Abstract
Purpose
The Communities, Households and SARS-CoV-2 Epidemiology (CHASING) COVID Cohort Study is a community-based prospective cohort study launched during the upswing of the USA COVID-19 epidemic. The objectives of the cohort study are to: (1) estimate and evaluate determinants of the incidence of SARS-CoV-2 infection, disease and deaths; (2) assess the impact of the pandemic on psychosocial and economic outcomes and (3) assess the uptake of pandemic mitigation strategies.
Participants
We began enrolling participants from 28 March 2020 using internet-based strategies. Adults≥18 years residing anywhere in the USA or US territories were eligible. 6740 people are enrolled in the cohort, including participants from all 50 US states, the District of Columbia, Puerto Rico and Guam. Participants are contacted regularly to complete study assessments, including interviews and dried blood spot specimen collection for serologic testing.
Findings to date
Participants are geographically and sociodemographically diverse and include essential workers (19%). 84.2% remain engaged in cohort follow-up activities after enrolment. Data have been used to assess SARS-CoV-2 cumulative incidence, seroincidence and related risk factors at different phases of the US pandemic; the role of household crowding and the presence of children in the household as potential risk factors for severe COVID-19 early in the US pandemic; to describe the prevalence of anxiety symptoms and its relationship to COVID-19 outcomes and other potential stressors; to identify preferences for SARS-CoV-2 diagnostic testing when community transmission is on the rise via a discrete choice experiment and to assess vaccine hesitancy over time and its relationship to vaccine uptake.
Future plans
The CHASING COVID Cohort Study has outlined a research agenda that involves ongoing monitoring of the incidence and determinants of SARS-CoV-2 outcomes, mental health outcomes and economic outcomes. Additional priorities include assessing the incidence, prevalence and correlates of long-haul COVID-19.
Keywords: infectious diseases, epidemiology, mental health, public health
Strengths and limitations of this study.
Prospective design, allowing direct observation of seroconversions and incident COVID-19 disease among those who were unexposed and/or disease free at enrolment. Launched in March 2020, the cohort spans every phase of the US COVID-19 pandemic.
Inclusion of a range of relevant measures, for a more comprehensive view of the impact of the pandemic and its response on several outcomes in addition to SARS-CoV-2 infection, including mental health and economic outcomes, vaccine hesitancy and uptake and long-haul COVID-19.
Potential to observe outcomes among those who recover from SARS-CoV-2 infection (asymptomatic or mild) and COVID-19 disease, and follow-up of these individuals over time to characterise their recovery, persistence of symptoms, onset and persistence of new symptoms and persistence of antibodies.
While our study is national in scope, it is unable to provide estimates of seroprevalence and cumulative incidence that are representative of that of the US population or individual states and cities.
We will likely underestimate the incidence of COVID-19-related hospitalisations.
Introduction
The COVID-19 pandemic has dramatically transformed life across the entire USA, resulting in medical and economic challenges and threats for individuals, households and communities. The earliest research efforts have focused on understanding the clinical course of COVID-19 and the most effective ways of treating people with severe symptoms or illness. As the pandemic progresses, however, we must also investigate COVID-19’s evolving epidemiology and the uptake/impact of non-pharmaceutical interventions (NPIs),1 such as physical distancing, health messaging and testing on the cumulative incidence of SARS-CoV-2. Researchers and public health practitioners called for cohort studies early on to describe the community attack rate, as well as how attack rates are influenced by different approaches to NPI implementation.2 There is also a need to characterise both the direct and indirect effects of the SARS-CoV-2 pandemic on mental health and economic outcomes. In response to the COVID-19 pandemic, the City University of New York Institute for Implementation Science in Population Health launched the prospective Communities, Households and SARS-CoV-2 Epidemiology (CHASING) COVID Cohort Study on 28 March 2020. We sought to prospectively recruit a geographically and sociodemographically diverse cohort of adults (18 years or older) in the USA and US territories in order to contribute to our understanding of the spread and impact of the SARS-CoV-2 pandemic. The purpose of this cohort profile paper is to describe the origin of the cohort, the study design and enrolment characteristics of the participants. We also present a sample of findings from the cohort to date.
Cohort description
Cohort objectives and study design
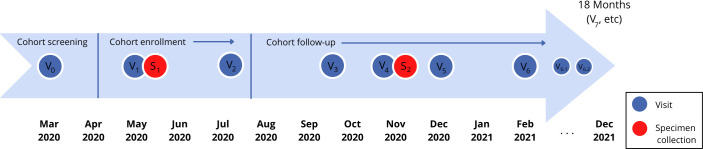
Key objectives of the cohort study are to: (1) estimate and evaluate determinants of the incidence of SARS-CoV-2 infection, disease and deaths; (2) assess the pandemic’s impact on psychosocial and economic outcomes (mental health, unemployment, food security) and (3) assess the uptake of pandemic mitigation strategies (NPIs, testing, contact tracing, isolation/quarantine, vaccination). The study design is shown in figure 1. Study visits (completion of questionnaires online and designated by Vx) are completed every 1–3 months following cohort screening and enrolment and will continue at least through December 2021, for a maximum of 21 months follow-up. Initial specimen collection (S1) occurred during April–June 2020 and the second specimen (S2) was collected from November 2020 to January 2021. Additional specimen collection may occur in 2021, depending on epidemic activity and availability of funding.
Figure 1.
Communities, Households and SARS-CoV-2 Epidemiology COVID Cohort enrolment and follow-up schedule.
