation, we observed clear homing of GFP+ Luc+ bone marrow cells to the site of ASC injection but no detectable homing to irradiated noninjected control. No detectable GFP+ Luc+ cells were detected at day 21 postirradiation in mice injected with saline (Fig. 6A). Although, in one of the mice, a Luc+ signal was observed in the leg at over 1 cm from the irradiated site, attributable to LUC positive cells in the bone marrow. GFP+ Luc+ cells were detected in all bone marrow sites after marrow transplant into these now chimeric mice. There were no marrow donor cells in nonirradiated tissue that were injected with ASCs (not shown).
Figure 6.
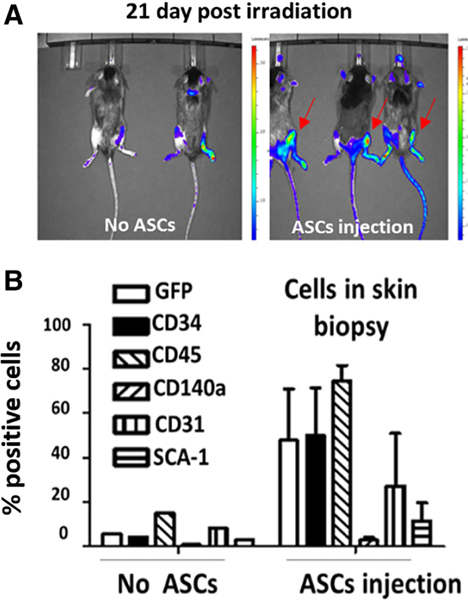
HGF promotes bone marrow cell homing to sites of injected ASCs in irradiated limbs. (A): C57bl/6 mice were total body irradiated and injected i.v. 24 hours later with 106 GFP+ Luc+ bone marrow cells. Four weeks post bone marrow transplant, the right hind limbs were irradiated to 35 Gy and ASCs (from other C57BL/6 mice) were injected at the irradiated sites (right panel) or received no ASCs (left panel). Homing of GFP+ Luc+ cells from the marrow to the site of irradiation was analyzed 21-days postirradiation. (B): Single cell suspensions were prepared following digestion of irradiated tissues at day 21 and subjected to fluorescence-activated cell sorting analyses using antibodies to CD34, CD45, CD140a, CD31, and SCA-1 to quantitate marrow origin cells. Abbreviations: ASC, adipose-derived stem cell.
FACS analyses of marrow origin cells at the RF site showed an increase in numbers of GFP+ cells and cells that expressed CD34, CD45, CD140a, CD31, and Sca 1 (Fig. 6B). The data indicated a paracrine mechanism of ASC injection, which caused migration of cells from the bone marrow to the site of ASC injection in tissues irradiated in vivo.
The results establish that HGF produced by ASCs at the irradiated site mitigate RIF—first, by directly downregulating the expression of the profibrotic genes in damaged cell to stop the progression of the disease and second, by recruiting help in the form of bone marrow cells to regenerate damaged tissue (Fig. 7).
Figure 7.
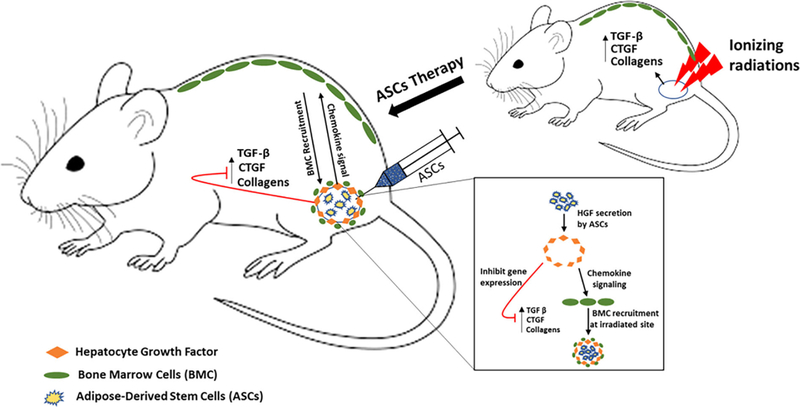
Graphical summary of mechanism by which ASCs mitigate radiation-induced fibrosis. Abbreviations: CTGF, connective tissue growth factor; TGF-β, transforming growth factor-β.
