Abstract
Background
To date, the coronavirus disease 2019 (COVID-19) pandemic remains ongoing and continues to affect millions of people worldwide. In the effort of fighting this pandemic, there has been an increasing interest in the potential of traditional, complementary, and integrative medicines (TCIMs) in engaging COVID-19. This study presents a bibliometric analysis of the research trends of TCIMs for COVID-19.
Methods
Six databases were searched on July 15, 2021, to retrieve all the citations on TCIM-focused randomized controlled trials (RCTs) available on COVID-19. Only RCTs that mentioned at least one TCIMs for the treatment and/or management or COVID-19 were eligible. Data such as number and countries of trials conducted, publication journal, research focus, study design, and sample size were extracted for analysis.
Results
The resulting 56 articles were authored by 553 unique authors, and included 28 English articles, 19 Chinese articles with English abstracts, and 9 Chinese articles without English abstract. Analyses had shown that China was the dominant country with TCIM related RCT publications, followed by India and the United States. The included articles were published across 24 English journals and 22 Chinese journals with a wide range of impact factors from 0.220 to 56.272. The most commonly studied TCIM modalities included Chinese herbal decoction (n=12) and Chinese patent medicine (n=16). In terms of study designs, TCIM interventions were integrated with standard medicine across the trials with most trials having a small to medium sample size and open-labeled.
Conclusion
This bibliometric analysis of RCTs demonstrated the research trends and characteristics of TCIM utilized in COVID-19 research. Although there are still many research gaps and limitations for pandemic research, the publication of TCIM-focused RCTs is anticipated to show a continuously increasing trend.
Keywords: Bibliometric, Clinical trials, Coronavirus, Pandemic, Research trends, Review, Study designs
1. Introduction
As of August 3, 2021, coronavirus disease 2019 (COVID-19) has affected more than 198 million people worldwide.1 While appropriate precautions such as social distancing, staying home, wearing a mask, and frequent handwashing are continuously taken, the world remains fully engaged and continues its effort to tackle the ongoing pandemic. Being part of the effort, there has been a growth in the interest to include traditional, complementary, and integrative medicines (TCIMs) in treating or preventing COVID-19. TCIM includes traditional medicine indigenous to different amorphous rationalities, traditions, and cultures, as well as a broad range of health care practices and supplements that are yet to be integrated into the dominant health care system. Existing systematic reviews and meta-analyses with a focus on TCIMs for COVID-19 have shown positive effects on improving clinical symptoms and complementing standard medications.2, 3, 4 Additional evidence has also shown that TCIMs may strengthen physical and psychological resilience which is worth to be studied further.5, 6
Despite the considerable number of TCIM-related studies published on COVID-19, there have been fairly few efforts taken to systematically analyze and describe the trends of these researches. The assessment of publication trends is necessary to understand the quantity and quality of these TCIM-focused COVID-19 studies while identifying their research gaps and avenue for future research. A bibliometric approach is useful in quantitatively analyzing literature characteristics and publication trends, where its findings can be used to identify strengths and limitations in the field of research.
This bibliometric study aimed to analyze the publication trends in TCIM randomized controlled trials (RCTs) since the outbreak of this ongoing pandemic, including (1) quantity of RCT publications, (2) most productive countries based on where the trials were conducted, (3) most common journals with their subject categories and recent impact factors, (4) most cited articles, (5) most commonly used TCIM modalities, and (6) characteristics of study designs.
2. Methods
2.1. Search strategies
Six bibliographic databases including PubMed, Embase, Allied and Complementary Medicine Database (AMED), China National Knowledge Infrastructure (CNKI), Wanfang, Chinese Science and Technology Periodical Database (VIP) were searched for publications related to TCIM approach for COVID-19. PubMed is the key database among health science researchers as it comprises extensive citations for biomedical literature with free access and user-friendliness. As PubMed alone does not cover all relevant literature, Embase is used as a supplement to identify studies that are not indexed in PubMed. AMED, on the other hand, is a specialized database designed on alternative and allied therapies-related subjects. Being the country that first considered TCIM approach for COVID-19 and issued relevant national guidelines, China has widely studied TCIM for its effectiveness in the treatment and prevention of COVID-19.7 Therefore, 3 major Chinese databases were also included in the literature search. CNKI is the largest Chinese journal database with constant updates and free access to wide-ranging coverage of citations in various fields. As a supplement to CNKI, Wanfang which is currently indexed in EBSCO Information Services (EBSCO), and VIP which has the highest indexing of Chinese journals, are both preferred leading Chinese databases for the literature search of clinical researches.
The literature search was performed on July 15, 2021. The authors used Medical Subject Heading (MeSH) terms, title/abstract words, and author keywords in PubMed, and equivalent indexed subject headings and terms were used in the other databases. The list of TCIM therapies provided by the World Health Organization8 and National Center for Complementary and Integrative Health9 were also referred to extend the search coverage. The complement search strategies are provided in the supplement. In addition to the comprehensive database search, relevant systematic reviews with or without meta-analysis were manually screened for additional eligible RCTs.