Cohort eligibility
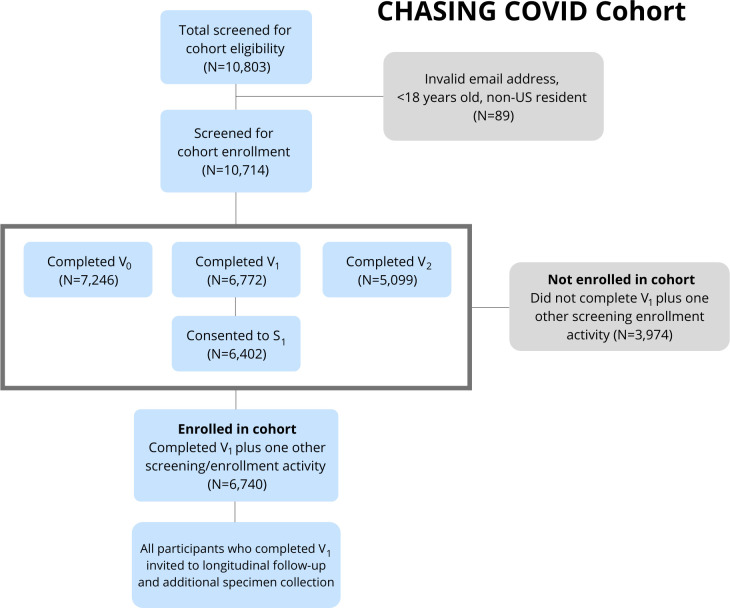
Eligibility was determined during cohort screening visits and cohort enrolment visits (figure 2). To be eligible for inclusion in the cohort, individuals had to: (1) reside in the USA or a US territory; (2) be aged 18 years or older; (3) provide valid email address and (4) demonstrate early engagement in longitudinal study activities, including: (a) completion of V1 (which provided the opportunity to consent for serologic testing) and (b) completion of at least one additional screening visit in addition to V1 (ie, V0 or V2) or provision of a baseline specimen for serologic testing (S1). Beginning in February 2021, participants began completing comprehensive interviews every 3 months and brief interviews to assess vaccination uptake and vaccine hesitancy among those who report being unvaccinated.
Figure 2.
Screening and enrolment in the Communities, Households and SARS-CoV-2 Epidemiology COVID Cohort.
Cohort screening and enrolment
Cohort screening and enrolment began on 28 March 2020, at that point there were 149 693 documented COVID-19 cases and 2 502 COVID-19 deaths reported in the USA.3 Enrolment ended on 21 August 2020, when there were 5 750 855 persons diagnosed with SARS-CoV-2, including 181 656 deaths in the USA.3
We used internet-based strategies that are effective for recruiting and following large and geographically diverse online cohorts, including at-home specimen collection.4–6 Study participants were recruited via ads on social media platforms (eg, Facebook, Instagram and Scruff), Qualtrics Panel or via referral to the study (anyone with knowledge of the study was allowed to invite others to participate). By relying on personal networks of participants through referrals, we aimed to bolster recruitment of persons >59 years of age, who were important to have represented in the cohort because of their risk, but may not be as active on social media as younger persons. Facebook and Instagram advertisements were developed in English and Spanish and were geographically targeted to people currently residing in the USA and US territories who were 18 years or older. Study staff systematically monitored cohort demographics and proactively adjusted advertisement strategies as needed to balance geographic and sociodemographic characteristics of respondents. For example, strategies could shift to prioritise recruitment of older persons if that demographic was poorly represented.
Interested participants were directed to a pre-enrolment survey (hosted by Qualtrics) to be completed in a web browser on their computer or mobile device.7 A consent form described the study, plans for follow-up assessments and future study opportunities, including the possibility to receive SARS-CoV-2 serologic testing as part of the study. The consent form also described the incentive schedule: a drawing for $100 for a pre-enrolment survey (V0) (with 20 winners) and gift cards ranging from $5 to $30 for all participants for completion of subsequent surveys and antibody testing.
Patient and public involvement
Patients were involved in the development of research questions and questionnaires, in that some members of the study team were also COVID-19 survivors. Study results are disseminated to participants during regular email and text communications as part of study updates.
Study measurements
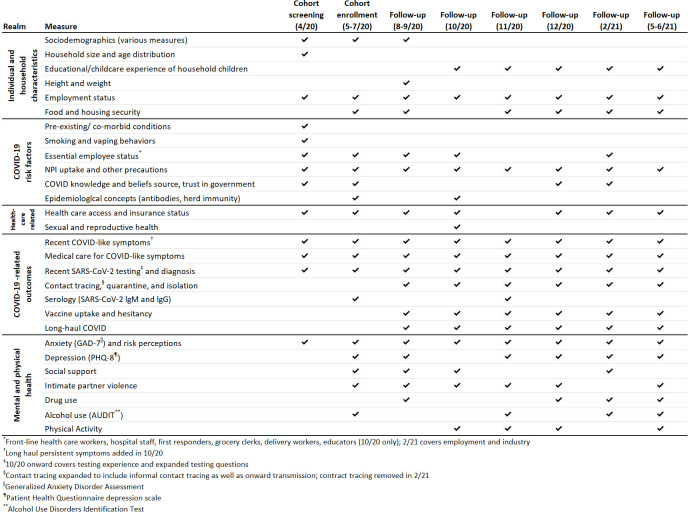
Measures included on the screening, enrolment and follow-up study questionnaires were derived from previously published research (eg, Together 5000,4 BRFSS and H1N1 influenza studies8 9) and from other researchers who had developed surveys for understanding COVID-19 (eg, Canadian COVID-19 Social Impacts Survey10 and Food Access and Food Security during COVID-1911). Measures were also developed de novo in response to the novel pandemic. The follow-up assessments gather data on symptoms, testing, hospitalisations and other time-varying factors (see figure 3 for key measurement realms). Copies of all study questionnaires are available on the CHASING COVID Cohort Study webpage.12
Figure 3.
Realms of measurement in the Communities, Households and SARS-CoV-2 Epidemiology COVID Cohort.
At-home specimen collection for serologic testing
As part of study assessments during May–August 2020 (period 1) and November 2020–January 2021 (period 2), participants were invited to participate in serologic testing using an at-home self-collected dried blood spot (DBS) specimen collection kit. To facilitate self-sampling procedures, all participants are provided printed instructions and a QR code to view a video demonstrating procedures for DBS collection and instructions to contact the study team, if they had questions.13 DBS cards were sent from and returned to the study laboratory (Molecular Testing Laboratories (MTL), Vancouver, Washington) via the US Postal Service (self-addressed, stamped envelope containing a biohazard bag).
