Abstract
Background
Liver cancer ranks the top four malignant cancer type worldwide, which needs effective and safe treatment. Ferroptosis is a novel form of regulated cell death driven by iron-dependent lipid peroxidation and has been regarded as a promising therapeutic target for cancers. In this work, we aimed to study the effects of anesthetic ketamine on proliferation and ferroptosis of liver cancer.
Methods
Cell viability and proliferation were detected by cell counting kit 8 (CCK-8), colony formation, and 5-ethynyl-2′-deoxyuridine (EdU) assay. Ferroptosis was determined by levels of Fe2+, lipid reactive oxygen species (ROS), and malondialdehyde (MDA). RNA levels of lncPVT1, miR-214-3p, and glutathione peroxidase 4 (GPX4) were checked by real-time PCR assay. Clinical liver tumor samples were collected to detect the levels of long noncoding RNA lncPVT1, miR-214-3p, and GPX4, and their correlation was evaluated by Pearson comparison test. Luciferase reporter gene assay and RNA pulldown were conducted to determine the binding between lncPVT1, miR-214-3p, and GPX4 3ʹUTR.
Results
Ketamine significantly suppressed viability and proliferation of liver cancer cells both in vitro and in vivo, as well as stimulated ferroptosis, along with decreased expression of lncPVT1 and GPX4. LncPVT1 directly interacted with miR-214-3p to impede its role as a sponge of GPX4. Depletion of lncPVT1 accelerated the ferroptosis of live cancer cells, whereas miR-214-3p inhibition and GPX4 overexpression reversed this effect. Ketamine-induced cell growth suppression and ferroptosis were also suppressed by miR-214-3p inhibition and GPX4 overexpression.
Conclusion
In this work, we determined that ketamine suppressed viability of liver cancer cells and induced ferroptosis and identified the possible regulatory mechanism of lncPVT1/miR-214-3p/GPX4 axis.
Keywords: liver cancer, ketamine, lncPVT1, miR-214-3p, GPX4
Introduction
Liver cancer ranks the top four malignant cancer type worldwide, among which hepatocellular carcinoma (HCC) is the mostly frequently occurred form.1 Most liver cancer cases are diagnosed at late stages due to the delayed symptoms and aggressive growth,2 which leads to less than ten months median survival and a 5-year overall survival rate of 10% of patients diagnosed with advanced liver cancer.3 Liver cancer is commonly generated from alcohol, non-alcoholic fatty liver disease (NAFLD), cirrhosis, and virus infection including hepatitis B virus (HBV) and hepatitis C virus (HCV).4 Surgical resection of tumors is currently the predominant treatment for liver cancer, and the multi-kinase inhibitor sorafenib is the only first-line drug.4 Hence, it is urgent to explore novel treatments for liver cancer.
Ferroptosis is a novel form of regulated cell death driven by iron-dependent lipid peroxidation, featured by increased free iron and accumulated lipid peroxides, which leads to accumulated intracellular reactive oxygen species (ROS) production and subsequent oxidative cell death.5,6 The phospholipid glutathione peroxidase 4 (GPX4) is an antioxidant that catalyzes the reduction of peroxide such as hydrogen peroxide and organic hydroperoxides, through co-acting with antioxidant glutathione (GSH).7 Accumulating evidences have demonstrated the critical inhibitory function of GPX4 during ferroptosis.7 Moreover, several small molecules, including Erastin and RSL3, have been recognized as inducers of ferroptosis, via suppressing the activity of GPX4.8,9
Ketamine is a commercially used rapid-acting anesthetic agent since 1970.10 Despite the risk of dissociative effects and abuse potential, ketamine is a desirable drug owing to its short half-life and lack of clinically significant respiratory depression.11–15 In addition to the anesthetic effect, accumulating researches have proved that ketamine exhibits anti-inflammatory activity, analgesic effect, and antidepressant effect.16–19 Although ketamine has been widely suggested as an adjunctive agent to deal with cancer-related pain,20–22 its effects on cancer therapy and the mechanisms are largely unknown.
Long noncoding RNAs (lncRNAs) are defined as a class of RNAs with a sequence longer than 200 nucleotides.23 Numerous studies have revealed the anticancer effects of lncRNAs involving almost every cellular process during carcinogenesis, such as proliferation, angiogenesis, and metastasis.24,25 Moreover, it is widely accepted that lncRNAs mainly function through interacting with microRNA (miRNA), which consequently target specific mRNAs and interfere gene expression.26 LncRNA plasmacytoma variant translocation 1 (PVT1) is found to be abnormally expressed in several cancers, such as gastric cancer, colorectal cancer, and cervical cancer.27–30 MiR-214-3p is a widely studied suppressive miRNA in cancers and has been proposed as a diagnostic and prognostic biomarker in several cancers.31–34
In this work, we aimed to determine the function of ketamine in liver cancer, and confirmed its role in activating ferroptosis, further identified the possible regulatory network of lncPVT1/miR-214-3p/GPX4. Our work presented ketamine as a promising therapeutic drug for liver cancer treatment.
Materials and Methods
Patient Samples
This study was ratified by the Ethics Committee of First Affiliated Hospital, China Medical University. We recruited 36 liver cancer patients who received surgical resection of tumors in First Affiliated Hospital, China Medical University between June 2018 and January 2020. The tumor and adjacent nontumor tissues were collected and stored at −80°C for further experiments. All participants have signed written informed consent. The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Cell Lines and Treatment
Human liver cancer cell lines HepG2 and Huh7 were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). All cells were cultured in high glucose-DMEM supplemented with 10% fetal bovine serum (FBS, Invitrogen, USA) and 1% penicillin/streptomycin (Sigma, USA), in a humidified 37°C incubator filled with 5% CO2. Ketamine hydrochloride was obtained from Narketan 10 (UK) and used at a concentration of 10 μg/mL for in vitro study. The ferroptosis activators, Erastin and RSL3, were purchased from Selleck (USA) and administrated to cells at a dose of 5 μM.