2.2. Selection of studies
2.2.1. Eligibility criteria
Only randomized controlled trials (RCTs) relating to TCIM for the management and/or treatment of COVID-19 were included in this bibliometric analysis. RCTs mentioning COVID-19 but not TCIM, or vice versa, were excluded. Other clinical studies such as case-controlled trials, observational studies, and case series/reports were also excluded. Research protocols, methodological studies, reviews, dissertations, conference proceedings, and abstracts were not eligible. As the COVID-19 is an emergency in public health, preprints indexed in the aforementioned electronic databases were considered for inclusion.
2.2.2. Screening and selection process
Titles and abstracts screening were performed by one author using reference manager software, Endnote 20.1. The full text of the potentially eligible RCTs were retrieved and assessed for inclusion by one author. All the screening and selection were verified by a second author and any disagreement was resolved by consensus with an arbiter.
2.2.3. Data retrieval, processing, and extraction
Bibliometric data were retrieved using export function of the electronic databases and were manually extracted for the following information: title, authors’ names, affiliations and affiliated countries, DOI, language of publication, publication type, publication month and year (issue online), publication journal, research focus, trial design, country of the trial conducted, sample sizes, TCIM modalities, intervention and comparison regimes, outcome measured, and trial registry number. All results were verified by a second author. The raw data were exported on the same day of the literature search to avoid discrepancies between the database's daily updates.
2.3. Bibliometric approach
Bibliometrics is the use of data from citation indexes to analyze, monitor, and visualize study publication trends, impacts, and research gaps. Specifically, the publications and citations, journal performance, and other information of interest such as types of TCIM modalities and outcome measures were analyzed in this study. This bibliometric analysis did not involve human research or protocol that require ethical approval from the relevant review board or committee.
2.4. Data analyses
Data were summarized using frequencies with percentages. For the analysis of study characteristics, the number of citations was retrieved from Clavirate Analytics' Web of Science (WoS) database for all studies regardless of their indexing in English or Chinese databases. To describe the journal's performance and whether the journal is TCIM-focused journal, the authors used Journal Citation Reports (JCR) impact factor and journal category for English articles, and CNKI reports for Chinese articles. Both JCR and CNKI defined the journal impact factor as the ratio between the number of all citations in a given year and the number of citable articles published during the previous 2 years. The research and publication trends associated with the eligible RCTs were also identified and presented. Visualization of the research trend using word clouds was created with wordart.com.
3. Results
3.1. Quantity of publication
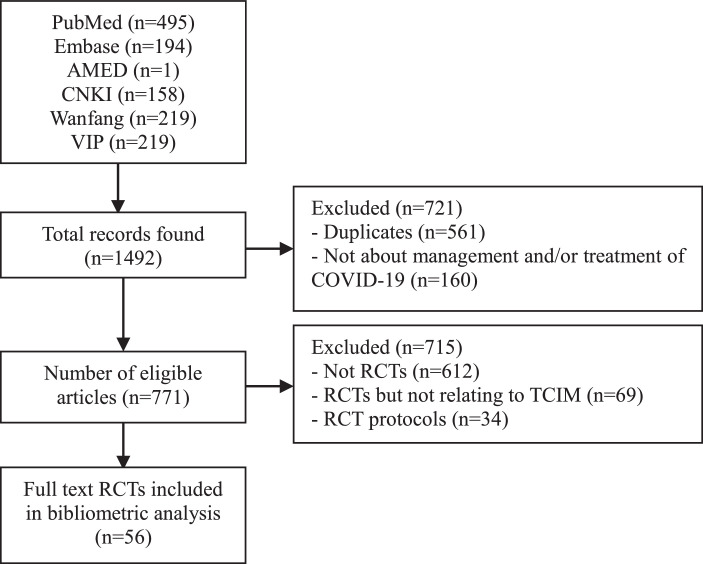
Results revealed 90 eligible publications on TCIM-focused RCTs. Among them, 56 (63%) were published as original full-length research articles and 34 (37%) were published as protocols that reported the study design and rationale. As the consistency of protocols with the final study report might not be definite, protocol publications were excluded in the final analysis. Therefore, only a total of 56 articles were included and reviewed in the bibliometric analysis. All the 56 RCTs mentioned at least one TCIM for the management and treatment of COVID-19. A flowchart on the study selection process is provided in Fig. 1.
Fig. 1.
Study selection and analysis flowchart.
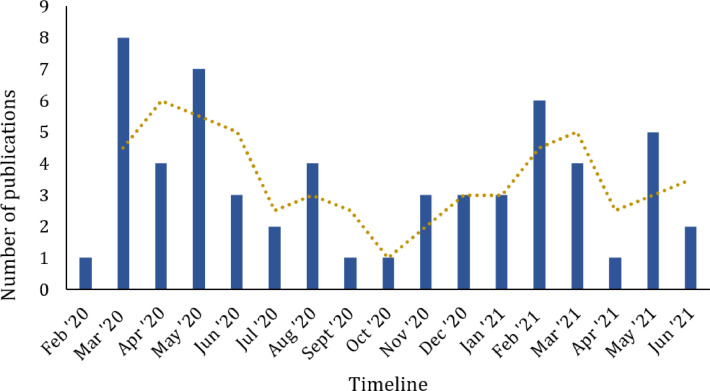
Across 574 authors of the included publications, 553 authors were unique and the number of publication per author ranged from 1 to 4 (1 article (n=537); 2 articles (n=12); 3 articles (n=3); and 4 articles (n=1)). In terms of language, 28 articles (50%) were published in English and 28 articles (50%) were published in Chinese of which English titles and abstracts were provided for 19 articles. The number of TCIM focused publications (Fig. 2) showed a fluctuation seesaw over the pandemic.