DISCUSSION
We used a mouse model of RF to determine the role of ASC injection in the process of radiation-induced skin/muscle fibrosis. We measured fibrosis in irradiated tissue using histological and molecular analysis. There was thickening of skin epithelium, edema, and influx of inflammatory cells to irradiated sites and upregulated expression of TGF-β1 and collagens 1–6 in irradiated skin tissue. In this study, using transwell coculture systems, we demonstrated paracrine-mediated antifibrotic effects of ASCs on irradiated fibroblasts. ASCs coculture resulted in downregulation of fibrotic genes in irradiated fibroblast cells. Thus, ASCs predominantly secrete HGF and downmodulate fibrosis-related TGF-β expression in irradiated fibroblasts in tissues showing RF. ASCs are known to secrete many paracrine factors including IL-10, basic fibroblast growth factor, vascular endothelial growth factor (VEGF), and TGF-β3. These factors along with HGF have been shown to exert antifibrotic effects and attenuate myocardial, pulmonary, hepatic, and renal fibrosis [32]. The coculture supernatants revealed a high HGF concentration, but it is likely that antifibrotic effects of ASCs therapy include effects of other paracrine factors. Our in vivo irradiation of GFP+ Luc+ bone marrow chimeric mice supported a likely role of HGF to promote migration of bone marrow cells to the irradiated site. Previous studies have demonstrated a role of HGF-c-Met axis in promoting migration of bone marrow origin CD45+ cells to skeletal muscle [29]. Studies in rats showed initial inflammation followed by development of fibrosis, characterized by thickening of epithelium, frequent necrosis and deposition of collagen [33]. Yet, another study demonstrated a higher expression of Smad3 in irradiated skin, indicating activation of the TGF-β/Smad3 pathway, which is in line with our observation that Smad 3 knockout mice having a defective TGF-β signaling do not develop radiation-induced skin fibrosis [34].
In our preclinical mouse model of radiation-induced skin fibrosis, we observed a loss of limb movement as a functional read out of fibrosis development. Previous studies have shown the regenerative potential of ASCs in wound healing and other radiation-induced tissue injuries [7, 8]. In the present study, we focused on the efficacy of ASCs in the treatment of RF in the skin/muscle by measurement of improved limb excursion. Furthermore, our ASCs-treated mice group showed thinning of epithelium and resolution of collagen deposition at the irradiated site compared with saline-treated group. Of note, irradiation of ASCs induces senescence and contributes to organismal aging [35], whereas a subset of ASCs expressing PDGFRα receptor is involved in obesity-related visceral ATs fibrosis [36]. These results indicate that health of the ASCs and tissue microenvironment dictate the profibrotic or antifibrotic role of adipose progenitor cells.
Autologous fat grafts enriched with ASCs are routinely used for reconstructive surgical procedures [37, 38]. There has been published clinical evidence demonstrating beneficial use of autologous fat for treatment of radiotherapy-induced tissue damage [32]. These data report an improvement in fibrosis and late effects normal tissue task force (LENT)-subjective, objective, management, analytic (SOMA) score attributed to enhanced vascularization and hydration of fibrotic tissue, an outcome mainly mediated by ASCs [39]. Subsequent studies verified the initial observations and an improvement in skin texture, and softening of fibrotic tissue was observed in childhood facial cancer treatment trial [40–42]. MSC therapy has demonstrated resolution of radiation-induced salivary gland dysfunction and xerostomia, and a clinical trial is ongoing to clinically asses these findings [43, 44]. Whether ASCs work in this regard is unknown. Systemic injection of autologous ASCs mitigates cutaneous damage induced by radiation [45]. A combination of intramuscular and subcutaneous-repeated ASCs injection in mini-pig model improved muscle regeneration upon radiation injury [46].