Cell Transfection
The small interfering NRA targeting lncPVT1 (siPVT1), GPX4 overexpressing vector pcDNA-GPX4 (OE-GPX40), lncPVT1 overexpressing vector pcDNA-PVT1 (OE-PVT1), miR-214-3p mimics and inhibitor, and corresponding negative control (NC) were synthesized by Gene-Pharma (Shanghai, China). Transfections were conducted at final concentrations of 500ng/mL overexpressing vectors or 50 nM of miRNA mimics and siRNAs by using the Lipofectamine 2000 reagent (Invitrogen, USA).
Cell Viability and Proliferation
To evaluate cell vitality, a Cell counting kit 8 (CCK-8) (Beyotime, China) was adopted. In short, cancer cells were transfected with indicated oligonucleotides, then seeded into 96-well plates at a density of 6×103 cells/well, and treated with ketamine, Erastin, or RSL3. At the indicated time, CCK-8 reagent (20 μL) was added into each well and incubated for 2 hours. The absorbance values (450 nm) were detected by a microplate spectrometer (Thermo, USA).
For colony formation assay, 1000 HepG2 or Huh7 cells were suspended in a complete medium as single cells and added to each well of a 6-well plate. The visible colonies were stained with Crystal Violet (Sigma) 3 weeks later, and a photographed under microscope (Nikon, Japan).
For 5-ethynyl-2′-deoxyuridine (EdU) assay, cells were seeded in 96-well plates, fixed, permeabilized, and incubated with EdU (50 µM) (Sigma) for 3 hours and DAPI (1 µg/mL) (Sigma) for 10 minutes. The cells were observed and captured under a fluorescence microscopy (Leica, Germany).
Real-Time PCR
Total RNAs were obtained from tissues or cells by using RNAiso Plus (TaKaRa, Japan). Then, cDNA was synthesized by using High-Capacity cDNA reverse transcription kits (Thermo), followed by quantification by SYBR Green/ROX qPCR Master Mix (Thermo). The relative gene expression was calculated by using the 2−ΔΔCT method. The lncPVT1 and GPX4 mRNA levels were normalized by GAPDH. The miR-214-3p level was normalized by U6.
Western Blotting
Liver cancer cells were lysed by ice-cold RIPA buffer (Thermo, USA) and added with a cocktail of protease inhibitors (Thermo). The lysates were then subjected to the SDS-PAGE and shifted to NC membranes. The protein bands were interacted with primary antibodies against GPX4 (1:1000, Abcam, USA) and β-actin (1:1000, Abcam), followed by secondary anti-mouse and anti-rabbit antibodies (1:2000, Abcam). The bands were then visualized by an ECL solution (Millipore, Germany) in a Gel Image system (Bio-Rad).
Xenograft Mice Model
All animal experiments were authorized by the Ethics Committee of the First Affiliated Hospital, China Medical University.
BALB/c nude mice (age 6 weeks) were brought from the Laboratory Animal Center of Chinese Academy of Sciences (China). HepG2 cell suspension (100 μL, 5×105 per site) was hypodermically inoculated into the fat pad of mice. Tumor volume was calculated as follows: tumor volume (mm3) = 0.5×width (mm)2 × length (mm). When tumor size reached 100 mm3, mice were treated with ketamine (20 mg/kg) or saline intraperitoneally. The mice were succumbed to death when tumor size reached 1000 mm3. Tumors were isolated and weighted. All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Detection of Ferroptosis
The relative level of intracellular iron was detected by an Iron Assay Kit (Abcam, USA) following manufacturer’s protocol. The lipid ROS was detected by using C11-BODIPY (Invitrogen) staining. In brief, cells were planted on 12-well plates and incubated overnight. After indicated incubation, cells were collected and resuspended in 200 μL PBS, stained with C11-BODIPY (5 μM) at 37°C for 30 minutes. Samples were examined by flow cytometer (BD, USA). The level of malondialdehyde (MDA) was assessed by using a lipid peroxidation (MDA) assay kit (Sigma). Cells were lysed by MDA lysis buffer on ice, followed by centrifugation at 13,000 × g for 5 minutes. The absorbance values were detected at 532 nm by a spectrophotometer (BD Biosciences).
Luciferase Reporter Gene Assay
The wild-type (WT) or mutant (MUT) lncPVT1 or GPX4 3ʹUTR was synthesized and cloned into pmirGLO vector, separately. Then, HepG2 and Huh7 cells were seeded on a 24-well plate, followed by co-transfected with WT or MUT and miR-214-3p or NC mimics. After transfection for 48 hours, the cells were lysed and a Dual-Luciferase Reporter Assay System (Promega, USA) was applied to detect the luciferase activity.
RNA Pulldown
Biotin-labeled wild-type miR-214-3p mimics (Bio-miR-214-3p-WT), biotin-labeled mutated miR-214-3p mimics (Bio-miR-214-3p-MUT), and negative control mimics (Bio-NC) were purchased from GenePharma (China). In short, cells were transfected with the probes for 48 hours, harvested and hatched with lysis buffer, followed by incubation with M280 streptavidin Dynabeads (Invitrogen) for 6 hours at 4°C. The mixture was washed and eluted. RNA level of lncPVT1 was determined by qRT-PCR assay.
Statistics
The data in this work were presented as means ± S. D, and analyzed by SPSS 22.0 software. The statistical differences among different groups were analyzed by Student’s t-test or one-way ANOVA. The correlation between lncPVT1, miR-214-3p, and GPX4 was analyzed by Pearson χ2 test. P values <0.05 were considered to be statistically significant.
Results
Ketamine Suppresses Viability and Proliferation of Liver Cells in vitro and in vivo
We first investigated the function of ketamine in viability and proliferation of liver cancer cells. As shown in Figure 1A and B, treatment with ketamine notably suppressed cell proliferation curve at 48 hours and 72 hours, comparing with the control group. The decreased colony formation ability (Figure 1C and D) and EdU-positive cell numbers (Figure 1E) further indicated the impaired proliferation of cells. Moreover, ketamine treatment notably inhibited in vivo tumor growth, as manifested by the suppressed growth curve (Figure 1F) and tumor weight (Figure 1G).
Figure 1.
Ketamine suppresses viability and proliferation of liver cells in vitro and in vivo. (A and B) HepG2 and Huh7 cells were treated with ketamine (Keta) for indicated time, cell viability was detected by CCK-8 assay. Colony formation (C and D) and EdU assay (E) were performed to detect cell proliferation. (F and G) Xenograft mice model was established by using HepG2 cells. Tumor growth curve (F) and tumor weight (G) were recorded. **p < 0.01.