Fig. 2.
Trends of TCIM focused randomized control trial research since COVID-19 outbreak.
3.2. Countries with high publications
Throughout the pandemic, China was the leading country with TCIM related RCT publications, followed by India, United States, and Iran. Publications were affiliated from the following 13 countries: China (n=41), India (n=5), United States (n=4), Iran (n=4), Belgium (n=2), Brazil (n=2), Australia (n=1), Canada (n=1), Germany (n=1), Pakistan (n=1), Saudi Arabia (n=1), Spain (n=1), and Turkey (n=1). The number of countries affiliated per publication only involved 1 (n=51) to 2 (n=7) countries.
3.3. Journal analysis
Most of the journals that published TCIM RCTs were from the field of general medicine, health care sciences, pharmacology, and integrative medicine. In total, 56 articles were published in 46 journals, of which 24 were English journals and 22 were Chinese journals. Only 5 journals (21%) were identified as TCIM-focused English journals and 12 journals (55%) as TCIM-focused Chinese journals. The impact factor of these journals ranged widely from 0.220 to 56.272. The number of articles published per journal ranged from 1 to 3. The articles that had been cited at least 5 times in Web of Science database, as of the search date, are provided in Table 1 and the details of the journals are provided in Table 2. The analysis of the terminology used for the article title showed the broad range of terms that were used to describe the title of the RCTs, as illustrated in Fig. 3.
Table 1.
Highly cited articles (>5 citations)
| Article title | Publication year | Citations (n) |
|---|---|---|
| Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study | 2020 | 131 |
| Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial | 2020 | 93 |
| Effects of progressive muscle relaxation on anxiety and sleep quality in patients with COVID-19 | 2020 | 50 |
| Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study) | 2020 | 40 |
| Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: A randomized clinical trial | 2020 | 37 |
| Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: The COVID A to Z randomized clinical trial | 2021 | 19 |
| Clinical study on 37 case of COVID-19 treated with integrated traditional Chinese and Western medicine | 2020 | 18 |
| Pilot trial of high-dose vitamin C in critically ill COVID-19 patients | 2021 | 17 |
| Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: A randomized controlled trial | 2020 | 15 |
| Clinical effect and mechanism of Qingfei Touxie Fuzheng recipe in the treatment of novel coronavirus pneumonia | 2020 | 13 |
| Effect of Xuebijing injection on inflammatory markers and disease outcome of coronavirus disease 2019 | 2020 | 10 |
| Guideline-based Chinese herbal medicine treatment plus standard care for severe coronavirus disease 2019 (G-CHAMPS): Evidence from China | 2020 | 8 |
| Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19: A pilot randomized clinical trial | 2020 | 8 |
| Exploring an integrative therapy for treating COVID-19: A randomized controlled trial | 2020 | 7 |
| Safety and effectiveness of high-dose vitamin C in patients with COVID-19: A randomized open-label clinical trial | 2021 | 6 |
| The combined therapy of Zhongyao Shufeng Jiedu capsule and arbidol hydrochloride tablets in treating COVID-19 patients | 2020 | 6 |
*Based on Web of Science data only
Table 2.
Journals that published TCIM-focused randomized controlled trials for COVID-19
| Journals | No. of articles | TCIM journal | 2020 Impact factor* |
|---|---|---|---|
| English | |||
| Phytomedicine | 3 | Yes | 5.340 |
| Biomedicine & Pharmacotherapy | 2 | No | 6.529 |
| Complementary Therapies in Clinical Practice | 2 | Yes | 2.446 |
| JAMA | 1 | No | 56.272 |
| JAMA Network Open | 1 | No | 8.483 |
| Pharmacological Research | 1 | No | 7.658 |
| Annals of Intensive Care | 1 | No | 6.925 |
| Phytotherapy Research | 1 | No | 5.878 |
| Frontiers in Pharmacology | 1 | No | 5.810 |
| Nutrients | 1 | No | 5.717 |