Hypoxia, poor vascularity, and lymphedema led to other pathological signs of RF. ASCs withstand hypoxic conditions and may enable the resolution of RF via ASC cytokines including IL-8, insulin-like growth factor 1 (IGF-1), and VEGF-D [47–49]. in vitro coculture of irradiated fibroblasts or endothelial cells with ASCs restored expression of inflammatory cytokines IL-6, fibroblast growth factor, intercellular adhesion molecule 1 (ICAM1), and vascular Cell Adhesion Molecule 1 (VCAM1) to normal levels and increased proliferation of irradiated cells [50, 51]. ASCs therapy modulates Th-17-mediated inflammatory responses in radiation-induced bowl disease model [52] and reduce TGF-β1 signaling by reducing the downstream target Smad3 expression in chronic radiation wound healing model [53, 54]. The present studies suggest that ASCs therapy of RF may be mediated in part by secreted HGF, which directly downregulate expression of profibrotic genes in damaged cells and enhance migration of bone marrow cells to the site of radiation injury for tissue regeneration. HGF plays an important role in cell proliferation, migration, and morphogenesis [55]. Previous studies have shown involvement of HGF in increased vascularization [56], decreased inflammation [57], downregulation of the profibrotic TGF-β-Smad signaling pathway [58], and promoting migration of bone marrow resident tissue-specific progenitor cells [29–31].
CONCLUSION
Radiation therapy is one of the most important tools in cancer treatment; however, iatrogenic comorbidities including RIF can significantly impair patient healing and life quality. Fractionated radiation therapy doses above 50 Gy to head and neck cancers, breast cancer, and soft tissue sarcomas can result in the late complication of RIF. Several published case studies suggest that application of autologous AT stem cells (ASCs) at the irradiation sites can ameliorate RIF, although the mechanism is not clear. In this study, we evaluated the efficacy of ASCs in management of RF in a mouse model and investigated the underlying molecular mechanism involved. Here, we show that irradiation of mouse hind limb using a single focused dose of 35 Gy result in visible fibrosis development in skin and significant reduction in limb excursion. The single fraction, electron beam radiation dose used in this study was that associated with reproducible skin fibrosis for experimental studies in this mouse strain and should be interpreted as relevant only to this animal model. The signs of fibrosis correlated well with high expression of TGF-β1 in the skin of irradiated mice, indicating an important role on this cytokine. The lack of RF in Smad 3–/– mice confirms the key role of TGF-β, as these mice cannot respond to TGF-β signaling. ASCs injection significantly improved limb excursion assessed by measuring degree of limb motion and restored normal epithelium architecture. Our in vitro transwell culture experiments with irradiated HFFs cocultured with ASCs showed a paracrine mechanism of mitigation. We identified HGF as a potential paracrine mediator released by ASCs which downregulates TGF-β in irradiated cells and also may act as a chemokine to attract bone marrow cells that are potentially involved in tissue repair.
SIGNIFICANCE STATEMENT.
Increased understanding of the etiology and progression of oncologic disease has resulted in advancements in effective treatments. External beam ionizing radiation therapy is a mainstay of cancer treatment, being used in more than 50% of all patients with a cancer diagnosis. However, it is associated with the late side effect of radiation-induced fibrosis. Currently, there are no effective treatments or prevention modalities available. The study evaluated the efficacy of adipose-derived stem cells (ASCs) in management of radiation-induced fibrosis in a mouse model. Furthermore, the study investigated the underlying molecular mechanisms involved. Using ASCs, the results show an improvement in the limb movement as a functional metric of successful mitigation postirradiation and observed an improvement in skin epithelium architecture. Present empirical evidence for the first time shows hepatocyte growth factor release by ASCs as a possible molecular mechanism of mitigation.
ACKNOWLEDGMENTS
This study was supported by CMCR NIAID/NIH Grant U19A168021 and NCI/NIH Grant R01-CA114246–08A1.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicated no potential conflicts of interest.