Serologic testing
All DBS specimens were tested by the study laboratory for total antibodies (total Ab) using the Bio-Rad Platelia test for IgA, IgM and IgG which targets the SARS-CoV-2 nucleocapsid protein (manufacturer sensitivity 98.0%, specificity 99.3%).14 This assay received Emergency Use Authorization from the US Food and Drug Administration and was further validated for use with DBS by the study laboratory using confirmed, true positive and negative patient specimens. In this local validation, the assay was found to have 100% sensitivity and 100% specificity (MTL, personal communication).
Data management and analysis
All data were imported and cleaned in R and SAS (V.9.4). Data were geocoded based on a self-reported ZIP code. Maps were created in ArcGIS 10.7. Data from V0 and V1 were used to compile summary statistics on enrolment characteristics. Enrolment variables missing <5% of data were imputed using regression-based simple imputation.15
Cohort eligibility
Of the 10 803 individuals who completed at least one study screening or enrolment visit, 10 714 were age 18 years or older, US residents and provided a valid email address for study follow-up. Of those, 7246 completed V0, 6772 completed V1, 5099 completed V2, 6402 consented to provide a baseline DBS specimen and 6740 met final study eligibility criteria of completing two of three screening/enrolment visits or consenting to provide a specimen as part of V1 and were considered enrolled in the cohort (figure 2). Participants who only completed V1 are routinely invited to complete additional study assessments and specimen collection. As of April 2021, we have completed six comprehensive follow-up interviews, two brief interviews and two rounds of serologic testing. Of the 6740 participants enrolling in the cohort during March–May 2020, 5675 (84.2%) had engaged in subsequent follow-up activities after enrolment and were considered retained in the study as of April 2021.
Findings to date
Cohort characteristics
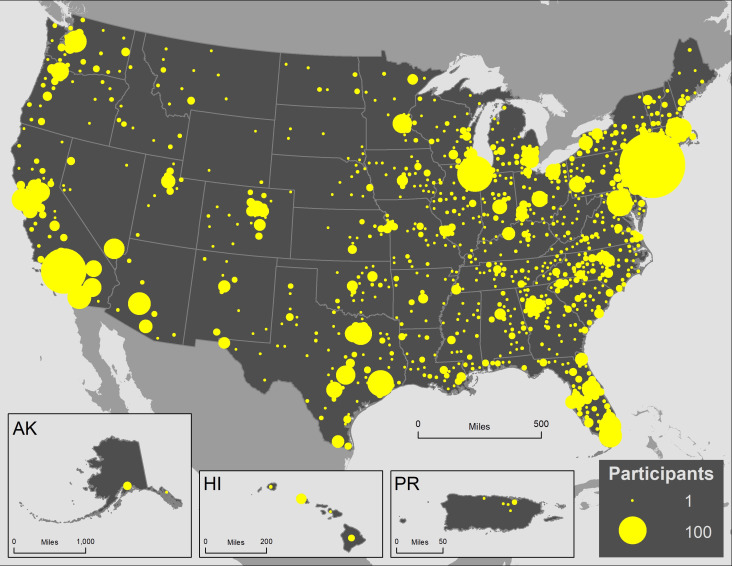
The cohort includes 6740 participants from all 50 states, the District of Columbia, Puerto Rico and Guam (figure 4). At V1, the median age of participants was 37 years (IQR: 29, 51); 73% were aged 18–49 years (including 5%<21 years (N=370)), 12% were aged 50–59 years and 15% were aged 60 years or older (table 1). Just under half (45%) of participants were male, 19% were Hispanic, 13% Black, non-Hispanic (NH), 7% Asian or Pacific Islander and 57% White, NH. A majority were currently employed (63%), 10% were retired, 13% were out of work and 9% were students.
Figure 4.
Geographic distribution of Communities, Households and SARS-CoV-2 Epidemiology COVID Cohort participants by county of residents, N=6740.
Table 1.
Characteristics of Communities, Households and SARS-CoV-2 Epidemiology COVID Cohort
| Total | 18–49 years | 50–59 years | 60+ years | |||||
| N | % | N | % | N | % | N | % | |
| Total | 6740 | 100.0 | 4922 | 73.0 | 792 | 11.8 | 1026 | 15.2 |
| Age (median, IQR) | 37 (29, 51) | 33 (27, 39) | 54 (51, 57) | 66 (63, 71) | ||||
| Gender | ||||||||
| Male | 3043 | 45.1 | 2253 | 45.8 | 370 | 46.7 | 420 | 40.9 |
| Female | 3526 | 52.3 | 2511 | 51.0 | 412 | 52.0 | 603 | 58.8 |
| Transgender/non-binary | 171 | 2.5 | 158 | 3.2 | 10 | 1.3 | 3 | 0.3 |
| Race/ethnicity | ||||||||
| Hispanic | 1308 | 19.4 | 1134 | 23.0 | 99 | 12.5 | 75 | 7.3 |
| Black, non-Hispanic (NH) | 899 | 13.3 | 755 | 15.3 | 90 | 11.4 | 54 | 5.3 |
| Asian/Pacific Islander, NH | 465 | 6.9 | 424 | 8.6 | 19 | 2.4 | 22 | 2.1 |
| White, NH | 3846 | 57.1 | 2440 | 49.6 | 559 | 70.6 | 847 | 82.6 |
| Other | 222 | 3.3 | 169 | 3.4 | 25 | 3.2 | 28 | 2.7 |
| Employment | ||||||||
| Employed | 4226 | 62.7 | 3365 | 68.4 | 524 | 66.2 | 337 | 32.8 |
| Out of work | 881 | 13.1 | 686 | 13.9 | 128 | 16.2 | 67 | 6.5 |
| Homemaker | 355 | 5.3 | 280 | 5.7 | 51 | 6.4 | 24 | 2.3 |
| Student | 582 | 8.6 | 570 | 11.6 | 10 | 1.3 | 2 | 0.2 |
| Retired | 696 | 10.3 | 21 | 0.4 | 79 | 10.0 | 596 | 58.1 |
| Income | ||||||||
| <$50 000 | 2804 | 41.6 | 2163 | 43.9 | 277 | 35.0 | 364 | 35.5 |
| $50 000 to $99 999 | 2080 | 30.9 | 1501 | 30.5 | 235 | 29.7 | 344 | 33.5 |
| ≥$100 000 | 1856 | 27.5 | 1258 | 25.6 | 280 | 35.4 | 318 | 31.0 |
| Region | ||||||||
| Midwest | 1138 | 16.9 | 816 | 16.6 | 136 | 17.2 | 186 | 18.1 |
| Northeast | 1894 | 28.1 | 1345 | 27.3 | 251 | 31.7 | 298 | 29.0 |
| South | 2063 | 30.6 | 1545 | 31.4 | 226 | 28.5 | 292 | 28.5 |
| West | 1637 | 24.3 | 1213 | 24.6 | 178 | 22.5 | 246 | 24.0 |
| US dependent | 8 | 0.1 | 3 | 0.1 | 1 | 0.1 | 4 | 0.4 |
| Urban | 2910 | 43.2 | 2239 | 45.5 | 316 | 39.9 | 355 | 34.6 |
| High risk for severe COVID-19* | 3853 | 57.2 | 2355 | 47.8 | 472 | 59.6 | 1026 | 100.0 |
| Any underlying health condition† | 2630 | 39.0 | 1549 | 31.5 | 411 | 51.9 | 670 | 65.3 |
| Smoker‡ | 1190 | 17.7 | 973 | 19.8 | 120 | 15.2 | 97 | 9.5 |
| Essential worker | 1248 | 18.5 | 1003 | 20.4 | 144 | 18.2 | 101 | 9.8 |
