Abstract
Introduction:
Chronic kidney disease (CKD) is a prevalent complication of sickle cell anemia (SCA). Hyperfiltration that delayed detection of CKD is common in SCA patients. Identification of novel urinary biomarkers correlating with glomerular filtration rates may help to detect and predict progression of renal disease.
Methods:
Re-analysis of mass spectra of urinary samples obtained from University of Illinois at Chicago identified kringle-domain-containing protein HGFL.
Results:
HGFL levels correlated with hyperfiltration; were significantly reduced at CKD stage 1 compared to stage 0; negatively correlated with progression of CKD and were suitable for differentiation of stage 1. Better prediction of CKD progression to stage 2 was observed for HGFL-based risk prediction compared to eGFR-based. Results from a Howard University patient cohort supported the utility of HGFL-based test for the differentiation of stage 1 of CKD.
Conclusion:
Urinary HGFL may contribute additional information beyond eGFR, and improve diagnosis of early stage CKD in SCA patients.
Keywords: sickle cell anemia, chronic kidney disease, HGFL, selected reaction monitoring, glomerular filtration rate, hyperfiltration
Introduction
Sickle-cell anemia (SCA) is a rare hereditary disorder that affects approximately 100,000 people in the USA, primarily of African descent. The E6V mutation in the β-globin gene leads to production of hemoglobin S that forms polymers under low oxygen conditions, inducing hemolysis of red blood cells, vaso-occlusion, and organ damage [1]. Chronic kidney disease (CKD) is a common complication of SCA that is observed in more than 50% of patients [2]. The prevalence of hyperfiltration defined as estimated glomerular filtration rate (eGFR)>130 ml/min/1.73m2 in SCA adult patients without albuminuria is more than 50% [3, 4]. Hyperfiltration delays detection of CKD. Whole kidney hyperfiltration results from single nephrons hyperperfusion which is associated with nephron damage before the onset of whole kidney hyperfiltration.The early detection of renal injury and prediction the CKD progression is essential to initiation of kidney protective therapy. Clinical biomarkers for early stage of CKD, such as proteinuria, albuminuria and increased eGFR, are not reliable in the presence of hyperfiltration and have low diagnostic value in SCA patients [3]. Hyperfiltration increases creatinine (Cr) clearance leading to overestimation of calculated eGFR. Because measurement of GFR in SCA patients is problematic, the identification and monitoring of CKD progression is challenging [5]. Thus, the unique pathophysiology of SCA may impede detection and risk of progression of CKD due to a high prevalence of hyperfiltration. Therefore, identification of novel reliable biomarkers to facilitate early diagnosis and predict the progression of renal disease in SCA patients is highly anticipated.
The purpose of the current study is to identify a urinary biomarker that correlates with hyperfiltration and/or eGFR, and may be beneficial for early detection of CKD and prediction of risk for disease progression. Urinary proteins are promising non-invasive biomarkers of renal impairment. Previously, we performed high-resolution liquid chromatography -Fourier transform mass spectrometry (LC-FT/MS) analysis of urine samples collected from SCA patients from University of Illinois at Chicago that revealed ceruloplasmin and orosomucoid as biomarkers of CKD reflecting abnormal iron homeostasis and inflammation [6, 7, 4]. Moreover, both ceruloplasmin and orosomucoid urinary levels positively correlated with the stages of CKD in SCA patients. In contrast, these biomarkers did not correlate with eGFR and hyperfiltration.Here we re-analyzed mass- spectrometry results stratifying them for hyperfiltration.
Methods
Fifty-four urine samples were obtained from University of Illinois at Chicago (Chicago cohort) and thirty samples were obtained from Sickle Cell Disease Registry study at Howard University (Howard cohort). Baseline demographic characteristics of subjects are shown in Table 1. Re-analysis of mass spectra of samples (Chicago cohort) was performed using SIEVE 2.1 software (Thermo Fisher, Waltham, MA, USA). Non-labeled and isotope labeled EDQTSPA[13C6, 15N4]PGLR HGFL peptides were obtained from AnaSpec Inc, and used for single reaction monitoring mass-spectrometry. Urinary levels of HGFL, creatinine and albumin were measured by ELISA kits (HGFL from MyBioSource, Cr from R&D Systems, and albumin from Bethyl Laboratories Inc.). Statistical analysis was performed using GraphPad Prizm version 6.07 software. Student’s t-test, Pearson correlation and Receiver operating characteristic (ROC) analysis were performed. Harrell’s c-statistic was used for analysis of risk prediction, p values <0.05 were considered significant.