Ketamine Induces Ferroptosis in Liver Cancer Cells
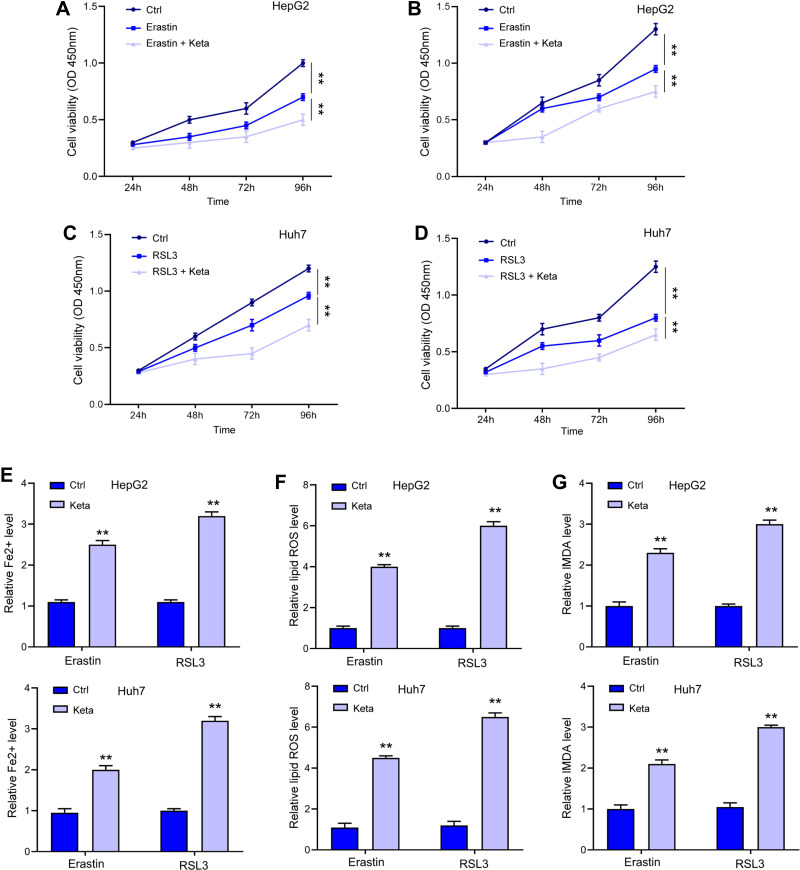
Erastin and RSL3 are widely applied ferroptosis stimulator. As shown in Figure 2A–D, treatment with ketamine significantly exacerbated Erastin- and RSL3-induced suppression of cell viability. Moreover, the signatures of ferroptosis, including the increased levels of Fe2+ (Figure 2E), lipid ROS (Figure 2F), and MDA (Figure 2G), were notably enhanced by ketamine, suggesting the onset of ferroptosis.
Figure 2.
Ketamine induced ferroptosis in liver cancer cells. (A–D) HepG2 and Huh7 cells were treated with Erastin or RSL3 alone, or with ketamine (Keta) for indicated time, cell viability was detected by CCK-8 assay. The levels of Fe2+ (E), lipid ROS (F), and MDA (G) were checked. **p < 0.01.
Ketamine Inhibits GPX4 Expression Through lncPVT1
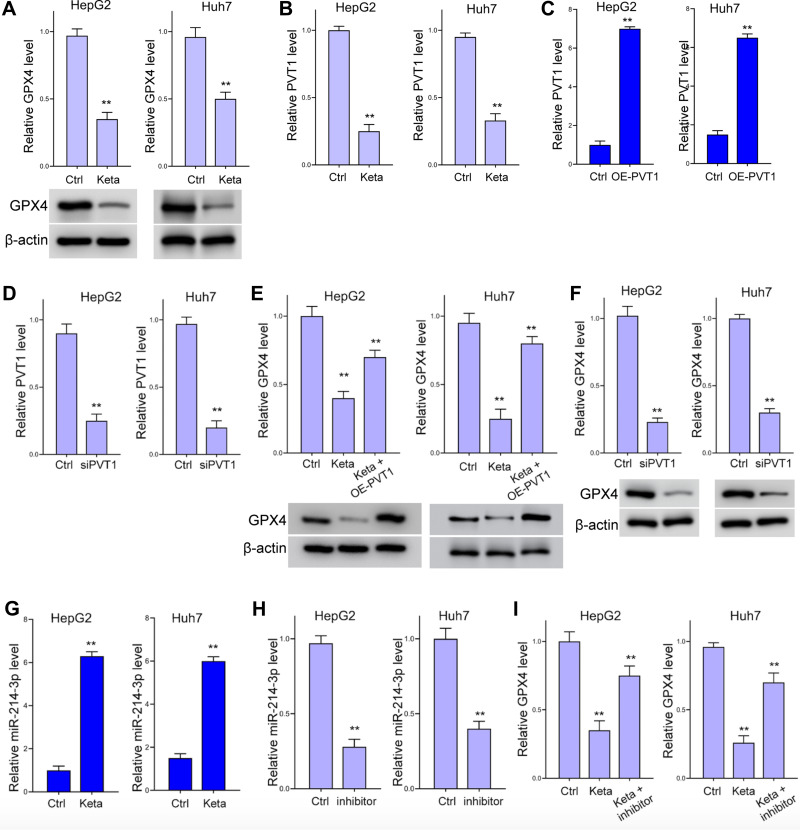
Treatment with ketamine notably impeded the expression of GPX4, the antioxidant agent during ferroptosis, comparing with the control group (Figure 3A). To explore the mechanisms underlying this regulation, we detected the expression of lnvPVT1, and revealed that the level of lncPVT1 was significantly suppressed by ketamine (Figure 3B). We next investigated if lncPVT1 modulate GPX4 expression by knockdown and rescue experiments. The transfection efficacy of lncPVT1 overexpressing vector (OE-PVT1) and siPVT1 is shown in Figure 3C and D. Ectopic expression of lncPVT1 reversed the suppressed GPX4 caused ketamine (Figure 3E). Knockdown of lncPVT1 decreased GPX4 expression (Figure 3F). Moreover, we discovered decreased level of miR-214-3p under ketamine administration (Figure 3G). Inhibition with miR-214-3p inhibitor relieved the decreased GPX4 level (Figure 3H and I).