| Journal of Translational Medicine | 1 | No | 5.531 |
| Frontiers in Medicine | 1 | No | 5.091 |
| International Immunopharmacology | 1 | No | 4.932 |
| Frontiers of medicine | 1 | No | 4.592 |
| Scientific Reports | 1 | No | 4.379 |
| Journal of Steroid Biochemistry and Molecular Biology | 1 | No | 4.292 |
| Annals of Palliative Medicine | 1 | No | 2.595 |
| Postgraduate Medical Journal | 1 | No | 2.401 |
| Integrative Medicine Research | 1 | Yes | 2.368 |
| Journal of medical virology | 1 | No | 2.327 |
| European Journal of Medical Research | 1 | No | 2.175 |
| Chinese Journal of Integrative Medicine | 1 | Yes | 1.978 |
| Journal of Complementary and Integrative Medicine | 1 | Yes | NA |
| Cureus | 1 | No | NA |
| Chinese | |||
| International Infectious Diseases | 2 | No | 0.047 |
| Hebei Journal of Traditional Chinese Medicine | 2 | Yes | 0.731 |
| Chinese Journal of Experimental Traditional Medical Formulae | 2 | Yes | 2.327 |
| Chinese Acupuncture and Moxibustion | 2 | Yes | 1.597 |
| Journal of Emergency in Traditional Chinese Medicine | 2 | Yes | 0.991 |
| Pharmacology and Clinics of Chinese Materia Medica | 2 | Yes | 1.079 |
| Chinese Critical Care Medicine | 1 | No | 1.820 |
| Journal of Traditional Chinese Medicine | 1 | Yes | 1.555 |
| Traditional Chinese Drug Research and Clinical Pharmacology | 1 | Yes | 1.277 |
| Chinese Pharmaceutical Journal | 1 | No | 1.141 |
| China Tropical Medicine | 1 | No | 1.122 |
| Journal of Xi'an Jiaotong University (Medical Sciences) | 1 | No | 1.071 |
| Journal of Hunan University of Chinese Medicine | 1 | Yes | 1.059 |
| Guangdong Medical Journal | 1 | No | 1.021 |
| Herald of Medicine | 1 | No | 0.954 |
| Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology | 1 | Yes | 0.761 |
| Chinese Journal of Virology | 1 | No | 0.753 |
| Jiangsu Journal of Traditional Chinese Medicine | 1 | Yes | 0.742 |
| China Pharmaceuticals | 1 | No | 0.689 |
| Zhejiang Journal of Integrated Traditional Chinese and Western Medicine | 1 | Yes | 0.457 |
| Guangming Journal of Chinese Medicine | 1 | Yes | 0.328 |
| Medical Journal of Communications | 1 | No | 0.220 |
*Latest impact factor as reported by Journal Citation Report (JCR) for English journals and by China National Knowledge Infrastructure (CNKI) for Chinese journals. The journals are listed in descending order of article number; for those with the same number of article(s), the order is by the impact factor.
Fig. 3.
The most frequent terms used in the title of TCIM-focused RCT publications.
3.4. Analysis of TCIM modalities
The most commonly studied TCIM modalities included Chinese herbal decoction (n=12), Chinese patent medicine (n=16), Chinese herbal injection (n=3), combined Chinese medicine intervention (n=5), moxibustion (n=1), vitamin D (n=5), vitamin C (n=3), herbal extractions (n=3), zinc (n=1), propolis (n=1), omega 3 fatty acid (n=1), zinc and ascorbic acid (n=1), Ayurvedic medicine (n=1), guided imagery (n=1), progressive muscle relaxation (n=1), and ozone therapy (n=1), as shown in Table 3. The details of the modalities used is provided in Table 4.
Table 3.
Types of TCIM modalities used in the trials for COVID-19 patients
| Type of TCIM | Modalities | No. of trials |
|---|---|---|
| Chinese medicine (n=37) | Chinese herbal decoction | 12 |
| Chinese patent medicine | 16 | |
| Chinese herbal injection | 3 | |
| Moxibustion | 1 | |
| Combined interventions | 5 | |
| Chinese herbal decoction and moxibustion | 1 | |
| Auricular acupuncture and Qigong | 1 | |
| Chinese medicine music therapy and Qigong | 1 | |
| Chinese herbal decoction and fumigation | 1 | |
| Chinese patent medicine and Qigong | 1 | |
| Health supplements (n=15) | Vitamin D | 5 |
| Vitamin C | 3 | |
| Herbs | 3 | |
| Zinc | 1 | |
| Propolis | 1 | |
| Omega-3 fatty acid | 1 | |
| Zinc and Ascorbic Acid | 1 | |
| Others (n=4) | Ayurvedic medicine | 1 |
| Guided imagery | 1 | |
| Progressive muscle relaxation | 1 | |
| Ozone therapy | 1 |
Table 4.
Details of the type of TCIM intervention used.
