Abstract
Background
The impact of COVID-19 on healthcare- associated infections (HCAI) caused by multidrug-resistant (MDR) bacteria that contribute to higher mortality is a growing area of study
Methods
This retrospective observational study compares the incidence density (ID) of HCAI caused by MDR bacteria (CRE, CRAB, CRP, MRSA and VRE) pre-COVID (2017-2019) and during the COVID-19 pandemic (2020) in overall hospitalized patients and in intensive care (ICU) units.
Results
We identified 8,869 HCAI, of which 2,641 (29.7%) were caused by bacterial MDR, and 1,257 (14.1%) were from ICUs. The overall ID of MDR infections increased 23% (P < .005) during COVID-19. The overall per-pathogen analysis shows significant increases in infections by CRAB and MRSA (+108.1%, p<0.005; +94.7%, p<0.005, respectively), but not in CRE, CRP, or VRE. In the ICU, the overall ID of MDR infections decreased during COVID, but that decline was not significant (-6.5%, P = .26). The ICU per-pathogen analysis of ID of infection showed significant increases in CRAB and MRSA (+42.0%, P = .001; +46.2%, P = .04), significant decreases in CRE and CRP (-26.4%, P = .002; -44.2%, P = 0.003, respectively) and no change in VRE.
Conclusions
The COVID-19 pandemic correlates to an increase in ID of CRAB and MRSA both in ICU and non-ICU setting, and a decrease in ID of CRE and CRP in the ICU setting. Infection control teams should be aware of possible outbreaks of CRAB and MRSA and promote rigorous adherence to infection control measures as practices change to accommodate changes in healthcare needs during and after the pandemic.
Key Words: Healthcare-associated infections, Epidemiology, Multidrug resistant infection, Multidrug resistant bacteria, Carbapenem-resistant Acinetobacter baumannii infection, Methicillin-resistant Staphylococcus aureus infection
Background
The COVID-19 pandemic is the greatest threat to global health since the Spanish Influenza pandemic in the 20th century. Following the World Health Organization's declaration of pandemic status in March 2020,1 health systems have been challenged to continuously adapt to the rapid changes in our understanding of the disease itself, abrupt policy changes related to pandemic management, the secondary health effects of the pandemic,2 the effects of the disease itself,3 and now evolving mutations of SARS-CoV-2.4 To protect patients and healthcare workers, hospitals and clinics implemented various measures to prevent contagion spread, such as the universal use of personal protective equipment (PPE), extensive training, and intensification of existing infection control policies. As the pandemic continues, these combined stresses have pushed many health systems to the limits of their functioning, threatening healthcare system collapse in addition to the unequivocal social and economic devastation that are emerging from this global crisis.5
During past pandemics and in outbreaks of respiratory viruses, secondary bacterial and fungal infections were common causes of overall morbidity and mortality, but the impact of these secondary infections during COVID-19 remains under study.6, 7 Some studies report that 14%-44% of patients develop secondary infection, which certainly contributes to worse clinical outcomes, longer hospital stays and higher mortality rates.6, 7 However, other studies suggest the actual rate of co-infection is relatively low at 3.5%, and the rate of secondary infection in hospitalized patients is 14.3%. Regardless of the actual rates of co-infection and secondary infection, antibiotics are almost certainly overprescribed, with an estimated prevalence of 74.6% in one meta-analysis. With long hospital stays and prolonged mechanical ventilation for some COVID-19 patients, healthcare-associated infections (HCAI) rates have increased, with an associated increase on infections by multidrug resistant (MDR) pathogens.5 Treatment for real and feared co-infection and HCAI have all contributed to a substantial increase in antibiotic prescribing globally, often using broad-spectrum antimicrobials.5 , 8 , 9 This, in turn, is associated with a major impact on the emergence of MDR bacterial infections worldwide.6, 7, 8, 9, 10
We hypothesize that the selective pressure from increased antibiotic use in combination with invasive support measures and continuous use of PPE will have a measurable impact on the overall incidence of MDR bacterial infections. The objective of this study is to assess the impact of the COVID-19 pandemic on the incidence of MDR bacterial infections in an acute care hospital in Brazil.
Methods
We performed an observational retrospective study to identify the incidence of all MDR bacterial infections from January-2017 to December-2020 at Instituto Central and analyzed all cases of HCAI caused by MDR bacteria. For detailed analysis, we also identified HCAI caused by any of these MDR bacteria: carbapenem-resistant Enterobacterales (CRE), carbapenem-resistant Pseudomonas aeruginosa (CRP), carbapenem-resistant Acinetobacter baumannii (CRAB), vancomycin-resistant Enterococcus (VRE), or methicillin-resistant Staphylococcus aureus (MRSA).
