Abstract
Background
The outcome of deploying balloon-mounted stents for symptomatic intracranial atherosclerotic stenosis (ICAS) has not been fully investigated. In this study we evaluate the safety and long-term outcome of using balloon-mounted stents to treat symptomatic ICAS in comparison with the WEAVE/WOVEN study.
Methods
In a multicenter registry study of stenting for symptomatic intracranial artery stenosis in China, 159 patients treated with an intracranial balloon-mounted stent approved by the China Food and Drug Administration were evaluated. The morphological features of the lesions were categorized by Mori classification. The endpoints, including periprocedural and long-term clinical and radiological outcomes, were the same as those in the WEAVE/WOVEN study.
Results
In the present study the mean percent stenosis before and after stenting was 84.0% and 6.1%, respectively. The proportions of Mori A, Mori B, and Mori C lesions were 33.3%, 52.2%, and 14.5%, respectively. The 72-hour rates of stroke and mortality after the procedure were 0%. The 1-year rates of any stroke, ischemic stroke, hemorrhagic stroke, and death were 6.3% (10/159), 5.7% (9/159), 0.6% (1/159), and 0.6% (1/159), respectively. The 1-year rate of in-stent restenosis (ISR) was 23.4% (15/64). The rate of ISR in Mori C lesions (53.8%, 7/13) was significantly higher than that in Mori A (15.8%, 3/19) or Mori B lesions (15.6%, 5/32) (p=0.024).
Conclusions
The short-term and long-term outcomes of using a balloon-mounted stent for symptomatic ICAS with focal and non-angular lesions (Mori A and B type) and smooth arterial access were comparable to the results of the WEAVE/WOVEN trial.
Keywords: atherosclerosis, stroke, stenosis, balloon, stent
Introduction
Stroke is a common and disabling disease worldwide, and intracranial atherosclerotic stenosis (ICAS) is one of the leading causes of ischemic stroke in Asian populations.1–5 Intracranial stenting has been evaluated for the treatment of patients with symptomatic ICAS unresponsive to aggressive medical management, to improve the cerebral perfusion irrigated by the culprit arteries and thereby reducing the risk of recurrent stroke and improving neurological outcome.6 Even though registry studies demonstrated low rates of perioperative complications (4.5–6.9%) for ICAS stenting using the self-expanding Wingspan stent system,7–10 high perioperative complication rates in the stenting group were reported by the SAMMPRIS (Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis) study (14.7%) and by the VISSIT (Vitesse Intracranial Stent Study for Ischemic Stroke Therapy) study (24.1%).7 11 The WEAVE (Wingspan Stent System Post Market Surveillance) and WOVEN (Wingspan One-year Vascular Events and Neurologic outcomes) study also demonstrated low rates of perioperative complications (2.6%) and long-term recurrent stroke (8.5%) for ICAS stenting using the self-expanding Wingspan stent system with experienced interventionalists and proper patient selection.12 13
Compared with the self-expanding Wingspan stent, balloon-mounted stents are placed at the same time as angioplasty, avoiding the time and risk of over-the-wire exchange. In addition, balloon-mounted stents have shorter cone tips and are easier to navigate through small and tortuous vessels distal to the target lesions, reducing the risks of vascular injury. Since the failed VISSIT study, studies on the feasibility and long-term efficacy of balloon-mounted stenting for symptomatic ICAS have been scarce.11 The Apollo stent (MicroPort Neuro Tech, Shanghai, China) is a balloon-mounted stent with a metallic surface area of about 14.0%, approved by the Chinese Food and Drug Administration for use in patients with symptomatic ICAS. Although several case series have supported its safety and efficacy since 2003,14–18 a prospective multicenter registry study has not been carried out.
We have previously reported the short-term and long-term outcomes of a prospective multicenter registry on intracranial angioplasty and stenting for symptomatic stenosis, which included both self-expanding and balloon-mounted stents. Many balloon-mounted stents were deployed for focal and non-angular lesions (Mori A and B type) with smooth arterial access. We therefore performed this study to re-evaluate the safety and long-term outcome of balloon-mounted stenting for symptomatic ICAS by using the same endpoints as the WEAVE/WOVEN study.
Materials and methods
Study design and population
This study is a retrospective review of a subgroup of patients from a prospective single-arm registry study with 20 participating sites. Details of the registry have been published elsewhere.19–21 Approval by each site’s institutional review board or ethics committee was obtained. Written informed consent was obtained from the patients or their legally authorized representatives. All reported endpoints were evaluated and confirmed by a central adjudication committee consisting of neurologists, neurosurgeons, and radiologists who were blinded to the treatment choices. An independent data and safety monitoring board oversaw the conduct, safety, and efficacy of the study.
In this subgroup analysis we focused on the clinical and radiological outcomes of the balloon-mounted stenting group in comparison with the WEAVE/WOVEN study. We used the same primary endpoints as in the WEAVE/WOVEN study, including any stroke, ischemic stroke, hemorrhagic stroke, and death within 72 hours and at 1 year after the procedure and restenosis at 1 year.