| Intervention category | Interventions used |
|---|---|
| Chinese herbal decoction | Jiawei Dayuan decoction, Maxing Xuanfei Jiedu decoction, Qingfei Paidu decoction, Qingfei Touxie Fuzheng prescription, Xuanfei Baidu decoction, Xuanfei Qingre decoction, Yidu-toxicity blocking lung decoction, Xiao Chaihu decoction with Maxingshigan or Sanren decoction, Herbal decoction by pattern identification, Self-prescribed decoction |
| Chinese patent medicine | Feiyan Yihao granules, Gegen Qinlian pill, Huoxiang Zhengqi drop pills, Jinhua Qinggan granules, Jinyinhua Oral Liquid, Keguan-1 powder, Lianhua qingwen granules, Qingre Kang oral liquid, Shenhuang Granule, Shenling Baizhu San, Shuanghuanglian oral liquids, Shufeng Jiedu Capsule, Toujie Quwen granules, Xianglan Jiedu, Yiqi Huoxue San, Yiqi Yangyin granules |
| Chinese herbal injections | Reduning injection, Xiyanping injection, Xuebijing injection |
| Other traditional Chinese medicine intervention | Taiji Liu Qigong, Baduanjin, TCM five elements music therapy, Liu Zhi Jue |
| Ayurvedic medicine | Giloy Ghanvati, Swasari Ras, Ashwagandha, Tulsi Ghanvati, Anu Taila |
| Health supplements | Ascorbic acid, Zinc gluconate, Calcifediol, Curcumin and piperine formulation, Diammonium Glycyrrhizinate enteric capsules, Intravenous vitamin C, Intravenous zinc, Omega-3 fatty acids fortified formula, Oral vitamin D3, Standardized Brazilian green propolis extract, Zingiber officinale and Echinacea |
| Others | Progressive muscle relaxation (PMR), Guided imagery, Ozone therapy (Ozonized rectal insufflation with minor auto haemotherapy) |
3.5. Study design and sample size
Among the retrieved data, 54 trials focused on the treatment and 2 trials focused on the rehabilitation of COVID-19 using TCIM approach. Due to the nature and complexity of the disease, all participants in the intervention group or the control group of the 54 trials that focused on COVID-19 treatment were given standard medications for COVID-19, such as antibiotics, antiviral and anti-inflammation drugs. None of the studies used inactive control (no intervention) except for 1 study that focused on the rehabilitation of discharged patients. In the other words, TCIM interventions were not used alone but integrated with standard treatment across the trials.
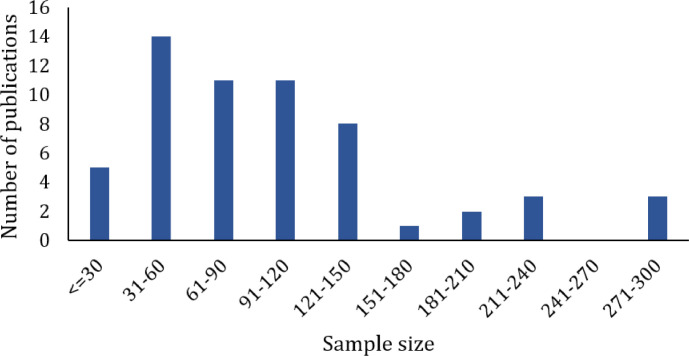
Most of the RCTs had 2 arms (n=51) or 3 arms (n=5), and only a small number of trials had 4 arms (n=2). All the RCTs had parallel designs. In terms of masking, most of the trials did not report on blinding methods (n=33), many were open-labeled (n=15), and few were double-blinded (n=6) or single-blinded (n=2). Most of the trials were conducted in China (n=41), followed by India (n=5), Iran (n=4), Brazil (n=2), Australia (n=1), Pakistan (n=1), Saudi Arabia (n=1), Spain (n=1), Turkey (n=1), and the United States (n=1). Twenty-five trials were registered in the trial registry while 31 trials did not report on the relevant information. For sample sizes, a total of 5,932 study participants were included across the RCTs. Small to medium sample-sized studies were dominant with only a few studies including larger sample size of more than 200 study participants (Fig 4).
Fig. 4.
Sample size of included COVID-19 randomized controlled trials relating to TCIM intervention.
3.6. Main research outcomes of TCIM related RCTs
Over the studied population, 5,169 (87%) participants were positive cases, 343 (6%) were suspected or positive cases, and 148 (2%) were suspected cases of COVID-19. The severity of the diseases ranged from mild to critical to recovery. After standardizing and merging similar outcomes, there were 33 outcomes reported in the included RCTs, including admission to intensive care unit, adverse events, acute physiology and chronic health evaluation (APACHE) II score, arterial blood gas parameters, changes in oxygenation index, chest X-ray or computed tomography (CT) findings, clinical symptoms score, duration of hospital admission, frequency and duration of mechanical ventilator assistance, Glasgow coma scale, health-related laboratory markers, hospitalization rate, incidents of acute respiratory distress syndrome (ARDS) development, inflammatory markers, medication status, mortality, need for vasoactive drugs, organ functions, overall improvement rate, overall rate of clinical cure, peak expiratory flow rate, prevalence of antibiotic, quality of life, rate and time to negative conversion of viral tests, rate of advancing to critical, rate of clinical deterioration, remission rate and time of clinical symptoms, retest of viral tests, severe adverse events, survival rate, traditional Chinese medicine (TCM) syndrome score, TCM tongue and pulse diagnosis score, and time to disease recovery. Details of the outcomes measured according to the severity of disease are provided in Table 5.
Table 5.