The rates of MDR bacterial infections are expressed in incidence density (ID) (Although we are prospectively collecting data to complete a time-series analysis of MDR infections when the pandemic concludes, to inform policy in the interim, in this study we look at median incidence change from baseline). The incidence density of infections caused by multidrug-resistant bacteria was calculated using the number of MDR infection cases in the period as numerator and the number of patient-days in the same period multiplied by 1,000 as denominator. In order to assess the trend curve, the incidence densities were aggregated for each 3-month period (quarters). To assess pre- and post-pandemic differences, the incidence densities were aggregated from the 2017 to 2019 (pre-pandemic) period and compared with the aggregated data from 2020 (the pandemic period). We analyzed total rates, rates per pathogen and rates of ICU units. In total rates are included the rates of ICUs.
Instituto Central is a tertiary care public teaching hospital affiliated with the Hospital das Clínicas of University of São Paulo, Brazil. It is housed in an 11-floor building and staffed with 6,000 healthcare workers (HCW) who manage care across 1,000 beds, 100 of which are intensive care (ICU). Instituto Central became a referral center for COVID-19 care in the state of São Paulo, and, in March of 2020, was dedicated solely to COVID patient care. In that setting HCW from other areas were dislocated do COVID-19 care and the number of ICU beds increased to 200 in total. However, with the remission of the pandemic in August 2020, the hospital returned to attend non-COVID cases, again becoming a general hospital. Personal protection equipment was provided to all healthcare workers taking care of COVID19 patients. Furthermore, all healthcare workers received training on the proper use of PPE, including N95 masks, disposable gloves and gowns.
The Department of Infection Control maintains a database and notifies all cases that meet the U.S. Centers for Disease Control and Prevention (CDC) definition of healthcare-associated infections - a healthcare-associated infection is a localized or systemic condition resulting from an adverse reaction to the presence of an infectious agent(s) or its toxin(s) that was not present on admission to the acute care facility and started after 72 hours of admission.11 For the ICU patients, HCAI is considered when the infectious evidence starts after 48 hours of admission in the ICU.12 All patients colonized or infected by MDR bacteria were under contact precautions. For COVID-19 infected patients with MDR bacteria infections, the use of a second gown for patient care was instituted, which was removed after each contact. The microbiology laboratory uses the MALDI-TOF methodology for identification of bacterial isolates, and the Vitek 2 (bioMerieux) system for antimicrobial sensitivity tests according to the "Clinical and Laboratory Standards Institute'' (CLSI) document.13
The incidence densities of infections were analyzed by quarters in all units and in the intensive care units to analyze trends. To analyze the differences between the pre-pandemic and pandemic periods, we used aggregated data and a comparison was made using the Mann-Whitney U test comparing medians in the two periods. A value of P ≤ .05 was considered statistically significant. The statistical analysis was performed using the statistical software Epi Info TM version 7.2 of the Centers for Diseases Control.
The study was approved by the ethics committee of the hospital under protocol number 4.414.708.
Results
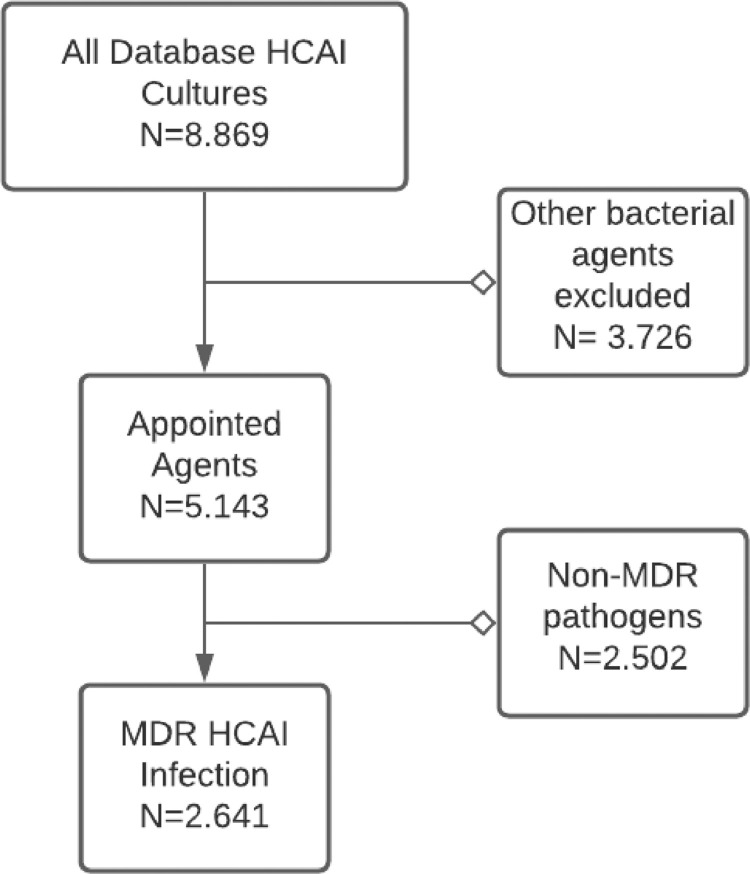
During the period of study, we identified 8,869 HCAI in our database; 5,143 (57.9%) were caused by one of the appointed bacterial agents and 2,641 (29.7%) were multidrug resistant and were selected for the analysis (as shown in Fig 1 ). Of these, 1,257 (14.1%) were from patients located in the intensive care unit. (See Table 1 ).