Enrollment of patients
Inclusion and exclusion criteria were established by the executive committee. Patients were aged 18–85 years and had a symptomatic ICAS of 70–99% with a lesion length of ≤15 mm and target vessel diameter of ≥2.0 mm in the intracranial internal carotid artery (ICA), middle cerebral artery (MCA), intracranial vertebral artery (VA), or basilar artery (BA) (table 1). The measurements were made on digital subtraction angiography (DSA) using the WASID (warfarin–aspirin symptomatic intracranial disease) method with normal distal vessels as the reference.22 The symptoms included transient ischemic attacks (TIA) or ischemic stroke within the past 90 days that were attributable to hypoperfusion in the territory of the target lesion. Ischemic stroke referred to a new focal neurologic deficit lasting ≥24 hours or lasting <24 hours with new infarction on imaging, and TIA was defined as acute onset of a focal neurologic deficit lasting <24 hours without new infarction on imaging. Hemodynamic impairment in the territory of the culprit artery was determined on imaging within 2 weeks before the endovascular procedure using any one of the following methods: (1) a decrease in cerebral blood flow of ≥30% compared with the perfusion on the contralateral side for an anterior circulation lesion or the anterior circulation territory for a posterior circulation lesion on computed tomography perfusion or single-photon emission computed tomography; (2) American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology Collateral Flow Grading System score of <3 on DSA23; (3) peak systolic velocity of ≥200 cm/s and ≤1 collateral vessel that could be insonated on transcranial Doppler examination24; and (4) hemodynamic ischemic lesion by MRI defined as small ischemic infarcts in a watershed distribution in the culprit vessel territory.6 The ischemic mechanisms that could be entirely explained as an embolic phenomenon or perforator occlusion were excluded from this registry. The images were centrally reviewed by at least two physicians, who were allowed to resolve the disagreement through discussion. The patients were excluded from the study if the raters could not agree on the classification.
Table 1.
Inclusion criteria
| Present study | WEAVE/WOVEN |
| Symptomatic ICAS of 70–99% | Symptomatic ICAS of 70–99% |
| Age 18–85 years | Age 22–80 years |
| Baseline mRS score ≤3 | Baseline mRS score ≤3 |
| Stenting of the lesion ≥3 weeks after the last stroke | Stenting of the lesion ≥8 days after the last stroke |
| Ischemic symptoms within the past 90 days attributable to hypoperfusion in the territory of the target lesion | ≥2 strokes in the territory of the target lesion with at least one stroke while on medical therapy |
ICAS, intracranial atherosclerotic stenosis; mRS, modified Rankin Scale.
Patients were excluded if they had acute infarcts within 3 weeks, severe vessel tortuosity precluding the employment of endovascular devices as determined by the executive committee, a non-atherosclerotic lesion confirmed by high-resolution MRI (HR-MRI), embolic or perforator stroke based on MRI or CT, or baseline modified Rankin Scale (mRS) score of >3. Only patients without risk factors for intracranial atherosclerosis or those with a lesion suspected to be non-atheroclerotic by regular CT, MRI, or DSA were subjected to HR-MRI. All clinical and imaging data were reviewed centrally by the executive committee to decide whether the patient was eligible for enrollment.
Endovascular treatment strategy and device selection
The procedures were performed under local or general anesthesia by experienced neurointerventionists (performing more than 100 intracranial endovascular procedures per year). Intravenous heparin was administered after the placement of vascular access using a bolus of 75 U/kg followed by half of the dose after an hour. Two endovascular strategies were used when treating ICAS, and the operators were instructed to choose the devices based on the following guideline, taking into consideration their experience and preference as well as selecting the device which they thought was best suited to the patient. The ICAS lesions were categorized into three types according to the Mori classification: Mori A, short (≤5 mm in length) concentric or moderately eccentric lesions with ready accessibility; Mori B, tubular (5–10 mm in length) extremely eccentric lesions with moderately angulated segment (≥45°, <90°) and moderate tortuosity of the proximal segment; and Mori C, diffuse lesions (>10 mm in length), extremely angulated (≥90°) lesions with excessive tortuosity of the proximal segment (figure 1).25 26 For patients with smooth arterial access and a Mori A lesion, the Apollo balloon-mounted stent was preferred. For patients with tortuous arterial access or a Mori C lesion or a lesion with a significant mismatch in the diameter between the proximal and distal segments, balloon dilatation plus a self-expanding stent (Gateway balloon plus Wingspan stent system; Stryker, Maple Grove, Minnesota, USA) was preferred, but the balloon-mounted stent could also be used based on the operators’ experience. Mori C lesions tend to be long and angular, requiring relatively longer stents. However, the longer the stent length, the more difficult it is to put the system in place. In cases with tortuous arterial access we used a double-microwire technique at the early stage and an intermediate catheter at the late stage to facilitate the delivery system over the lesion.
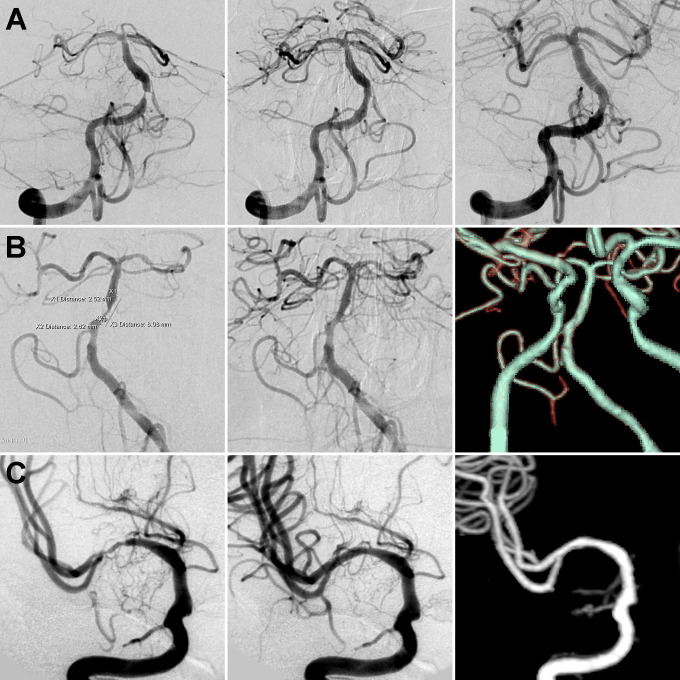
Figure 1.