Figure 3.
Ketamine inhibits GPX4 expression through lncPVT1. (A and B) Levels of GPX4 (A) and lncPVT1 in HepG2 and Huh7 cells after treatment with ketamine (short as Keta) were detected by Western blot analysis or RT-PCR. (C and D) Transfection efficacy of lncPVT1 overexpressing vector (OE-PVT1) (C) and siPVT1 (D) were detected by RT-PCR. (E and F) The level of GPX4 under treatment of ketamine and OE-PVT1 (E), or transfection with siPVT1 (F) were detected by RT-PCR and Western blot analysis. (G) RNA level of miR-214-3p in HepG2 and Huh7 cells after treatment with ketamine was detected by RT-PCR. (H) Transfection efficacy of miR-214-3p inhibitor (short as inhibitor) was detected by RT-PCR. (I) The relative RNA level of GPX4 under treatment of ketamine and miR-214-3p inhibitor. **p < 0.01.
LncPVT1 Regulates GPX4 Expression via Suppressing miR-214-3p
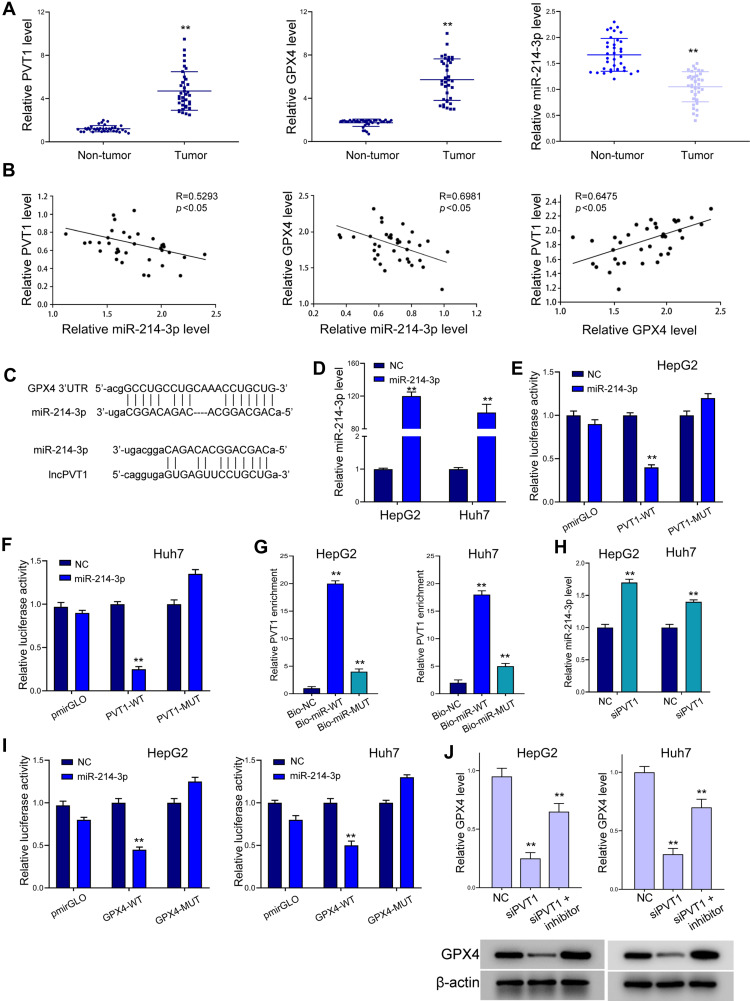
We next investigated the regulatory correlation between lncPVT1, miR-214-3p and GPX4. Analysis on clinical liver cancer patient samples revealed elevated expression of lncPVT1 and GPX4 and decreased level of miR-214-3p in tumor tissues, comparing with the adjacent non-tumor parts (Figure 4A and B). Results from Pearson correlation analysis indicated negative correlation between miR-214-3p with lncPVT1 and GPX4, and positive correlation between lncPVT1 and GPX4 in liver cancer tissues. As shown in Figure 4C, there exists potential interaction between miR-214-3p with lncPVT1 and 3ʹUTR of GPX4. Results from luciferase reporter gene assay revealed decreased activity of wild-type lncPVT1, rather than the mutated type, under transfection of miR-214-3p mimics (Figure 4D–F). RNA pulldown assay further identified the direct binding between miR-214-3p and lncPVT1 (Figure 4G). Decreased expression of miR-214-3p under lncPVT1 knockdown confirmed their correlation (Figure 4H). Similarly, results from luciferase reporter gene assay demonstrated miR-214-3p as a sponge of GPX4 (Figure 4I). The inhibition of miR-214-3p recovered the downregulated level of GPX4 caused by lncPVT1 depletion (Figure 4J).
Figure 4.
LncPVT1 regulates GPX4 expression via suppressing miR-214-3p. (A and B) Tumor (n=36) and non-tumor (n=36) tissues were collected, then levels of lncPVT1, GPX4, and miR-214-3p were detected by RT-PCR (A). Their correlation was analyzed by Pearson test (B). (C) Binding sites of miR-214-3p on lncPVT1 and 3ʹUTR of GPX4 predicted by ENCORI. (D) RT-PCR to determine miR-214-3p level in liver cancer cells under transfection of miR-214-3p mimics. (E and F) Luciferase activity of wild type (WT) and mutated type (MUT) lncPVT1 reporter gene vector. (G) RNA pulldown assay to detect enrichment of lncPVT1 by miR-214-3p. Bio-NC, biotin-labeled negative control mimics; Bio-miR-WT, biotin-labeled wild-type miR-214-3p mimics; Bio-miR-MUT, biotin-labeled mutated miR-214-3p mimics. (H) RT-PCR to determine miR-214-3p level. (I) Luciferase activity of wild type (WT) and mutated type (MUT) GPX4 3ʹUTR reporter gene vector. (J) Level of GPX4 detected by RT-PCR and Western blot analysis. **p < 0.01.