Outcomes measured in the TCIM-focused randomized controlled trials for COVID-19
| Population studied | Severity of COVID-19 | Outcomes measured |
|---|---|---|
| Positive cases (n=5169) | Mild | Remission rate and time of clinical symptoms Inflammatory markers Hospitalization rate Chest CT findings Overall rate of clinical cure Adverse events |
| Mild or asymptomatic | Health-related laboratory markers Inflammatory markers Rate and time to negative conversion of viral tests Adverse events |
|
| Moderate | Remission rate and time of clinical symptoms Health-related laboratory markers Inflammatory markers Duration of hospital admission Chest CT findings TCM syndrome score Time to negative conversion of viral tests Rate of clinical deterioration Overall rate of clinical cure Adverse events |
|
| Severe | Remission rate and time of clinical symptoms Inflammatory markers Duration of hospital admission APACHE II score Prevalence of antibiotic Chest X-ray and CT findings Frequency of mechanical ventilator assistance Mortality Time to negative conversion of viral tests Rate of clinical deterioration Overall rate of clinical cure Adverse events |
|
| Critical | Health-related laboratory markers Duration of hospital admission Admission to intensive care unit Duration of mechanical ventilator assistance Changes in oxygenation index Arterial blood gas parameters Glasgow coma scale Organ functions Survival rate Mortality Severe adverse events |
|
| Recovery | Remission rate of clinical symptoms Inflammatory markers Peak expiratory flow rate TCM syndrome score Quality of life Chest CT findings Retest of viral tests Overall rate of clinical cure Adverse events |
|
| Mild to moderate | Remission rate and time of clinical symptoms Health-related laboratory markers Inflammatory markers Duration of hospital admission Admission to intensive care unit Changes in oxygenation index Chest CT findings TCM syndrome score Time to negative conversion of viral tests Rate of clinical deterioration Overall rate of clinical cure Adverse events |
|
| Mild to severe | Remission rate and time of clinical symptoms Health-related laboratory markers Inflammatory markers Duration of hospital admission Admission to intensive care unit Changes in oxygenation index Chest X-ray or CT findings Frequency and duration of mechanical ventilator assistance Mortality TCM syndrome score TCM tongue and pulse diagnosis score Time to negative conversion of viral tests Rate of clinical deterioration Overall rate of clinical cure Adverse events |
|
| Moderate to severe | Health-related laboratory markers Inflammatory markers Duration of hospital admission Admission to intensive care unit Frequency and duration of mechanical ventilator assistance Clinical symptoms score Mortality Adverse events |
|
| Mild to critical | Remission rate and time of clinical symptoms Health-related laboratory markers Inflammatory markers Changes in oxygenation index Chest CT findings Organ functions Time to disease recovery Rate and time to negative conversion of viral tests Adverse events |
|
| Severe to critical | Inflammatory markers Frequency and duration of mechanical ventilator assistance Rate of advancing to critical Mortality Organ functions Overall improvement rate Adverse events |
|
| Not specified | Remission rate and time of clinical symptoms Inflammatory markers Duration of hospital admission Admission to intensive care unit Frequency and duration of mechanical ventilator assistance Need for vasoactive drugs TCM syndrome score Chest CT findings Organ functions Rate of clinical deterioration Mortality Medication status Overall improvement rate Overall rate of clinical cure Time to negative conversion of viral tests Adverse events |
|
| Suspected cases (n=148) | Ambulatory | Remission rate and time of clinical symptoms Incidents of ARDS development Hospitalization rate Admission to intensive care unit Frequency and duration of mechanical ventilator assistance Chest CT findings Mortality Time to negative conversion of viral tests Adverse events |
APACHE, acute physiology and chronic health evaluation; ARDS, acute respiratory distress syndrome; CT, computed tomography; TCM, traditional Chinese medicine.
Inflammatory markers, including levels of cluster of differentiation (CD) 3+, CD4+, CD4+/CD8+, CD45+, CD8+, creatinine, C-reactive protein (CRP), D-Dimer, erythrocyte sedimentation rate (ESR), ferritin, fibrinogen, high-sensitivity-CRP, interferon gamma (IFN-γ), interleukin-2 receptor (IL-2R), interleukin(IL)-4, IL-6, IL-8, lactate dehydrogenase (LDH), partial pressure of carbon dioxide (PCO2), partial pressure of oxygen (PO2), procalcitonin (PCT), tumor necrosis factor alpha (TNF-α), 25-hydroxy-cholecalciferol [25(OH)D]; lymphocyte (LYM) cell counts, LYM%, neutrophils (NEU), NEU%, neutrophil-to-lymphocyte (N/L) ratio, and white blood cell (WBC) counts.
Organ functions, including myocardial, liver, kidney, and Sequential Organ Failure Assessment (SOFA) score.
Medication status, including Chinese medicine, antibiotics, anti-viral drug, anti-inflammatory drug etc.
Emotional wellbeing and quality of life outcomes including 12-Item Short Form Survey (SF-12), Burnout Scale, Hamilton Anxiety Rating Scale (HAM-A), Hamilton Depression Rating Scale (HDRS), Pittsburgh Sleep Quality Index (PSQI), Self-Rating Depression (SDS), Sleep State Self-Rating Scale (SRSS), Spielberger State-Trait Anxiety Inventory (STAI), Pain quality (Short-Form McGill Pain Questionnaire (SF-MPQ), Pain intensity (Visual Analogue Scale), Subjective Units of Distress Scale (SUDs), Zung Self-Rating Anxiety Scale (SAS).
The full information of all RCTs containing all of the aforementioned characteristics is available in the supplement for the convenience of clinical researchers who are interested in pursuing the original publication or foster further researches.
