Abstract
Objective:
Cholesterol efflux capacity (CEC), the ability of extracellular acceptors to pick up cholesterol from macrophages, is a clinically relevant cardiovascular biomarker. CEC is inversely associated with incident ASCVD events. However, CEC is only modestly associated with HDL-C levels, which may explain the failure of HDL-C raising therapies to improve ASCVD outcomes. Determinants of variation in CEC are not well understood. Thus, we sought to establish whether extreme high and low CEC is a robust persistent phenotype and to characterize associations with cholesterol, protein, and phospholipids across the particle size distribution.
Approach and Results:
CEC was previously measured in 2,924 participants enrolled in the Dallas Heart Study, a multi-ethnic population-based study from 2000–2002. We prospectively recruited those who were below the 10th and above 90th percentile of CEC. Our study revealed that extreme low and high CEC are persistent, robust phenotypes after 15 years of follow up. Utilizing size exclusion chromatography, CEC to fractionated plasma depleted of apolipoprotein B (fraction-specific CEC) demonstrated significant differences in CEC patterns between persistent high and low efflux groups. Fraction-specific CEC was correlated with fraction-specific total phospholipid but not apolipoprotein A-I, cholesterol, or total protein. These correlations varied across the size distribution and differed among persistent high vs. low efflux groups.
Conclusion:
Extreme high and low CEC are persistent and robust phenotypes. CEC patterns in fractionated plasma reveal marked variation across the size distribution. Future studies are warranted to determine specific molecular species linked to CEC in a size-specific manner.
Keywords: atherosclerosis, cholesterol, cardiovascular research, cardiovascular disease risk factors, cardiovascular disease, Lipids and Cholesterol, Biomarkers, Basic Science Research
Introduction
Reverse cholesterol transport (RCT) is an important mechanism for maintaining intracellular lipid homeostasis and is deemed a key anti-atherosclerotic function1. However, almost all interventions to date have targeted limiting the influx and deposition of circulating cholesterol into the arterial wall instead of focusing on promotion of RCT as an anti-atherosclerotic strategy. Appropriate balance of cholesterol influx and efflux are crucial in preventing overloading of macrophages with cholesterol and transformation into foam cells, which ultimately leads to atherosclerotic plaque formation. As a circulating biomarker for this pathway, macrophage-specific cholesterol efflux capacity (CEC) to plasma or serum depleted of apolipoprotein B-containing particles has been shown to be inversely associated with prevalent coronary atherosclerosis, incident myocardial infarction, and recurrent cardiovascular (CV) events 2–8. These associations are independent of HDL cholesterol levels and apolipoprotein A-1 levels, the main acceptors of cholesterol from the periphery, suggesting the CEC trait is clinically relevant as a prognostic indicator and may be a viable therapeutic target.
However, despite multiple large studies of CEC, it remains unclear what risk factors, lipid species, or other molecular entities drive variation in the CEC trait in humans. HDL-C, Apo-AI, and total HDL particle concentration only modestly correlate with CEC in most cohorts, suggesting other unidentified factors may be more relevant. Some studies suggest that the CEC can persist over time, suggesting a biologically relevant trait 9. Whether CEC is truly a persistent and robust phenotype remains unclear. Understanding variation in CEC in a diverse human population may lead to insights regarding the molecular drivers of CEC and may reveal new therapeutic targets to prevent and treat atherosclerosis.
To better understand variation in CEC, we sought to study individuals at the extremes of CEC at baseline who persisted in their cholesterol efflux trait over 15 years of follow up. We utilized participants enrolled in the Dallas Heart Study due to the multi-ethnic composition of the study population, lack of selection bias with respect to chronic conditions, and normal distribution of CEC at baseline. To elucidate patterns of variation in extreme CEC trait we used size exclusion chromatography (SEC, which also lends itself as a tool with which the broad molecular classes of lipids and proteins can be correlated with differing sizes of particles and degrees of efflux.