Fig 1.
Healthcare associated infection (HCAI) database selection diagram.
MDR = Multidrug resistant
Table 1.
Comparison of incidence density between pre-pandemic and pandemic period
| Incidence density |
DIR* | Percent change | P† | ||
|---|---|---|---|---|---|
| Pre-pandemic period (2017-2019) | Pandemic Period (2020) | ||||
| Overall MDR infections | |||||
| Total | 3.14 | 3.89 | 1.59 | 23.9% | <.005 |
| CRE | 1.29 | 1.38 | 1.20 | 6.5% | .42 |
| CRP | 0.42 | 0.36 | 0.99 | -15.1% | .28 |
| CRAB | 0.53 | 1.10 | 2.95 | 108.1% | <.005 |
| MRSA | 0.24 | 0.46 | 2.84 | 94.7% | <.005 |
| VRE | 0.65 | 0.59 | 1.43 | -10.6% | .34 |
| ICU MDR Infections | |||||
| Total ICU | 9.22 | 8.62 | 1.19 | -6.5% | .26 |
| CRE | 3.82 | 2.81 | 0.84 | -26.4% | <.005 |
| CRP | 1.21 | 0.67 | 0.60 | -44.2% | <.005 |
| CRAB | 2.07 | 2.94 | 2.08 | 42.0% | <.005 |
| MRSA | 0.76 | 1.12 | 1.94 | 46.2% | .04 |
| VRE | 1.36 | 1.08 | 1.39 | -20.5% | .17 |
Boldp values are significant.
CRE, Carbapenem Resistant Enterobacterales; CRAB, Carbapenem Resistant Acinetobacter baumannii, CRP, Carbapenem Resistant Pseudomonas aeruginosa, MRSA, Methicillin Resistant Staphylococcus aureus, VRE, Vancomycin resistant Enterococcus, ID, Incidence Density
Density incidence Ratio
Chi square test to 95% confidence.
There is no statistical difference in incidence between 2017 and 2018, 2018 and 2019, or 2017 and 2019 (unpaired 2 tailed t-test to 95% confidence, P = .962, 0.156, and .207, respectively; combined simple linear regression trendline R^2 = 0.233). Hence, the combined data from 2017 to 2019 serves as an appropriate baseline for incidence of MDR infections.
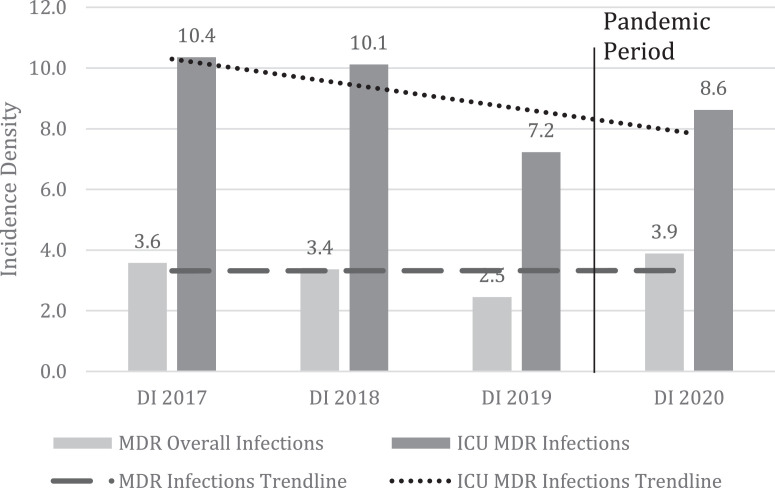
When we analyze the incidence density per year, we can note that the overall incidence density remained stable during the years 2017 to 2020, but when we analyzed the incidence density only in intensive care units, we noticed a decline (Fig 2 ). Although the increase in MDR incidence between baseline years (2017-19) and the pandemic year (2020) is not significant (6 vs 8.4, P = .063), the difference between the baseline years and the peak of the pandemic (Second and third trimesters, 2020) is significant (6 vs 11.4, P<.001). This is easily visualized in Fig. 2 and 3 . When the incidence of MDR is considered only in the ICU setting, there is a decrease in incidence from 9.2 to 8.6, but this is not significant (P > .05).
