Abstract
Aims:
This study aimed to compare three approaches of blood glucose monitoring (BGM) frequency attainment and to examine their associations with glycemic control in youth with type 1 diabetes (T1D).
Methods:
Cross-sectional data was derived from the baseline assessment in three clinical trials. Clinical and demographic characteristics of youth with T1D was obtained by chart review. BGM frequency was assessed by parent-youth interview, chart review, and meter downloads. To examine the relationship between A1c and frequency of BGM we performed analysis of variance.
Results:
In youth with T1D (N=385, 50% female, age 13.6±2.5 years, 74% pump users), the 3 methods of assessing BGM frequency were significantly correlated. Frequency by self-report (6.4±2.3 times/day) was significantly higher than both meter download (5.6±2.4 times/day, p<.0001) and clinician report (5.7±2.4 times/day, p<.0001). For all methods, more frequent BGM was associated with lower A1c and lower mean glucose (p<.0001). For each additional daily blood glucose check, there was a 0.2% decrease in A1c (p<.0001).
Conclusion:
BGM remains a potent predictor of glycemic control, warranting continued targeting in clinical efforts to improve glycemic management in youth with T1D.
Keywords: Type 1 Diabetes, Blood Glucose Monitoring, Glycemic Control, A1c, Self-care, Pediatrics
INTRODUCTION
Global estimates state over 1,110,100 people under 19 years old have type 1 diabetes. 1 Youth with type 1 diabetes need daily insulin injections and regular blood glucose monitoring to ensure glucose values remain within a safe range (>70mg/dL and <180md/dL). With appropriate education and support, youth with type 1 diabetes can live healthy lives and prevent complications associated with sub-optimal glycemic control. 2
Despite greater penetration of technology in diabetes treatment for many persons with type 1 diabetes, such as continuous glucose monitoring (CGM), the overwhelming majority of people with diabetes still rely on blood glucose monitoring. 3 Blood glucose monitoring (BGM) remains the fundamental approach to assessing glucose levels in many persons with type 1 and type 2 diabetes. 4 Further, the costs of CGM may be prohibitive for many developing countries, especially those in which there are large numbers of people with diabetes, like Brazil. 5, 6 When assessing BGM data, multiple reports have consistently demonstrated that increased frequency of BGM is associated with lower A1c levels in type 1 diabetes. 7–10
Challenges related to assessing BGM frequency in youth are based upon the reliability of available BGM data. Some biased sources of BGM data are self-maintained logbooks, self-reports, or downloads obtained from one meter when multiple meters are in use (e.g home meter and school meter). 11 Previous studies have shown that self-report potentially overestimates BGM frequency, likely due to cofounders such as patient age, parental involvement and depressive symptoms. 12 Moreover, blood glucose diaries occasionally leads to misreports of BGM data, and downloading devices can be considered time consuming for some clinicians. 13, 14
Given the importance of reliable BGM frequency assessment in clinical practice, especially in patients not using CGM, we aimed to robustly compare BGM frequency ascertained by three different methods in order to inform clinical evidence-based practice. Further, we examined the associations between frequency of BGM by the three methods and glycemic control across ages to add to existing literature with clinical implications of our findings.
METHODS
Design
Cross-sectional data was derived from the baseline assessment in three clinical trials.15–17 All studies took place around 2010–2015 in a tertiary care pediatric diabetes center in Boston, Massachusetts, U.S. Study reporting was consistent with the EQUATOR guidelines, using the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) checklist (Supplementary File).
Participants
Researchers recruited youth 8–18 years of age, English speakers, type 1 diabetes duration ≥1year, daily insulin dose ≥0.5 units/kg, A1c 6.5–10%, not using CGM in the past 3 months.
Data collection
Only participants with BGM frequency data for all three methods were included. Parents completed a demographics survey. Meter BGM frequency, mean glucose, and standard deviation glucose were assessed by downloaded meter and pump data for the previous 2 weeks. Self-report of BGM frequency was assessed by parent-youth interviews. Chart review provided additional clinical and demographic data as well as the clinician report of BGM frequency. Clinician providing the BG frequency was the participant’s primary diabetes provider. The clinician report of BGM frequency was obtained on the same day as the HbA1c measurement in nearly all participants. A1c was measured using a DCCT-standardized assay (reference range 4–6%).
Ethical considerations
The Institutional Review Board approved the study protocols and parents/youth provided written informed consent/assent prior to completing any study procedures.