Materials & Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
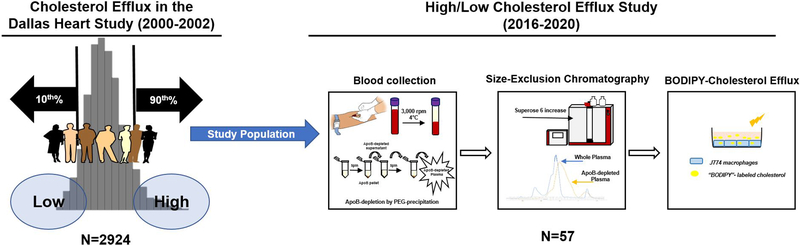
Study population included participants from the Dallas Heart Study (DHS), a multi-ethnic, population-based cohort of Dallas County residents 10. We previously reported baseline cholesterol efflux capacity on 2924 DHS participants 2. For this study, we prospectively recruited a random subset of 57 DHS participants that were below 10th or above 90th percentile of cholesterol efflux distribution (Figure 1 and Supplemental Figure I A–B). Participants with existing malignancy, HIV, chronic kidney disease and/or pregnancy were excluded. One persistent efflux participant was recruited for assessment of technical and biologic reproducibility of the assays.
Figure 1:
Study Design
The study protocol was approved by the Institutional review Board of the University of Texas Southwestern Medical Center and was conducted in compliance with institutional guidelines. At enrollment, all participants provided written informed consents, completed study questionnaire (demographic and medical history), a blood sample and time of last meal information. Participants who reported their time of last meal as less than nine hours at the time of sample collection were defined as non-fasting.
Blood collection
Blood was collected in standard blood collection tubes containing EDTA (BD 366643), maintained at 4°C for ≥3 hour and then centrifuged at 3000 rpm for 15 minutes at 4°C to isolate plasma. Plasma was removed and immediately used for lipoprotein separation and efflux assays or stored at −80°C. Whole blood (BD367844) and serum (367988) aliquots were sent to Quest Diagnostics for standard lipid, metabolic panel and complete blood count measurements.
Preparation of ApoB-depleted plasma (PEG-precipitation)
EDTA plasma collected from participants was depleted of ApoB-containing lipoproteins using polyethylene glycol precipitation method. 20% PEG (molecular weight 6000, Sigma-Aldrich) was prepared in 200mM, pH 7.4 glycine buffer. For every 10μL of whole plasma 4μL of PEG was added (Plasma:PEG, 10:4 ratio). The mixture was incubated for 20 minutes at room temperature and then centrifuged at 16,000 rpm for 30 minutes at 4°C. Following centrifugation, the supernatant was transferred to a fresh tube and centrifuged a second time to ensure complete removal of ApoB-containing lipoproteins. This process is outlined in our study design (Figure 1).
Lipoprotein separation by fast-performance liquid chromatography
500μL of fresh apoB-depleted plasma was applied to a single Superose 6 (10/300 GL; GE Healthcare) size-exclusion column on an ÄKTA pure protein purification system (GE Healthcare). The sample was processed at a flow rate of 0.4 mL/min, detection wavelength 280nm, and 4°C. Samples were eluted in filtration buffer (20mM NaH2PO4, 30mM Na2HPO4,150mM NaCl). A total of 77 fractions, 300μL each, were eluted using fraction collector F9-C (GE healthcare). To approximate the size distribution of eluted fractions, a series of standard gel filtration chromatography molecular weight markers (Sigma, MWGF200 and MWGF1000) were separated on our Superose 6 column (Supplemental Figure IV).
BODIPY-cholesterol efflux
Cholesterol efflux capacity (CEC) was determined by measuring the efflux of a fluorophore tagged cholesterol, BODIPY (Avanti polar lipids), from J774 murine macrophages (ATCC) to an appropriate acceptor.
J774 macrophages were grown with RPMI supplemented with 10%FBS in T75 flasks until they reached confluency. Once confluent, these cells were seeded into 96-well plates at a density of 70,000 cells/well and allowed to divide for an additional 24 hours until reaching 90–100% confluency. The following day, cells were incubated with a mixture containing fluorescence-labeled cholesterol, BODIPY (Avanti polar lipids), 20% BSA, and ACAT inhibitor for one hour. Subsequently, cells were washed two times with MEM-hepes buffer and then incubated with cAMP media for a period of 24 hours to enhance expression of the ABCA1 transporter on the surface of macrophages. The following day, cells were washed once more with MEM-hepes buffer and then incubated at 37°C without CO2 with a cholesterol acceptor for four hours. In our study, cholesterol acceptors included fresh ApoB-depleted plasma of participants along with individual fresh FPLC fractions obtained after each sample was subjected to size exclusion chromatography. Following the four-hour incubation, efflux media was removed and analyzed with a microplate reader for fluorescence intensity (excitation 482nm, emission 515nm). Cell monolayers were solubilized using NaOH and H2O and then fluorescence intensity recorded to account for BODIPY-cholesterol remaining inside of the cells. The sum of cholesterol removed from cells (efflux) and remaining inside of the cells accounts for total BODIPY-cholesterol incorporated into the cells at time zero.
