Dear Editor
The novel findings by Molfino et al. [1] and subsequent remarks by Kara et al. [2] highlight the potential contribution of sarcopenia to disease severity in COVID-19 infection. Although exploratory in nature, the study by Molfino et al. evaluates the importance of muscle wasting as a critical prognostic marker of COVID-19-related complications. The remarks by Kara et al. suggest additional measures to improve the evaluation of sarcopenia. We congratulate the authors for the excellent work. However, we would like to add some remarks and share our novel exciting data to broaden the perspective on the interface of sarcopenia and Covid-19.
First, both studies did not consider the standard definition of sarcopenia by the European Working Group on Sarcopenia in Older People (EWGSOP2), which includes muscle weakness (Handgrip strength; HGS < 27 kg), muscle wasting (appendicular skeletal mass index; ASMI < 7 kg/m2), and low physical performance (gait speed ≤ 0.8 m/s) in men [3]. Importantly, low ASMI measures systemic muscle wasting and is superior to measurements of individual muscles or groups of muscles, performed by Molfino et al. [1]. This is because isolated muscle(s) may be preferentially atrophied or relatively spared in aging due to their distinct fiber-type composition, as pointed by Kara et al. [2]. The EWGSOP2 definition is also a useful measure of functional independence in ambulant patients. Second, since a substantial population of elderly COVID-19 patients are non-ambulant and cannot provide gait speed data, there is a need to investigate biochemical markers of sarcopenia, preferably in plasma. Third, several lines of evidence suggest a coupling between sarcopenia and COVID–19, which necessitate the dynamic evaluation of crosstalk between two conditions. For example, sarcopenia shares several pathogenic mechanisms with the disease severity in COVID–19 infection, including intrinsic (systemic inflammation, oxidative stress, tissues hypoxia) and extrinsic (physical inactivity, malnutrition, comorbidities) factors [4]. Additionally, patients with sarcopenia develop respiratory muscles weakness, which is associated with poor performance on spirometry [5] and hence the chances of acquiring and/or exacerbating the COVID–19 infection. Conversely, nosocomial pneumonia and community-acquired pneumonia, analogs to COVID–19, are independent risk factors for sarcopenia [6]. In this context, it is essential to evaluate the bi-directional crosstalk between sarcopenia and COVID-19 infection.
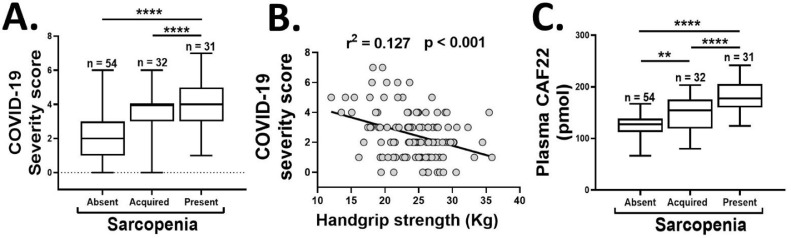
We conducted a prospective study on a group of 53—65-year-old male participants (n = 117), who were initially recruited as healthy controls for an ongoing sarcopenia project but were followed up for this study after testing positive for COVID–19 infection with the real-time PCR method. The patients were evaluated for EWGSOP2 definition of sarcopenia before and after acquiring the COVID-19 infection over 17—53 days. We classified COVID-19 disease severity based on cardiovascular, neurological, and metabolic decline by monitoring the alterations in pulse rate, blood pressure, respiratory rate, body temperature, and levels of consciousness [7]. Overall, 32 participants (27.35%) were sarcopenic, and 85 (72.64%) participants were non-sarcopenic at baseline. Among the non-sarcopenics, 31 participants (26.49%) subsequently developed sarcopenia after acquiring COVID-19 infection. Notably, the patients with sarcopenia at baseline or following the COVID-19 infection had higher disease severity scores than the non-sarcopenic patients ( figure 1 A). This data confirm and extend the earlier findings by Molfino et al. [1], and indicate a potential crosstalk between the COVID-19 infection and sarcopenia phenotype. Conversely, the presence of sarcopenia is associated with more severe COVID-19 infection than the non-sarcopenic individuals.
Fig. 1.
The association of COVID-19 severity scores with sarcopenia status (A) and HGS (B), and the plasma CAF22 levels (C) of the participants in the absence or presence of sarcopenia at baseline, or development of sarcopenia during the disease course of COVID-19 infection (n = 117). One-way analysis of variance (A and C), **p < 0.01, ****p < 0.0001.
