Abstract
Background
The World Trade Center (WTC) attacks on 11 September 2001 created a hazardous environment with known and suspected carcinogens. Previous studies have identified an increased risk of prostate cancer in responder cohorts compared with the general male population.
Objectives
To estimate the length of time to prostate cancer among WTC rescue/recovery workers by determining specific time periods during which the risk was significantly elevated.
Methods
Person-time accruals began 6 months after enrolment into a WTC cohort and ended at death or 12/31/2015. Cancer data were obtained through linkages with 13 state cancer registries. New York State was the comparison population. We used Poisson regression to estimate hazard ratios and 95% CIs; change points in rate ratios were estimated using profile likelihood.
Results
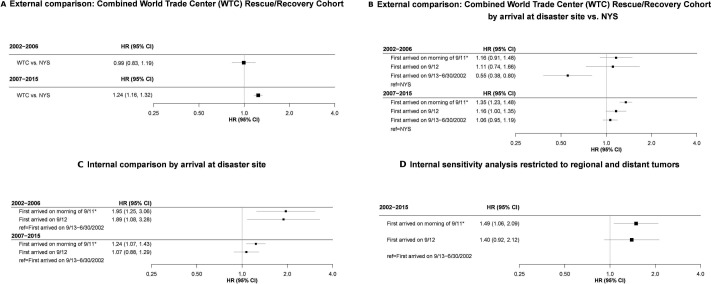
The analytic cohort included 54 394 male rescue/recovery workers. We observed 1120 incident prostate cancer cases. During 2002–2006, no association with WTC exposure was detected. Beginning in 2007, a 24% increased risk (HR: 1.24, 95% CI 1.16 to 1.32) was observed among WTC rescue/recovery workers when compared with New York State. Comparing those who arrived earliest at the disaster site on the morning of 11 September 2001 or any time on 12 September 2001 to those who first arrived later, we observed a positive, monotonic, dose-response association in the early (2002–2006) and late (2007–2015) periods.
Conclusions
Risk of prostate cancer was significantly elevated beginning in 2007 in the WTC combined rescue/recovery cohort. While unique exposures at the disaster site might have contributed to the observed effect, screening practices including routine prostate specific antigen screening cannot be discounted.
Keywords: materials, exposures or occupational groups, medical oncology, longitudinal studies, environmental exposure, risk assessment
Key messages.
What is already known about this subject?
Prior studies have identified an increased risk of prostate cancer among World Trade Center exposed rescue/recovery workers.
What are the new findings?
Just over 5 years after the World Trade Center attacks, prostate cancer incidence was elevated among rescue/recovery workers, demonstrating a shorter period from occupational exposure to disease onset when compared with other non-World Trade Center research.
How might this impact on policy or clinical practice in the foreseeable future?
It increases the understanding of long-term consequences of World Trade Center exposures, informs surveillance efforts for future environmental disasters and may stimulate further research into environmental risk factors for cancer in this and other cohorts.
Introduction
The period between potential exposure to carcinogens and the development of prostate cancer is of interest from both clinical and public health perspectives. The exact amount of time between an exposure and the development of cancer in an individual is dependent on many factors including the type and dose of exposure. While estimates vary considerably, for prostate cancer, studies have demonstrated it can take between 10 and 20 years following exposure for cancers to be diagnosed.1 2 These findings, however, are based on outdated reviews of evidence and do not account for advancements in screening.3 4 This is particularly challenging given the high sensitivity of prostate-specific antigen (PSA) testing, and prolonged lead time of disease which has resulted in early detection of presymptomatic individuals.5–7
Recent studies have identified an increased incidence of prostate cancer among World Trade Center (WTC)-exposed responders (rescue/recovery workers) when compared with the general male population.8–11 However, the length of time between exposure to the toxic environment at the disaster site beginning on 11 September 2001 (11 September) and onset of cancer remains unknown. Carcinogens known to be present in relatively high quantities at the primary WTC site included asbestos, benzene, chromium, dioxins and polychlorinated biphenyls.12–14 Some have suggested that these contaminants may increase the likelihood of prostate cancer and expedite oncogenesis,15 16 however, this result was not found by all.17 Building on this hypothesis, one study which compared inflammatory gene expression among WTC and non-WTC-exposed prostate cancer patients found a higher differential expression of genes related to damage to DNA repair pathways and glycolysis among tissue samples of WTC-exposed patients.18 The study also found a Th17 inflammatory response in the prostate tissue of rats exposed to WTC dust.
The current prospective cohort study will expand on previous research revealing an increased incidence of prostate cancer among WTC-exposed workers by combining three WTC-exposed responder cohorts (the Fire Department of the City of New York (FDNY),19 the General Responder Cohort (GRC),20 and the World Trade Center Health Registry (WTCHR)21) to provide the statistical power needed to explore the length of time to prostate cancer diagnosis using piecewise exponential change point models. This methodology has been previously used in studies of respiratory diseases in WTC-exposed subjects22 23 and in other research.24 The study aim was to determine the average time interval between 11 September exposure and observed increases in prostate cancer incidence, taking into account changes expected from ageing and secular trends in New York State (NYS). The amount of time from WTC exposure to the diagnosis of prostate cancer was estimated by determining the specific time periods between 2002 and 2015 during which the risk was significantly elevated compared with the NYS rate.