REFERENCES
- 1.Arcangeli G, Friedman M, Paoluzi R. A quantitative study of late radiation effect on normal skin and subcutaneous tissues in human beings. Br J Radiol 1974;47:44–50. [DOI] [PubMed] [Google Scholar]
- 2.Dorr W, Hendry JH. Consequential late effects in normal tissues. Radiother Oncol 2001;61:223–231. [DOI] [PubMed] [Google Scholar]
- 3.Bentzen SM. Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nat Rev Cancer 2006;6:702–713. [DOI] [PubMed] [Google Scholar]
- 4.Feng X-H, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev Biol 2005;21:659–693. [DOI] [PubMed] [Google Scholar]
- 5.Citrin DE, Prasanna PGS, Walker AJ et al. Radiation-induced fibrosis: Mechanisms and opportunities to mitigate. Report of an NCI Workshop, September 19, 2016. Radiat Res 2017;188:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trojahn Kølle S-F, Oliveri RS, Glovinski PV et al. Importance of mesenchymal stem cells in autologous fat grafting: A systematic review of existing studies. J Plast Surg Hand Surg 2012;46:59–68. [DOI] [PubMed] [Google Scholar]
- 7.Shukla L, Morrison WA, Shayan R. Adipose-derived stem cells in radiotherapy injury: A new frontier. Front Surg 2015;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizuno H, Tobita M, Uysal AC. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 2012;30:804–810. [DOI] [PubMed] [Google Scholar]
- 9.Minteer DM, Marra KG, Rubin JP. Adipose stem cells: Biology, safety, regulation, and regenerative potential. Clin Plast Surg 2015;42:169–179. [DOI] [PubMed] [Google Scholar]
- 10.Horl S, Ejaz A, Ernst S et al. CD146 (MCAM) in human cs-DLK1(–)/cs-CD34(+) adipose stromal/progenitor cells. Stem Cell Res. 2017;22:1–12. [DOI] [PubMed] [Google Scholar]
- 11.Ejaz A, Mitterberger MC, Lu Z et al. Weight loss upregulates the small GTPase DIRAS3 in human white adipose progenitor cells, which negatively regulates adipogenesis and activates autophagy via Akt–mTOR inhibition. EBioMedicine 2016;6:149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minteer D, Marra KG, Rubin JP. Adipose-derived mesenchymal stem cells: Biology and potential applications. Adv Biochem Eng Bio-technol 2013;129:59–71. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y, Liu T, Song K et al. Adipose-derived stem cell: A better stem cell than BMSC. Cell Biochem Funct 2008;26:664–675. [DOI] [PubMed] [Google Scholar]
- 14.Brand RM, Epperly MW, Stottlemyer JM et al. A topical mitochondria-targeted redox-cycling nitroxide mitigates oxidative stress-induced skin damage. J Invest Dermatol 2017; 137:576–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalash R, Epperly MW, Goff J et al. Amelioration of radiation-induced pulmonary fibrosis by a water-soluble bifunctional sulfoxide radiation mitigator (MMS350). Radiat Res 2013;180: 474–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ejaz A, Mattesich M, Zwerschke W. Silencing of the small GTPase DIRAS3 induces cellular senescence in human white adipose stromal/progenitor cells. Aging (Albany NY) 2017;9: 860–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavinato M, Koziel R, Romani N et al. UVB-induced senescence of human dermal fibroblasts involves impairment of proteasome and enhanced autophagic activity. J Gerontol Ser A 2017;72:632–639. [DOI] [PubMed] [Google Scholar]
- 18.Berhane H, Epperly MW, Cao S et al. Radio-resistance of bone marrow stromal and hematopoietic progenitor cell lines derived from Nrf2–/– homozygous deletion recombinant-negative mice. In Vivo 2013;27:571–582. [PMC free article] [PubMed] [Google Scholar]
- 19.Greenberger JS, Sakakeeny MA, Humphries RK et al. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/ basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci USA 1983;80:2931–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Randall K, Coggle J. Expression of transforming growth factor-β1 in mouse skin during the acute phase of radiation damage. Int J Radiat Biol 1995;68:301–309. [DOI] [PubMed] [Google Scholar]