(A) A patient presented with dizziness for 40 days. Digital subtraction angiography (DSA) showed a basilar arterial stenosis classified as Mori A type, which was treated with a 2.5 mm × 8 mm Apollo stent (MicroPort Neuro Tech, Shanghai, China). One-year DSA suggested no significant in-stent restenosis. (B) A patient presented with dizziness for 48 days. A basilar arterial stenosis classified as Mori B type was treated with a 2.5 mm × 8 mm Apollo stent. One-year computed tomography angiography (CTA) suggested no significant in-stent restenosis. (C) A patient presented with weakness of the left limbs for 36 days. A right middle cerebral arterial stenosis classified as Mori C type was treated with a 2.5 mm × 13 mm Apollo stent. Five-year CTA suggested no significant in-stent restenosis.
Periprocedural management
Perioperative systolic blood pressure was kept between 100 and 120 mmHg during the procedure and for 3 days after the procedure. Non-contrast head CT was obtained to exclude hemorrhage after the procedure. All patients were given a weight-based dose of 0.4–0.6 mL nadroparin (Fraxiparine; Sanofi Winthrop Industry) every 12 hours subcutaneously for 3 days and monitored closely until discharge.
Medical treatment
All patients received aspirin (100 mg/day) and clopidogrel (75 mg/day) for more than 5 days before the procedure. Resistance testing for antiplatelet drugs was not performed. Other aggressive medical therapy was implemented to achieve the following goals: systolic blood pressure <140 mmHg (or <130 mm Hg in patients with diabetes mellitus), low-density lipoprotein <70 mg/dL (1.81 mmol/L) or a decrease by 50%, smoking cessation, lifestyle modification for obesity and sedentary state.
Clinical and radiological outcomes
Follow-up information on clinical outcomes was collected and reviewed by trained personnel who were blinded to treatment assignment at study entry, the day of discharge, 30-day follow-up, and face-to-face interview every 3 months. All follow-up visits were in person unless the patient could not return for a visit, in which case telephone follow-up was completed. If necessary, brain imaging studies including MR angiography (MRA) and CT angiography (CTA) were obtained in patients who developed neurological symptoms. DSA was recommended to patients at 12 months follow-up.
In this subgroup analysis we focused on the short-term outcomes including any stroke, ischemic stroke, hemorrhagic stroke, and death within 72 hours after stenting, and long-term outcomes including any stroke, ischemic stroke, hemorrhagic stroke, and death within 1 year. The definitions of ischemic stroke, hemorrhagic stroke, and TIA were the same as in the previous studies.19 20 27 For patients evaluated with DSA, in-stent restenosis (ISR) was defined as >70% stenosis within or immediately adjacent (within 5 mm) to the implanted stent (figure 2). For patients evaluated with CTA, the stents were considered as ISR if the stented segment or the proximal and distal parent vessel could not be well visualized or showed an apparent filling defect on CTA (figure 1).9
Figure 2.
(A) A patient presented with weakness of the right limbs for 73 days. A left middle cerebral arterial stenosis classified as Mori B type was treated with a 2.5 mm × 8 mm Apollo stent. An in-stent total occlusion was found on the 1-year DSA. (B) A patient presented with dizziness for 70 days. A basilar arterial stenosis classified as Mori C type was treated with a 2.5 mm × 13 mm Apollo stent. An in-stent total occlusion was found on the 1-year DSA.
Statistical analysis
Continuous variables were presented as mean±SD (normal distribution data) or median with interquartile range (skewed distribution data), as appropriate. Categorical variables were presented as number and percentage. The χ2 test was used to analyze differences between the present study and the WEAVE/WOVEN study and differences in ISR rates among patients with different Mori types of lesion and between patients with anterior and posterior circulation lesions. The statistical analysis was performed using a commercial statistical software package (SPSS for Windows, Version 25.0, IBM-SPSS, Chicago, Illinois, USA).
Results
Patient baseline characteristics
From September 2013 to January 2015 a total of 300 patients were recruited in 15 sites in China, including 159 patients treated with balloon-mounted stenting and 141 patients treated with balloon dilatation plus self-expanding stenting. In this study the 159 patients (aged 59.4±9.5 years) treated with balloon-mounted stenting (recruited in 14 sites) were analyzed, including 120 (75.5%) men and 39 (24.5%) women. Of the 159 patients, 110 (69.2%) had hypertension, 53 (33.3%) had diabetes mellitus, 60 (37.7%) had hyperlipidemia, and 45 (28.3%) were current smokers. The treated lesions were located in the ICA in 25 cases (15.7%), MCA in 38 cases (23.9%), VA in 48 cases (30.2%), and BA in 48 cases (30.2%). The main qualifying event included stroke in 52.8% and TIA in 47.2% of the patients. The median time to stenting from the qualifying event was 20 days (12 days for TIA and 26 days for stroke). The baseline characteristics are shown in table 2.