Methods
Study population
The source population for this study included rescue/recovery workers (n=69 102) from three WTC-exposed responder cohorts: the FDNY,19 the GRC,20 and the WTCHR.21 Responders included firefighters, emergency medical service providers, police, construction and communication workers, volunteers and cleanup workers. While the WTCHR also includes persons who resided, went to school, or worked (in non-rescue/recovery positions) at the WTC disaster site, this study included only rescue/recovery workers. Additional details regarding consolidation of the WTC combined rescue/recovery cohort and data harmonisation have been described elsewhere.25 To avoid selection bias, persons who enrolled into any of the cohorts after October 1, 2012, when cancer coverage began under the James Zadroga 9/11 Health and Compensation Act,26 were excluded from all analyses. Under the provisions of this enactment, WTC Health Programme (WTCHP) members have access to diagnostic procedures and treatment for numerous cancers and thus, to avoid differential self-selection into the cohort were removed. Figure 1A is a flow diagram of study participants for the WTC combined rescue recovery cohort (n=54 394), enumerating the exclusionary criteria.
Figure 1.
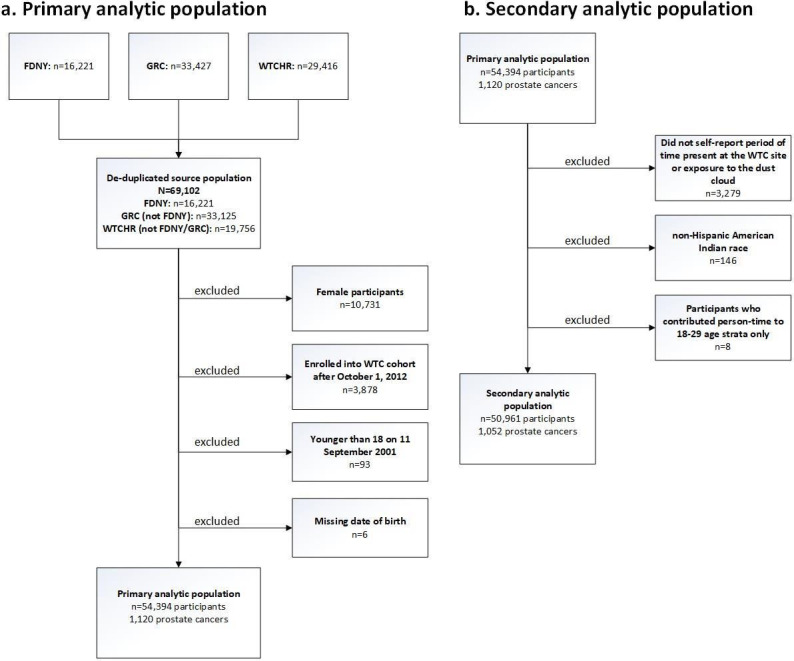
Flow of study participants. FDNY, Fire department of the City of New York; GRC, General Responder Cohort; WTCHR, World Trade Centre Health Registry.
Outcome assessment
Prostate cancer cases defined as ICD-O-3 site C619 with malignant behaviour code 3, using the Surveillance, Epidemiology and End Results (SEER) site recode table, were obtained by matching the WTC combined rescue/recovery cohort to data from 13 state cancer registries. These states included Arizona, California, Connecticut, Florida, Massachusetts, New Jersey, New York, North Carolina, Ohio, Pennsylvania, Texas, Virginia, and Washington. We have examined out-migration patterns from New York City based on US Census Bureau American Community Survey data, a summary by the New York City Independent Budget Office,27 and our records for the rescue/recovery workers, and have concluded that the 13 registries resulted in approximately 90% coverage of the population. Information on tumour characteristics such as diagnosis date and stage were also provided by state cancer registries. Cancer cases obtained from multiple states registries for the same participant were reconciled and included as one case. Cancers diagnosed within 6 months of enrolment into any of the responder cohorts were excluded from all analyses to ensure that prevalent cases were removed and to account for potential self-selection bias (i.e., selectively enrolling into a WTCHP cohort to receive augmented cancer coverage).
Exposure measures and demographic characteristics
Researchers from the three participating cohorts developed common exposure metrics to ensure that results from collaborative studies, such as this one, be comparable. The exposure measure for our primary external analysis was presence at the WTC site, or WTC rescue/recovery work at other sites such as the Fresh Kills Landfill in Staten Island, at any time from 11 September 2001 to 30 June 2002, the time when work on the recovery effort ended. We also used a three-level exposure variable according to self-reported first arrival at a WTC disaster site: on 11 September 2001 or being caught in the dust cloud resulting from the collapse of the towers on the morning of the attacks; or on 12 September 2001; or on any other time from 13 September 2001 to 30 June 2002. Some of the hazardous substances found to be present at the disaster site are known carcinogenic agents including, but not limited to, asbestos, sulfuric acid, benzo(a)pyrene, benzene, and arsenic,13 28 29 which we have posited were most ubiquitous on 11 September 2001, and gradually dissipated thereafter. Basic demographic characteristics such as sex, birth month, birth year, race/ethnicity, and smoking status were available in each cohort. Since only birth month and year were available for some study participants, the 15th of each month was used to calculate age. When both birth month and day were missing, 15 June of the birth year was used. Death dates were obtained by each cohort and duplicate patients as well as discordant dates of enrolment, diagnosis and death were resolved by the New York State Cancer Registry to ensure accurate ascertainment and person-time contributions. Death ascertainment by cancer registries is commensurate with data from the National Death Index. In a subset of our data, we looked at employment and pension records, and observed excellent completeness of mortality matches.
NYS comparison population
NYS population rates were used for the comparison population for our primary external analysis and were calculated using SEER*Stat software. Data were summarised in strata of person-time and cases by race/ethnicity (non-Hispanic White, non-Hispanic Black, non-Hispanic Asian/Pacific Islander, non-Hispanic American Indian and Hispanic), age (18–19, and 20 to 85+ in 5-year increments) and calendar year (2002–2015).
Statistical analyses
The outcome for all multivariable analyses was incident prostate cancer. Both comparison rates and observed cancer counts included multiple primary cancers. That is, every identified prostate cancer was counted as a case regardless of a patient’s cancer history (other than prostate). WTC combined rescue/recovery cohort data were grouped in strata of person-time and cases in the same way as the NYS comparison population. Person-time accruals began 6 months after enrolment into a WTC rescue/recovery cohort. This date was chosen so that prevalent cancers which had likely developed prior to 11 September 2001 were not misclassified as incident cases. The follow-up period ended at the earlier of date of death and 31 December 2015.