| All healthcare workers (HCW) | 796 | 12.0 | 679 | 14.0 | 71 | 9.0 | 46 | 4.5 |
| HCW who screens or cares for patients with COVID-19 | 396 | 49.7 | 350 | 51.5 | 32 | 45.0 | 14 | 30.4 |
| Law enforcement | 33 | 0.5 | 23 | 0.5 | 8 | 1.0 | 2 | 0.2 |
| Emergency response | 23 | 0.3 | 19 | 0.4 | 3 | 0.4 | 1 | 0.1 |
| Delivery (eg, food) | 105 | 1.6 | 99 | 2.0 | 4 | 0.5 | 2 | 0.2 |
| Transportation (eg, taxi or airline) | 65 | 1.0 | 41 | 0.8 | 15 | 1.9 | 9 | 0.9 |
*High risk for severe illness included people aged 60 years or older or reporting any underlying health condition, daily smoking or screening or providing care for patients with COVID-19.
†Any underlying health condition include people who reported chronic lung disease, asthma (current), type 2 diabetes, serious heart conditions, kidney disease, HIV or immunocompromised.
‡People who report they smoke cigarettes or e-cigarettes daily.
More than half (57%) were considered to be at increased risk for severe COVID-19 disease should they become infected with SARS-CoV-2, on the basis of age (60+), presence of an underlying health condition (chronic lung disease, asthma (current), type 2 diabetes, serious heart condition, kidney disease, or an immunocompromised status), or daily smoking (table 1). The proportion of persons with an underlying health condition increased with age category (32% among 18–49 years olds and 65% among 60+ years olds) and the proportion of participants who were daily smokers decreased with increasing age category (20% and 9%, respectively).
Overall, 19% of participants reported being an essential worker (table 1). By employment category, 12% were healthcare workers (HCW) (half of whom reported screening or caring for patients with COVID-19), 1.6% worked in delivery services (eg, food) and 1% worked in transportation (eg, taxis).
NPI/physical distancing behaviors stratified by age categories
At cohort enrolment, a high proportion of participants reported avoiding large groups with >20 people in the prior 2 weeks and avoiding handshakes or hugs (90% and 90%, respectively) (table 2). Almost two-thirds (60%) reported working from home. A majority reported wearing gloves (58%) and masks (93%). Almost one in three (29%) participants reported stockpiling personal protective equipment and 40% reported stockpiling food. The proportion of participants who reported stockpiling Personal Protective Equipment (PPE) or food decreased with increasing age categories.
Table 2.
Behaviours and symptoms by age category of Communities, Households and SARS-CoV-2 Epidemiology COVID Cohort
| Total | 18–49 years | 50–59 years | 60+ years | |||||
| N | % | N | % | N | % | N | % | |
| Total | 6740 | 100.0 | 4922 | 73.0 | 792 | 11.8 | 1026 | 15.2 |
| Behaviours in the past month | ||||||||
| Avoided groups>20 | 6014 | 90.3 | 4331 | 89.1 | 730 | 92.6 | 953 | 94.4 |
| Avoided handshakes or hugs | 5958 | 90.4 | 4265 | 88.9 | 742 | 95.0 | 951 | 94.2 |
| Bought firearm | 369 | 5.5 | 345 | 7.0 | 14 | 1.8 | 10 | 1.0 |
| Worked from home | 4015 | 59.6 | 3294 | 66.9 | 414 | 52.3 | 307 | 29.9 |
| Wore gloves | 3912 | 58.0 | 2825 | 57.4 | 473 | 59.7 | 614 | 59.8 |
| Wore mask | 6234 | 93.3 | 4510 | 92.6 | 748 | 94.6 | 976 | 95.5 |
| Stockpiled personal protective equipment (PPE) | 1917 | 28.9 | 1509 | 31.3 | 168 | 21.4 | 240 | 23.5 |
| Stockpiled food | 2644 | 39.7 | 2078 | 42.9 | 264 | 33.4 | 302 | 29.6 |
| Quarantined | 4270 | 66.0 | 3229 | 68.3 | 444 | 58.3 | 597 | 60.8 |
| Symptoms since last survey/in past month | ||||||||
| COVID-19-like illness | 1129 | 16.8 | 902 | 18.3 | 117 | 14.8 | 110 | 10.7 |
| New cough | 653 | 9.7 | 543 | 11.1 | 56 | 7.1 | 54 | 5.3 |
| Fever | 302 | 4.5 | 249 | 5.1 | 32 | 4.1 | 21 | 2.1 |
| Shortness of breath | 481 | 7.2 | 369 | 7.5 | 52 | 6.6 | 60 | 5.9 |
| Coughing blood | 47 | 0.7 | 45 | 0.9 | 0 | 0.0 | 2 | 0.2 |
COVID-19 symptoms and care outcomes
One in six (17% or N=1129) reported any COVID-19-like symptoms in the month prior to visit 1 (cough, fever or shortness of breath) and this decreased with age (18% vs 11% among 18–49 and 60+ years olds, respectively) (table 2). The most common symptoms reported were new cough (10%) followed by shortness of breath (7%) and fever (5%). Among the 17% of participants reporting COVID-19-like symptoms (N=1129), 34% (N=385) said they called or saw a physician/healthcare professional and 7% (N=82) were hospitalised (table 3). Compared with participants at lower risk for COVID-19 illness, participants with higher risk for severe COVID-19 illness were more likely to report seeing a physician or being hospitalized for their symptoms. Among all participants, 12% (N=813) reported being tested for COVID-19 and 4% (N=256) reported receiving a COVID-19 diagnosis. Participants at higher risk for severe COVID-19 were more likely to report testing or receiving a diagnosis than participants at lower risk for severe COVID-19 illness (testing: 14% vs 10% and diagnosis: 5% vs 2%, respectively).