Fig 2.
Incidence densities for MDR infections in pre-pandemic and pandemic period.
MDR = Multidrug resistant bacteria; ICU = Intensive Care Unit; ID = Incidence Density
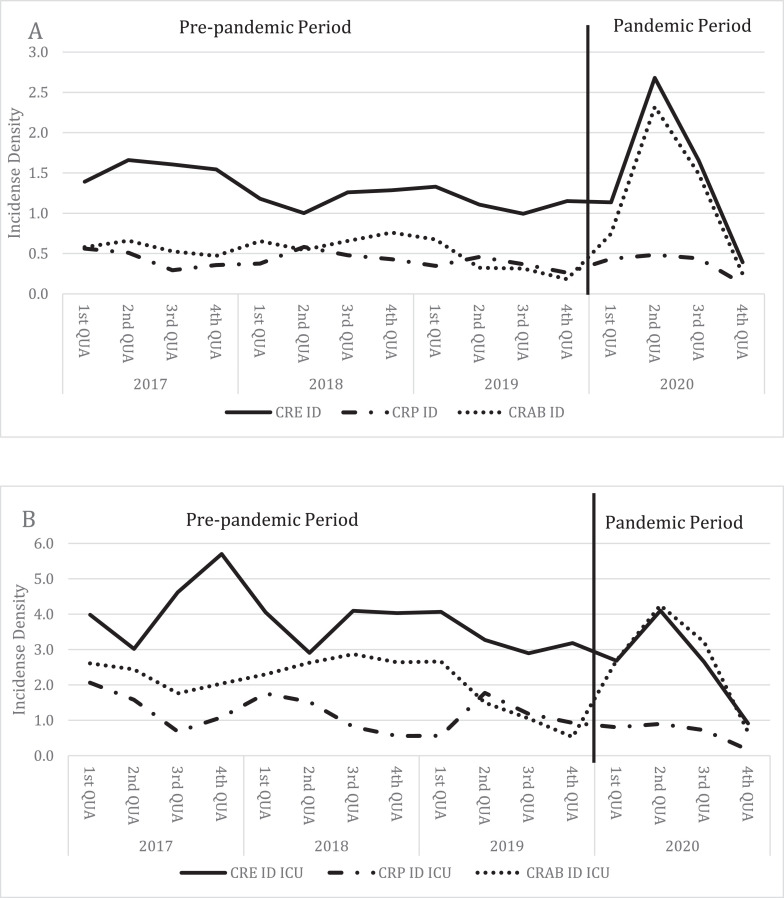
Fig 3.
Distribution of Incidence densities of Gram negative MDR - CRE, CRAB and CRP – infections (A = overall; B = ICU).
CRE = Carbapenem Resistant Enterobacterales; CRAB = Carbapenem Resistant Acinetobacter baumannii; CRP = Carbapenem Resistant Pseudomonas aeruginosa; ID = Incidence Density; QUA = Quarter
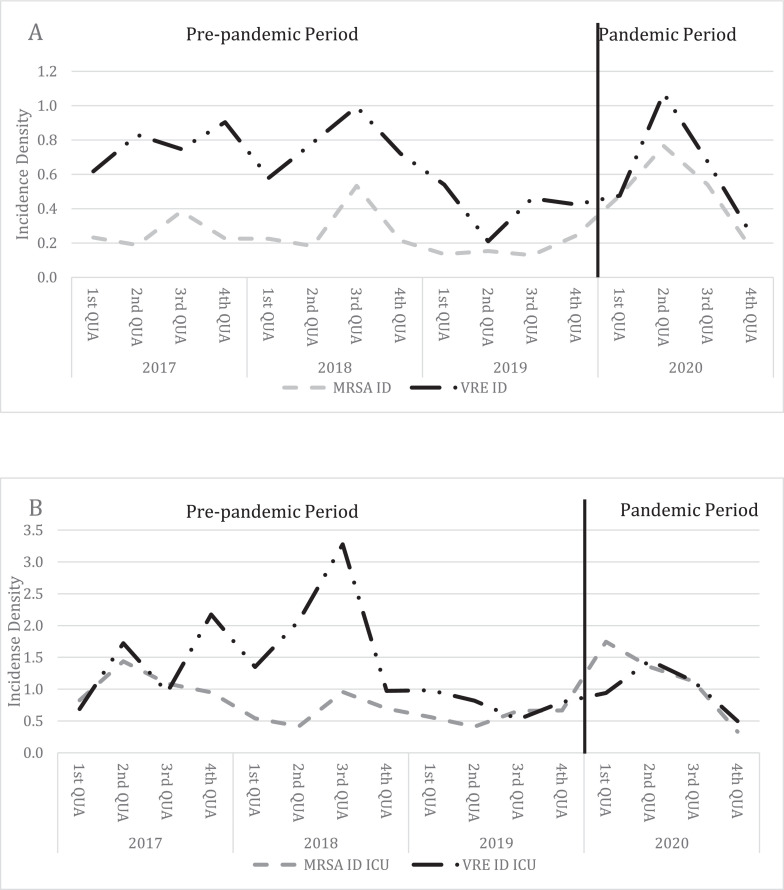
Fig. 3 and 4 show the incidence densities by pathogen. Fig 3 shows the incidence densities of carbapenem-resistant gram-negative bacilli (CRE, CRP, CRAB) in all hospital units (A) and intensive care units only (B). Fig 4 shows the incidence densities of resistant gram-positive cocci (MRSA and VRE) in the same manner.