LncPVT1 Depletion Accelerates Ferroptosis in Liver Cancer Cells
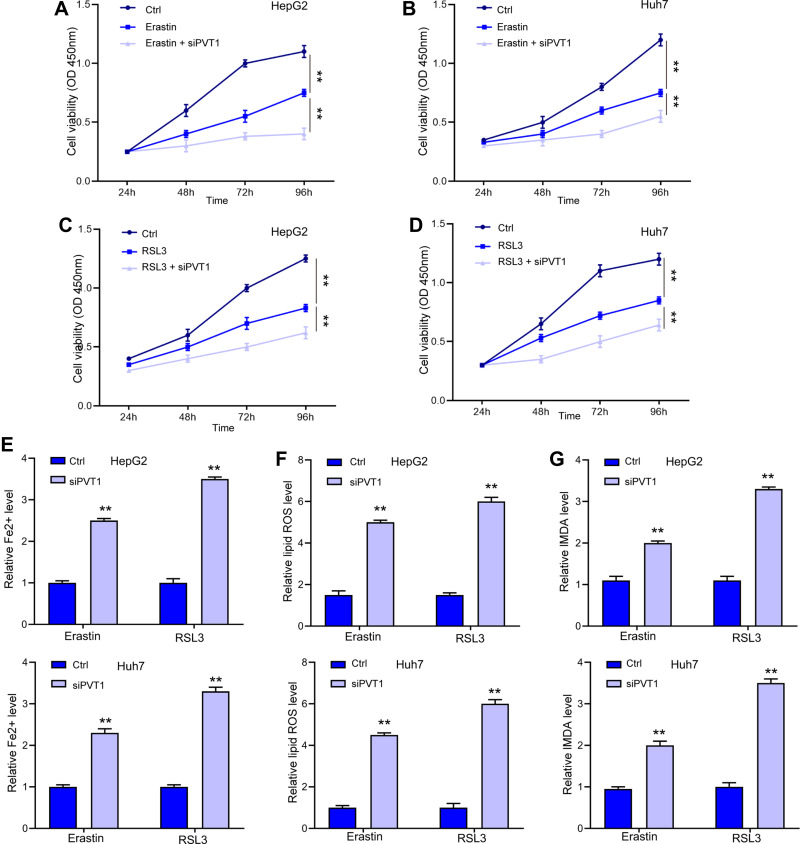
After identifying the direct regulation of lncPVT1 on GPX4 expression, we investigated if lncPVT1 modulated ferroptosis in liver cancer cells. We observed that depletion of lncPVT1 enhanced Erastin- and RSL3-inhibited cell viability in HepG2 (Figure 5A and C) and Huh7 cells (Figure 5B and D). Besides, the elevated levels of Fe2+ (Figure 5E), lipid ROS (Figure 5F), and MDA (Figure 5G), significantly elevated by siPVT1 also suggested the occurrence of ferroptosis.
Figure 5.
LncPVT1 promotes ferroptosis in liver cancer cells. (A–D) HepG2 and Huh7 cells were treated with Erastin or RSL3 alone, or with transfection of siPVT1 for indicated time, cell viability was detected by CCK-8 assay. The levels of Fe2+ (E), lipid ROS (F), and MDA (G) were checked. **p < 0.01.
LncPVT1 Modulates Ferroptosis via miR-214-3p/GPX4 Axis
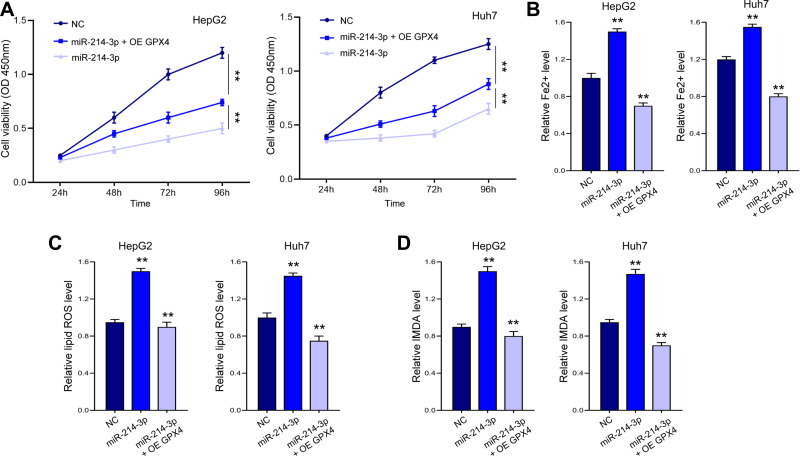
Subsequently, we explored whether lncPVT1 modulates ferroptosis via miR-214-3p/GPX4 axis. As shown in Figure 6A, miR-214-3p notably inhibited cell proliferation, whereas overexpression of GPX4 alleviated this effect. Moreover, miR-214-3p promoted ferroptosis, manifested by elevated levels of Fe2+ (Figure 6B), lipid ROS (Figure 6C), and MDA (Figure 6D), whereas ectopic expression of GPX4 abolished this activation.
Figure 6.
MiR-214-3p promotes ferroptosis in liver cancer cells via targeting GPX4. HepG2 and Huh7 cells were transfected with miR-214-3p mimics or NC, with or without GPX4 overexpressing vectors (OE GPX4). (A) Cell viability was detected by CCK-8 assay. The levels of Fe2+ (B), lipid ROS (C), and MDA (D) were checked. **p < 0.01.