Table 3.
Communities, Households and SARS-CoV-2 Epidemiology COVID Cohort care outcomes stratified by risk for severe COVID-19 illness
| Total | High risk | Not high risk | ||||
| N | % | N | % | N | % | |
| Total | 6740 | 100.0 | 3853 | 57.2 | 2887 | 42.8 |
| Tested | ||||||
| Yes | 813 | 12.1 | 542 | 14.1 | 271 | 9.4 |
| No, but tried | 666 | 9.9 | 421 | 10.9 | 245 | 8.5 |
| No, did not need or try | 5203 | 77.2 | 2855 | 74.1 | 2348 | 81.3 |
| Don't know | 58 | 0.9 | 35 | 0.9 | 23 | 0.8 |
| COVID-19 diagnosis | ||||||
| Yes | 256 | 3.8 | 199 | 5.2 | 57 | 2.0 |
| No | 577 | 8.6 | 358 | 9.3 | 219 | 7.6 |
| Don't know | 27 | 0.4 | 14 | 0.4 | 13 | 0.5 |
| Not tested | 5880 | 87.2 | 3282 | 85.2 | 2598 | 90.0 |
| All with COVID-19-like symptoms: cough, fever or shortness of breath | 1129 | 16.8 | 785 | 20.4 | 344 | 11.9 |
| Saw physician - among those with COVID-19-like symptoms (N = 1129) | ||||||
| Yes | 385 | 34.1 | 297 | 37.8 | 88 | 25.6 |
| No | 739 | 65.5 | 485 | 61.8 | 254 | 73.8 |
| Missing/Don't know | 5 | 0.4 | 3 | 0.4 | 2 | 0.6 |
| Hospitalised - among those with COVID-19-like symptoms (N = 1129) | ||||||
| Yes | 82 | 7.3 | 76 | 9.7 | 6 | 1.7 |
| No | 1046 | 92.6 | 709 | 90.3 | 337 | 98.0 |
| Missing/Don't know | 1 | 0.1 | 0 | 0.0 | 1 | 0.3 |
Household factors and the risk of severe COVID-19-like illness early in the US pandemic
Early in the US pandemic, crowded indoor settings and sustained close indoor contact without masks were associated with an increased likelihood of SARS-CoV-2 spread.16 The role of such exposures as well as the role of the presence of children in the household have not been investigated as risk factors for severe COVID-19-like illness (ie, hospitalisation). We found that the risk of hospitalisation due to COVID-19 was higher among participants who had (vs those who did not have) children in the home, with an adjusted OR (aOR) of hospitalisation of 10.5 (95% CI: 5.7 to 19.1) among study participants living in multiunit dwellings and 2.2 (95% CI: 1.2 to 6.5) among those living in single-unit dwellings.16 Among participants living in multiunit dwellings, the aOR for COVID-19 hospitalisation among participants with more than four persons in their household (vs one person) was 2.5 (95% CI: 1.0 to 6.1) and 0.8 (95% CI: 0.15 to 4.1) among those living in single-unit dwellings. This work demonstrated that, early in the US SARS-CoV-2 pandemic, certain household exposures likely increased the risk of both SARS-CoV-2 acquisition and the risk of severe COVID-19 disease requiring hospitalisation.
The relationship between anxiety, health and potential stressors among adults in the USA during the early phase of the COVID-19 pandemic
Epidemiologic data on the mental health impact of the COVID-19 pandemic in the USA remain limited. We found that more than one-third (35%) of individuals reported moderate or severe anxiety symptoms at cohort screening/enrolment visits. Having lost income due to COVID-19 (adjusted prevalence ratio (aPR) 1.27 (95% CI: 1.16 to 1.30), having recent COVID-19-like symptoms (aPR 1.17 (95% CI: 1.05 to 1.31) and having been previously diagnosed with depression (aPR 1.49, (95% CI: 1.35 to 1.64) were positively associated with moderate or severe anxiety symptoms.17 This work demonstrated that anxiety symptoms were common and appeared to be elevated among adults in the US during the early phase of the COVID-19 pandemic. Strategies to screen and treat individuals at increased risk of anxiety, such as individuals experiencing financial hardship and individuals with prior diagnoses of depression, should be developed and implemented.
SARS-CoV-2 testing service preferences of adults in the USA: discrete choice experiment (DCE) and patterns of SARS-CoV-2 testing preferences in a national cohort in the USA
A DCE was used to assess the relative importance of type of SARS-CoV-2 test, specimen type, testing venue and results turnaround time.18 19 Turnaround time for test results had the highest relative importance (30.4%), followed by test type (28.3%), specimen type (26.2%) and venue (15.0%). Participants preferred fast results on both past and current infection and using a non-invasive specimen type, preferably collected at home. Simulations suggested that providing immediate or same day test results, providing both PCR and serology or collecting oral specimens would substantially increase testing uptake over the current typical testing option. Using latent class analysis, we also found five distinct patterns in testing preferences in the cohort, including groups of ‘comprehensive testers’, ‘fast-track testers’, ‘dual testers’, ‘non-invasive, dual testers’ and ‘home testers’.19 We concluded that testing strategies that account for preferences and their heterogeneity would likely have the most uptake and engagement among residents in communities with increasing community transmission of SARS-CoV-2.