Table 1.
Baseline demographic and renal function characteristics of subjects from University of Illinois at Chicago (Chicago) and Howard University sickle cell disease registry (Howard) cohorts
| Variable | Chicago | Howard |
|---|---|---|
| Number of patients | 54 | 30 |
| Sex (female/male)1 | 48% (26)/52% (28) | 50% (15)/50% (15) |
| Age (years) | 37 (range 20-58) | 35 (range 18-67) |
| Prevalence of CKD (total)2 | 57.4% (31/54) | 20% (6/30) |
| Stage of CKD2 | ||
| Stage 1 | 71% (22/31) | 100% (6/6) |
| Stage 2 | 3.2% (1/31) | |
| Stage 3 | 22.6% (7/31) | |
| Stage 4 | 3.2% (1/31) | |
| Hyperfiltration at CKD stage 03 | 60.9% (14/23) | 67% (16/24) |
| Hyperfiltration at CKD stage 13 | 50% (11/22) | 83% (5/6) |
Data are shown as percentage (number of subjects).
Data are shown as percentage (number of subjects/total number in the group).
Hyperfiltration was defined as eGFR > 130 ml/min/1.73 m2 for females and eGFR > 140 ml/min/1.73 m2 for males. Data are shown as percentage (number of subjects with hyperfiltration/total number of subjects with the stage).
Results
Sixty urine spectra were generated from 20 samples in triplicates. Samples were collected from SCA patients (Chicago cohort) without renal disease and were separated into two groups: subjects with hyperfiltration (N=13) and subjects with normal filtration rate (N=7). Several urinary proteins were up or down regulated in the urine of subjects with hyperfiltration compared to subjects with normal filtration (suppl. Fig. 1A, volcano plot). Among these proteins, kringle domain containing proteinHGFL levels were increased in the samples collected from subjects with hyperfiltration. Two samples were selected from each group based on the reproducibility of spectra between triplicates and label-free quantification was performed using SIEVE 2.1 software. HGFL levels were normalized to urine Cr levels using SIEVE2.1 built-in normalization tool. The label-free quantification demonstrated higher HGFL levels (2.4-fold) in the subjects with hyperfiltration (suppl. Fig. 1B).
Urinary HGFL levels were further quantified by selected reaction monitoring (SRM) analysis in nineteen samples (Chicago cohort) and twelve control samples (Howard cohort). As HGFL-derived peptide (EDQ.TSPAPGLR) was identified in all samples, it was selected for isotope labeling. High resolution mass spectrometry showed an ion peak for non-labeled HGFL peptide at m/z=585.79 and heavy isotope-labeled peptide at m/z=590.80 (suppl. Fig. 2A). A calibration curve for isotope labeled peptide was generated using 0.5-100 nM concentrations (suppl. Fig. 2B), and was used for quantification of HGFL levels. The labeled peptide (50 nM) was added to each urine sample as internal standard prior sample preparation for mass-spectrometry, and SRM was performed.. HGFL/Cr levels were lower for SCA subjects without kidney disease compared to healthy controls (suppl. Fig. 2C, 6.83±3.01 ng/g vs. 10.67±3.85 ng/g, p=0.0043). Higher levels of HGFL/Cr were observed in SCA subjects with hyperfiltration compared to SCA subjects with normal filtration rates (Fig. 1A, 7.96±2.86 ng/g vs. 4.86±1.93 ng/g, p=0.02). Moreover, HGFL/Cr demonstrated strong positive correlation with eGFR values calculated using the CKD-EPI equation (Fig. 1B, r=0.5117; 95% CI 0.074 to 0.7838; R2=0.2619). No significant correlations were found between HGFL/Cr and urinary albumin or hemoglobin (suppl. Fig. 3A and 3B).
Fig. 1.