Fig 4.
Distribution of incidence densities of Gram positive MDR - MRSA and VRE – infections (A = overall; B = ICU).
MRSA = Methicillin Resistant Staphylococcus aureus; VRE = Vancomycin resistant Enterococcus; ID = Incidence Density; QUA = Quarter
Analysis by MDR pathogens in overall units shows an increase in the incidence density of CRE, CRAB and MRSA and a decrease in the incidence density of CRP and VRE. The increase in the incidence density of CRAB was 108% (P < .005) and MRSA was 94% (P < .005; Table 1).
Analysis by MDR pathogens in ICU units also shows an increase in the incidence density of MRSA and CRAB and also shows a decrease in the incidence density of CRE, CRP and VRE. In the ICU, the increase in the incidence density of CRAB and MRSA 42% (P = .001) and 46.2% (P = .040), respectively (Table 1).
This increase was the result of two outbreaks in the ICU units. The MRSA outbreak occurred at a medical ICU in isolates of tracheal secretions and it was investigated by collecting nasal surveillance cultures from all patients and healthcare workers. A pulsed-field gel electrophoresis (PFGE) analysis was made and demonstrated a polyclonal pattern, showing that cross transmissions probably were not the cause of this outbreak. After implementation of the patient´s cohort and reinforced hand hygiene, the outbreak was controlled. The CRAB outbreak occurred at a surgical ICU both in blood cultures and tracheal secretions but it was not microbiologically investigated, so unfortunately we do not have clonality analysis. After the introduction of a second gown for the care of infected/colonized patients there was a decrease in the number of cases.
Discussion
To our knowledge this is the first Brazilian study to evaluate incidence densities of bacterial MDR infections during the COVID-19 pandemic. We found significant differences in the density incidence of MDR infections when comparing the pre-pandemic and pandemic period, especially on MRSA and CRAB infections. Our results show how the transition of a big general hospital to a COVID-19 dedicated health service alters the incidence of MDR infections.
COVID-19 has rapidly grown from a pneumonia outbreak in Wuhan, China13 to a global catastrophe. The Americas are currently regarded as the epicenter of the pandemic, and Brazil has 9 million confirmed cases and about 240,000 deaths through February 2021. [Ministério da Saúde]
Our hospital responded to the pandemic by rapidly developing and implementing a massive operation to transform all its beds for the exclusive care of COVID-19 patients and converting 200 regular beds to enable ICU-level care. As part of enhanced precautions in the hospital, and increased awareness and concern for COVID-19 spread, we expected a decrease in the incidence density of bacterial MDR infections, consistent with contact precautions for COVID-19 patients.
In recent years, isolates of CRE have been reported worldwide and has dramatically increased in Brazil,14 so our Department of Infection Control implemented admission and cross-sectional surveillance cultures on all ICU patients to screen carrier status and ensure appropriate precautions to avoid cross-transmission and to avoid infections by MDR. Since 2014, this strategy has been associated with year-by-year decreases in incidence density by CRE infection in the ICUs of our hospital (internal data). The CRE infection surveillance program may explain pre-COVID decline since its main objective is to prevent cross-transmission and potentially reduce infection rates. CRE surveillance was suspended during the pandemic period due to the universal contact precautions measures in the complex, and that confounds comparison of pre- and post-COVID CRE results.