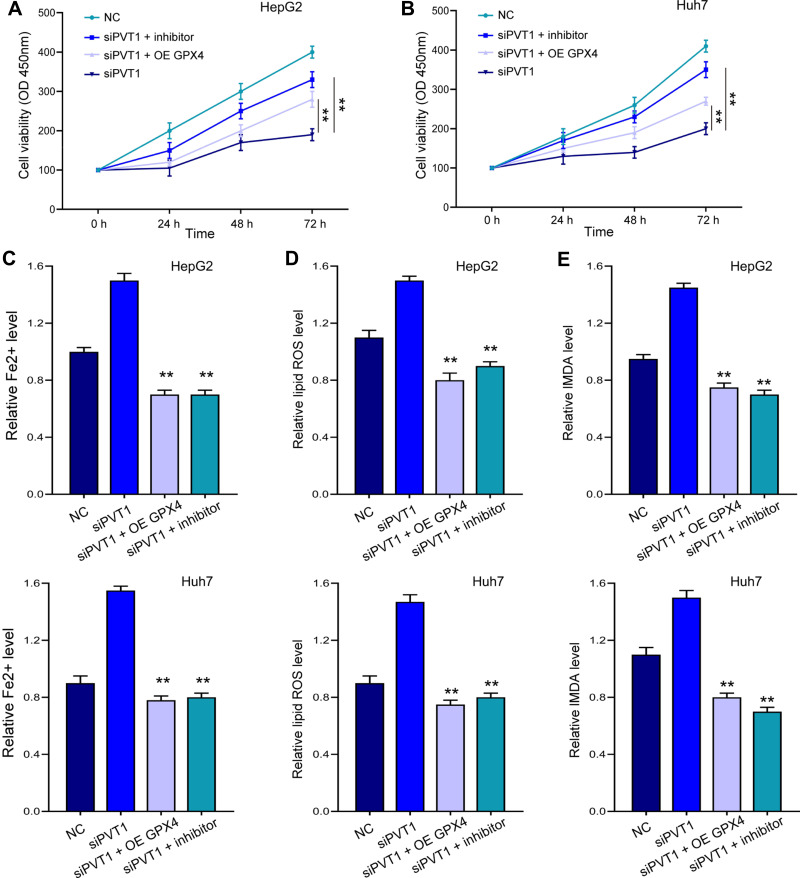
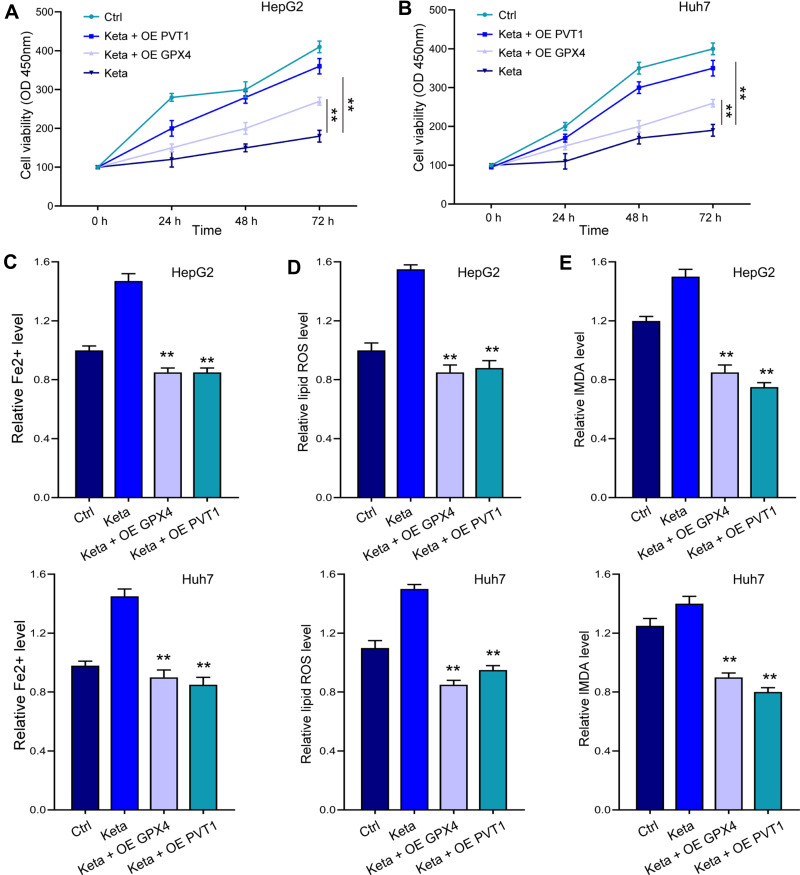
Ferroptosis Induced by siPVT1 or Ketamine Could Be Reversed by miR-214-3p Inhibition and GPX4 Overexpression
Noteworthy, overexpression of GPX4 and inhibition of miR-214-3p relieved siPVT1-suppressed cell viability (Figure 7A and B) in HepG2 and Huh7 cells. The siPVT1-stimulated ferroptosis was also suppressed by miR-214-3p inhibitor and GPX4 overexpression, suggested by downregulated levels of Fe2+ (Figure 7C), lipid ROS (Figure 7D), and MDA (Figure 7E). Coincidently, suppressed cell viability and stimulated ferroptosis under ketamine treatment were also remarkably reversed by GPX4 overexpression and miR-214-3p inhibitor (Figure 8).
Figure 7.
MiR-214-3p inhibition and GPX4 overexpression reversed siPVT1-stimulated ferroptosis. HepG2 and Huh7 cells were transfected with siPVT1 alone, or with miR-214-3p inhibitor (inhibitor) or GPX4 overexpressing vectors (OE GPX4). (A and B) Cell viability was detected by CCK-8 assay. The levels of Fe2+ (C), lipid ROS (D), and MDA (E) were checked. **p < 0.01.
Figure 8.
Overexpression of lncPVT1 and GPX4 impeded ketamine-induced ferroptosis. HepG2 and Huh7 cells were treated with ketamine alone, or with transfection of miR-214-3p inhibitor (inhibitor) or GPX4 overexpressing vectors (OE GPX4). (A and B) Cell viability was detected by CCK-8 assay. The levels of Fe2+ (C), lipid ROS (D), and MDA (E) were checked. **p < 0.01.