Data Analyses
Analyses included descriptive statistics to examine sample characteristics, using mean±standard deviations, and percentages. To examine associations among study variables we used Pearson correlations and paired t-tests. To examine the relationship between A1c and frequency of BGM we performed analysis of variance (ANOVA). All statistical procedures used SAS software (version 9.4, SAS Institute Inc., Cary, NC). P values <.05 were considered statistically significant.
RESULTS
Participant Characteristics
Participants in this study were 385 youth (50% female), 13.6±2.5 (mean±SD) years old, with diabetes duration 6.5±3.5 years. The majority (74%) were on insulin pump therapy and mean A1c was 8.2±0.9%. Additional characteristics are shown in Table 1.
Table 1.
Participant Characteristics (N=385)
| Age (years) | 13.6±2.5 |
| Sex (% female) | 50 |
| z-BMI | 0.7±0.8 |
| Race/ethnicity (% non-white) | 9 |
| Family structure (% 2-parent) | 90 |
| Diabetes duration (years) | 6.5±3.5 |
| Insulin regimen (% pump therapy) | 74 |
| Daily insulin dose (Units/kg) | 0.9±0.3 |
| A1c (%) | 8.2±0.9 |
Data presented are mean±SD or percent.
Blood Glucose Monitoring Frequency by Method
BGM frequency ranged from 0.5 to 14.1 times/day. While the majority of youth (58%) reported using 1 meter, a substantial proportion (42%) used >1 meter. BGM frequency from self-report (6.4±2.3 times/day) was significantly higher than BGM frequency from both clinician report (5.7±2.4 times/day, p<.0001) and meter download (5.6±2.4 times/day, p<.0001). The distributions of BGM frequency according to method of ascertainment are shown in Figure 1.
Figure 1.

Distribution of BGM Frequency by Ascertained Method for BGM.
In addition, in all three methods, more frequent BGM was associated with lower mean glucose (meter download, r= −.36; clinician report, r= −.32; self-report, r= −.22; all p<.0001).
BGM frequency was significantly correlated between all 3 methods (meter download/clinician report, r=0.82, p<.0001; meter download/self-report, r=0.71, p<.0001; clinician report/self-report, r=0.75, p<.0001).
Association between Blood Glucose Monitoring Frequency and A1c
For all methods, more frequent BGM was significantly associated with lower A1c (meter download, r= −.40, p<.0001; clinician report, r= −.38, p<.0001; self-report, r= −.29, p<.0001) (Figure 2).
Figure 2.

Associations between BGM Frequency and HbA1c by Ascertained Method for BGM.
Similar associations of BGM frequency with A1c were present across age strata (<13 years [n=133, r= −.26 to −.45, all p≤.002] and ≥13 years [n=252, r= −.25 to −.32, all p<.0001]), diabetes duration strata (<5 years [n=148, r= −.34 to −.38, all p<.0001] and ≥5 years [n=237, r= −.26 to −.39, all p<.0001]), sex (male [n=192, r= −.34 to −.47, all p<.0001] and female [n=193, r= −.27 to −.35, all p≤.0001]), and insulin regimen (injections [n=100, r= −.29 to −.49, all p≤.003] and pump [n=285, r= −.27 to −.35, all p<.0001]).
As BGM frequency from meter download presented the most objective data and had the strongest correlations with A1c, we used BGM frequency from meter download to confirm the relationship of BGM frequency with A1c. For each additional daily blood glucose check, there was a 0.2% decrease in A1c (p<.0001) (Figure 3). Among those checking 0–5 times/day, for each additional daily BGM check, there was a 0.3% decrease in A1c (p<.0001).
Figure 3.