Recent SARS-CoV-2 seroconversion in a national, community-based prospective cohort of US adults
A total of 303 of 4459 individuals showed serologic evidence of past SARS-CoV-2 infection (cumulative incidence of 6.8%; 95% CI: 6.1% to 7.6%).20 Among 303 seropositive individuals, 27.4% had asymptomatic infection and 32% reported a positive SARS-CoV-2 PCR test or provider diagnosis of COVID-19. Among 3280 initially seronegative participants with a subsequent serologic test, we observed 145 seroconversions during 1562 person years of follow-up (incidence rate of 9.3 per 100 person-years (95% CI: 7.9 to 11.0)). Race/ethnicity, region of residence, household crowding and inconsistent mask use in indoor public spaces such grocery stores, salons, gyms, restaurants and gathering in large groups indoors and outdoors were identified as risk factors for seroconversion.
Determinants of COVID-19 vaccine hesitancy and vaccine uptake in a national cohort of US adults
We estimated trends in and correlates of vaccine hesitancy and its association with subsequent vaccine uptake among cohort participants. Vaccine hesitancy and resistance decreased from 51% and 8% in September 2020 to 35% and 5% in December 2020, respectively.21 We found that racial/ethnic gaps in vaccine hesitancy widened, despite overall decrease in hesitancy. Compared with White, non-Hispanic (NH) participants, Black, NH and Hispanic participants had higher adjusted odds ratios (aORs) for both vaccine hesitancy (aOR: 3.3 (95% CI: 2.6 to 4.2) for Black, NH and 1.8 (95% CI: 1.5 to 2.2) for Hispanic) and vaccine resistance (aOR: 6.4 (95% CI: 4.3 to 9.4) for Black, NH and 1.9 (95% CI: 1.3 to 2.7) for Hispanic). Willingness to vaccinate was associated with a lower odds of subsequent vaccine uptake among 65+ years olds (aOR: 0.4, 95% CI: 0.3 to 0.6 for hesitancy; aOR: 0.1, 95% CI: 0.01 to 0.6 for resistance) and HCW (aOR: 0.2, 95% CI: 0.1 to 0.3 for hesitancy; aOR: 0.04, 95% CI: 0.006 to 0.2 for resistance). We concluded that health education and awareness and implementation efforts should focus on vaccine hesitant low income and minority individuals to address vaccine-related concerns and barriers to better ensure more equitable vaccine uptake.
Discussion
This longitudinal, community-based cohort study enrolled 6740 persons from all 50 US states, the District of Columbia, Puerto Rico and Guam and was rapidly established during the upswing of the SARS-CoV-2 pandemic in the USA. The cohort is geographically and sociodemographically diverse and includes participants from many pandemic hotspots, as well as HCW and other essential workers and individuals who are vulnerable to severe outcomes associated with SARS-CoV-2 infection. Initial serologic testing indicates that the cohort overwhelmingly had no evidence of prior SARS-CoV-2 infection at the time of specimen collection and high rates of subsequent seroconversion were observed.20
While SARS-CoV-2 is understood to be transmitted from person to person via respiratory droplets and exhaled aerosols, the incidence of SARS-CoV-2 infection and risk factors for incident infection have not been well characterised by routine case-based surveillance of SARS-CoV-2 diagnoses or by cross-sectional seroprevalence studies to date.22–24 Globally, few prospective epidemiologic studies of SARS-CoV-2 have been published. One recent global systematic review of observational studies of SARS-CoV-2 that employed serologic or PCR testing found only 18 prospective studies.25 Most were focused on HCW or other occupational groups, individuals in congregate settings, evacuees or cruise ships, none were community based (ie, focused on risk factors in communities vs other higher risk populations/settings).25 A greater understanding of SARS-CoV-2 incidence and risk factors and other pandemic-related outcomes in community samples can substantially complement routine case-based surveillance of new SARS-CoV-2 diagnoses and cross-sectional serosurveys, serving to inform aspects of implementation of the public health response and policies for the current and future pandemics.
The observed cumulative incidence in our cohort may be lower than the true cumulative incidence in our cohort because of waning of SARS-CoV-2 antibodies. Recent studies suggest waning of antibodies to both nucleocapsid and spike proteins,23 which combined with the timing of specimen collection relative to infection for many participants in our cohort (median of 190 days),20 could mean that we have underestimated the true cumulative incidence. Limitations of serological assays notwithstanding26 cross-sectional serosurveys done prior to the relaxing of physical distancing have reported cumulative incidence estimates ranging from 1.7% in Indiana to 21% in New York City and <10% nationally as of the end of September 2020.23 27–31
Strengths of our cohort study include its prospective design, allowing direct observation of seroconversions and incident COVID-19 disease among those who were unexposed and/or disease free at enrolment. We also ascertained a range of relevant measures, for a more comprehensive view of the impact of the pandemic and its response on several outcomes in addition to SARS-CoV-2 infection, including mental health and economic outcomes, vaccine hesitancy and uptake and long-haul COVID-19. Prospective studies are complementary to and offer some strengths over cross-sectional studies and case-based surveillance, especially in the context of rapidly evolving emergencies and the associated public health response. While repeat cross-sectional surveys are valuable in a pandemic, including their ability to assess trends in many important outcomes, they cannot assess what factors may influence change over time in an individual’s or a household’s exposures and outcomes (eg, seroincidence, vaccine hesitancy/uptake).