The increase in MDR bacterial infections for CRAB, MRSA and CRE are substantial, significant, and consistent with other published literature,15, 16 which show increases in MDR infections of 6%-15%, with associated increases in higher mortality and length of hospital stay in COVID-19 patients.17, 18, 19, 20
We found an increase of 108% in HCAI by CRAB in all units and 42% in ICUs units. Outbreaks of CRAB have been well documented in acute care hospitals, particularly among critically ill patients, and are often driven by factors that include breaches in infection control and persistent environmental contamination.21 During the COVID-19 pandemic, inexperienced healthcare workers (HCW), reduced capacity for overall auditing practices and both intentional and unintentional changes in infection control practices likely contributed to this CRAB increase. We were unable to investigate this outbreak microbiologically. The large number of CRAB isolates, in combination with work overload, precluded a robust investigation into the causes of the CRAB increase. However, containment measures were implemented and training on the use of personal protection equipment (PPE) were performed, mainly in intensive care units, where in some COVID-19 cases the gown was used for more than one patient and discarded only at the end of the work shift. After this outbreak, we instituted the use of a second gown for patients colonized or infected by CRAB and this second gown was discarded after patient care.
Regarding the increase of 94% in HCAI by MRSA in all units and 46% in ICUs units, because of the smaller number of isolates and infrequency of MRSA in our ICUs we were able to investigate causes. MRSA infections are known to be associated with invasive devices and breach of prevention measures such as proper hand hygiene and use of inadequate PPE.18, 19, 20 Other publications show a significant increase in bloodstream infection with an impact on mortality, with a report of 86% increase in the number of MRSA infections.22
We collected surveillance cultures (nasal swabs) from patients and HCW of a specific ICU where the cases were concentrated and found no secondary colonization. We were able to investigate the outbreak microbiologically since the number of isolates was smaller and the pathogen, until then infrequent and mainly in the ICUs, drew earlier attention to the increase in the number of cases.
We noted a stabilization in CRE rates and a decrease in CRP and VRE rates. A possible explanation for why some MDR rates increase while others decrease is the biological plausibility of these agents or the consumption of antimicrobials by selective pressure.23
Despite campaigns reinforcing the absence of antibiotic action in viral diseases it is noted that there is a trend in more antibiotic prescriptions in these situations and that it should continue in the current context, leading to a greater risk of selection of resistant microbiota, a serious contemporary problem that spreads as well as a pandemic, already with complex and serious consequences.8, 24, 25, 14, 26 The analysis of antimicrobial consumption is a concern of another study.
Several antibiotics are (over)prescribed for secondary infection in COVID-19 patients, but there is a tendency to prescribe macrolides in the outpatient setting, likely because of inpatient associations with better outcomes that have not been proven in outpatient settings.21 Inpatient clinicians tend to prescribe third generation cephalosporins.27, 28 In a recent meta-analysis, it was noted that up to two thirds (62.4%) of patients who received some medical care because of SARS-COV-2 infection received some antibiotic, the choice was heterogeneous and dependent on the region evaluated with microbiological evidence of presumptive bacterial infection in 5% to 8% of the patients studied.27, 29, 30, 31 The need to stay ahead of infection in the critically ill pushes clinicians towards a greater use of broad-spectrum antimicrobials in mechanically ventilated patients due to the difficulty in distinguishing the inflammatory phase of COVID-19 with the appearance of secondary infection.8, 32, 33 Future studies are warranted to evaluate the changes in incidence of MDR infections during COVID-19 in Brazil with changes in antibiotic prescribing patterns.34, 35
Another consideration is that COVID-19 critically ill patients tend to have longer mechanical ventilation periods and longer hospital stays. That can lead to important sequels like pressure ulcers with secondary infections by MDR agents and other HCAI in non-ICU patients, a reasonable explanation on the MDR rise both in ICU and non-ICU settings.36
Limitations of this study include its single center data and the center's focus on COVID-19 care during part of the pandemic period, which may have introduced a selection bias that limits generalizability.
Recently there is a large scientific production on COVID-19 but few publications directly assessing the changes occurring in health services. The majority of articles and reviews focus on theoretical postulations. Thus, our study shows the impact of the COVID-19 pandemic on the incidence of multidrug-resistant bacterial infections in an acute care hospital in Brazil.
Our study highlights two risks of modifying standard practice to address a pandemic: (1) infection control measures and (2) antibiotic over prescription based on the difficulty in accurate etiological diagnosis. Consistency in practice is a hallmark of high-quality medical care; practice modifications may be necessary and appropriate but should never be taken lightly.37, 38
In conclusion, COVID-19 is associated with an increase in incidence of CRAB and MRSA infections both in ICUs and non-ICUs units. Infection control teams should be aware of the risk of outbreaks from these pathogens. Strategies to reduce antimicrobial consumption in hospitalized COVID-19 patients and ensure proper PPE use may mitigate these increases. Research is needed to explore the relationship between antimicrobial prescription patterns during COVID-19 and increases in MDR incidence.
