Abstract
Coronavirus infection disease 2019 (COVID-19) has been linked to the development of various autoimmune disorders. Lofgren syndrome, consisting of bilateral pulmonary hilar lymphadenopathy, erythema nodosum and polyarthritis, is a rare autoimmune disease that represents an acute form of sarcoidosis. We present the case of Lofgren syndrome developing in close temporal association with COVID-19. Clinical presentation consisted of fever, bilateral lung lymphadenopathy, arthralgias and erythema nodosum. Hilar lymph node biopsy revealed pathology consistent with sarcoidosis. Three weeks prior to presentation, the patient experienced respiratory symptoms. Serological examination at the time of presentation revealed positive IgM and IgG antibodies against SARS-CoV-2 nucleocapsid protein. Most symptoms resolved following a course of oral prednisone. This case report suggests a possible link between COVID-19 and the development of sarcoidosis, however, further studies are needed to conclude this association.
Keywords: Lofgren syndrome, sarcoidosis, erythema nodosum, COVID-19
Introduction
Coronavirus disease 2019 (COVID-19) is a systemic viral infection caused by the novel, zoonotic coronavirus SARS-CoV-2 [1]. SARS-CoV-2 originated in Hubei province, China, and quickly spread across the globe; a pandemic was declared by the WHO in spring 2020 [2]. Associations between COVID-19 and the risk of developing an autoimmune disorder as a sequel to the acute infection have been proposed by various authors [3], [4], [5], [6]. Soon after the onset of the pandemic, the links between COVID-19 and the development of Kawasaki-like disease and pediatric multi-system inflammatory syndrome of children were clearly established [6]. Since then, possible associations between COVID-19 and other autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis and multi-system inflammatory syndrome in adults, have been investigated [3], [4], [5], [6], [7], [8]. Lofgren syndrome is a rare acute autoimmune syndrome consisting of bilateral pulmonary hilar lymphadenopathy, erythema nodosum and polyarthritis and is associated with the new development of sarcoidosis [9]. We present the case of Lofgren syndrome developing in close temporal association with COVID-19.
Case
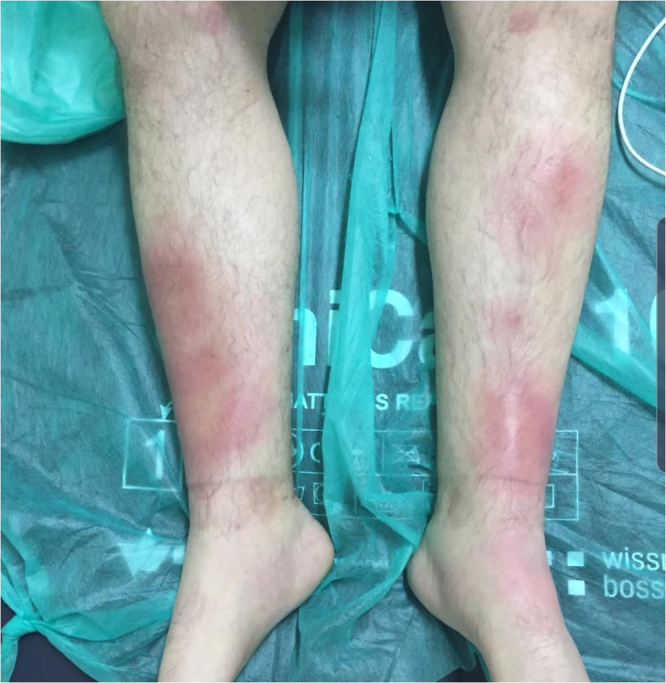
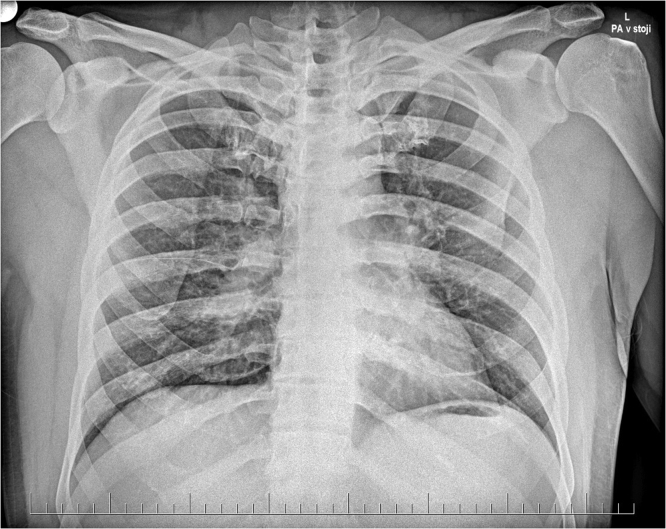
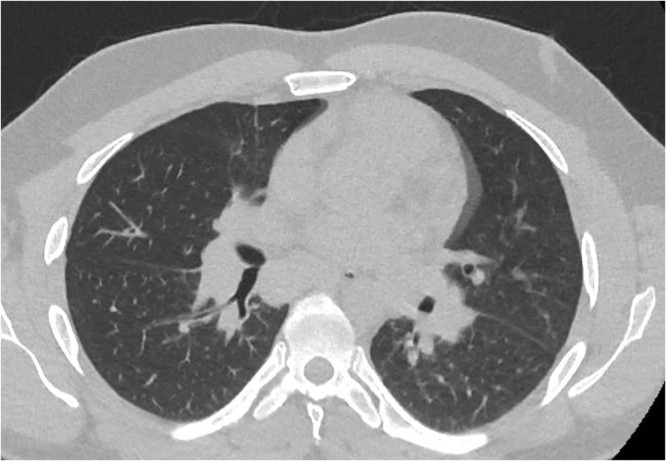
A 30-year old, previously healthy caucasian male (entnic group – Slovak, West Slavic), presented to the Emergency Office of the Clinic of Infectology and Geographical medicine, University Hospital in Bratislava, with a 10-day history of low grade fever up to 37.6 degrees Celsius and pain in both ankles, wrists and right elbow as well as a 4-day history of dry cough, dull pain over the sternum and exertional dyspnea. Before admission, the patient was treated with azithromycin and amoxicillin-clavulanate for four days without any marcable effect on symptoms. The physical examination revealed bilateral multiple erythematous and indurated warm plaques