On each plate, a set of controls were included to account for BODIPY-cholesterol background (media containing no acceptors). Additionally, an apoB-depleted plasma sample from pooled healthy volunteers was used on each plate to account for inter-assay variability. In addition, a recombinant human ApoA-I (apolipoprotein A-I) protein (Sigma, SRP4693) at seven increasing concentrations (1–100ug/mL) was used as a cholesterol acceptor to create a standard curve on each plate (Supplemental Figure IIB). Percent cholesterol efflux was calculated by subtracting background from efflux measurements of all acceptors and then dividing by the total BODIPY-cholesterol incorporated in each cell at time zero. Efflux measurements were then normalized to efflux elicited by ApoA-I concentration of 50ug/mL. Bioinformatics analysis prior to proposed association analyses revealed that normalization to this concentration produced the most robust signal; yielding minimal signal to noise ratio. Detailed calculations are provided in supplemental figure IIA. From preliminary studies, only fractions #30–69 elicited efflux, thus, all fraction-specific measurements were performed only in these fractions.
Reproducibility of efflux elicited by FPLC fractions
To evaluate reproducibility of efflux elicited by FPLC fractions, we conducted biological and technical replicates in one participant. For biological replicates, blood was collected on two separate fasting blood draws 24 hours apart and separated on Superose 6 column prior to efflux measurements. For technical replicates, we obtained two plasma samples from a single blood draw each of which were separated using SEC (superose 6 column). Results of technical and biological replicates are detailed in supplemental figure IIIA–B respectively, demonstrating excellent technical and biological reproducibility.
Lipoprotein profile, total protein, and ApoA-I ELISA
Each FPLC fraction was evaluated for total cholesterol, protein, and phospholipid levels by colorimetric assay. Cholesterol (Infinity cholesterol and triglyceride reagent; ThermoFisher). Bradford protein assay (Pierce™ Coomassie Bradford protein assay kit; ThermoFisher). Phospholipid (Wako). ApoA-I was measured using human ApoA-I ELISA kit (Cayman Chemical- catalog #501080 and Abcam- ab189576).
Statistical Analysis
All statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc. version 8.0) and R (version 3.5.1). For comparison of demographics and risk factors between high and low groups, P-values for continuous variables were calculated using Kruskal-Wallis test and those for categorical variables using Chi-square test. For comparisons of cholesterol efflux and other measurements between high and low groups, results of two group comparisons were assessed using 2-tailed Student’s t-test and statistical significance was assessed at p ≤ 0.05. Data have been analyzed for normality and equal variance as a justification for using parametric analysis such as Student’s t-test. Spearman correlation was applied in scatter plot and 99% confidence interval is shown for each regression line. Multiple regression analysis for adjustment of demographics and risk factors was performed with R.
Results
Study Design and Population
Demographics, risk factors, along with clinical lab values and medical history are reported for the 36 participants with persistent high and low cholesterol efflux (Table 1). The median age was 60 years with 70% women and 61% Black participants. There were no significant differences in traditional cardiovascular risk factor prevalence. Triglyceride levels were significantly lower in the persistent low cholesterol efflux group (median High vs. Low: 1.36 vs, 1.1 mmol/L, p=0.04; Table 1). HDL-C levels (median High vs. Low: 1.24 vs. 1.42 mmol/L) and apolipoprotein A-I levels (median High vs. Low: 1.54 vs. 1.55 g/L) were not significantly different (p>0.05 for both, Table 1).
Table 1.