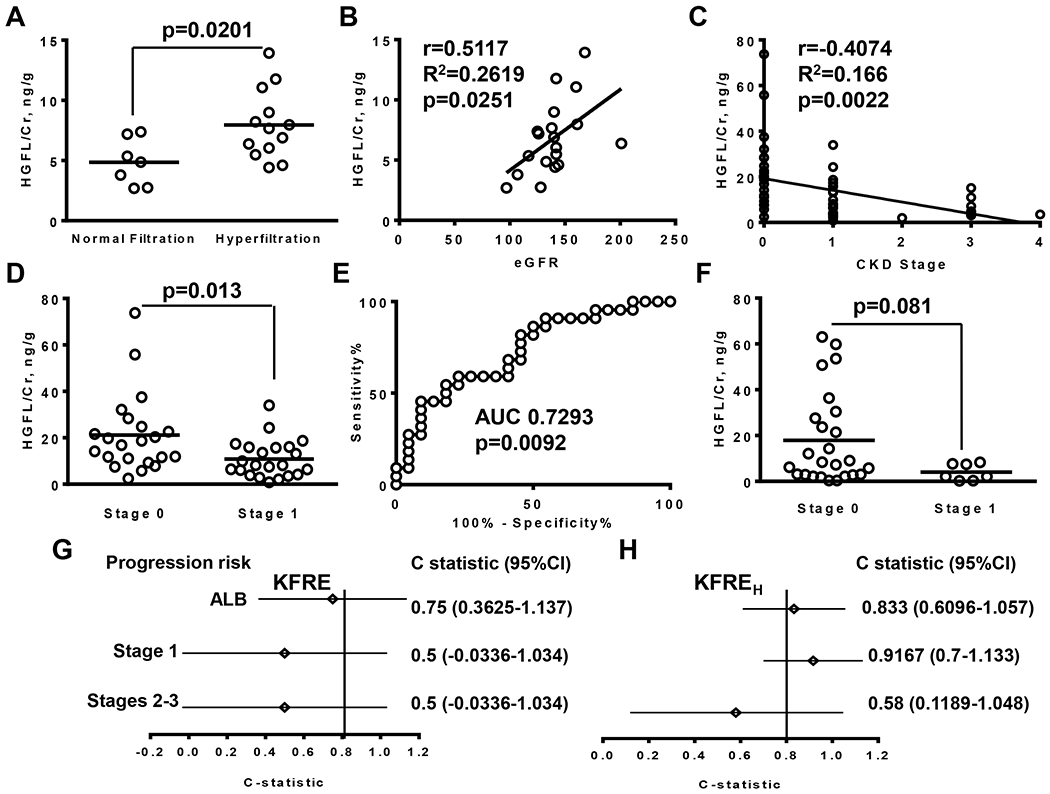
Urinary HGFL levels increase in the SCA subjects with hyperfiltration, correlate with eGFR, allow of differentiation of CKD stage 1, and better predict progression of albuminuria and CKD. (A) HGFL/Cr levels in subjects with normal filtration and hyperfiltration quantified by single reaction monitoring (SRM) mass-spectrometry, performed in 19 samples collected from Chicago cohort patients without kidney disease. Hyperfiltration was defined as eGFR>130 ml/min/1.73m2 for women and >140 ml/min/1.73m2 for men. Results on the graph are shown for each subject and as mean for group. (B) Pearson correlation of HGFL/Cr levels quantified by SRM with eGFR calculated based on CKD-EPI equation. (C). Pearson correlation between HGFL/Cr levels quantified by ELISA and stages of CKD. The 54 samples were collected from the Chicago cohort patients with stages 0-4 CKD. (D) HGFL/Cr levels at stage 1 of CKD and in subjects without kidney disaese quantified by ELISA in the Chicago cohort samples (N=22). Results on the graph are shown for each subject and as mean for group. (E) Receiver operating characteristic curve analysis for differentiation of stage 1 of CKD from no kidney disease for the Chicago cohort samples. AUC is an area under curve. (F) HGFL/Cr levels at stage 1 of CKD and in subjects without kidney disease quantified by ELISA in the Howard cohort samples (25 sarnies with stage 0 and 7 samples with stage 1). Results on the graph are shown for each subject and as mean for group. (G-H) Fifty-four patients from the Chicago cohort were followed up for a median length of 26 months (range 6-68 months) and risk of disease progression was calculated using 4 variables kidney failure risk equation. (G) C-statistic, calculated using eGFR-based equation. (H) C-statistic, calculated using HGFL-based equation. Area under curve (AUC) and 95% confident intervals (95% Cl) are shown. ALB- risk of progression from microalbuminurria (30mg/g<ALB/Cr<300 mg/g) to macroalbuminuria (ALB/Cr>300 mg/g), Stage 1 - risk of progression from stage 1 to stage 2 CKD, Stage 2-3 – risk of progression from stage 2 and 3 to the next stage of renal disease. Cr – creatinin.