Our study includes those who recover from SARS-CoV-2 infection (asymptomatic or mild) and COVID-19 disease, and these individuals are followed up over time to characterise their recovery, including persistence of symptoms, onset and persistence of new symptoms and persistence of antibodies. This will allow us to characterise the epidemiology of long-haul COVID-19 (aka postacute COVID-19 syndrome).32 We will also be able to assess mortality and related risk factors via matching with the US National Death Index.33
The ongoing pandemic necessitated fully online recruitment of study participants with at home specimen collection. Our approach employs protocols for overcoming common pitfalls of fully online studies (eg, repeat/duplicate participation). Our online, volunteer recruitment approach allows us to sample individuals who may not be reached by traditional telephone recruitment approaches, which can have very low response rates. As part of our enrolment procedures, we record IP address, email addresses, participant contact information and require participants to have valid US mailing addresses (required for those who opt to receive an at-home SARS-CoV-2 specimen collection kit). Participants are ‘known’ to the research team (name, email, address), thus averting some of the traditional shortcomings of online-only studies (particularly anonymous, cross-sectional online studies).
Our cohort study has limitations worth noting, as they inform what can and cannot be assessed and inferred. First, while our study is national in scope, it was not designed to provide estimates of seroprevalence and cumulative incidence that are representative of that in the US population. Second, we will likely underestimate the incidence of COVID-19-related hospitalisations. Most research studies deployed in the middle of a pandemic, including ours, will by definition produce some biased estimates since they will not include some information on participants who died from COVID-19, were hospitalised with COVID-19 after recruitment and were either lost to follow-up or were too sick to participate in the research activities. We will ascertain deaths in our participants using the National Death Index. Additionally, from published studies, we will routinely assess bias in our estimates due to these factors and adjust them accordingly when possible. Finally, although our sample is not representative of the entire US population, it is geographically representative and sociodemographically diverse. Our study will complement other efforts and approaches to address similar research questions, such as the NIH’s national study34 and the COVIDVu Study.35 Indeed, it will be important to assess the extent to which the different strategies used in each of these cohort studies reach similar or divergent conclusions about the SARS-CoV-2 pandemic.
Future plans
The CHASING COVID Cohort Study will continue to conduct ongoing monitoring of the cumulative incidence and determinants of SARS-CoV-2 outcomes (including mortality), mental health outcomes (eg, anxiety and depression) and economic outcomes (eg, income loss, food security), as well as overall and cause-specific mortality. A number of longitudinal analyses are ongoing or planned using the data that have already been collected, with priorities including those related to COVID-19 vaccine hesitancy, uptake and effectiveness; NPI engagement before and after vaccination; incidence, prevalence and correlates of long-haul COVID-19 and extent and duration of protective effect of SARS-CoV-2 antibodies. We plan to conduct a match with the National Death Index33 36 to identify deaths and associated causes among participants who are lost to follow-up.
Supplementary Material
Acknowledgments
The authors wish to thank the participants of the Communities, Households and SARS-CoV-2 Epidemiology COVID Cohort Study. The authors are grateful to the participants for their contributions to the advancement of science around the SARS-CoV-2 pandemic. The authors also want to acknowledge the role of Sarah Kulkarni as a COVID-19 survivor in the study team who, in addition to her usual role on our projects, also brought first-hand patient experience and perspectives to the study design, questionnaire development and scientific agenda for the project.
Footnotes
Twitter: @epi_dude
Contributors: MMR, SGK, CG and DN conceptualised the study. SK, MMR, MC and MR performed statistical analyses. MMR and DN wrote the first draft of the paper. MMR and DN supervised the statistical analyses. MMR, MR, SGK, AB, CM, SK, WY, AM, RZ, DW, CG, AMP, LW and DN contributed to interpreting the data and to the writing and revising of the manuscript.
Funding: Funding for this project was provided by the National Institute for Allergies and Infectious Diseases, award number 3UH3AI133675-04S1 (MPIs: DN and CG), the City University of New York (CUNY) Institute for Implementation Science in Population Health, the CUNY Graduate School of Public Health and Health Policy (COVID-19 Grant Program) and NICHD grant P2C HD050924 (Carolina Population Center).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. The authors will post a deidentified, HIPAA compliant, public use version of visit 1 and follow-up data on Backblaze, a secure cloud storage provider. Data will be presented as flat text files (CSV) formatted for compatibility with county-level longitudinal case load datasets, including date, county, state and FIPS code. The authors will exclude counties with <20 000 residents to protect participant privacy. The authors will continue to provide direct feedback to their cohort and other stakeholders who have signed up for updates via follow-up emails to participants and the City University of New York Institute for Implementation Science in Population Health Study website.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study protocol was approved by the Institutional Review Board at the City University of New York Graduate School for Public Health and Health Policy.
References
- 1.Hutchins HJ, Wolff B, Leeb R, et al. COVID-19 mitigation behaviors by age group - United States, April-June 2020. MMWR Morb Mortal Wkly Rep 2020;69:1584–90. 10.15585/mmwr.mm6943e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19 - studies needed. N Engl J Med 2020;382:1194–6. 10.1056/NEJMp2002125 [DOI] [PubMed] [Google Scholar]
- 3.Johns Hopkins Coronavirus Resource Center . COVID-19 United States cases by County, 2020. Available: https://coronavirus.jhu.edu/us-map
- 4.Grov C, Westmoreland DA, Carneiro PB, et al. Recruiting vulnerable populations to participate in HIV prevention research: findings from the together 5000 cohort study. Ann Epidemiol 2019;35:4–11. 10.1016/j.annepidem.2019.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nash D, Stief M, MacCrate C, et al. A web-based study of HIV prevention in the era of pre-exposure prophylaxis among vulnerable HIV-negative gay and bisexual men, Transmen, and Transwomen who have sex with men: protocol for an observational cohort study. JMIR Res Protoc 2019;8:e13715. 10.2196/13715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grov C, Westmoreland D, Rendina HJ, et al. Seeing is believing? unique capabilities of internet-only studies as a tool for implementation research on HIV prevention for men who have sex with men: a review of studies and methodological considerations. J Acquir Immune Defic Syndr 2019;82 Suppl 3:S253–60. 10.1097/QAI.0000000000002217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qualtrics . Qualtrics XM - experience management software, 2015. Available: https://www.qualtrics.com/
- 8.Santibanez TA, Singleton JA, Santibanez SS, et al. Socio-Demographic differences in opinions about 2009 pandemic influenza A (H1N1) and seasonal influenza vaccination and disease among adults during the 2009-2010 influenza season. Influenza Other Respir Viruses 2013;7:383–92. 10.1111/j.1750-2659.2012.00374.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar S, Quinn SC, Kim KH, et al. The impact of workplace policies and other social factors on self-reported influenza-like illness incidence during the 2009 H1N1 pandemic. Am J Public Health 2012;102:134–40. 10.2105/AJPH.2011.300307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy E, Vikse J, Chaufan C, et al. Canadian COVID-19 social impacts survey - summary of results #1: risk perceptions, trust, impacts, and responses 2020.