with palpable subcutaneous nodules in the malleolar and pretibial areas (Fig. 1) in addition to mild inspiratory crepitations over the basal lung areas. Laboratory examination revealed elevated C-reactive protein (147 mg/L), interleukin-6 (160.5 pg/mL), D-dimer (2.15 mg/L) and fibrinogen (6.2 g/L) in addition to leukocytosis (10,870 cells/mL) with absolute neutrophilia (8770 cells/mL). Chest X-ray revealed bilateral hilar enlargement and inflammatory infiltrations in the lower right lung field (Fig. 2). As the patient presented during the peak of the COVID-19 winter epidemic, COVID-19 was suspected and oropharyngeal and nasopharyngeal swabs for SARS-CoV-2 were examined by RNA polymerase chain reaction (PCR), however, these tests were negative. Therefore we examined antibodies against the nucleocapsid protein of SARS-COV-2 using enzyme-linked immunosorbent assay (ELISA), which was positive for both IgM and IgG (NovaLisa, NovaTec Immundiagnostica GmbH, Dietzenbach, Germany). The patient later recalled a short episode of mild fatigue and the sensation of swollen nasal mucosa three weeks before the onset of symptoms. Contact with a colleague with respiratory symptoms preceded the mild respiratory illness by several days. Serology for human immunodeficiency virus, hepatitis B, hepatitis C, cytomegalovirus, Epstein-Barr virus, Chlamydia pneumoniae and Mycoplasma pneumoniae infection, borreliosis, syphilis, tularemia, yersiniosis and bartonelosis were all negative, while blood, sputum and urine cultures and nasal and tonsilar swabs were unremarkable. The patient was admitted and treatment was continued with amoxicillin-clavulanate. Due to the patient‘s arthritis, treatment with slow release diclofenac was also started. However, after three days of treatment, there were no signs of regression of the erythema and the joint pain progressed while the patient developed a fever up to 38.5 degrees Celsius and C-reactive protein increased to 212 mg/L. To elucidate the X-ray findings of hilar enlargement, computed tomography was performed, which revealed bilateral hilar and mediastinal lymphadenopathy with largest lymph nodes up to 16 mm in diameter, consistent with stage I pulmonary sarcoidosis (Fig. 3). Further laboratory workup revealed slightly elevated plasmatic angiotensin converting enzyme (1.27 ukat/L) as well as elevated thymidine kinase (30.6 units/L) and complement component 3 (1.95 g/L). On the basis of these findings, the diagnosis of Lofgren syndrome was proposed and we started corticotherapy with 40 mg of prednisone daily. After just two days of treatment, the fever and joint pain resolved and there was marcable regression of exanthema. C-reactive protein decreased to 95 mg/L and then to 18 mg/L over the next three days. The patient was discharged and continued the corticotherapy with 20 mg of prednisone daily. The patient later underwent diagnostic video assisted thoracoscopy with extirpation of hilar lymph nodes. The histology revealed noncaseating granulomas, concluding the diagnosis of sarcoidosis.