SRM detection is highly sensitive and reproducible, but has a limited application in the clinical setting. Thus commercially available enzyme-linked immunosorbent assay was used for the quantification of HGFL, as ELISA assays are widely available and more suitable for clinical diagnostics. The HGFL/Cr values measured by ELISA were 4.1-fold higher and demonstrated a weak correlation with the values determined by SRM (suppl. Fig. 4A, r=0.1952; 95% CI −0.2842 to 0.5965, R2=0.0381). The higher HGFL values and weak correlation with SRM might result from non-specific binding of other urine proteins to ELISA plates. No significant difference was found between SCA and control groups (suppl. Fig. 4B, 21.92±21.55 ng/g vs. 22.99±17.18 ng/g, p=0.8978).
To further test the utility of HGFL ELISA for detection of CKD in SCA patients, we extended analysis including additional 34 samples collected from SCA patients (Chicago cohort) with different CKD stages. HGFL/Cr levels negatively correlated with progressive CKD stage (Fig. 1C, r=−0.4074; 95% CI −0.6087 to 0.1567; R2=0.1660). HGFL/Cr levels at stage 1 were significantly lower than in SCD subjects without renal disease (Fig. 1D, 10.83±8.13 ng/g vs. 21.162±16.85 ng/g, p=0.013, N=22). In contrast, eGFR values were similar for subjects without renal disease and with stage 1 (137±25.4 ml/min/1.73m2 vs. 137.1±14.2 ml/min/1.73m2, p=0.9876). Receiver operating characteristic curve (ROC) analysis demonstrated moderate sensitivity and specificity (Fig. 1E, sensitivity 59.09%, 95% CI 48.63 to 83.32%; specificity 77.27%, 95% CI 54.63 to 92.18%; OD 2.6; AUC 0.7293) for CKD stage 1 differentiation at a cutoff HGFL/Cr value 10.5 ng/g. Taken together, HGFL ELISA assay was successfully used for the differentiation of stage 1 CKD.
In addition we examined the utility of HGFL/Cr levels for differentiation of CKD stage 1 using 30 samples obtained from Howard cohort. HGFL/Cr levels in subjects without kidney disease in Howard cohort were similar to the levels detected in Chicago cohort (suppl, Fig. 4B, 18.67±20.15 ng/g vs. 23±17.65 ng/g; p=0.4782). The HGFL/Cr in the Howard cohort were also reduced at stage 1 compared to subjects without kidney disease but the difference was not statistically significant (Fig. 1F, 4.02±3.62 ng/g vs. 18.67±20.15 ng/g; p=0.081). ROC analysis demonstrated high sensitivity but low specificity for differentiation of stage 1 at a cutoff HGFL/Cr value less than 8 ng/mg (sensitivity 100%, 95%CI 54.07 to 100%; specificity 54.17%, 95% CI 32.82 to 74.45%; OR 2.182; AUC 0.7565). Areas under courve were similar for Chicago and Howard cohorts (0.7293 vs. 0.7565). Thus the Howard cohort study supported the utility of HGFL/Cr based test for the differentiation of stage 1 of CKD from no kidneyl disease.
Next we test the ability of HGFL to predict the risk of CKD progression in SCA patients. Quantification of risk was performed using 4 variables kidney failure risk equation (KFRE) [8]: KFRE=1-0.9240^[exp(−0.2201*(age/10-7.036)+0.2467*(gender-0.5642)−0.5567*(eGFR/5-7.222)+0.5642)+0.4510*(ln(ALB/Cr)-5.137))], where age was in years; gender was 1 for male and 0 for female; eGFR was in ml/min/1.73m2; and albumin/ creatinine ratio (ALB/Cr) was in mg/g.
We developed a modified KFREH equation based on the HGFL/Cr values using linear regression equation for eGFR and HGFL/Cr (HGFL/Cr=0.06705*eGFR-2.556): KFREH=1-0.9240A[exp(−0.2201*(age/10-7.036)+0.2467*(gender-0.5642)-0.5567*(HGFL/Cr/0.6705-3.4099)+0.5642)+0.4510*(ln(ALB/Cr)-5.137))], where HGFL/Cr ratio was in ng/g. Coefficients for age, gender and ALB/Cr were the same for both equations.