Table 2.
Demographic and clinical characteristics
| Variable | Present study (n=159) |
WEAVE/WOVEN (n=152) |
P value |
| Age, mean±SD | 59.4±9.5 | 61.9±10.5 | – |
| Female, n (%) | 39 (24.5%) | 71 (46.7%) | <0.001 |
| Hypertension, n (%) | 110 (69.2%) | 140 (92.1%) | <0.001 |
| Diabetes mellitus, n (%) | 53 (33.3%) | 91 (59.9%) | <0.001 |
| Hyperlipidemia, n (%) | 60 (37.7%) | 131 (86.2%) | <0.001 |
| Smoking, n (%) | 0.004 | ||
| Current smoker | 45 (28.3%) | 21 (13.8%) | |
| Previous smoker | 43 (27.0%) | 59 (38.8%) | |
| Never smoked | 71 (44.7%) | 72 (47.4%) | |
| BMI, mean±SD | 25.66±3.14 | 30.93±7.03 | – |
| Lesion distribution | <0.001 | ||
| Anterior circulation | 63 (39.6%) | 102 (65.0%) | |
| Posterior circulation | 96 (60.4%) | 55 (35.0%) | |
| Target artery distribution | <0.001 | ||
| ICA | 25 (15.7%) | 40 (25.5%) | |
| MCA | 38 (23.9%) | 62 (39.5%) | |
| VA | 48 (30.2%) | 32 (20.4%) | |
| BA | 48 (30.2%) | 22 (14.0%) | |
| PCA | 0 (0.0%) | 1 (0.6%) | |
| Qualifying ischemic events | <0.001 | ||
| TIA | 75 (47.2%) | 0 (0.0%) | |
| Ischemic stroke | 84 (52.8%) | 152 (100.0%) | |
| Qualifying events on the basis of antiplatelet therapy, n (%) | 75 (47.2%) | – | – |
| Time to stent from qualifying event (days), median (IQR) | 20 (9–34) | 22 | – |
| Baseline NIHSS, median (IQR) | 0 (0–2) | – | – |
| Baseline mRS, n (%) | <0.001 | ||
| 0 | 96 (60.4%) | 20 (13.2%) | |
| 1 | 48 (30.2%) | 37 (24.3%) | |
| 2 | 15 (9.4%) | 52 (34.2%) | |
| 3 | 0 (0.0%) | 43 (28.3%) |
BA, basilar artery; BMI, body mass index; ICA, internal carotid artery; MCA, middle cerebral artery; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; PCA, posterior cerebral artery; TIA, transient ischemic attack; VA, vertebral artery.
Periprocedural characteristics
Of the 159 patients, 88 (55.3%) were operated under general anesthesia and 71 (44.7%) under local anesthesia. The proportions of Mori A, Mori B, and Mori C lesions were 33.3% (53/159), 52.2% (83/159), and 14.5% (23/159), respectively. The mean percent stenosis before and after stenting was 84.0% and 6.1%, respectively, and the median percent stenosis before and after stenting was 85.0% and 5.0%, respectively. The mean stent diameter and stent length were 2.8±0.4 mm and 10.2±2.9 mm, respectively, and the median stent diameter and stent length were 2.5 mm and 8.0 mm, respectively. The technical procedural characteristics are shown in table 3.
Table 3.
Periprocedural and technical characteristics
| Variable | Present study | WEAVE/WOVEN | P value |
| Type of anesthesia, n (%) | <0.001 | ||
| General anesthesia | 88 (55.3%) | 148 (97.4%) | |
| Local anesthesia | 71 (44.7%) | 4 (2.6%) | |
| Mori types, n (%) | – | ||
| Mori A | 53 (33.3%) | – | |
| Mori B | 83 (52.2%) | – | |
| Mori C | 23 (14.5%) | – | |
| Percent (%) stenosis baseline | – | ||
| Mean±SD | 84.0±7.4 | 83.2±8.3 | |
| Median (IQR) | 85.0 (80.0–90.0) | 82.0 (77.0–91.0) | |
| Range (min, max) | (70.0, 99.0) | (40.0, 97.0) | |
| Percent (%) stenosis after stenting | – | ||
| Mean±SD | 6.1±7.0 | 28.3±16.9 | |
| Median (IQR) | 5.0 (0.0–10.0) | 27.5 (14.0–41.0) | |
| Range (min, max) | (0.0, 40.0) | (0.0, 84.0) | |
| Stent diameter (mm) | – | ||
| Mean±SD | 2.8±0.4 | – | |
| Median (IQR) | 2.5 (2.5–3.0) | – | |
| Range (min, max) | (2.5, 4.0) | – | |
| Stent length (mm) | – | ||
| Mean±SD | 10.2±2.9 | – | |
| Median (IQR) | 8.0 (8.0–13.0) | – | |
| Range (min, max) | (8.0, 20.0) | – |
Clinical and radiological outcomes
In the present study the stroke rates at 72 hours, 30 days, and 1 year after the procedure were 0% (0/159), 2.5% (4/159), and 6.3% (10/159), respectively; the rates of ischemic stroke at 72 hours, 30 days, and 1 year after the procedure were 0% (0/159), 2.5% (4/159), and 5.7% (9/159), respectively; the rates of hemorrhagic stroke at 72 hours, 30 days, and 1 year after the procedure were 0% (0/159), 0% (0/159), and 0.6% (1/159), respectively; and the mortality rates at 72 hours, 30 days, and 1 year after the procedure were 0% (0/159), 0% (0/159), and 0.6% (1/159), respectively (table 4). Even though follow-up vascular imaging was not a requirement of the registry, 64 patients had follow-up DSA or CTA at 1 year. The 1-year rates of ISR and symptomatic ISR were 23.4% (15/64) and 3.1% (2/64), respectively (table 4). The rate of ISR in Mori C lesions (53.8%, 7/13) was significantly higher than that in Mori A (15.8%, 3/19) or Mori B lesions (15.6%, 5/32) (p=0.024). The rates of ISR in lesions of the anterior and posterior circulation were (26.7%, 4/15) and (22.4%, 11/49) respectively.