We used piecewise exponential survival models to estimate hazard ratios (HRs) and associated 95% CIs. These models are similar to Cox regression models, but they allow the baseline hazard to change at numerous specified time intervals rather than with every event; we used 1-year time intervals. The models also allow for incidence to be estimated in the reference group. The HRs also have rate ratio interpretations. These rate ratios were allowed to vary over follow-up using change points. Specifically, these change point analyses were conducted to estimate the yearly intervals for which relative incidences significantly changed from one period to the next.
We first fit a model with no change points which assumed constant incidence rates for both the WTC and NYS groups throughout the entire follow-up period. Then, we fit a series of models with one change point, allowing each model to change incidence rates at a different follow-up time, at yearly intervals, with change points varying from 2003 to 2014, resulting in a total of 12 possible models. The change points were estimated using profile likelihood and we reported estimates and 95% CIs for the HRs which have relative rate interpretation.24 30–32 Best fitting models were selected using likelihood ratio tests. The same process was used for models comparing two change points to one change point. In each case the combination of change points was run in separate models and then compared by profile likelihood. The specific model was
Yik is the number of incident cases of prostate cancer, modelled to follow a Poisson distribution given the covariates, Tik is the total person time at risk, each for a particular stratum i and time interval k, and tik is the time since exposure. The exposures are indicated by the values of xi , a binary variable taking the value of 1 for the WTC-exposed cohort, and 0 for the comparison NYS general population, and zil’s represent demographic covariates age, and race/ethnicity. The wik’s are dummy variables representing the 1-year time intervals. βj is the log HR, also having log-relative-rate interpretation, comparing incidence in the WTC-exposed cohort to the NYS reference population for time interval j, which is the period between change points tj-1 and tj . The ak’s were parameters representing the baseline hazard as a function of time which has cases per person time interpretation. The equation is described in greater detail, elsewhere.23 This primary external analysis using NYS as a comparison controlled for race/ethnicity, age and calendar year.
Three secondary analyses were conducted. The first was an external analysis and also used NYS as the comparison population but employed time of arrival at the WTC site as the exposure variable. This analysis excluded participants who did not report either a specific period of time they were present at the WTC site or exposure to the dust cloud (n=3279). Additionally, non-Hispanic American Indian (n=146) participants were removed because of low power. Person-time for the 18–19, 20–24 and 25–29 age strata were excluded due to small numbers (n=8 participants, 1 prostate cancer) which led to unstable parameter estimates. The final restricted population included 50 961 participants for this secondary external analysis (figure 1B).
The second was an internal analysis that used the same exclusionary criteria but evaluated those who arrived at the WTC site between 13 September 2001 and 30 June 2002 as the referent category. This model controlled for smoking status at baseline (ever vs never) as well as the additional covariates used in the primary analysis. The same model equation applied to this internal analysis as to the primary analysis, except that there were three exposure categories (first arrival at WTC site on 11 September or caught in dust cloud; first arrived on 12 September; first arrived on 13 September 2001–30 June 2002). We also calculated adjusted baseline incidence rates stratified by exposure category. These graphs were created by including an interaction term between the exposure variable and calendar year, such that each group could vary with time. For this analysis, we applied a locally-weighted polynomial regression (LOESS)33 smoothing function for point estimates and associated 95% CI.
Third, we conducted several sensitivity analyses to address the potential for surveillance bias by restricting to late-stage prostate cancers (regional and distant tumours), only. That is, regional and distant tumours are defined as those cancers that have metastasised from beyond the primary tumour (i.e., prostate) to neighbouring and distant lymph nodes or organs, respectively. Categorisations were made using SEER Summary Staging Manual 2000 coding instructions.34 For each analysis, change points were evaluated, separately.
Analyses were conducted using SAS V.9.4 (SAS Institute) and R V.3.6.0.29. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines and was approved by IRB at Albert Einstein College of Medicine, New York City Department of Health and Mental Hygiene, the New York State Department of Health, and all thirteen cancer registries. The Icahn School of Medicine at Mount Sinai IRB ruled the research exempt. Data that support the findings of the study may be obtained from the corresponding author (CBH) on reasonable request after approval by the Steering Committee for ‘Incidence, Latency and Survival of Cancer Following World Trade Center Exposure’ (NIOSH Cooperative Agreement U01 OH011932) in accordance with the study’s official data sharing plan.
Results
The 54 394 participants in the final analytic cohort yielded 1120 prostate cancer cases in 1120 participants diagnosed between 12 March 2002 and 31 December 2015, with 573 512 person-years of follow-up. During the same period, and using the same inclusion criteria, 207 252 cases were diagnosed in the NYS comparison population, with 105 413 892 person-years of follow-up. Demographic characteristics for the analytic cohort are presented in table 1. The mean age at diagnosis among prostate cancer cases was 60.3 (SD=7.9) and ranged from 35 to 93 years old. Compared with WTC responders without prostate cancer, WTC responders with prostate cancer were significantly more likely to be non-Hispanic White (75.1% vs 71.7%; p<0.001) or non-Hispanic Black (17.4% vs 8.3%; p<0.001) and were significantly less likely to be Hispanic (7.0% vs 14.8%; p<0.001). There were significantly more ever smokers among prostate cancer cases when compared with persons without prostate cancer (46.9% vs 38.9%; p<0.001). Among persons with and without prostate cancer, 92 (8.2% of 1,120) and 2166 (4.1% of 53 274) had another (non-prostate) malignancy within the study period, respectively. Among persons with prostate cancer, the median (IQR) time from 11 September to diagnosis was 9.4 years (7.3, 12.1). The majority of cases were diagnosed between 2009–2015 (n=734; 66%).