Fifty-four patients from the Chicago cohort were followed up for a median length of 26 months (range 6-68 months). During the follow-up, two patients with CKD stage 1 progressed with albuminuria status from microalbuminuria (30 mg/g<ALB/Cr<300 mg/g) to macroalbuminuria (ALB/Cr>300 mg/g); two patients with stage 1 progressed to stage 2; and two patients with stages 2-3 CKD progressed to the next stage. KFRE and KFREH equations were used for calculation the risk of disease progression, and c-statistic (discrimination statistics) was calculated for three groups of patients separately. C-statistics was calculated for 14 patients with stage 1 CKD and microalbuminuria, 8 patients with stage 1 CKD and macro-albuminuria, and 8 patients with CKD stages 2-3 (Fig. 1G and H). The eGFR-based calculation of KFRE showed a relatively good prediction of progression from micro to macro-albuminuria at stage 1 (Fig. 1G, 0.75, 95% CI 0.3625-1.117), and poor prediction for CKD progression from stage 1 to stage 2 (Fig. 1G, 0.5, 95% CI 0.0336-1.034). In contrast, HGFL-based equation provided strong prediction for progression from micro to macro-albuminuria at stage 1 (Fig. 1H, 0.833, 95% CI 0.6096-1.057) and CKD progression from stage 1 to stage 2 (Fig. 1H, 0.9167, 95% CI 07-1.133). Both eGFR and HGFL-based equations showed poor prediction for CKD progression for stage 2-3 patients (Fig. 1G and H, 0.5, 95% CI-0.0336-1.034 for KFER; and 0.58, 95% CI 0.1189-1.048 for KFREH). Thus the kidney risk equation based on the urinary HGFL/Cr is similar or marginally superior to the eGFR-based prediction and may be suitable for the prediction of disease progression at the early stage of CKD in SCA patients
Discussion
In this study we identify HGFL protein as a potential biomarker of renal disease in SCA patients. To our knowledge, this is the first study identifying a potential urinary biomarker that correlates with hyperfiltrationand eGFR in SCA patients, differentiates stage 1 of CKD from no kidney disease, and predicts renal disease progression.
Cohorts from the large interventional studies were used previously for the identification of urinary biomarkers to improve prediction of renal disease progression [9]. However this aproach is not feasible for rare diseases. Because large amounts of non-specific proteins are released into the urine during advanced CKD stages, we did not compare samples from SCA patients with and without proteinuria. Instead, we evaluated relationship of a relatively small number of urinary proteins identified in the samples from SCA patients without proteinuria with hyperfiltration, that is the risk factor for renal disease. A similar approach has been used by our group for identification of urinary biomarkers that correlated with hemoglobinuria [6, 7]. Using this approach we identified HGFL as a potential biomarker correlating with hyperfiltration.
Interestingly, HGFL levels did not correlate with albumin and hemoglobin levels. Furthermore, HGFL levels were decreased at stage 1 CKD. Thus, HGFL levels in urine did not correlate with increased renal filtration of plasma proteins.
HGFL (PIK3IP1) is a phosphoinositide-3-kinase-interacting protein 1, also known as kringle domain- containing protein. PIK3IP1 is among thirty most abundant proteins in normal human urine [10] and its levels are increased in diabetic renal disease [11]. PIK3IP1 was shown to negatively regulate PI3K activity and block activation of Akt [12]. PI3K/Akt pathway is involved in different renal pathologies including hypoxia-induced renal fibrosis [13], polycystic renal disease[14], and renal carcinoma [15]. Further investigation is needed to determine HGFL role in the renal function and CKD.
Additionally, we considered whether the urinary HGFL levels in SCA patients with mild renal dysfunction can predict kidney function decline using risk equation [8]. The kidney risk equation based on the urinary HGFL is similar to the eGFR-based prediction and suitable for the prediction of progression of both albuminuria and stage 1 CKD even in a very small cohort. Because of the low number of progressors in the patient cohort, the results obtained here will need further validation in large longitudinal cohort.
There are several limitations to our study. The first limitation is the small number of patients in both discovery and validation cohorts. Even smaller number of patients progress with stage of renal diseases. The small number of samples used for calculation mayt produce larger ranges for c-statistic 95% confidential ratios. The second limitation is that the GFR is calculated. Thus, further validation is needed to fully correlate urinary HGFL with measured GFR in SCA patients. Finally, the role of HGFL in the renal function and the cause of its reduction in CKD urine should be further investigated. In conclusion, HGFL may be used as diagnostic and prognostic biomarker of CKD in addition to microalbuminuria and eGFR in SCA patients.