Additionally, the remarks by Kara et al. [2] and the revised definition of sarcopenia by EWGSOP2 [3] place increasing emphasis on the importance of HGS as the predictor of sarcopenia. Likewise, a recent study by Kara et al. found a positive correlation between low HGS and disease severity in COVID-19 [8]. However, this study was not exclusive to sarcopenia and recruited a wide range of patients from 20—90 years of age. The narrow age-range relevant to sarcopenia in our study population may further dissect the association of age-related decline in HGS to COVID-19 disease severity. Notably, we confirm and extend the findings by Kara et al. [8] that low HGS may be an independent predictor of disease severity in COVID-19 patients ( figure 1 B).
Lastly, the diagnosis and assessment of sarcopenia could be a significant problem in bedridden and/or comatose patients with COVID–19 infection. The measurement of plasma biomarkers offers a cost-effective and objective tool to evaluate sarcopenia in such cases. We have recently shown that the plasma levels of c-terminal agrin fragment 22 (CAF22), a breakdown product of neuromuscular junction (NMJ), are elevated in sarcopenic patients with respiratory diseases [9]. Additionally, elevated plasma CAF22 levels can accurately predict all three indexes of sarcopenia, including reduced HGS, ASMI, and walking speed [9]. Consistent with these findings, we found elevated baseline plasma CAF22 levels in sarcopenic vs. non-sarcopenic patients with COVID-19 (34.13% higher, p < 0.01) ( figure 1 C). Interestingly, among the non-sarcopenic patients at baseline, the cohort which subsequently developed sarcopenia had higher baseline CAF22 levels than the remaining participants (34.13% higher, p < 0.01) ( figure 1 C). This exciting finding hints that the disruption of NMJ may precede the development of sarcopenia in COVID-19 patients. To our knowledge, no direct involvement of NMJ is known in COVID-19. However, our findings are consistent with clinical and biochemical presentations mimicking myasthenia crises and other neuromuscular diseases in COVID–19 patients [10].
The study has some limitations. The control group is absent, although the development of sarcopenia in 26% of participants over a 17—53 days period is likely not a physiological consequence of aging and is primarily driven by COVID-19 infection. All patients were males, so we could not evaluate sex differences. 13 patients died during the study, and selective survival of the patients must be considered as any other cohort study.
Altogether, our study extends the earlier findings by Molfino et al. [1] and provides valuable insight into the interplay of sarcopenia and COVID–19 infection. Considering that both diseases can potentially aggravate each other, we recommend assessing sarcopenia in the high-risk population and the COVID–19 patients. Additionally, the treatment of sarcopenia can be a therapeutic target to promote the prevention or treatment of COVID–19. Further studies are required to thoroughly characterize skeletal muscle wasting and treatment modalities in COVID–19 infection.
Declaration of competing interests
None
Acknowledgment
This work was supported by Target (1901090168) and competitive grants (1901090157) from the University of Sharjah to Rizwan Qaisar.
References
- 1.Molfino A. The link between nutritional status and outcomes in COVID-19 patients in ICU: is obesity or sarcopenia the real problem? Eur J Intern Med. 2021 doi: 10.1016/j.ejim.2021.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murat Kara A.M.A., Özçakar Levent. Sarcopenic obesity is the real problem in COVID-19 ! Eur J Internal Med. 2021 doi: 10.1016/j.ejim.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft A.J. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):601. doi: 10.1093/ageing/afz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang T. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21(4):543–559. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qaisar R., Karim A., Muhammad T. Circulating biomarkers of handgrip strength and lung function in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2020;15:311–321. doi: 10.2147/COPD.S225765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okazaki T. Association between sarcopenia and pneumonia in older people. Geriatr Gerontol Int. 2020;20(1):7–13. doi: 10.1111/ggi.13839. [DOI] [PubMed] [Google Scholar]
- 7.Son K.B., Lee T.J., Hwang S.S. Disease severity classification and COVID-19 outcomes, Republic of Korea. Bull World Health Organ. 2021;99(1):62–66. doi: 10.2471/BLT.20.257758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kara O. Grip strength as a predictor of disease severity in hospitalized COVID-19 patients. Heart Lung. 2021;50(6):743–747. doi: 10.1016/j.hrtlng.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qaisar R., Karim A., Muhammad T. Plasma CAF22 levels as a useful predictor of muscle health in patients with chronic obstructive pulmonary disease. Biology (Basel) 2020;9(7) doi: 10.3390/biology9070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paliwal V.K. Neuromuscular presentations in patients with COVID-19. Neurol Sci. 2020;41(11):3039–3056. doi: 10.1007/s10072-020-04708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]