Table 1.
Select demographic characteristics of WTC combined rescue and recovery cohort and other characteristics of prostate cancer cases
| Characteristics | No (%) of responders | ||
| Prostate cancer cases (n=1120) | Other rescue/recovery workersa (n=53 274) | Total (n=54 394) | |
| Age at study entry | |||
| 18–19 | 0 (0.00) | 28 (0.05) | 28 (0.05) |
| 20–24 | ≤5 (≤1.00) | 868 (1.63) | 869 (1.60) |
| 25–29 | 0 (0.00) | 3227 (6.06) | 3227 (5.93) |
| 30–34 | 12 (1.07) | 6714 (12.60) | 6726 (12.37) |
| 35–39 | 32 (2.86) | 10 109 (18.98) | 10 141 (18.64) |
| 40–44 | 127 (11.34) | 11 161 (20.95) | 11 288 (20.75) |
| 45–49 | 217 (19.38) | 8903 (16.71) | 9120 (16.77) |
| 50–54 | 233 (20.80) | 5661 (10.63) | 5894 (10.84) |
| 55–59 | 238 (21.25) | 3315 (6.22) | 3553 (6.53) |
| 60–64 | 161 (14.38) | 1757 (3.30) | 1918 (3.53) |
| 65–69 | 55 (4.91) | 854 (1.60) | 909 (1.67) |
| 70–74 | 34 (3.04) | 472 (0.89) | 506 (0.93) |
| 75–79 | 8 (0.71) | 159 (0.30) | 167 (0.31) |
| 80–84 | ≤5 (≤1.00) | 38 (0.07) | 40 (0.07) |
| 85+ | 0 (0.00) | 8 (0.02) | 8 (0.01) |
| Race/Ethnicity | |||
| Non-Hispanic White | 841 (75.09) | 38 171 (71.65) | 39 012 (71.72) |
| Non-Hispanic Black | 195 (17.41) | 4418 (8.29) | 4613 (8.48) |
| Non-Hispanic American Indian/Alaska Native | ≤5 (≤1.00) | 153 (0.29) | 154 (0.28) |
| Non-Hispanic Asian/Pacific Islander | ≤5 (≤1.00) | 922 (1.73) | 926 (1.70) |
| Hispanic | 78 (6.96) | 7900 (14.83) | 7978 (14.67) |
| Non-Hispanic, with unknown/unclassifiable race | ≤5 (≤1.00) | 1710 (3.21) | 1711 (3.15) |
| Smoking status at enrolment | |||
| Current | 142 (12.68) | 8372 (15.71) | 8514 (15.65) |
| Former | 383 (34.20) | 12 351 (23.18) | 12 734 (23.41) |
| Never | 570 (50.89) | 31 279 (58.71) | 31 849 (58.55) |
| Unknown/missing | 25 (2.23) | 1272 (2.39) | 1297 (2.38) |
| Vital status by the end of follow-up (12/31/2015) | |||
| Alive | 1076 (96.07) | 51 594 (96.85) | 52 670 (96.83) |
| Deceased | 44 (3.93) | 1680 (3.15) | 1724 (3.17) |
| First arrival at WTC site | |||
| 11/9/2001 | 515 (45.98) | 24 019 (45.09) | 24 534 (45.10) |
| 12/9/2001 | 189 (16.88) | 9541 (17.91) | 9730 (17.89) |
| 13/9/2001 to 30/6/2002 | 349 (31.16) | 16 496 (30.96) | 16 845 (30.97) |
| Not at WTC site | 37 (3.30) | 2593 (4.87) | 2630 (4.84) |
| Unknown | 30 (2.68) | 625 (1.17) | 655 (1.20) |
| Year of diagnosis | n (%) |
| 2002 | ≤5 (≤1.0) |
| 2003 | 12 (1.1) |
| 2004 | 23 (2.1) |
| 2005 | 42 (3.8) |
| 2006 | 42 (3.8) |
| 2007 | 70 (6.3) |
| 2008 | 82 (7.3) |
| 2009 | 110 (9.8) |
| 2010 | 120 (10.7) |
| 2011 | 123 (11.0) |
| 2012 | 121 (10.8) |
| 2013 | 136 (12.1) |
| 2014 | 119 (10.6) |
| 2015 | 115 (10.3) |
| Stage | |
| Localised | 867 (77.4) |
| Regional | 171 (15.3) |
| Distant | 28 (2.5) |
| Unknown | 54 (4.8) |
| Other descriptive statistics | Mean (SD) |
| Age at diagnosis (years) | 60.3 (7.9) |
| Age at end of follow-up (years) | 65.1 (7.8) |
| Length of follow-up (years) | 11.2 (2.5) |
a, Rescue/recovery workers that did not have a prostate cancer diagnosis in the study period; WTC, World Trade Center.
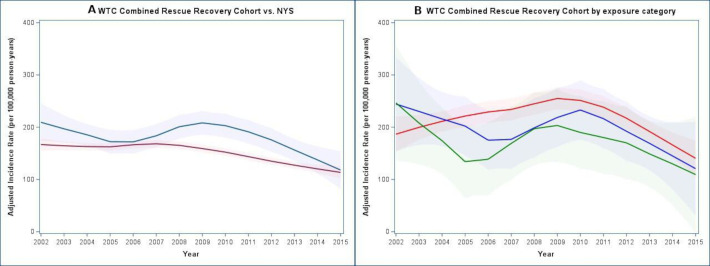
Figure 2 displays adjusted incidence rates for the study. Models were centred at non-Hispanic white participants age 50–54 for both graphs and additionally, never smokers in the secondary models. Figure 2A illustrates an increased adjusted incidence among the WTC combined rescue/recovery cohort coinciding with a decreased incidence among the NYS comparison rates beginning in 2006. From 2009–2015, prostate cancer incidence decreased for both groups. Similarly, figure 2A illustrates an increased incidence beginning in 2006 for all exposure categories, followed by a gradual decline from 2009 to 2015. A dose–response trend was observed; however, rates were not significantly different for most calendar years, individually. We estimated change points in 2006 for the primary external analysis (95% CI: 2006 to 2008) (figure 3A), as well as the secondary external (95% CI: 2006 to 2008) (figure 3B), and internal analyses (95% CI: 2006 to 2007) (figure 3C). The majority of cases had localised (n=867; 77%) tumours, 15.3% were regional (n=171) and 2.5% were distant (n=28). Our sensitivity analysis restricting to only regional and distant tumours yielded no significant change points. However, when evaluating HRs over the entire study period (2002–2015), rates were significantly elevated (figure 3D).