4. Discussion
This study quantitatively explored the current publication trends of TCIM-focused RCTs, as of July 2021. The 56 publications retrieved from 6 databases demonstrated the efforts taken to investigate the potential of TCIM in treating and/or managing COVID-19. The publications during the past 18 months suggest that TCIM RCT publication fluctuates and is yet to be leveled off, having possibilities of continued growth of publication in the near future. The country with the highest productivity is dominated by China, given that TCIM approach is initiated by China through the usage of traditional Chinese medicine in the management of COVID-19. Globally, the number of distributing countries remains limited and scattered. Although TCIM-focused RCTs are partially published in Chinese, most studies are open access with English titles and abstracts, provided by CNKI for non-Chinese readers. Notably, CNKI has developed an open-access specialized platform in English that currently indexed more than 7,500 COVID-19 related studies from 2,000 Chinese and non-Chinese journals to disseminate research findings.10 The included RCTs were found to be published in biomedical journals in a wide range of fields, with only a little more than one-third of the articles being published in TCIM-focused journals. On the other hand, a little less than half of the articles were published in journals indexed in Science Citation Index Expanded (SCIE) which raises potential questions on the credibility and quality of the studies.
This analysis found that the TCIM modalities mentioned in the included articles are highly disproportionate. It is foreseeable that a vast majority of TCIMs mentioned are related to traditional Chinese medicine as a high proportion of these trials were conducted in China and published by Chinese researchers. However, TCIMs mentioned in articles that are published in high impact factors journals or have a high number of citations are mostly on health supplements such as vitamin C, vitamin D, and zinc. This indicates that health supplements, with a relatively smaller proportion of studies published, gain more attention and recognition among researchers and journals. In the meantime, much fewer studies focused on TCIM modalities such as Ayurveda and mind-body practices, and there are yet to have any studies published on other modalities, showing the potential avenue for future research.
Looking at the analysis of the retrieved data, 2-armed parallel RCTs were highly dominant, suggesting that study investigators were conducting comparison studies where the related clinical question was focused on the effectiveness of the treatment arm (i.e comparing the effectiveness of TCIM interventions with established interventions for the treatment and/or management of COVID-19). Meanwhile, reporting on the masking (also known as blinding) of the RCTs is a huge issue that should not be ignored. Blinding is an important methodologic component of RCTs to reduce bias and increase the validity of the results, and is equally important to the randomization method. However, a high proportion of the studies did not mention blinding, causing their study findings to be questionable. It is also found that too many trials were open and too few were double-blinded, which is generally considered more common in trials related to COVID-19. This bibliometric analysis also observed that small to medium-sized studies were dominant, which is a common limitation that leads to a certain degree of bias in study findings. In terms of outcome measures, it is seen that the outcomes measured varied across the trials with many of less critical outcomes in each intended population. Implications of such heterogeneity should not be ignored in future studies, and researchers should consider selecting their outcomes by referring to the core outcome set developed by Core Outcome Measures in Effectiveness Trials (COMET) Initiative.11 There is also a lack of consistency in the terminology for the study design in the title, indicating lack of proper reporting as suggested by CONsolidated Standards Of Reporting Trials (CONSORT) guidelines to include identification of a randomized trial in the title.
In current situation, there is no doubt that clinical research on COVID-19 faces many ethical challenges due to the complexity of the disease. Yet, well-designed trials are immensely necessary as badly-designed trials are likely to produce potentially misleading or exaggerated information; in the meantime, exposing trial participants to a higher risk of harm. Given the urgency of COVID-19 situation, the perception that core methodological components of high-quality RCTs, such as adequate sample size, proper randomization or blinding, and validated outcome measures, are expendable is highly inappropriate. Several rapidly published research trials with suboptimal designs have faced retraction, but they are not free from affecting clinical practice to face the consequences risen from the results of such studies. As exemplified by hydroxychloroquine, its usage had increased after the rapid publication of an open-label, non-randomized, small-sampled trial claiming its effectiveness and was later shown to be no different from placebo and revoked in a well-designed RCT with a large sample size.12, 13 Therefore, RCTs with suboptimal designs will not help either patients or clinicians, and may result in significant ethical lapse. Instead of allowing exceptionalism, researchers should coordinate their studies to uphold the gold standard of RCT to contribute to the advancement of clinical evidence base for COVID-19.14
To the authors’ knowledge, this study is the first bibliometric analysis of TCIM-focused RCTs. In contrast to a number of COVID-19 bibliometric analyses published over the past year, the authors centered their study to RCTs only, and manually screened and extracted all the data to provide a comprehensive review on the state of TCIM clinical research. Particularly, a bibliometric analysis on the global trends of overall TCIM research providing a larger view of the publication landscape15 and a bibliometric analysis focusing on traditional Chinese medicine16 have been published in recent months. It is worth mentioning that several general comparisons can be drawn to the findings of this study in comparison with these bibliometric analyses. One study15 identified that the vast majority proportion of publications included traditional Chinese medicine and vitamin D supplementation, whereas the other study16 found that research direction is highly concentrated in the clinical researches focusing on traditional Chinese medicine in the treatment of COVID-19. These findings are highly in line with our findings where traditional Chinese medicine has the greatest proportion in TCIM-focused RCTs publications, followed by vitamin D supplementation.