Acknowledgments
We are grateful to all following members of Infection Control Department of the Instituto Central of Hospital das Clínicas for their hard and precise work: Adolfo Edison Illanes Manrique, Aleia Faustina Campos, Icaro Boszczovski, Laína Bubach de Carvalho, Maria Luiza do Nascimento Moura, Maristela Pinheiro Freire, Matias Salomão, Ana Natiele da Silva Barros, Fernanda de Sousa Spadão, Isabel Cristina Vilela Oshiro, Laura Maria Brasileiro Gomes, Cristina Ramalho e Sueli Maria da Silva Bernardo. We would also like to thank the microbiology laboratory for their excellence in performing the tests.
Footnotes
Funding/Support: No funding was received for this work.
Prior presentations: This submission was not prior presented at any meeting
Conflict of interest: The authors declare no conflicts of interest.
References
- 1.World Health Organization. Rational use of personal protective equipment (PPE) for coronavirus disease (COVID-19): interim guidance, 19 March 2020. No. WHO/2019-nCoV/IPC PPE_use/2020.2. World Health Organization, 2020.
- 2.Douglas M, Katikireddi SV, Taulbut M, McKee M, McCartney G. Mitigating the wider health effects of covid-19 pandemic response. BMJ. 2020;369:m1557. doi: 10.1136/bmj.m1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet (British edition) 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray AK. The Novel Coronavirus COVID-19 Outbreak: global implications for antimicrobial resistance. Front Microbiol. 2020;11:1020. doi: 10.3389/fmicb.2020.01020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cookson C. Scientists alarmed at spread of Covid mutant. December 20, 2020. Available at: https://advance.lexis.com/api/document?collection=news&id=urn:contentItem:61JV-8481-F039-600D-00000-00&context=1516831. Accessed February 18, 2021
- 6.Hsu J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ. 2020;369:m1983. doi: 10.1136/bmj.m1983. [DOI] [PubMed] [Google Scholar]
- 7.Munita JM, Arias CA. Mechanisms of antibiotic resistance. Microbiol Spectr. 2016;4:481–511. doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paterlini M. On the front lines of coronavirus: the Italian response to covid-19. BMJ. 2020;368:m1065. doi: 10.1136/bmj.m1065. [DOI] [PubMed] [Google Scholar]
- 9.Reardon S. Antibiotic treatment for COVID-19 complications could fuel resistant bacteria. Science. 2020 [Google Scholar]
- 10.Rawson TM, Ming D, Ahmad R, Moore LSP, Holmes AH. Antimicrobial use, drug-resistant infections and COVID-19. Nat Rev Microbiol. 2020;18:409–410. doi: 10.1038/s41579-020-0395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brasil . Medidas de Prevenção de Infecção Relacionada à Assistência à Saúde. Anvisa; Brasília: 2017. Agência Nacional de Vigilância Sanitária. [Google Scholar]
- 12.National Healthcare Safety Network. CDC/NHSN Surveillance Definitions for Specific Types of Infections. 2021. Available at: https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf. Accessed October 15, 2021.