Fig. 1.
Photography of the skin lesions visible as bilateral multiple erythematous and indurated plaques in the malleolar and pretibial areas.
Fig. 2.
Chest X-ray - posteroanterior view. Native chest X-ray patient chest revealing bilateral enlargement of hilar lymph nodes.
Fig. 3.
Tomogram - transversal plane. Native tomogram in the transversal plane of the patient chest revealing bilateral enlargement of hilar lymph nodes.
Discussion
We present the case of new onset of Lofgren syndrome in close temporal association with resolved SARS-CoV-2 infection. Lofgren syndrome is characterized by the presence of bilateral pulmonary hilar lymphadenopathy, fever, erythema nodosum and polyarthritis and is considered to be an acute form of sarcoidosis [9].
Sarcoidosis is a multisystem inflammatory disease that can affect any organ and may lead to significant morbidity and disability. The hallmark of sarcoidosis is the presence of noncaseating granulomas in the affected tissues. The most common pathologies associated with sarcoidosis are bilateral pulmonary infiltrates and bilateral hilar lymphadenopathy, however, musculoskeletal, nervous and lymphatic tissues are often also affected. Sarcoidosis is a rare disease with an incidence of 5–12 per 100,000 people of caucasian race, typically affecting young and middle aged adults [10], [11]. Erythema nodosum is autoimmune septal panniculitis that is associated with sarcoidosis as well as other autoimmune and various infectious diseases [12]. The clinical presentation of Lofgren syndrome may be quite severe with high fever, shortness of breath and debilitating arthralgias, however, the overall prognosis is favorable with up to 90% of cases remitting spontaneously [9], [10], [11].
An etiology of Lofgren syndrome and sarcoidosis itself has not been clearly established, however, most theories suggest an interplay between genetic factors and environmental factors, such as exposure to various exogenous antigens. The heritability of sarcoidosis is polygenic with more than 10 locuses having been linked to development of the disease [13]. The pathogenesis of sarcoidosis is based on dysregulated T-cell responses to the presence of various antigens, including bacteria and viruses. Dendritic cells and alveolar macrophages present these antigens to CD4+ T-cells that can differentiate into two distinct lines based on their cytokine profiles, specifically T helper 1 (Th1) and T helper 2 (Th2) cells. Resolution or maintenance of granulomas in affected tissues is determined by the proportion of Th1 and Th2 cells. A predominance of Th2 cells stimulates fibroblast proliferation and the accumulation of collagen, which is the hallmark of progressive fibrosis and granuloma formation [14]. Various infections have been identified as possible precipitating factors in the development of sarcoidosis and may also contribute to the development of Lofgren syndrome, particularly by dysregulating the interplay of antigen presenting cells, alveolar macrophages and T-cells [9], [10], [11].