Supplementary Material
Acknowledgement
Songping Wang and Dr. Tatiana Ammosova (Howard University Center for Sickle Cell Disease) provided help with preparing samples for mass-spectrometry. Dr. Xiaomei Niu (Howard University Center for Sickle Cell Disease) helped to select samples from HUH CSCD repository.
Funding Sources
This work was supported by NIH Research Grants SC1HL150685, P50HL118006, R01HL125005, 5U54MD007597, R01HL153161 and R03HL146788. AT was supported by ASH MMSAP (American Society of Hematology Minority Medical Student Award Program). The content is solely the responsibility of the authors, and does not necessarily represent the official view of NHLBI, NIMHD or NIH.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Statement of Ethics
All studies were conducted in complience with the principles of the Declaration of Helsinki. The study was approved by the institutional review boards (IRBs) of the University of Illinois at Chicago (UIC) and Howard University (HU). All subjects provided written informed consent prior to urine sample collection.
References
- 1.Niu X, Nouraie M, Campbell A, Rana S, Minniti CP, Sable C, et al. Angiogenic and inflammatory markers of cardiopulmonary changes in children and adolescents with sickle cell disease. PloS one. 2009Nov 23;4(11):e7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Airy M, Eknoyan G. The kidney in sickle hemoglobinopathies. Clinical nephrology. 2017Feb;87 (2017)(2):55–68. [DOI] [PubMed] [Google Scholar]
- 3.Haymann JP, Stankovic K, Levy P, Avellino V, Tharaux PL, Letavernier E, et al. Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clinical journal of the American Society of Nephrology : CJASN. 2010May;5(5):756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jerebtsova M, Taye A, Smith N, Afangbedji N, Stokes D, Niu X, et al. Association between plasma and urinary orosomucoid and chronic kidney disease in adults with sickle cell disease. British journal of haematology. 2020May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheinman JI. Sickle cell disease and the kidney. Nature clinical practice Nephrology. 2009Feb;5(2):78–88. [DOI] [PubMed] [Google Scholar]
- 6.Jerebtsova M, Saraf SL, Lin X, Lee G, Adjei EA, Kumari N, et al. Identification of ceruloplasmin as a biomarker of chronic kidney disease in urine of sickle cell disease patients by proteomic analysis. American journal of hematology. 2018Feb;93(2):E45–E47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jerebtsova M, Saraf SL, Soni S, Afangbedji N, Lin X, Raslan R, et al. Urinary orosomucoid is associated with progressive chronic kidney disease stage in patients with sickle cell anemia. American journal of hematology. 2018Aug;93(4):E107–E09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tangri N, Grams ME, Levey AS, Coresh J, Appel LJ, Astor BC, et al. Multinational Assessment of Accuracy of Equations for Predicting Risk of Kidney Failure: A Meta-analysis. Jama. 2016Jan 12;315(2):164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombo M, McGurnaghan SJ, Bell S, MacKenzie F, Patrick AW, Petrie JR, et al. Predicting renal disease progression in a large contemporary cohort with type 1 diabetes mellitus. Diabetologia. 2020Mar;63(3):636–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao M, Li M, Yang Y, Guo Z, Sun Y, Shao C, et al. A comprehensive analysis and annotation of human normal urinary proteome. Scientific reports. 2017Jun 8;7(1):3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marikanty RK, Gupta MK, Cherukuvada SV, Kompella SS, Prayaga AK, Konda S, et al. Identification of urinary proteins potentially associated with diabetic kidney disease. Indian journal of nephrology. 2016Nov-Dec;26(6):434–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Z, He X, Johnson C, Stoops J, Eaker AE, Stoffer DS, et al. PI3K is negatively regulated by PIK3IP1, a novel p110 interacting protein. Biochemical and biophysical research communications. 2007Jun 22;358(1):66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu M, Ning X, Li R, Yang Z, Yang X, Sun S, et al. Signalling pathways involved in hypoxia- induced renal fibrosis. Journal of cellular and molecular medicine. 2017Jul;21(7):1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margaria JP, Campa CC, De Santis MC, Hirsch E, Franco I. The PI3K/Akt/mTOR pathway in polycystic kidney disease: A complex interaction with polycystins and primary cilium. Cellular signalling. 2020Feb;66:109468. [DOI] [PubMed] [Google Scholar]
- 15.De Santis MC, Sala V, Martini M, Ferrero GB, Hirsch E. PI3K Signaling in Tissue Hyper-Proliferation: From Overgrowth Syndromes to Kidney Cysts. Cancers. 2017Mar 29; 9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