Figure 2.
Adjusted prostate cancer incidence rates. (a) WTC combined rescue recovery cohort versus NYS. WTC, NYS, comparison population; models are controlled for race/ethnicity and age; rates are centred at non-Hispanic white race/ethnicity and ages 50–54; rates are displayed per 100 000 person-years; blue lines: smoothed adjusting incidence curves for WTC combined rescue/recovery cohort; red lines: smoothed adjusting incidence curves for NYS comparison population. line shadows represent 95% CI. (b) WTC combined rescue recovery cohort by exposure category models are controlled for race/ethnicity and age; rates are centred at non-Hispanic white race/ethnicity and ages 50–54; rates are displayed per 100 000 person-years; red lines: smoothed adjusting incidence curves for point estimates for each year of WTC combined rescue/recovery cohort who first arrived at the WTC disaster site on 11 September or were caught in the dust cloud; blue lines: smoothed adjusting incidence curves for WTC combined rescue/recovery cohort who first arrived at the WTC disaster site on 12 September; green lines: smoothed adjusting incidence curves for WTC combined rescue/recovery cohort who first arrived at the WTC disaster site on 13 September – 30 June 2002. Line shadows represent 95% CIs. There were 515/24 478 (2.1%), 188/9692 (1.9%) and 349/16 791 (2.1%) cases among participants in the 11 September or dust cloud, 12 September and 13 September or later exposure categories, respectively. NYS, New York State; WTC, World Trade Centre.
Figure 3.
Change Point Models for Incident Prostate Cancer among World Trade Centre rescue/recovery workers: 12 March 2002–31 December 2005. ”9/11” = 11 September 2001. Best-fitting models for 3a, 3b and 3c all had one change point in 2006. All analyses control for age and race/ethnicity. (c) Also controls for ever-smoking. (a) Includes the entire Combined WTC rescue/recovery cohort (n=54 394). (b, c, d) Are restricted to those who self-reported an arrival time or dust-cloud exposure at the WTC sites, were at least 30 years old when diagnosed with prostate-cancer and were not non-Hispanic American Indian race/ethnicity (n=50 961). *Arrived on morning of 11 September or self-reported dust cloud exposure. NYS, New York State.
Discussion
In the current study, we observed an increased incidence of prostate cancer beginning 5.25 years postexposure among participants in the WTC combined rescue/recovery Cohort.25 Starting in 2007 and continuing through the end of the study period in 2015, we found a 24% increased risk of prostate cancer relative to NYS rates. In internal analyses, a dose–response trend was observed in both the early (2002–2006) and later (2007–2015) periods of follow-up, with the largest risk estimated in the early period. The increased hazard among those who responded to the disaster earliest or were caught in the dust cloud suggests that a high intensity of exposure may have played some role in premature oncogenesis. Relative to NYS, however, a lower relative risk was observed in the initial period, particularly among those responders who arrived at the WTC disaster site latest.
Earlier assessments among participants followed at the FDNY, GRC, and WTCHR have all independently established an elevated risk for prostate cancer.8–11 Our findings were in support of the previous studies, despite removing duplicate cases between the cohorts that could have artificially inflated risk estimates. This study combined the three cohorts, providing statistical power and identifying the specific period (2007–2015) when the risk appears to be greatest. We found the period from exposure to onset of cancer to be just over 5 years, shorter than prior non-WTC studies,1 35 which may reflect the carcinogenicity of exposures experienced at the main WTC disaster site. The finding of an internal positive, monotonic dose–response association during the early (2002–2006) and late (2007–2015) periods is noteworthy. This is especially relevant because certain occupational groups may be at additional risk of prostate cancer, irrespective of WTC exposure. In addition, after controlling for cigarette smoking, an important confounder and one of the few known behavioural risk factors linked to prostate cancer, the association was upheld for those who arrived at the disaster site earliest or were caught in the dust cloud. The results of our sensitivity analysis evaluating WTC-exposure and regional/distant tumours, argue against surveillance bias. We continued to observe a dose-response association despite the absence of a change point. This observation, in conjunction with our previous report of favourable survival outcomes among WTCHP participants, supports further use of this cohort for studying prostate cancer treatments and interventions.
Higher than general population rates of PSA screening among WTC-exposed responders may have played a role in the increase in prostate cancer incidence. It is noteworthy that although PSA testing is not a formal part of WTC medical monitoring examination, it is offered to older participants at FDNY exams. Moreover, disaster-related services including diagnostic evaluations for WTC-related cancers and treatment were added to the FDNY responders’ routine screening after 11 September, while other responders may be tested in other primary healthcare settings that offered comparable medical evaluation and treatment. Arguing against surveillance bias are prior studies which have demonstrated similar findings in WTC-exposed cohorts without known formal PSA testing.8 36 The findings of this combined cohort study are consistent with a study from the WTC-exposed GRC, which found: (1) an internal dose-response association using a well-defined exposure metric; (2) high rates of advanced stage tumours which argue against lead-time bias and (3) an association among young and old participants but no association among participants aged 55–69, a group which is historically screened most frequently.9 However, because we are unable to assess association between exposure and number of PSA monitoring visits, screening cannot be completely discounted for the observed effect.