- 13.Clinical and Laboratory Standards Institute . Vol. 40. CLSI; Wayne, PA: 2020. Performance Standards for Antimicrobial Susceptibility Testing; 30th Informational Supplement. (CLSI Publication M100-S18). [Google Scholar]
- 14.World Health Organization; Geneve: 2020. WHO Pneumonia of Unknown Cause—China. Disease Outbreak News: 5 January 2020.https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/ Available at: Accessed March 17, 2020. [Google Scholar]
- 15.World Health Organization Implementation manual to prevent and control the spread of carbapenem-resistant organisms at the national and health care facility level: interim practical manual supporting implementation of the Guidelines for the prevention and control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in health care facilities. No. WHO/UHC/SDS/2019.6. World Health Organization. 2019 [Google Scholar]
- 16.Perez S, Innes GK, Walters MS, et al. Increase in hospital-acquired carbapenem-resistant acinetobacter baumannii infection and colonization in an acute care hospital during a surge in covid-19 admissions — New Jersey, February–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1827–1831. doi: 10.15585/mmwr.mm6948e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clancy CJ, Nguyen MH. Coronavirus Disease 2019, superinfections, and antimicrobial development: what can we expect? Clin Infect Dis. 2020;71:2736–2743. doi: 10.1093/cid/ciaa524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-Meniño I, Forcelledo L, Rosete Y, García-Prieto E, Escudero D, Fernández J. Spread of OXA-48-producing Klebsiella pneumoniae among COVID-19-infected patients: The storm after the storm. J Infect Public Health. 2021;14:50–52. doi: 10.1016/j.jiph.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasperini B, Cherubini A, Lucarelli M, Espinosa E, Prospero E. Multidrug-resistant bacterial infections in geriatric hospitalized patients before and after the covid-19 outbreak: results from a retrospective observational study in two geriatric wards. Antibiotics. 2021;10:95. doi: 10.3390/antibiotics10010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez-Simmonds A, Annavajhala MK, Mcconville TH, et al. 371. Cluster of carbapenemase-producing Enterobacterales secondary infections during the COVID-19 crisis at a New York City hospital. Open Forum Infect Dis. 2020;7(suppl 1):S256. doi: 10.1093/jac/dkaa466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez S, Innes GK, Walters MS, et al. Increase in Hospital-Acquired Carbapenem-Resistant Acinetobacter baumannii Infection and Colonization in an Acute Care Hospital During a Surge in COVID-19 Admissions — New Jersey, February–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1827–1831. doi: 10.15585/mmwr.mm6948e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support covid-19 antimicrobial prescribing. Clin Infect Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucien MAB, Canarie MF, Kilgore PE, et al. Antibiotics and antimicrobial resistance in the COVID-19 era: perspective from resource-limited settings. Int J Infect Dis. 2021;104:250–254. doi: 10.1016/j.ijid.2020.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rawson TM, Hernandez B, Moore LSP, et al. A Real-world Evaluation of a Case-based Reasoning Algorithm to Support Antimicrobial Prescribing Decisions in Acute Care. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa383. [DOI] [PubMed] [Google Scholar]
- 25.Rossato L, Negrão FJ, Simionatto S. Could the COVID-19 pandemic aggravate antimicrobial resistance? Am J Infect Control. 2020;48:1129–1130. doi: 10.1016/j.ajic.2020.06.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yam ELY. COVID-19 will further exacerbate global antimicrobial resistance. J Travel Med. 2020;27:taaa098. doi: 10.1093/jtm/taaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharland M, Pulcini C, Harbarth S, et al. Classifying antibiotics in the WHO Essential Medicines List for optimal use—be AWaRe. Lancet Infect Dis. 2018;18:18–20. doi: 10.1016/S1473-3099(17)30724-7. [DOI] [PubMed] [Google Scholar]
- 28.Antimicrobial resistance in the age of COVID-19. Nat Microbiol. 2020;5:779. doi: 10.1038/s41564-020-0739-4. [DOI] [PubMed] [Google Scholar]
- 29.Tumbarello M, Viale P, Viscoli C, et al. Predictors of Mortality in Bloodstream Infections Caused by Klebsiella pneumoniae Carbapenemase-Producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 30.Tiri B, Sensi E, Marsiliani V, et al. Antimicrobial Stewardship Program, COVID-19, and Infection Control: Spread of Carbapenem-Resistant Klebsiella Pneumoniae Colonization in ICU COVID-19 Patients. What Did Not Work? J Clin Med. 2020;9:2744. doi: 10.3390/jcm9092744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guisado-Gil A, Infante-Domínguez C, Peñalva G, et al. Impact of the COVID-19 pandemic on antimicrobial consumption and hospital-acquired candidemia and multidrug-resistant bloodstream infections. Antibiotics. 2020;9:816. doi: 10.3390/antibiotics9110816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guimarães T, Nouér SA, Martins RCR, et al. Ceftazidime-avibactam as salvage therapy for infections caused by enterobacteriales coresistant to carbapenems and polymyxins. Antimicrob Agents Chemother. 2019;63:e00528–19. doi: 10.1128/AAC.00528-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collignon P, Beggs JJ. CON: COVID-19 will not result in increased antimicrobial resistance prevalence. JAC-Antimicrob Resist. 2020;2:dlaa051. doi: 10.1093/jacamr/dlaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langford BJ, So M, Raybardhan S, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021;27:520–531. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rawson TM, Moore LSP, Castro-Sanchez E, et al. COVID-19 and the potential long-term impact on antimicrobial resistance. J Antimicrob Chemother. 2020;75:1681–1684. doi: 10.1093/jac/dkaa194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Getahun H, Smith I, Trivedi K, Paulin S, Balkhy HH. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull. World Health Organ. 2020;98 doi: 10.2471/BLT.20.268573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arakawa Y. Systematic research to overcome newly emerged multidrug-resistant bacteria. Microbiol Immunol. 2020;64:231–251. doi: 10.1111/1348-0421.12781. [DOI] [PubMed] [Google Scholar]