Demographic and Clinical Characteristics in those with Persistent High and Low Cholesterol Efflux
| VARIABLE | PERSISTENT HIGH EFFLUX (N=19) | PERSISTENT LOW EFFLUX (N=17) | P-VALUE |
|---|---|---|---|
|
| |||
| AGE, YEARS | 56 (51–67) | 64 (58–71) | 0.12 |
| FEMALE SEX (%) | 68% (13) | 71% (12) | 0.89 |
| HISPANIC ETHNICITY (%) | 21% (4) | 6% (1) | 0.24 |
| BLACK RACE (%) | 63% (12) | 59% (10) | 0.79 |
| HYPERCHOLESTEROLEMIA (%) | 50% (9) | 53% (9) | 0.86 |
| DIABETES (%) | 33% (6) | 24% (4) | 0.52 |
| HEART DISEASE (%) | 11% (2) | 0% (0) | 0.15 |
| HYPERTENSION (%) | 44% (8) | 71% (12) | 0.12 |
| MENOPAUSAL (%) | 84% (11) | 92% (11) | 0.59 |
| CURRENT SMOKER (%) | 21% (4) | 6% (1) | 0.19 |
| HISTORY OF ALCOHOL INTAKE (%) | 68% (13) | 71% (12) | 0.89 |
| BLOOD PRESSURE MEDICATION (%) | 47% (9) | 69% (11) | 0.20 |
| GLUCOSE MEDICATION (%) | 26% (5) | 25% (4) | 0.93 |
| LIPID MEDICATION (%) | 42% (8) | 37% (6) | 0.78 |
| BMI | 30.4 (26.3–35.4) | 29.8 (26.0–34.6) | 0.96 |
| TOTAL CHOLESTEROL, mmol/L | 4.61 (4.11–6.39) | 3.98 (3.78–5.10) | 0.27 |
| LDL-C, mmol/L | 2.64 (2.12–4.22) | 2.27 (2.09–3.26) | 0.21 |
| NON-HDL-C, mmol/L | 3.10 (2.66–5.12) | 2.77 (2.46–3.78) | 0.09 |
| TRIGLYCERIDES, mmol/L | 1.36 (1.1–2.55) | 1.1 (1.07–1.32) | 0.04 |
| HDL-C, mmol/L | 1.24 (1.06–1.50) | 1.42 (1.27–1.58) | 0.27 |
| APOLIPOPROTEIN A-I, G/L | 1.54 (1.48–1.82) | 1.55 (1.49–1.71) | 0.92 |
| GLUCOSE, mmol/L | 5.72 (4.94–6.94) | 5.27 (4.99–5.77) | 0.59 |
| GLYCOHEMOGLOBIN, Fraction of Total Hb | 0.057 (0.055–0.068) | 0.058 (0.055–0.059) | 0.61 |
| CREATININE, UMOL/l | 77.79 (61.88–86.63) | 71.60 (51.27–81.33) | 0.33 |
| TOTAL PROTEIN, G/L | 73 (70–75) | 73 (69–73) | 0.38 |
| ALBUMIN, G/L | 44 (42–45) | 45 (41–46) | 0.34 |
| HEMOGLOBIN, G/L | 0.136 (0.129–0.149) | 0.137 (0.13–0.143) | 0.81 |
Continuous variables reported as median (interquartile range) and categorical as % (N). P-values for continuous variables calculated using Kruskal-Wallis test; those for categorical variables using Chi-square test.
Persistence of Extreme High and Low Cholesterol Efflux Trait
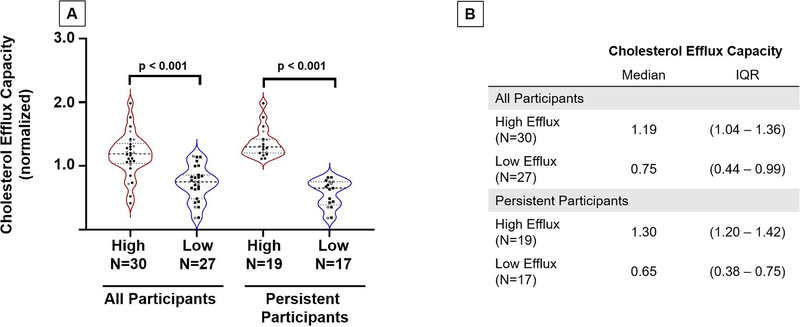
Among the recruited participants who were originally below the 10th (low, n=27) and above the 90th percentile (high, n=30) of the efflux distribution at baseline, there was a 2.5-fold difference in baseline apo B-depleted plasma CEC (high vs. low: median CEC 1.50 [1.44–1.65] vs. 0.60 [0.51–0.66], p < 0.0001; Supplemental Figure I) between the high and low efflux groups at the original baseline visit. After 14–18 years follow up, the overall group maintained a significant difference in apo B-depleted plasma CEC (high vs. Low: 1.19 [1.04 – 1.36] vs. 0.75 [0.44–0.99]; p < 0.0001; Figure 2). About two-thirds of these participants (N= 36 out of 57; 63%) remained persistent in their efflux trait, as defined by the two-fold difference in apo B-depleted plasma CEC (median 1.30 [1.20–1.42] vs. 0.65 [0.38 – 0.75], p < 0.0001; Figure 2). These differences remained significant when adjusted for demographics and risk factors (p < 0.0001).