This current study has several strengths. It is the first to analyse the time from exposure to onset of cancer, in detail, among WTC-exposed responders. In addition to our efforts to reduce the impacts of surveillance bias, another strength of this work is the rigorous efforts to address self-selection bias by removing cancers that occurred within 6 months of enrolment in a WTC cohort and by requiring enrolment prior to the initiation of cancer coverage in 2012.26 We also believe that this work will be of interest in the broader of context of disaster management, particularly when analysing time to cancer diagnosis among responders who attended signal events with similar exposures such as the Deepwater Horizon Oil explosion. This study used parametric survival modelling with change points to model the HR as a nonlinear function of time, similar to those used in non-WTC cancer research24 and in WTC-related respiratory research.22 23 In general, the amount of time for prostate cancer to develop in relation to carcinogens known to be present is understudied and thus, this work may begin to fill an important gap in the scientific literature. The use of relative rates rather than standardised incidence ratios avoids reliance on assumptions of proportional incidence across strata that may not be met. This method is also not unduly influenced by small strata.
Finally, a major strength of this study is the continued follow-up of three cohorts of WTC rescue/recovery workers, which has provided a unique opportunity to better understand time from exposure to cancer diagnoses in a human population after a time-delimited exposure to a broad spectrum of environmental carcinogens. The WTC combined rescue/recovery cohort allows for analytic flexibility by augmenting the statistical power needed for studying cancer in longitudinal survival analyses. Researchers from the cohorts following these workers had previously agreed on a common exposure definition37 and identical case ascertainment using thirteen state cancer registries. The NYS Cancer Registry served as an ‘honest broker’ by consolidating data from the three surveillance groups to ensure data security and confidentiality, harmonising data formats and deduplicating data from rescue/recovery workers followed by multiple cohorts so that they were counted once for analyses.25
This study is not without limitations. First, we cannot determine the extent to which other behavioural, occupational, and environmental exposures beyond cigarette smoking might have contributed to prostate cancer risk. Other studies of firefighters and police officers with no known exposure to the WTC disaster have also reported increased incidence compared with national rates.38–41 Unfortunately, we did not have access to information about years of employment or potentially important occupational exposures. Another limitation is the absence of PSA testing data on the WTC combined rescue/recovery cohort among those who were tested, although a study on GRC responders failed to find a significant association between frequency of screening exams and prostate cancer incidence.9 Additionally, while we were unable to explore alternate biological pathways for prostate tumorigenesis such as those mediated by stress,42 we believe that it is beyond the scope of this study but warrants further exploration in the future. Finally, the WTC combined rescue/recovery cohort was likely a healthy subset of the general population prior to exposure and resided mostly in the greater New York region, which limits generalisability of these findings to less healthy participants in other regions of the country.
In summary, we observed an increased incidence of prostate cancer beginning 5.25 years postexposure, substantially shorter than findings from non-WTC studies.1 35 A dose–response trend was observed in both the early (2002–2006) and later (2007–2015) periods of follow-up. Evidence suggests a relationship between WTC exposure and prostate cancer not fully explained by random or systematic error. This research may increase understanding of the period between environmental exposure and cancer incidence in human populations. It increases the understanding of long-term consequences of WTC-exposure, informs surveillance efforts for future environmental disasters and may stimulate further research into environmental risk factors for cancer in this and other cohorts. Our findings support the need for continued research evaluating the burden of prostate cancer in WTC responders.
Acknowledgments
We thank the 13 state cancer registries for carrying out record linkages: Bureau of Cancer Epidemiology, New York State Department of Health (DOH); Arizona Cancer Registry Department of Health Services (DOHS); California Cancer Registry, Department of Public Health (DPH); Connecticut Tumor Registry, Connecticut DPH; Florida Cancer Registry, Florida DOH; Massachusetts Cancer Registry, Massachusetts DPH; New Jersey State Cancer Registry, New Jersey DOH and Rutgers Cancer Institute of New Jersey; North Carolina Central Cancer Registry, State Center for Health Statistics; Ohio Cancer Incidence Surveillance System, Ohio DOH; Bureau of Health Statistics and Research, Pennsylvania DOH; Texas Cancer Registry, Texas Department of State Health Services, Virginia Cancer Registry, Virginia DOH, and Washington State Cancer Registry, Washington DOH. Additional Acknowledgements and Disclaimers from individual State Cancer Registries: The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP006344; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors. The Connecticut Department of Public Health Human Investigations Committee approved this research project, which used data obtained from the Connecticut Department of Public Health. The Connecticut Department of Public Health does not endorse or assume any responsibility for any analyses, interpretations or conclusions based on the data. The authors assume full responsibility for all such analyses, interpretations and conclusions. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (FCDS), the statewide cancer registry funded by the Florida Department of Health (DOH) and the Centers for Disease Control and Preventions National Program of Cancer Registries (CDC-NPCR). The views expressed herein are solely those of the author(s) and not necessarily reflect those of the DOH or CDC-NPCR. Cancer incidence data used in these analyses were obtained from the Ohio Cancer Incidence Surveillance System (OCISS), Ohio Department of Health (ODH), a cancer registry partially support by the National Program of Cancer Registries at the Centers for Disease Control and Prevention (CDC) through Cooperative Agreement Number NU58DP006284. Use of these data does not imply that ODH or CDC agrees or disagrees with the analyses, interpretations or conclusions in this report (or publication/presentation). These data were supplied by the Bureau of Health Statistics & Registries, Pennsylvania Department of Health, Harrisburg, Pennsylvania. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions.