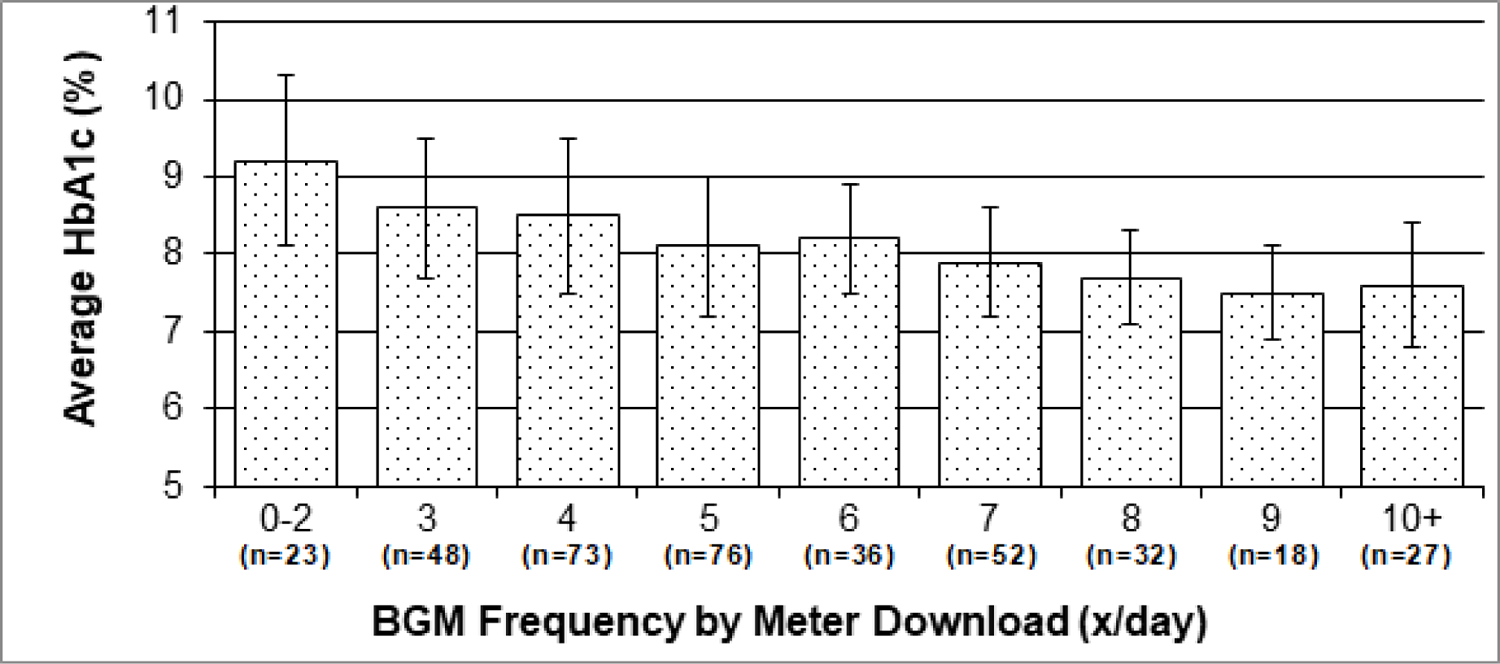
Mean HbA1c According to BGM Frequency by Meter Download. Error bars depict standard deviations.
Similar associations were present across insulin regimen (injections and pump) (Figure 4). For each additional BGM check, there was a 0.2% decrease in A1c among injection users (p<.0001) and a 0.1% decrease in A1c (p<.0001) among pump users. Among those checking 0–5 times/day, for each additional BGM check, there was a 0.3% decrease in A1c among injection users (p=.03) and a 0.2% decrease in A1c among pump users (p=.005).
Figure 4.

Mean HbA1c According to BGM Frequency by Meter Download and insulin delivery method (injection and pump). Error bars depict standard deviations.
DISCUSSION
We compared BGM frequency ascertained by three different methods in a sample of 385 youth with type 1 diabetes. Our results indicate BGM frequency by all methods was significantly correlated. However, self-report of BGM levels were significantly higher than both meter download and clinician report by almost 1 time/day. When self-reporting BGM frequency, youth and families might overestimate number of BGM checks per day likely due to social desirability bias (e.g. presenting a logbook that will be approved by the health care team). 18, 19
BGM frequency by clinician report was the only one highly correlated to the gold standard of meter download. However, clinician report is often derived from family report, school reports, meter download or patient logbook. Therefore, reliability of clinician report may be limited by the biases associated with the data on which it is based. To this end, our findings add to the existing literature supporting the need for more meter download use in clinical practice. 12, 13
Furthermore, previous studies have confirmed a tight inverse relationship between BGM frequency and A1c levels. In data from >20,000 persons with type 1 diabetes, more frequent daily BGM was strongly associated with lower A1c (p<.001) across all age groups and in both insulin pump and injection users. 9 Our study found more frequent BGM was significantly associated with lower A1c and lower mean glucose in all three approaches. Similar associations were present across age (<13 years and ≥13 years), diabetes duration (<5 years and ≥5 years), sex, and insulin regimen (injections and pump therapy). Also, youth using injections, as well as youth checking BGM levels 0–5 times/day, benefitted from a greater decrease in A1c for each additional BGM check. Therefore, providing youth with more BGM strips aids glycemic control and must be considered for evidence-based practice. Furthermore, strategies could be used in clinical practice to increase the frequency of BGM such as text message reminders17 and interventions designed to increase parent-adolescent communication18.