Discussion
As a frequently occurred malignancy, liver cancer severely threatened human lives, which makes seeking effective and safe therapeutic manners an urgent issue.1 Ketamine is a common N-methyl-d-aspartate receptor (NMDAR) antagonist, and an ideal option to deal with cancer-related pain in clinical practice. Noteworthy, ketamine has shown the capacity to attenuate cancer cell malignancy in recent studies.35 It has been reported that carbon tetrachloride is reversed by natural products in mammals.36,37 Standardized extract of ginger relieves liver cancer via enhancing apoptosis and repressing proliferation by inhibiting oxidative stress and inflammation signaling.38 Induced reproductive toxicity can be associated with chemotherapeutics.39 In this research, we observed the anti-cancer effect of ketamine against liver cancer cells, manifested by suppressed cell viability and activated ferroptosis. Ferroptosis is a novel form of regulated cell death that differs from classic cell apoptosis. Ferroptosis is characterized by accumulated intracellular reactive oxygen species (ROS) production and subsequent oxidative cell death.5,6 Accumulating evidences have confirmed ferroptosis as a promising target for cancer therapy. Several small molecules, such as Erastin and RSL3, are identified as stimulators of ferroptosis, which suppress the antioxidant activity of GPX4.8,9 We observed elevated accumulation of Fe2+ iron, lipid ROS and MDA, along with decreased level of GPX4 after ketamine treatment, which suggested lipid peroxidation.
To investigate the mechanisms underlying ketamine-induced ferroptosis, we investigated the lncPVT1/miR-214-3p/GPX4 regulatory axis. Over the past decades, the participation of lncRNAs in cancer development has been well established.23–25 We found that ketamine treatment downregulated the expression of lncPVT1 and GPX4, while upregulated the level of miR-214-3p. Analysis on clinical liver tumor tissues confirmed the negative correlation between miR-214-3p with lncPVT1 and GPX4, and positive correlation between lncPVT1 and GPX4. Further, luciferase reporter gene assay and RNA pulldown assay manifested the direct interaction between lncPVT1 and miR-214-3p, as well as the sponge of miR-214-3p to GPX4 3ʹUTR. The role of lncPVT1 in cancer has been previously reported. For instance, lncPVT1 was upregulated in gastric cancer cells and directly interacted with FOXM1 to promote tumor progression.27 Overexpression of lncPVT1 is a poor prognostic biomarker and facilitates migration and invasion in colorectal cancer, which may be correlated with c-Myc function.28 Moreover, lncPVT1 also activates cervical cancer development via acting as a molecular sponge of miR-424.29 Ren et al recently reported that lncPVT1 was overexpressed in renal cell carcinoma and targeted miR-16-5p to promote cell proliferation, invasion, and epithelial mesenchymal transition (EMT).30
MiR-214-3p overexpression repressed proliferation and cancer cell stemness in vitro and in vivo in lung squamous cell cancer, via targeting YAP1,31 and also targeted the fibroblast growth factor/MAPK signaling.32 Han et al suggested that miR-214-3p modulated breast cancer cell proliferation and apoptosis by targeting Survivin.33 MiR-214-3p also interacted with TWIST1 to suppress epithelial-to-mesenchymal transition of endometrial cancer cells.34 We spotted that the overexpression of GPX4 and inhibition of miR-214-3p abolished the ferroptosis induced by siPVT1 or ketamine treatment. There are still some limitations in this study. Despite we analyzed the correlation of lncRNA PVT1, miR-214-3p, and GPX4 in liver cancer, the relationship of ketamine and lncRNA PVT1/miR-214-3p/GPX4 axis in liver cancer progression, especially the mechanism underlying ketamine-mediated lncRNA PVT1, remains unclear and needs to be explored in the future investigation. In this study, we identified that ketamine inhibited GPX4 expression by reducing lncPVT1 and lncPVT1 promoted GPX4 expression by sponging miR-214-3p. LncPVT1/miR-214-3p axis was one of the potential mechanisms by which ketamine regulated GPX4 expression to regulate ferroptosis of liver cancer cells. Our finding provides a novel mechanism of treatment of liver cancer. In addition, there is the less likely possibility that ketamine is approved as a therapeutic approach for liver cancer with the concerns of abuse potential. It is reported that ketamine became commercially available for human use in 1970 as a rapid-acting anesthetic. Ketamine was derived from phencyclidine (PCP) with the aim of lessening the serious psychotomimetic/psychodysleptic side effects and abuse potential of the parent drug, which was subsequently removed from the market in 1978. However, ketamine still induces dissociative effects and has abuse potential although to a lesser extent than PCP. Of course, the value of ketamine for chronic pain management in patients with cancer is being recognized, and the patient may benefit from both analgesic and antitumor triggered by ketamine.
Conclusion
To summarize, this work clarified the tumor-suppressive role of ketamine in liver cancer, and determined the involvement of ferroptosis during this process. Analysis on mechanisms identified the potential regulatory axis of lncPVT1/miR-214-3p/GPX4 in ketamine-stimulated ferroptosis. Our work may provide a novel therapeutic approach for liver cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152:745–761. doi: 10.1053/j.gastro.2016.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 4.Wang C, Vegna S, Jin H, et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature. 2019;574:268–272. doi: 10.1038/s41586-019-1607-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yagoda N, von Rechenberg M, Zaganjor E, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–868. doi: 10.1038/nature05859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon SJ, Patel DN, Welsch M, et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. doi: 10.7554/eLife.02523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shintoku R, Takigawa Y, Yamada K, et al. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci. 2017;108:2187–2194. doi: 10.1111/cas.13380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dundee JW, Knox JW, Black GW, et al. Ketamine as an induction agent in anaesthetics. Lancet. 1970;295(7661):1370–1371. doi: 10.1016/S0140-6736(70)91273-0 [DOI] [PubMed] [Google Scholar]