Nevertheless, there are limitations of this study. First, the authors did not include explicit search terms related to each TCIM modalities such as specific herb/product names or technique used (e.g. curcumin, calcifediol, Baduanjin); however, these interventions were included if they were described as TCIM modalities. Therefore, there may be other RCTs on these TCIM interventions that were missed out. Second, a number of non-English or non-Chinese journals may not be indexed in the aforementioned databases; hence, it is possible that TCIM-focused RCTs that were published in their native language or local journals may have been neglected. Regrettably, such articles are often hard to capture as their accessibility is highly limited to authors, and there could be other forms of less known TCIM modalities that were overlooked in this analysis.
In conclusion, this bibliometric analysis of RCTs demonstrated the research trends of TCIMs mentioned in COVID-19 research. There are still many research gaps in the pandemic research of TCIMs which requires evaluation of study designs for more rigorous research from various countries. The burgeoning literature on TCIM-focused clinical trials for COVID-19 in the future is highly anticipated.
Author contributions
Conceptualization: MSL. Methodology: LA, ES. Software: LA. Validation: MSL. Formal analysis: LA, ES. Investigation: MSL. Data curation: LA. Writing – Original Draft: LA. Writing – Review & Editing: MSL, ES. Visualization: MSL. Supervision: MSL. Project administration: MSL. Funding acquisition: MSL.
Conflict of interests
LA, ES, and MSL are editors of IMR but their status had no bearing on editorial consideration. This article was externally peer-reviewed. There is no other conflict of interests.
Funding
This research was supported by Korea Institute of Oriental Medicine (KSN2013210).
Ethical statement
Not applicable.
Data availability
The data that support the findings of this study are available as supplementary material.
References
- 1.World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard, <https://covid19.who.int/table>; Published 2020.
- 2.Ang L, Song E, Lee HW, Lee MS. Herbal medicine for the treatment of Coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2020;9(5) doi: 10.3390/jcm9051583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou LP, Wang J, Xie RH, Pakhale S, Krewski D, Cameron DW. The effects of traditional Chinese medicine as an auxiliary treatment for COVID-19: a systematic review and meta-analysis. J Altern Complement Med. 2021;27(3):225–237. doi: 10.1089/acm.2020.0310. [DOI] [PubMed] [Google Scholar]
- 4.Liang S-B, Fang M, Liang C-H, Lan H-D, Shen C, Yan L-J. Therapeutic effects and safety of oral Chinese patent medicine for COVID-19: A rapid systematic review and meta-analysis of randomized controlled trials. Complem Ther Med. 2021;60 doi: 10.1016/j.ctim.2021.102744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seifert G, Jeitler M, Stange R, Michalsen A, Cramer H, Brinkhaus B. The relevance of complementary and integrative medicine in the COVID-19 pandemic: a qualitative review of the literature. Front Med. 2020;7(946) doi: 10.3389/fmed.2020.587749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tillu G, Chaturvedi S, Chopra A, Patwardhan B. Public health approach of Ayurveda and Yoga for COVID-19 prophylaxis. J Altern Complement Med. 2020;26(5):360–364. doi: 10.1089/acm.2020.0129. [DOI] [PubMed] [Google Scholar]
- 7.Ang L, Lee HW, Choi JY, Zhang J, Soo Lee M. Herbal medicine and pattern identification for treating COVID-19: a rapid review of guidelines. Integr Med Res. 2020;9(2) doi: 10.1016/j.imr.2020.100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO). Health topic: Traditional, complementary and integrative medicine, <https://www.who.int/health-topics/traditional-complementary-and-integrative-medicine#tab=tab_1>; Published 2021 Accessed July 10.
- 9.National Center for Complementary and Integrative Health. Health Topics A–Z, <https://www.nccih.nih.gov/health/atoz>; Published 2021 Accessed July 10.
- 10.China National Knowledge Infrastructure (CNKI). Open access (OA) Online-first publishing of research papers on COVID-19, <https://xgbteng.oversea.cnki.net/xgbt/en>; Published 2021 Accessed August 1.
- 11.Core Outcome Measures in Effectiveness Trials (COMET) Initiative. Core outcome set developers’ response to COVID-19, <https://www.comet-initiative.org/studies/details/1538>; Published 2021 Accessed August 1.
- 12.The RECOVERY Collaborative Group Effect of Hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383(21):2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saag MS. Misguided use of Hydroxychloroquine for COVID-19: The infusion of politics into science. JAMA. 2020;324(21):2161–2162. doi: 10.1001/jama.2020.22389. [DOI] [PubMed] [Google Scholar]
- 14.London AJ, Kimmelman J. Against pandemic research exceptionalism. Science. 2020;368(6490):476–477. doi: 10.1126/science.abc1731. [DOI] [PubMed] [Google Scholar]
- 15.Ng JY. Global research trends at the intersection of coronavirus disease 2019 (COVID-19) and traditional, integrative, and complementary and alternative medicine: a bibliometric analysis. BMC Complem Med Therapies. 2020;20(1):353. doi: 10.1186/s12906-020-03151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang K-L, Jin X-Y, Gao Y, Xie J, Liu M, Zhang J-H. Bibliometric analysis of researches on traditional Chinese medicine for coronavirus disease 2019 (COVID-19) Integr Med Res. 2020;9(3) doi: 10.1016/j.imr.2020.100490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available as supplementary material.