Infection with SARS-CoV-2 seems to be a potent promoter of the development of various autoimmune disorders, however, causality has yet to be determined. A clear link has been established between COVID-19 and Kawasaki-like disease in children [6]. Additionally, there is evidence of possible associations between COVID-19 and the development of systemic lupus erythematosus, rheumatoid arthritis, ANCA-associated vasculitis and other autoimmune systemic diseases [3], [4], [5], [6], [7], [8].
To our knowledge, this is the first documented case of newly developed Lofgren syndrome or sarcoidosis in close temporal association with COVID-19 published to this date. Behbahani et al. reported the case of sarcoid-like reactions in patients recovering from COVID-19. The skin involvement in the previously reported patient, however, resembled erythema nodosum and was present at atypical locations, such as the face, while biopsy revealed sarcoidal granulomas; additionally, there is no mention of lung or joint involvement in their case report [15]. Our patient presented with bilateral erythematous, subcutaneous nodules localized to the pretibial areas, which is a typical clinical presentation of erythema nodosum [16]. Regarding the lungs, our patient presented with bilateral hilar lymphadenopathy consistent with typical involvement in pulmonary sarcoidosis [9], [10], [11]. The presence of joint pain, erythema nodosum and hilar lymphadenopathy in our patient made up the complete clinical picture of Lofgren syndrome. Histologic examination of excidet hilar lymph nodes revealed noncaseating granulomas typical for sarcoidosis, thus concluding the diagnosis. Lofgren syndrome is typically an acute presentation of sarcoidosis [8]. Therefore, we concluded that our patient presented with Lofgren syndrome as the presentation of a new onset of sarcoidosis. The SARS-CoV-2 infection in our patient that preceded the development of Lofgren syndrome was documented by the presence of mild respiratory symptoms and positive antibodies against SARS-CoV-2 using ELISA, with documented specificity for IgM exceeding 96% and for IgG exceeding 98% [17]. The presence of IgM antibodies suggested recent past infection. The positivity rate of IgM antibodies against the nucleocapsid protein of SARS-CoV-2 is highest during the eight to 30 day period following onset of symptoms, after which it declines sharply [18]. Other diseases proposed to be linked to autoimmune processes triggered by COVID-19, such as multi-system inflammatory syndrome and systemic lupus erythematosus, have been seen to occur within the time period from 0 to 12 weeks after a positive test for COVID-19 [3], [4], [5], [6], [7], [8]. On the basis of respiratory symptoms and the presence of IgM and IgG antibodies, we timed the SARS-CoV-2 infection within this time period, thus suggesting a potential causal association.
Limitations
COVID-19 infection was diagnosed according to the presence of respiratory symptoms and antibodies and not by direct methods, such as PCR for viral RNA. However, according to the high specificity of ELISA for anti-SARS-CoV-2 antibodies used and the presence of respiratory symptoms during the large concurrent outbreak of COVID-19, the false positivity of antibodies is extremely unlikely. As noted above, while associations between infections and the development of sarcoidosis have been clearly established [9], [10], [11], causality is difficult to prove and the development of sarcoidosis preceded by COVID-19 may just be pure coincidence.
Conclusion
We documented the first case of Lofgren syndrome and sarcoidosis developing shortly after mild COVID-19, suggesting a possible link between COVID-19 and the development of sarcoidosis. However, further studies are needed to conclude whether COVID-19 infection increases the risk of developing lofgren syndrome and new onset sarcoidosis.
Funding
This case study did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
CRediT authorship contribution statement
Peter Mihalov: Writing – original draft, Visualization. Eliška Krajčovičová: Writing – original draft. Helena Káčerová: Conceptualization. Peter Sabaka: Writing – original draft, Conceptualization.
Consent
This case study has been conducted in accordance with the Declaration of Helsinki and approved by the local ethics committee of the University Hospital Bratislava. Written informed consent was obtained from the patient for publication of this case report and the accompanying images. A copy of the written consent is available for review by the Editor-in Chief of this journal on request.
Final statement
All authors read the revised manuscript and accepted all the changes to the original manuscript.
Declaration of competing interest
The authors have no conflict of interest to declare.