- 11.Niles MT, Neff R, Biehl E. Food access and security during coronavirus Survey- version 1.0, 2020. Available: 10.7910/DVN/RQ6NMG [DOI]
- 12.ISPH . Chasing COVID research study, 2021. Available: https://cunyisph.org/chasing-covid/
- 13.Molecular Testing Labs . How to collect blood with dry blood spot kit - molecular testing labs, 2020. Available: https://moleculartestinglabs.com/how-to-collect/
- 14.Center for Devices,, Radiological Health . EUA authorized serology test performance, 2021. Available: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance
- 15.Donders ART, van der Heijden GJMG, Stijnen T, et al. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol 2006;59:1087–91. 10.1016/j.jclinepi.2006.01.014 [DOI] [PubMed] [Google Scholar]
- 16.Nash D, Qasmieh S, Robertson M, et al. Household factors and the risk of severe COVID-like illness early in the US pandemic. medRxiv 2020. 10.1101/2020.12.03.20243683. [Epub ahead of print: 04 Dec 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parcesepe AM, Robertson M, Berry A, et al. The relationship between anxiety, health, and potential stressors among adults in the United States during the COVID-19 pandemic. medRxiv 2020. 10.1101/2020.10.30.20221440. [Epub ahead of print: 04 Nov 2020]. [DOI] [Google Scholar]
- 18.Zimba R, Kulkarni S, Berry A, et al. SARS-CoV-2 testing service preferences of adults in the United States: discrete choice experiment. JMIR Public Health Surveill 2020;6:e25546. 10.2196/25546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romo ML, Zimba R, Kulkarni S, et al. Patterns of SARS-CoV-2 testing preferences in a national cohort in the United States. medRxiv 2020. 10.1101/2020.12.22.20248747. [Epub ahead of print: 24 Dec 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nash D, Rane M, Chang M, et al. Recent SARS-CoV-2 seroconversion in a national, community-based prospective cohort of U.S. adults. medRxiv 2021. [Google Scholar]
- 21.Rane MS, Kochhar S, Poehlin E. Determinants of COVID-19 vaccine hesitancy and vaccine uptake in a national cohort of US adults. medRxiv 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angulo FJ, Finelli L, Swerdlow DL. Estimation of US SARS-CoV-2 infections, symptomatic infections, hospitalizations, and deaths using seroprevalence surveys. JAMA Netw Open 2021;4:e2033706. 10.1001/jamanetworkopen.2020.33706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bajema KL, Wiegand RE, Cuffe K, et al. Estimated SARS-CoV-2 seroprevalence in the US as of September 2020. JAMA Intern Med 2021;181:450–60. 10.1001/jamainternmed.2020.7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CDC . COVID data tracker, 2020. Available: https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- 25.Oran DP, Topol EJ. The proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review. Ann Intern Med 2021;174:655–62. 10.7326/M20-6976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milani GP, Dioni L, Favero C, et al. Serological follow-up of SARS-CoV-2 asymptomatic subjects. Sci Rep 2020;10:20048. 10.1038/s41598-020-77125-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.New York Times . Cuomo says 21% of those tested in N.Y.C. had virus antibodies, 2020. Available: https://www.nytimes.com/2020/04/23/nyregion/coronavirus-new-york-update.html
- 28.Bendavid E, Mulaney B, Sood N. COVID-19 antibody seroprevalence in Santa Clara County, California. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Country Los Angeles Public Health . USC-LA County study: early results of antibody testing suggest number of COVID-19 infections far exceeds number of confirmed cases in Los Angeles County, 2020. Available: http://publichealth.lacounty.gov/phcommon/public/media/mediapubhpdetail.cfm?prid=2328
- 30.Menachemi N, Yiannoutsos CT, Dixon BE, et al. Population point prevalence of SARS-CoV-2 infection based on a statewide random sample - Indiana, April 25-29, 2020. MMWR Morb Mortal Wkly Rep 2020;69:960–4. 10.15585/mmwr.mm6929e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biggs HM, Harris JB, Breakwell L, et al. Estimated community seroprevalence of SARS-CoV-2 antibodies - two Georgia counties, April 28-May 3, 2020. MMWR Morb Mortal Wkly Rep 2020;69:965–70. 10.15585/mmwr.mm6929e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis HE, Assaf GS, McCorkell L. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. bioRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wentworth DN, Neaton JD, Rasmussen WL. An evaluation of the social security administration master beneficiary record file and the National death index in the ascertainment of vital status. Am J Public Health 1983;73:1270–4. 10.2105/AJPH.73.11.1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NIH: National Institute of Allergy and Infectious Diseases . Nih begins study to quantify undetected cases of coronavirus infection, 2020. Available: https://www.niaid.nih.gov/news-events/nih-begins-study-quantify-undetected-cases-coronavirus-infection
- 35.Emory Univercity . Emory launches national COVID-19 home testing study to estimate infection and antibody rates in the U.S, 2020. Available: https://sph.emory.edu/news/news-release/2020/06/covid-vu-study.html
- 36.Center for Disease Control and Prevention . National death index, 2021. Available: https://www.cdc.gov/nchs/ndi/index.htm
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. The authors will post a deidentified, HIPAA compliant, public use version of visit 1 and follow-up data on Backblaze, a secure cloud storage provider. Data will be presented as flat text files (CSV) formatted for compatibility with county-level longitudinal case load datasets, including date, county, state and FIPS code. The authors will exclude counties with <20 000 residents to protect participant privacy. The authors will continue to provide direct feedback to their cohort and other stakeholders who have signed up for updates via follow-up emails to participants and the City University of New York Institute for Implementation Science in Population Health Study website.