Table 4.
Clinical and radiological outcomes
| Present study | WEAVE/WOVEN | P value | |
| N | 159 | 152/129 | – |
| 72-hour any stroke | 0.0% (0/159) | 1.3% (2/152) | 0.458 |
| 72-hour ischemic stroke | 0.0% (0/159) | 1.3% (2/152) | 0.458 |
| 72-hour hemorrhagic stroke | 0.0% (0/159) | 0.0% (0/152) | – |
| 72-hour death | 0.0% (0/159) | 1.3% (2/152) | 0.458 |
| 30-day any stroke | 2.5% (4/159) | 1.3% (2/152) | 0.721 |
| 30-day ischemic stroke | 2.5% (4/159) | 1.3% (2/152) | 0.721 |
| 30-day hemorrhagic stroke | 0.0% (0/159) | 0.0% (0/152) | – |
| 30-day death | 0.0% (0/159) | 1.3% (2/152) | 0.458 |
| 1-year any stroke | 6.3% (10/159) | 8.5% (11/129) | 0.468 |
| 1-year ischemic stroke | 5.7% (9/159) | 8.5% (11/129) | 0.341 |
| 1-year hemorrhagic stroke | 0.6% (1/159) | 0.0% (0/129) | >0.999 |
| 1-year death | 0.6% (1/159) | 1.6% (2/129) | 0.855 |
| 1-year ISR | 23.4% (15/64) | 17.6% (18/102) | 0.363 |
| ISR in Mori A lesions | 15.8% (3/19) | – | – |
| ISR in Mori B lesions | 15.6% (5/32) | – | – |
| ISR in Mori C lesions | 53.8% (7/13) | – | – |
| ISR in anterior circulation | 26.7% (4/15) | 18.8% (13/69) | 0.742 |
| ISR in posterior circulation | 22.4% (11/49) | 15.2% (5/33) | 0.414 |
| 1-year symptomatic ISR | 3.1% (2/64) | 6.9% (7/102) | 0.495 |
| 1-year asymptomatic ISR | 20.3% (13/64) | 10.8% (11/102) | 0.089 |
ISR, in-stent restenosis.
Discussion
The VISSIT study reported high rates of perioperative complications (24.1%) and 1-year recurrent stroke or hard TIA (36.2%) for ICAS stenting using a balloon-mounted stent.11 We found that the short-term and long-term outcomes of balloon-mounted stenting for symptomatic ICAS with focal and non-angular lesions (Mori A and B type) and smooth arterial access were comparable to the results of the WEAVE/WOVEN trial.
Balloon-mounted stents have been used in symptomatic ICAS treatment since 2001. Initially, off-label coronary balloon-mounted stents (including bare stents and drug-eluting stents) were used, but the rigidity of the delivery system impeded their clinical application.14 15 28 The Apollo balloon-mounted stent was designed for treating ICAS lesions and has been approved for use in China since 2004. The Apollo stent system offers 40 different stents of various combinations of six different lengths (8, 10, 13, 15, 18, and 23 mm) and seven different diameters (2.5, 2.75, 3.0, 3.5, 4.0, 4.5, and 5.0 mm). Other parameters of the Apollo stent system are as follows: the metallic surface area is about 14.0%; the nominal expansion pressure is 6 atm; the length of the delivery system is 1450 mm; the maximum guide wire diameter is 0.014 inch; and the minimum guide catheter is 6 French. The delivery system has a longer distance from the tip to the rapid-exchange outlet (300 mm) than that of the coronary stent systems (usually about 250 mm). Moreover, the Apollo balloon material (Pebax 70D) is softer than that of typical coronary balloons (usually Pebax 72D or 74 D). These two features make the Apollo stent more flexible than typical coronary stent delivery systems. Several single-center studies have shown its safety and efficacy for patients with ICAS.14–18 29 These improved features of the Apollo stents could have contributed to the low complication rate compared with the Vitesse stents.