There are ways in which the current report can help inform understanding of CGM use in the current era and in areas where CGM penetration is high. For example, use of professional CGM may encourage persons with diabetes to “perform” differently during the 7, 10, or 14 days of CGM wear when the person with diabetes is aware that the results will be carefully reviewed afterwards by providers. 19 Those using personal CGM may also be more likely to maintain better self-care behaviors in the 2 weeks that precede a clinic appointment. At such times when the 2 weeks of CGM data do not match the measured laboratory A1c value, the clinician might want to review CGM glucose data from the previous 30, or even 90, days. Of course, in these cases, there may be other ‘fallacies’ to interpreting the A1c data that experienced clinicians understand. 20
Finally, acknowledging the importance of frequent BGM among low-adherent youth might also justify the use of technologies to support glycemic vigilance.20 Policy changes in public health and insurance coverage can benefit youth with deteriorating glycemic control with technology advantages and aid access to CGM systems.
Limitation and Strengths
A notable limitation of this study is that when analyzing BGM frequency impact, the influence of non-modifiable factors was not take into account, such as youth race, family socioeconomic status, and youth and parent cognitive function on youth’s A1c levels 21. Moreover, frequency of blood glucose monitoring is but one metric of family and youth adherence to diabetes management. There is also a potential for missing glucose meter data, reflecting devices left at school, sports or daycare, potentially underestimating the meter download report. Clinicians likely accounted for such meters in their reports. Next, this investigation did not collect information related to how BGM data were used in diabetes management, for example, if more frequent BGM supported the timely delivery of insulin correction doses. Last, the study participants were part of clinical trials, potentially reducing generalizability. Future research is needed to understand how adolescents and families respond to numbers from BGM and how that influences BGM frequency.
Nonetheless, strengths of the present study include its use of a central laboratory for measuring A1c, a large sample size of an age group at risk for suboptimal glycemic control, and multiple sources of BGM reports for comparison.
CONCLUSIONS
The three methods of assessing BGM frequency (meter download, clinician report, and self-report) were significantly correlated. BGM frequency by self-report was significantly higher than by both meter download and clinician report. Overall, meter download had the strongest correlations with A1c.
In youth with T1D, more frequent BGM was associated with lower A1c across age, diabetes duration, sex, and insulin regimen. In the modern era of intensive insulin therapy and advanced diabetes technologies, BGM remains a potent predictor of glycemic control. Meter download should be continuously encouraged in order to ensure nursing evidence-based practice. Frequency of BGM is a modifiable component of diabetes self-management that warrants continued targeting in efforts to improve glycemic control in youth with type 1 diabetes.
Highlights.
Our findings support the need for more meter download use in clinical practice
Frequent BGM was associated with lower A1c and lower mean glucose
Acknowledging BGM importance among low-adherent youth justify the use of technologies
Acknowledgments
Financial support: This research was supported by NIH grants R01DK089349, R01DK095273, and P30DK036836, JDRF grant 2-SRA-2014–253-M-B, the Katherine Adler Astrove Youth Education Fund, the Maria Griffin Drury Pediatric Fund, the Eleanor Chesterman Beatson Fund, and a Mary Iacocca Research Fellowship by the Iacocca Family Foundation.
Footnotes
Guarantor Statement: Dr. Lori M. Laffel is the guarantor of this work and, as such, had full access to all information and takes responsibility for the integrity and accuracy of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-Of-Interest Disclosure: None of the authors have any potential conflicts of interest to disclose.