- 21.Barcellos-Hoff M Radiation-induced transforming growth factor p and subsequent extracellular matrix reorganization in murine mammary gland. Cancer Res 1993;53:3880–3886. [PubMed] [Google Scholar]
- 22.Flanders KC, Sullivan CD, Fujii M et al. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol 2002;160:1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epperly MW, Franicola D, Zhang X et al. Reduced irradiation pulmonary fibrosis and stromal cell migration in Smad3–/– marrow chimeric mice. In Vivo 2006;20:573–582. [PubMed] [Google Scholar]
- 24.Akita S Treatment of radiation injury. Adv Wound Care 2014;3:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mou S, Wang Q, Shi B et al. Hepatocyte growth factor suppresses transforming growth factor-beta-1 and type III collagen in human primary renal fibroblasts. Kaohsiung J Med Sci 2009;25:577–587. [DOI] [PubMed] [Google Scholar]
- 26.Inoue T, Okada H, Kobayashi T et al. Hepatocyte growth factor counteracts transforming growth factor-β1, through attenuation of connective tissue growth factor induction, and prevents renal fibrogenesis in 5/6 nephrectomized mice. FASEB J 2003;17:268–270. [DOI] [PubMed] [Google Scholar]
- 27.Suga H, Eto H, Shigeura T et al. IFATS collection: Fibroblast growth factor-2-induced hepatocyte growth factor secretion by adipose-derived stromal cells inhibits postinjury fibrogenesis through ac-Jun N-terminal kinase-dependent mechanism. Stem Cells 2009;27:238–249. [DOI] [PubMed] [Google Scholar]
- 28.Collino M, Mastrocola R, Nigro D et al. Variability in myosteatosis and insulin resistance induced by high-fat diet in mouse skeletal muscles. Biomed Res Int 2014;2014:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosu-Myles M, Stewart E, Trowbridge J et al. A unique population of bone marrow cells migrates to skeletal muscle via hepatocyte growth factor/c-met axis. J Cell Sci 2005;118:4343–4352. [DOI] [PubMed] [Google Scholar]
- 30.Kucia M, Wojakowski W, Reca R et al. The migration of bone marrow-derived non-hematopoietic tissue-committed stem cells is regulated in an SDF-1-, HGF-, and LIF-dependent manner. Arch Immunol Ther Exp (Warsz) 2006; 54:121–135. [DOI] [PubMed] [Google Scholar]
- 31.Son BR, Marquez-Curtis LA, Kucia M et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells 2006;24: 1254–1264. [DOI] [PubMed] [Google Scholar]
- 32.Borovikova AA, Ziegler ME, Banyard DA et al. adipose-derived tissue in the treatment of dermal fibrosis: Antifibrotic effects of adipose-derived stem cells. Ann Plast Surg 2018;80: 297–307. [DOI] [PubMed] [Google Scholar]
- 33.Gallet P, Phulpin B, Merlin JL et al. Long-term alterations of cytokines and growth factors expression in irradiated tissues and relation with histological severity scoring. PLoS One 2011;6: e29399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thanik VD, Chang CC, Zoumalan RA et al. A novel mouse model of cutaneous radiation injury. Plast ReconstrSurg 2011;127:560–568. [DOI] [PubMed] [Google Scholar]
- 35.Xu M, Pirtskhalava T, Farr JN et al. Senolytics improve physical function and increase lifespan in old age. Nat Med 2018;24:1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcelin G, Ferreira A, Liu Y et al. A PDGFRα-mediated switch toward CD9 high adipocyte progenitors controls obesity-induced adipose tissue fibrosis. Cell Metab 2017;25: 673–685. [DOI] [PubMed] [Google Scholar]
- 37.Khonji N Breast reconstruction using autologous fat. Br J Surg 2010;97:795–796. [DOI] [PubMed] [Google Scholar]
- 38.Kolle SF, Fischer-Nielsen A, Mathiasen AB et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: A randomised placebo-controlled trial. Lancet 2013;382:1113–1120. [DOI] [PubMed] [Google Scholar]
- 39.Rigotti G, Marchi A, Gali?? M et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: A healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg 2007;119:1409–1422. [DOI] [PubMed] [Google Scholar]