In this study the inclusion criteria in the degree of symptomatic stenosis (ranging from 70% to 99%), patient age (18–85 years vs 22–80 years), baseline mRS score (≤3), and time to stenting from last stroke (≥3 weeks vs ≥8 days) were similar to the WEAVE/WOVEN study. The median time to stenting from the qualifying event was comparable in the present study and the WEAVE/WOVEN study (20 days vs 22 days). However, there were several differences between the present study and the WEAVE/WOVEN study. First, in the WEAVE/WOVEN study all of the qualifying ischemic events were ischemic stroke, while in this study the qualifying ischemic events also included TIA (47.2%). The present study enrolled the patients with ischemic symptoms within the past 90 days due to hypoperfusion in the territory of the target lesion, while the WEAVE/WOVEN study enrolled the patients with a recurrent stroke in the territory of the same lesion after receiving medical therapy. Second, regarding atherosclerotic risk factors of the enrolled patients, in the WEAVE/WOVEN study there was a higher prevalence of hypertension, hyperlipidemia, and diabetes mellitus (p<0.001) but a lower proportion of smokers (p=0.004). Third, compared with the WEAVE/WOVEN study, the proportion of anterior circulation stenting (39.6% vs 55.0%, p<0.001) and baseline mRS ≥2 (9.4% vs 62.5%, p<0.001) were lower.
In this study, technical success was achieved in 100% (159/159) of the patients. The preprocedural mean percent stenosis was comparable to that in the WEAVE/WOVEN study (84.0% vs 83.2%) but the postprocedural mean percent stenosis was relatively lower than that in the WEAVE/WOVEN study (6.1% vs 28.3%), which indicated that the technical success achieved using the balloon-mounted stent was not inferior to the self-expanding Wingspan stent system.12 Jiang et al performed a multicenter analysis of stenting in symptomatic ICAS which suggested that the 30-day periprocedural stroke rate was 6.0% in patients treated with balloon-mounted stents.30 The proportion of Mori A type was 32.0% (147/454) in the balloon-mounted group, which is similar to that in the present study (33.3%, 53/159). It should be noted that their study also included patients in the acute phase of stroke, which may increase the risk of perioperative complications. In the present study, both the stroke rate and mortality rate within 72 hours after the procedure were 0% while, in the WEAVE study, both the stroke rate and mortality rate within 72 hours after the procedure were 1.3%. Compared with the results of the WEAVE study, the safety of balloon-mounted stenting for symptomatic ICAS was acceptable.
It should be noted that, in this study, the proportion of Mori A or Mori B lesions was relatively high (85.5%) whereas Mori C lesions accounted for only 14.5%. However, in the WEAVE/WOVEN study, the morphological features of the ICAS lesions were not described. Balloon-mounted stenting requires the stent and the balloon to be in place at the same time, so it is more suitable for lesions that are relatively simple in shape, short in length, and with approximate diameters in the distal and proximal vessels, such as Mori A or Mori B lesions.25 This study suggests that endovascular treatment for patients with symptomatic ICAS (especially Mori A or Mori B lesions with smooth arterial access) using a balloon-mounted stent was feasible and safe.
In the VISSIT study the 1-year primary outcome of stroke or hard TIA occurred in 36.2% (21/58) of the patients in the stent group. However, few studies have investigated the long-term outcomes after balloon-mounted stenting for symptomatic ICAS with experienced interventionalists and proper patient selection.17 18 The 1-year rates of any stroke, ischemic stroke, hemorrhagic stroke, and death were 8.5%, 8.5%, 0%, and 1.6% in the WOVEN study, and comparable values were found using balloon-mounted stents in the present study.13
In the present study the 1-year rate of ISR was 23.4% (15/64) with a follow-up rate of 40%, which was higher than that in the VISSIT study (2.9%, 1/34) with a follow-up rate of 58.6% and in the WOVEN study (17.6%, 18/102) with a follow-up rate of 79.1%.11 13 However, the 1-year rate of ISR in this study was similar to the 6-month rate of ISR in patients treated with balloon-mounted stents in the study by Jiang et al (20.3%, 60/295).30 The rate of ISR in Mori C lesions (53.8%, 7/13) was significantly higher than that in Mori A (15.8%, 3/19) or Mori B lesions (15.6%, 5/32), which has not been described in the VISSIT or WOVEN studies. The 1-year rate of symptomatic ISR was 3.1% (2/64) in this study, which might have been underestimated because only near half of the patients with recurrent stroke had available follow-up images (44.4%, 4/9). This study indicated that the long-term efficacy of endovascular treatment using balloon-mounted stents for symptomatic ICAS with focal and non-angular lesions (Mori A and B type) and smooth arterial access were comparable to the results of the WEAVE/WOVEN trial. Previous studies have indicated that the long length of ICAS lesions or stents was associated with an increased risk of ISR.31 32 In this study, the higher rate of ISR in Mori C lesions may also be related to the longer length of the ICAS lesions and the placed stents.
Recently, balloon-mounted stents (including the Apollo stent) and self-expanding stents have been reported to be used for acute intracranial large vessel occlusion due to presumed atherosclerotic disease.33–36 These studies suggest that intracranial stent implantation may be one of the rescue treatments for acute ischemic stroke due to ICAS, but its safety and efficacy require confirmation in future studies.
This study has several potential limitations. First, the defining criteria for hemodynamic impairment were heterogeneous. Four different imaging modalities were allowed to evaluate patients, which made a homogenous evaluation of subgroups of patients difficult. Second, as a 1-year angiogram was performed in only 40% of the recruited patients on a voluntary basis, potential selection bias may be inevitable, especially for evaluation of ISR. Third, the patients were all Chinese, so the results cannot be generalized to other ethnicities. Whether the results of this registry would stand examination by a randomized trial remains to be seen.
Conclusions
The short and long-term outcomes of balloon-mounted stenting for symptomatic ICAS with focal and non-angular lesions (Mori A and B type) and smooth arterial access were comparable to the results of the WEAVE/WOVEN trial.