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. ([Epub ahead of print]) [DOI] [PubMed] [Google Scholar]
- 2.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. PMID: 32191675; PMCID: PMC7569573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galeotti C., Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16(8):413–414. doi: 10.1038/s41584-020-0448-7. PMID: 32499548; PMCID: PMC7271827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J., Liu H.H., Yin X.D., Li C.C., Wang J. COVID-19 illness and autoimmune diseases: recent insights. Inflamm Res. 2021;70(4):407–428. doi: 10.1007/s00011-021-01446-1. Epub 2021 Feb 28. PMID: 33640999; PMCID: PMC7914392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y., Sawalha A.H., Lu Q. COVID-19 and autoimmune diseases. Curr Opin Rheumatol. 2021;33(2):155–162. doi: 10.1097/BOR.0000000000000776. PMID: 33332890; PMCID: PMC7880581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P. PIMS-TS Study Group and EUCLIDS and PERFORM Consortia. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324(3):259–269. doi: 10.1001/jama.2020.10369. PMID: 32511692; PMCID: PMC7281356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris S.B., Schwartz N.G., Patel P., Abbo L., Beauchamps L., Balan S. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection – United Kingdom and United States, March-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(40):1450–1456. doi: 10.15585/mmwr.mm6940e1. PMID: 33031361; PMCID: PMC7561225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zamani B., Moeini Taba S.M., Shayestehpour M. Systemic lupus erythematosus manifestation following COVID-19: a case report. J Med Case Rep. 2021;15(1):29. doi: 10.1186/s13256-020-02582-8. PMID: 33494816; PMCID: PMC7832415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown F., Modi P., Tanner L.S. Lofgren Syndrome. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. PMID: 29493940. [PubMed]
- 10.Mañá J., Gómez-Vaquero C., Montero A., Salazar A., Marcoval J., Valverde J. Löfgren’s syndrome revisited: a study of 186 patients. Am J Med. 1999;107(3):240–245. doi: 10.1016/s0002-9343(99)00223-5. PMID: 10492317. [DOI] [PubMed] [Google Scholar]
- 11.Statement on sarcoidosis Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160(2):736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 12.Chowaniec M., Starba A., Wiland P. Erythema nodosum – review of the literature. Reumatologia. 2016;54(2):79–82. doi: 10.5114/reum.2016.60217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer A., Ellinghaus D., Nutsua M., Hofmann S., Montgomery C.G., Iannuzzi M.C. Identification of immune-relevant factors conferring sarcoidosis genetic risk. Am J Respir Crit Care Med. 2015;192(6):727–736. doi: 10.1164/rccm.201503-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain R., Yadav D., Puranik N., Guleria R., Jin J.O. Sarcoidosis: causes, diagnosis, clinical features, and treatments. J Clin Med. 2020;9(4):1081. doi: 10.3390/jcm9041081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behbahani S., Baltz J.O., Droms R., Deng A.C., Amano S.U., Levin N.A. Sarcoid-like reaction in a patient recovering from coronavirus disease 19 pneumonia. JAAD Case Rep. 2020;6(9):915–917. doi: 10.1016/j.jdcr.2020.07.026. Epub 2020 Jul 24. PMID: 32837988; PMCID: PMC7378473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung A.K.C., Leong K.F., Lam J.M. Erythema nodosum. World J Pediatr. 2018;14(6):548–554. doi: 10.1007/s12519-018-0191-1. Epub 2018 Sep 29. PMID: 30269303. [DOI] [PubMed] [Google Scholar]
- 17.Tré-Hardy M., Wilmet A., Beukinga I., Favresse J., Dogné J.M., Douxfils J. Analytical and clinical validation of an ELISA for specific SARS-CoV-2 IgG, IgA, and IgM antibodies. J Med Virol. 2021;93(2):803–811. doi: 10.1002/jmv.26303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins R.L., Rawlings S.A., Case J., Lee F.Y., Chan C.W., Barrick B. Longitudinal SARS-CoV-2 antibody study using the Easy Check COVID-19 IgM/IgGTM lateral flow assay. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0247797. PMID: 33661960; PMCID: PMC7932143. [DOI] [PMC free article] [PubMed] [Google Scholar]