- 40.Akita S, Yoshimoto H, Ohtsuru A et al. Autologous adipose-derived regenerative cells are effective for chronic intractable radiation injuries. Radiat Prot Dosimetry 2012;151:656–660. [DOI] [PubMed] [Google Scholar]
- 41.Mizuno H, Hyakusoku H. Fat grafting to the breast and adipose-derived stem cells: Recent scientific consensus and controversy. Aesthet Surg J 2010;30:381–387. [DOI] [PubMed] [Google Scholar]
- 42.Faghahati S, Delaporte T, Toussoun G et al. Treatment by fat tissue transfer for radiation injury in childhood facial cancer. Ann Chir Plast Esthet 2010;55:169–178. [DOI] [PubMed] [Google Scholar]
- 43.Jensen DH, Oliveri RS, Trojahn Kølle SF et al. Mesenchymal stem cell therapy for salivary gland dysfunction and xerostomia: A systematic review of preclinical studies. Oral Surg Oral Med Oral Pathol Oral Radiol 2014;117: 335e1–342e1. [DOI] [PubMed] [Google Scholar]
- 44.Gronhoj C, Jensen DH, Glovinski PV et al. First-in-man mesenchymal stem cells for radiation-induced xerostomia (MESRIX): Study protocol for a randomized controlled trial. Trials 2017;18:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riccobono D, Agay D, Scherthan H et al. Application of adipocyte-derived stem cells in treatment of cutaneous radiation syndrome. Health Phys 2012;103:120–126. [DOI] [PubMed] [Google Scholar]
- 46.Riccobono D, Agay D, François S et al. Contribution of INTRAMUSCULAR autologous adipose tissue-derived stem cell injections to treat cutaneous radiation syndrome: Preliminary results. Health Phys 2016;111:117–126. [DOI] [PubMed] [Google Scholar]
- 47.Ohnishi S, Sumiyoshi H, Kitamura S et al. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Lett 2007; 581:3961–3966. [DOI] [PubMed] [Google Scholar]
- 48.Hsiao ST, Asgari A, Lokmic Z et al. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev 2012;21:2189–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suga H, Eto H, Aoi N et al. Adipose tissue remodeling under ischemia: Death of adipocytes and activation of stem/progenitor cells. Plast Reconstr Surg 2010;126:1911–1923. [DOI] [PubMed] [Google Scholar]
- 50.Haubner F, Leyh M, Ohmann E et al. Effects of external radiation in a co-culture model of endothelial cells and adipose-derived stem cells. Radiat Oncol 2013;8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haubner F, Muschter D, Pohl F et al. A co-culture model of fibroblasts and adipose tissue-derived stem cells reveals new insights into impaired wound healing after radiotherapy. Int J Mol Sci 2015;16:25947–25958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bessout R, Demarquay C, Moussa L et al. TH17 predominant T-cell responses in radiation-induced bowel disease are modulated by treatment with adipose-derived mesenchymal stromal cells. J Pathol 2015;237:435–446. [DOI] [PubMed] [Google Scholar]
- 53.Huang SP, Huang CH, Shyu JF et al. Promotion of wound healing using adipose-derived stem cells in radiation ulcer of a rat model. J Biomed Sci 2013;20:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sultan SM, Stern CS, Allen RJ Jr et al. Human fat grafting alleviates radiation skin damage in a murine model. Plast Reconstr Surg 2011;128:363–372. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura T, Sakai K, Nakamura T et al. Hepatocyte growth factor twenty years on: Much more than a growth factor. J Gastroenterol Hepatol 2011;26:188–202. [DOI] [PubMed] [Google Scholar]
- 56.Xin X, Yang S, Ingle G et al. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am J Pathol 2001;158:1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giannopoulou M, Dai C, Tan X et al. Hepatocyte growth factor exerts its anti-inflammatory action by disrupting nuclear factor-κB signaling. Am J Pathol 2008;173:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y Hepatocyte growth factor in kidney fibrosis: Therapeutic potential and mechanisms of action. Am J Physiol 2004;287: F7–F16. [DOI] [PubMed] [Google Scholar]