Footnotes
Twitter: @CharlesBHallPhD
Contributors: The manuscript was conceptualised by: DG, RZ-O, DK, CBH and PB. The acquisition, analysis or interpretation of data for the work was done by: DG, RZ-O, DK, JL, RMB, MRF, JEC, CBH and PB. All authors had a role in drafting this work or revising it critically for important intellectual content.All authors have read and agreed for this version to be published.
Funding: This research was supported through the National Institute for Occupational Safety and Health (NIOSH) cooperative agreements (U01OH011315, U01 OH011932, U01 OH011681, U01 OH011931, U01 OH011480) and contracts (200-2011-39378, 200-2017-93325 and 200-2017-93326). This publication was supported by Cooperative Agreement Numbers U50/OH009739 from the National Institute for Occupational Safety and Health (NIOSH) of the Centers for Disease Control and Prevention (CDC); U50/ATU272750 from the Agency for Toxic Substances and Disease Registry (ATSDR), CDC, which included support from the National Center for Environmental Health, CDC; and by the New York City Department of Health and Mental Hygiene (NYC DOHMH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIOSH, CDC or the Department of Health and Human Services. This research was also supported by grant P30 CA013330 from the National Cancer Institute (NCI), NIH. National Cancer Institute (U58 DP006309).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request. Data that support the findings of the study may be obtained from the corresponding author (CBH) upon reasonable request after approval by the Steering Committee for 'Incidence, Latency and Survival of Cancer Following World Trade Center Exposure' (NIOSH Cooperative Agreement U01 OH011932) in accordance with the study’s official Data Sharing Plan.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
All responders who were members of any of the three cohorts and either provided informed consent for research or were covered under a research ethics board, or Institutional Review Board (IRB)-approved waiver of consent were eligible for inclusion in analyses. These include: the Albert Einstein College of Medicine/Montefiore Medical Center: IRB#2016-6692; the NYC Department of Health and Mental Hygiene: IRB #16-130; the NYS Department of Health: IRB #16-052; and the Icahn School of Medicine at Mt. Sinai: IRB#: 16-00814.
References
- 1.Haas GP, Delongchamps N, Brawley OW, et al. The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol 2008;15:3866–71. [PMC free article] [PubMed] [Google Scholar]
- 2.Bostwick DG. Prostatic intraepithelial neoplasia (PIN): current concepts. J Cell Biochem Suppl 1992;16H:10–19. 10.1002/jcb.240501205 [DOI] [PubMed] [Google Scholar]
- 3.Breslow NE, Day NE. Statistical methods in cancer research. Volume I - The analysis of case-control studies. IARC Sci Publ 1980;32:5–338. [PubMed] [Google Scholar]
- 4.Armenian HK, Lilienfeld AM. The distribution of incubation periods of neoplastic DISEASES1. Am J Epidemiol 1974;99:92–100. 10.1093/oxfordjournals.aje.a121599 [DOI] [PubMed] [Google Scholar]
- 5.Welch HG, Schwartz LM, Woloshin S. Are increasing 5-year survival rates evidence of success against cancer? JAMA 2000;283:2975–8. 10.1001/jama.283.22.2975 [DOI] [PubMed] [Google Scholar]
- 6.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst 2009;101:374–83. 10.1093/jnci/djp001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auvinen A, Määttänen L, Stenman U-H, et al. Lead-time in prostate cancer screening (Finland). Cancer Causes Control 2002;13:279–85. 10.1023/A:1015040231402 [DOI] [PubMed] [Google Scholar]
- 8.Shapiro MZ, Wallenstein SR, Dasaro CR. Cancer in general responders participating in world Trade center health programs, 2003–2013. JNCI Cancer Spectr 2020;4. 10.1093/jncics/pkz090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashim D, Boffetta P, Galsky M, et al. Prostate cancer characteristics in the world Trade center cohort, 2002-2013. Eur J Cancer Prev 2018;27:347–54. 10.1097/CEJ.0000000000000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boffetta P, Zeig-Owens R, Wallenstein S, et al. Cancer in world Trade center responders: findings from multiple cohorts and options for future study. Am J Ind Med 2016;59:96–105. 10.1002/ajim.22555 [DOI] [PubMed] [Google Scholar]
- 11.Zeig-Owens R, Webber MP, Hall CB, et al. Early assessment of cancer outcomes in New York City firefighters after the 9/11 attacks: an observational cohort study. Lancet 2011;378:898–905. 10.1016/S0140-6736(11)60989-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lioy PJ, Georgopoulos P. The anatomy of the exposures that occurred around the world Trade center site: 9/11 and beyond. Ann N Y Acad Sci 2006;1076:54–79. 10.1196/annals.1371.002 [DOI] [PubMed] [Google Scholar]
- 13.Lioy PJ, Weisel CP, Millette JR, et al. Characterization of the dust/smoke aerosol that settled East of the world Trade center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ Health Perspect 2002;110:703–14. 10.1289/ehp.02110703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claudio L. Environmental aftermath. Environ Health Perspect 2001;109:A528–36. 10.1289/ehp.109-a528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun XL, Kido T, Honma S, et al. The relationship between dioxins exposure and risk of prostate cancer with steroid hormone and age in Vietnamese men. Sci Total Environ 2017;595:842–8. 10.1016/j.scitotenv.2017.04.013 [DOI] [PubMed] [Google Scholar]
- 16.Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Ninth Biennial Update) . Board on the Health of Select Populations; Institute of Medicine. In: Veterans and agent orange. Washington, DC: National Academies Press, 2014. [Google Scholar]