REFERENCES
- 1.Patterson CC, Karuranga S, Salpea P, et al. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. November 2019;157:107842. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Children and Adolescents: Standards of Medical Care in Diabetes-2020. Diabetes Care. January2020;43(Suppl 1):S163–S182. [DOI] [PubMed] [Google Scholar]
- 3.Foster NC, Beck RW, Miller KM, et al. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther. February2019;21(2):66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan W, Zheng H, Wei N, Nathan DM. Estimating HbA1c from timed Self-Monitored Blood Glucose values. Diabetes Res Clin Pract. July 2018;141:56–61. [DOI] [PubMed] [Google Scholar]
- 5.Gomes MB, Tannus LR, Cobas RA, et al. Determinants of self-monitoring of blood glucose in patients with Type 1 diabetes: a multi-centre study in Brazil. Diabet Med. October 2013;30(10):1255–1262. [DOI] [PubMed] [Google Scholar]
- 6.Garg SK, Hirsch IB. Self-Monitoring of Blood Glucose. Diabetes Technol Ther. February 2019;21(S1):S4–S12. [DOI] [PubMed] [Google Scholar]
- 7.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Hemoglobin A1c and mean glucose in patients with type 1 diabetes: Analysis of data from the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Care. 3/2011. 2011;34(3):540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan DM, Kuenen J, Borg R, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. August2008;31(8):1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller KM, Beck RW, Bergenstal RM, et al. Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care. 7/20132013;36(7):2009–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler R, Heidtmann B, Hilgard D, et al. Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2/2011. 2011;12(1):11–17. [DOI] [PubMed] [Google Scholar]
- 11.Blackwell M, Tomlinson PA, Rayns J, Hunter J, Sjoeholm A, Wheeler BJ. Exploring the motivations behind misreporting self-measured blood glucose in adolescents with type 1 diabetes - a qualitative study. J Diabetes Metab Disord. 2015;15:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guilfoyle SM, Crimmins NA, Hood KK. Blood glucose monitoring and glycemic control in adolescents with type 1 diabetes: meter downloads versus self-report. Pediatr Diabetes. September 2011;12(6):560–566. [DOI] [PubMed] [Google Scholar]
- 13.Chae M, Reith DM, Tomlinson PA, Rayns J, Wheeler BJ. Accuracy of verbal self-reported blood glucose in teenagers with type I diabetes at diabetes ski camp. J Diabetes Metab Disord. January 8 2014;13(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sjoeholm A, Gray A, Rayns J, Tomlinson PA, Wheeler BJ. Prior knowledge of blood glucose meter download improves the accuracy of verbal self-reported blood glucose in teenagers with type I diabetes at ski camp. Acta Diabetol. August 2016;53(4):637–642. [DOI] [PubMed] [Google Scholar]
- 15.Commissariat PV, Volkening LK, Guo Z, ElBach JL, Butler DA, Laffel LM. Associations between major life events and adherence, glycemic control, and psychosocial characteristics in teens with type 1 diabetes. Pediatr Diabetes 2018;19:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Telo GH, Volkening LK, Butler DA, Laffel LM. Salient characteristics of youth with type 1 diabetes initiating continuous glucose monitoring. Diabetes Technol Ther 2015;17:373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caccavale LJ, Nansel TR, Quick V, Lipsky LM, Laffel LM, Mehta SN. Associations of Disordered Eating Behavior With the FamilyDiabetes Environment in Adolescents With Type 1 Diabetes. J Dev Behav Pediatr 2015;36:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monaghan M, Clary L, Mehta P, Stern A, Sharkey C, Cogen FR, Vaidyanathan P, Streisand R. Checking In: A Pilot of a Physician-Delivered Intervention to Increase Parent-Adolescent Communication About Blood Glucose Monitoring. Clin Pediatr (Phila). 2015. December;54(14):1346–53. 10.1177/0009922815581833. Epub 2015 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Driscoll KA, Johnson SB, Tang Y, Yang F, Deeb LC, Silverstein JH. Does blood glucose monitoring increase prior to clinic visits in children with type 1 diabetes? Diabetes Care. 10/20112011;34(10):2170–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delamater AM, Kurtz SM, White NH, Santiago JV. Effects of Social Demand on Reports of Self-Monitored Blood Glucose in Adolescents with Type 1 Diabetes Mellitus. Journal of Applied Social Psychology. 1988;18(6):491–502. [Google Scholar]
- 21.Blumer I The Contemporary Role of Masked Continuous Glucose Monitoring in a Real-Time World. J Diabetes Sci Technol. May 2016;10(3):790–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The Fallacy of Average: How Using HbA1c Alone to Assess Glycemic Control Can Be Misleading. Diabetes Care. August2017;40(8):994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalew S, Gomez R, Vargas A, et al. Hemoglobin A1c, frequency of glucose testing and social disadvantage: Metrics of racial health disparity in youth with type 1 diabetes. J Diabetes Complications. December 2018;32(12):1085–1090. [DOI] [PubMed] [Google Scholar]