- 11.Pomarol-Clotet E, Honey GD, Murray GK, et al. Psychological effects of ketamine in healthy volunteers. Phenomenological study. Br J Psychiatry. 2006;189:173–179. doi: 10.1192/bjp.bp.105.015263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newcomer JW, Farber NB, Jevtovic-Todorovic V, et al. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118. doi: 10.1016/S0893-133X(98)00067-0 [DOI] [PubMed] [Google Scholar]
- 13.Bowdle TA, Radant AD, Cowley DS, Kharasch ED, Strassman RJ, Roy-Byrne PP. Psychedelic effects of ketamine in healthy volunteers: relationship to steady-state plasma concentrations. Anesthesiology. 1998;88:82–88. doi: 10.1097/00000542-199801000-00015 [DOI] [PubMed] [Google Scholar]
- 14.Stewart CE. Ketamine as a street drug. Emerg Med Serv. 2001;30:30, 2, 4 passim. [PubMed] [Google Scholar]
- 15.Morgan CJ, Curran HV; Independent Scientific Committee on D. Ketamine use: a review. Addiction. 2012;107:27–38. doi: 10.1111/j.1360-0443.2011.03576.x [DOI] [PubMed] [Google Scholar]
- 16.Weisman H. Anesthesia for pediatric ophthalmology. Ann Ophthalmol. 1971;3:229–232. [PubMed] [Google Scholar]
- 17.Roytblat L, Talmor D, Rachinsky M, et al. Ketamine attenuates the interleukin-6 response after cardiopulmonary bypass. Anesth Analg. 1998;87:266–271. doi: 10.1213/00000539-199808000-00006 [DOI] [PubMed] [Google Scholar]
- 18.Zarate CA Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856 [DOI] [PubMed] [Google Scholar]
- 19.Wolff K, Winstock AR. Ketamine: from medicine to misuse. CNS Drugs. 2006;20:199–218. doi: 10.2165/00023210-200620030-00003 [DOI] [PubMed] [Google Scholar]
- 20.Singh V, Gillespie TW, Harvey RD. Intranasal ketamine and its potential role in cancer-related pain. Pharmacotherapy. 2018;38:390–401. doi: 10.1002/phar.2090 [DOI] [PubMed] [Google Scholar]
- 21.Bell RF, Eccleston C, Kalso EA. Ketamine as an adjuvant to opioids for cancer pain. Cochrane Database Syst Rev. 2017;6:CD003351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fallon MT, Wilcock A, Kelly CA, et al. Oral ketamine vs placebo in patients with cancer-related neuropathic pain: a randomized clinical trial. JAMA Oncol. 2018;4:870–872. doi: 10.1001/jamaoncol.2018.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839:1097–1109. doi: 10.1016/j.bbagrm.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 24.Chen C, Zhou L, Wang H, et al. Long noncoding RNA CNALPTC1 promotes cell proliferation and migration of papillary thyroid cancer via sponging miR-30 family. Am J Cancer Res. 2018;8:192–206. [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Ding L, Wang L, et al. LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancer. Oncotarget. 2015;6:41045–41055. doi: 10.18632/oncotarget.5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong YW, Cannell IG, de Moor CH, et al. The mechanism of micro-RNA-mediated translation repression is determined by the promoter of the target gene. Proc Natl Acad Sci U S A. 2008;105:8866–8871. doi: 10.1073/pnas.0800650105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu MD, Wang Y, Weng W, et al. A positive feedback loop of lncRNA-PVT1 and FOXM1 facilitates gastric cancer growth and invasion. Clin Cancer Res. 2017;23:2071–2080. doi: 10.1158/1078-0432.CCR-16-0742 [DOI] [PubMed] [Google Scholar]
- 28.Guo K, Yao J, Yu Q, et al. The expression pattern of long non-coding RNA PVT1 in tumor tissues and in extracellular vesicles of colorectal cancer correlates with cancer progression. Tumour Biol. 2017;39:1010428317699122. doi: 10.1177/1010428317699122 [DOI] [PubMed] [Google Scholar]
- 29.Gao YL, Zhao ZS, Zhang MY, Han LJ, Dong YJ, Xu B. Long noncoding RNA PVT1 facilitates cervical cancer progression via negative regulating of miR-424. Oncol Res. 2017;25:1391–1398. doi: 10.3727/096504017X14881559833562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren Y, Huang W, Weng G, Cui P, Liang H, Li Y. LncRNA PVT1 promotes proliferation, invasion and epithelial-mesenchymal transition of renal cell carcinoma cells through downregulation of miR-16-5p. Onco Targets Ther. 2019;12:2563–2575. doi: 10.2147/OTT.S190239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu T, Yang Y, Li Z, Lu S. MicroRNA-214-3p inhibits the stem-like properties of lung squamous cell cancer by targeting YAP1. Cancer Cell Int. 2020;20:413. doi: 10.1186/s12935-020-01506-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Li Z, Yuan H, et al. Reciprocal regulatory mechanism between miR-214-3p and FGFR1 in FGFR1-amplified lung cancer. Oncogenesis. 2019;8:50. doi: 10.1038/s41389-019-0151-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han LC, Wang H, Niu FL, Yan JY, Cai HF. Effect miR-214-3p on proliferation and apoptosis of breast cancer cells by targeting survivin protein. Eur Rev Med Pharmacol Sci. 2019;23:7469–7474. [DOI] [PubMed] [Google Scholar]
- 34.Fang YY, Tan MR, Zhou J, et al. miR-214-3p inhibits epithelial-to-mesenchymal transition and metastasis of endometrial cancer cells by targeting TWIST1. Onco Targets Ther. 2019;12:9449–9458. doi: 10.2147/OTT.S181037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duan W, Hu J, Liu Y. Ketamine inhibits colorectal cancer cells malignant potential via blockage of NMDA receptor. Exp Mol Pathol. 2019;107:171–178. doi: 10.1016/j.yexmp.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 36.El-Dakhly SM, Salama AAA, Hassanin SOM, Yassen NN, Hamza AA, Amin A. Aescin and diosmin each alone or in low dose- combination ameliorate liver damage induced by carbon tetrachloride in rats. BMC Res Notes. 2020;13:259. doi: 10.1186/s13104-020-05094-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamza AA, Lashin FM, Gamel M, Hassanin SO, Abdalla Y, Amin A. Hawthorn herbal preparation from Crataegus oxyacantha attenuates in vivo carbon tetrachloride -induced hepatic fibrosis via modulating oxidative stress and inflammation. Antioxidants (Basel). 2020;9:1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamza AA, Heeba GH, Hamza S, Abdalla A, Amin A. Standardized extract of ginger ameliorates liver cancer by reducing proliferation and inducing apoptosis through inhibition oxidative stress/ inflammation pathway. Biomed Pharmacother. 2021;134:111102. doi: 10.1016/j.biopha.2020.111102 [DOI] [PubMed] [Google Scholar]
- 39.Amin A, Mahmoud-Ghoneim D, Syam MI, Daoud S. Neural network assessment of herbal protection against chemotherapeutic-induced reproductive toxicity. Theor Biol Med Model. 2012;9:1. doi: 10.1186/1742-4682-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]