- 17.Prins GS. Endocrine disruptors and prostate cancer risk. Endocr Relat Cancer 2008;15:649–56. 10.1677/ERC-08-0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong Y, Wang L, Yu H, et al. Prostate cancer in world Trade center responders demonstrates evidence of an inflammatory cascade. Mol Cancer Res 2019;17:1605–12. 10.1158/1541-7786.MCR-19-0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webber MP, Glaser MS, Weakley J, et al. Physician-diagnosed respiratory conditions and mental health symptoms 7-9 years following the world Trade center disaster. Am J Ind Med 2011;54:661–71. 10.1002/ajim.20993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbert R, Moline J, Skloot G, et al. The world Trade center disaster and the health of workers: five-year assessment of a unique medical screening program. Environ Health Perspect 2006;114:1853–8. 10.1289/ehp.9592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farfel M, DiGrande L, Brackbill R, et al. An overview of 9/11 experiences and respiratory and mental health conditions among world Trade center health registry enrollees. J Urban Health 2008;85:880–909. 10.1007/s11524-008-9317-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Yip J, Zeig-Owens R, et al. The effect of World Trade center exposure on the timing of diagnoses of obstructive airway disease, chronic rhinosinusitis, and gastroesophageal reflux disease. Front Public Health 2017;5:2. 10.3389/fpubh.2017.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glaser MS, Webber MP, Zeig-Owens R, et al. Estimating the time interval between exposure to the world Trade center disaster and incident diagnoses of obstructive airway disease. Am J Epidemiol 2014;180:272–9. 10.1093/aje/kwu137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodman MS, Li Y, Tiwari RC. Detecting multiple change points in piecewise constant hazard functions. J Appl Stat 2011;38:2523–32. 10.1080/02664763.2011.559209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brackbill RM, Kahn AR, Li J, et al. Combining three cohorts of World Trade center Rescue/Recovery workers for assessing cancer incidence and mortality. Int J Environ Res Public Health 2021;18. 10.3390/ijerph18041386. [Epub ahead of print: 03 02 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention . Covered Conditions - World Trade Center Health Program, 2020. Available: https://www.cdc.gov/wtc/conditions.html [Accessed 27 May 2020].
- 27.Office N. When new Yorkers move out of new York City where do they go? 2014.
- 28.NTP 12th Report on Carcinogens . Report on carcinogens : carcinogen profiles 2011;12:iii–499. [PubMed] [Google Scholar]
- 29.International Agency for Research on Cancer . IARC Monographs on the evaluation of carcinogenic risks to humans: painting, firefighting, and shiftwork. Lyon, France: IARC, 2010. [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen U, Lütkebohmert C. A Cox-type regression model with change-points in the covariates. Lifetime Data Anal 2008;14:267–85. 10.1007/s10985-008-9083-3 [DOI] [PubMed] [Google Scholar]
- 31.Hall CB, Lipton RB, Sliwinski M, et al. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer's disease. Stat Med 2000;19:1555–66. [DOI] [PubMed] [Google Scholar]
- 32.Hall CB, Ying J, Kuo L, et al. Bayesian and profile likelihood change point methods for modeling cognitive function over time. Comput Stat Data Anal 2003;42:91–109. 10.1016/S0167-9473(02)00148-2 [DOI] [Google Scholar]
- 33.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 1979;74:829–36. 10.1080/01621459.1979.10481038 [DOI] [Google Scholar]
- 34.Young JL, Roffers SD, Ries LAG. SEER Summary Staging Manual - 2000: Codes and Coding Instructions. Bethesda, MD: National Cancer Institute, NIH Pub, 2001: 1–4969. [Google Scholar]
- 35.Watanabe KK, Kang HK. Mortality patterns among Vietnam veterans: a 24-year retrospective analysis. J Occup Environ Med 1996;38:272–8. 10.1097/00043764-199603000-00012 [DOI] [PubMed] [Google Scholar]
- 36.Li J, Brackbill RM, Liao TS, et al. Ten-Year cancer incidence in rescue/recovery workers and civilians exposed to the September 11, 2001 terrorist attacks on the world Trade center. Am J Ind Med 2016;59:709–21. 10.1002/ajim.22638 [DOI] [PubMed] [Google Scholar]
- 37.Weakley J, Maslow C, WTC Working Group . Defining common categories of exposure among four cohorts of rescue/recovery workers who responded to the world Trade center disaster. Council of state and territorial epidemiologists (CSTE) annual conference. Pittsburgh, PA, 2011. [Google Scholar]
- 38.Lee DJ, Koru-Sengul T, Hernandez MN, et al. Cancer risk among career male and female Florida firefighters: evidence from the Florida Firefighter cancer registry (1981-2014). Am J Ind Med 2020;63:285–99. 10.1002/ajim.23086 [DOI] [PubMed] [Google Scholar]
- 39.Sritharan J, MacLeod J, Harris S, et al. Prostate cancer surveillance by occupation and industry: the Canadian census health and environment cohort (CanCHEC). Cancer Med 2018;7:1468–78. 10.1002/cam4.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniels RD, Kubale TL, Yiin JH, et al. Mortality and cancer incidence in a pooled cohort of US firefighters from San Francisco, Chicago and Philadelphia (1950-2009). Occup Environ Med 2014;71:388–97. 10.1136/oemed-2013-101662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demers PA, Checkoway H, Vaughan TL, et al. Cancer incidence among firefighters in Seattle and Tacoma, Washington (United States). Cancer Causes Control 1994;5:129–35. 10.1007/BF01830258 [DOI] [PubMed] [Google Scholar]
- 42.Clouston SAP, Kuan P, Kotov R, et al. Risk factors for incident prostate cancer in a cohort of World Trade center responders. BMC Psychiatry 2019;19:389. 10.1186/s12888-019-2383-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request. Data that support the findings of the study may be obtained from the corresponding author (CBH) upon reasonable request after approval by the Steering Committee for 'Incidence, Latency and Survival of Cancer Following World Trade Center Exposure' (NIOSH Cooperative Agreement U01 OH011932) in accordance with the study’s official Data Sharing Plan.