Acknowledgments
We thank all of the patients and healthcare providers who participated in this study.
Footnotes
Collaborators: The list of Study Group of Registry Study of Stenting for Symptomatic Intracranial Artery Stenosis in China: Ligang Song, Xuan Sun, Lian Liu, Xiaotong Xu, Xiaoqing Li, Xingquan Zhao, Liping Liu, Kehui Dong, Peiyi Gao, Jiong Zhan, Shaowu Li, Xianjun Zhang, Zhirong Zhang, Xiaojun Ji, Xiaohua Pan, Tianyou Zhang, Xia Li, Shugui Shi, Zhenghua Zhou, Yi An, Zhijun Dong, Xiaomei Gao, Jun Wang, Zhiqiang Zhang, Guozhen Zhang, Shaoju Shao, Xiongjun He, Kaifeng Li, Liang Zhang, Yongsheng Liu, Ke Li, Ruiyan Li, Minghui Chen, Yan Feng, Muke Zhou, Fayun Hu
Contributors: NM and ZM had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: YL, YW, KK, NM, and ZM. Acquisition of clinical data: KK, NM, YZ, JS, CJ, QZ, KC, LL, BL, XS, LG, YL, FW, YL, TL, HZ, DM, FG, and the Study Group of Registry Study of Stenting for Symptomatic Intracranial Artery Stenosis in China. Analysis and interpretation of data: KK, LF, NM, and ZM. Drafting of the manuscript: KK. Critical revision of the manuscript for important intellectual content: NM and ZM. Statistical analysis: KK and NM.
Funding: This work was supported by the National Natural Science Foundation of China (81371290), Beijing High-level Personnel Funds (2013-2-019). This study is also funded by the National Science and Technology Support Program of ‘The 12th Five-Year Plan’ of the Ministry of Science and Technology (2011BAI08B02).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Liu L, Wang D, Wong KSL, et al. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke 2011;42:3651–4. 10.1161/STROKEAHA.111.635755 [DOI] [PubMed] [Google Scholar]
- 2.Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet 2019;394:1145–58. 10.1016/S0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redon J, Olsen MH, Cooper RS, et al. Stroke mortality and trends from 1990 to 2006 in 39 countries from Europe and central Asia: implications for control of high blood pressure. Eur Heart J 2011;32:1424–31. 10.1093/eurheartj/ehr045 [DOI] [PubMed] [Google Scholar]
- 4.Wong KS, Li H, Lam WWM, et al. Progression of middle cerebral artery occlusive disease and its relationship with further vascular events after stroke. Stroke 2002;33:532–6. 10.1161/hs0202.102602 [DOI] [PubMed] [Google Scholar]
- 5.Ssi-Yan-Kai G, Nasr N, Faury A, et al. Intracranial artery stenosis or occlusion predicts ischemic recurrence after transient ischemic attack. AJNR Am J Neuroradiol 2013;34:185–90. 10.3174/ajnr.A3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol 2013;12:1106–14. 10.1016/S1474-4422(13)70195-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993–1003. 10.1056/NEJMoa1105335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaidat OO, Klucznik R, Alexander MJ, et al. The NIH registry on use of the Wingspan stent for symptomatic 70-99% intracranial arterial stenosis. Neurology 2008;70:1518–24. 10.1212/01.wnl.0000306308.08229.a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiorella DJ, Turk AS, Levy EI, et al. U.S. Wingspan registry: 12-month follow-up results. Stroke 2011;42:1976–81. 10.1161/STROKEAHA.111.613877 [DOI] [PubMed] [Google Scholar]
- 10.Bose A, Hartmann M, Henkes H, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke 2007;38:1531–7. 10.1161/STROKEAHA.106.477711 [DOI] [PubMed] [Google Scholar]
- 11.Zaidat OO, Fitzsimmons B-F, Woodward BK, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA 2015;313:1240–8. 10.1001/jama.2015.1693 [DOI] [PubMed] [Google Scholar]
- 12.Alexander MJ, Zauner A, Chaloupka JC, et al. WEAVE trial: final results in 152 on-label patients. Stroke 2019;50:889–94. 10.1161/STROKEAHA.118.023996 [DOI] [PubMed] [Google Scholar]
- 13.Alexander MJ, Zauner A, Gupta R, et al. The WOVEN trial: Wingspan one-year vascular events and neurologic outcomes. J Neurointerv Surg 2020. 10.1136/neurintsurg-2020-016208. [Epub ahead of print: 19 Jun 2020]. [DOI] [PubMed] [Google Scholar]
- 14.Yu W, Jiang W-J. Stenting for intracranial stenosis: potential future for the prevention of disabling or fatal stroke. Stroke Vasc Neurol 2018;3:140–6. 10.1136/svn-2018-000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang WJ, Xu XT, Du B, et al. Comparison of elective stenting of severe vs moderate intracranial atherosclerotic stenosis. Neurology 2007;68:420–6. 10.1212/01.wnl.0000252939.60764.8e [DOI] [PubMed] [Google Scholar]
- 16.Jiang W-J, Xu X-T, Jin M, et al. Apollo stent for symptomatic atherosclerotic intracranial stenosis: study results. AJNR Am J Neuroradiol 2007;28:830–4. [PMC free article] [PubMed] [Google Scholar]
- 17.Tang L, Hu P, Liu Y, et al. The experiences of balloon-expandable stent in symptomatic stenosis of middle cerebral artery. Springerplus 2016;5:1413. 10.1186/s40064-016-3078-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang L, Wang L, Li C, et al. Treatment of basilar artery stenosis with an Apollo balloon-expandable stent: a single-centre experience with 61 consecutive cases. Acta Neurol Belg 2020;371. 10.1007/s13760-020-01311-8. [Epub ahead of print: 25 Feb 2020]. [DOI] [PubMed] [Google Scholar]
- 19.Miao Z, Zhang Y, Shuai J, et al. Thirty-day outcome of a multicenter registry study of stenting for symptomatic intracranial artery stenosis in China. Stroke 2015;46:2822–9. 10.1161/STROKEAHA.115.010549 [DOI] [PubMed] [Google Scholar]
- 20.Ma N, Zhang Y, Shuai J, et al. Stenting for symptomatic intracranial arterial stenosis in China: 1-year outcome of a multicentre registry study. Stroke Vasc Neurol 2018;3:176–84. 10.1136/svn-2017-000137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Miao Z, Wang Y, et al. Protocol for a prospective, multicentre registry study of stenting for symptomatic intracranial artery stenosis in China. BMJ Open 2014;4:e005175. 10.1136/bmjopen-2014-005175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samuels OB, Joseph GJ, Lynn MJ, et al. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol 2000;21:643–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intraarterial cerebral thrombolysis for acute ischemic stroke. J Vasc Interv Radiol 2003;14:E1–31. 10.1016/S1051-0443(07)60431-X [DOI] [PubMed] [Google Scholar]
- 24.Shuaib A, Butcher K, Mohammad AA, et al. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol 2011;10:909–21. 10.1016/S1474-4422(11)70195-8 [DOI] [PubMed] [Google Scholar]
- 25.Mori T, Fukuoka M, Kazita K, et al. Follow-up study after intracranial percutaneous transluminal cerebral balloon angioplasty. AJNR Am J Neuroradiol 1998;19:1525–33. [PMC free article] [PubMed] [Google Scholar]
- 26.Mori T, Mori K, Fukuoka M, et al. Percutaneous transluminal cerebral angioplasty: serial angiographic follow-up after successful dilatation. Neuroradiology 1997;39:111–6. 10.1007/s002340050376 [DOI] [PubMed] [Google Scholar]
- 27.Miao Z, Song L, Liebeskind DS, et al. Outcomes of tailored angioplasty and/or stenting for symptomatic intracranial atherosclerosis: a prospective cohort study after SAMMPRIS. J Neurointerv Surg 2015;7:331–5. 10.1136/neurintsurg-2014-011109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qureshi AI, Kirmani JF, Hussein HM, et al. Early and intermediate-term outcomes with drug-eluting stents in high-risk patients with symptomatic intracranial stenosis. Neurosurgery 2006;59:1044–51. 10.1227/01.NEU.0000245593.54204.99 [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Rajah GB, Liu P, et al. Balloon-mounted versus self-expanding stents for symptomatic intracranial vertebrobasilar artery stenosis combined with poor collaterals. Neurol Res 2019;41:704–13. 10.1080/01616412.2019.1610837 [DOI] [PubMed] [Google Scholar]
- 30.Jiang W-J, Cheng-Ching E, Abou-Chebl A, et al. Multicenter analysis of stenting in symptomatic intracranial atherosclerosis. Neurosurgery 2012;70:25–31. 10.1227/NEU.0b013e31822d274d [DOI] [PubMed] [Google Scholar]
- 31.Zhu SG, Zhang RL, Liu WH, et al. Predictive factors for in-stent restenosis after balloon-mounted stent placement for symptomatic intracranial atherosclerosis. Eur J Vasc Endovasc Surg 2010;40:499–506. 10.1016/j.ejvs.2010.05.007 [DOI] [PubMed] [Google Scholar]
- 32.Zheng D, Mingyue Z, Wei S, et al. The incidence and risk factors of in-stent restenosis for vertebrobasilar artery stenting. World Neurosurg 2018;110:e937–41. 10.1016/j.wneu.2017.11.112 [DOI] [PubMed] [Google Scholar]
- 33.Gross BA, Desai SM, Walker G, et al. Balloon-mounted stents for acute intracranial large vessel occlusion secondary to presumed atherosclerotic disease: evolution in an era of supple intermediate catheters. J Neurointerv Surg 2019;11:975–8. 10.1136/neurintsurg-2019-014877 [DOI] [PubMed] [Google Scholar]
- 34.Wareham J, Flood R, Phan K, et al. A systematic review and meta-analysis of observational evidence for the use of bailout self-expandable stents following failed anterior circulation stroke thrombectomy. J Neurointerv Surg 2019;11:675–82. 10.1136/neurintsurg-2018-014459 [DOI] [PubMed] [Google Scholar]
- 35.Yang D, Lin M, Wang S, et al. Primary angioplasty and stenting may be superior to thrombectomy for acute atherosclerotic large-artery occlusion. Interv Neuroradiol 2018;24:412–20. 10.1177/1591019918763380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jia B, Feng L, Liebeskind DS, et al. Mechanical thrombectomy and rescue therapy for intracranial large artery occlusion with underlying atherosclerosis. J Neurointerv Surg 2018;10:746–50. 10.1136/neurintsurg-2017-013489 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author upon reasonable request.