Figure 2: Cholesterol efflux capacity of recruited participants 15–18 years after baseline enrollment.
Panel A. Cholesterol efflux of participants at follow-up visit (2016–2020) n=57; 30 high efflux group, 27 low efflux group. Of those recruited, we narrowed down our list to those who remained persistent over time (at least two-fold difference). Efflux was normalized to a human ApoA-I protein standard at a concentration of 50ug/mL. Men are represented by open circles, and women by closed circles.
Panel B. Table with median cholesterol efflux values along with IQR for each group (high vs. low) obtained at follow-up visit
Characterization of Extreme High and Low Cholesterol Efflux Trait by Size-Exclusion-Fast Performance Liquid Chromatography (SEC-FPLC)
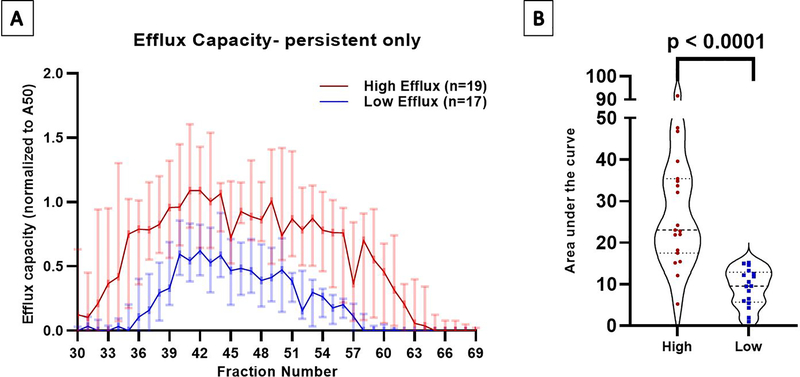
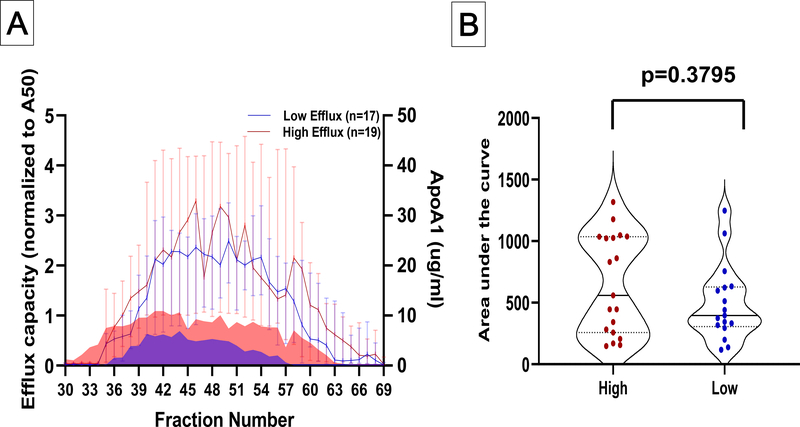
Those with persistent extreme high efflux elicited cholesterol efflux across a broader size range than those with persistent extreme low efflux, beginning over five fractions earlier (#31.5 vs 36; p=0.007) and ending over four fractions later (#61.5 vs. 57; p=0.005) (Figure 3A). At almost every fraction, those with extreme high efflux had significantly higher efflux than those with extreme low efflux (Figure 3A). The total area under the curve reflected a three-fold difference in total fraction-specific CEC (Figure 3B). These differences remained significant when adjusted for demographics and risk factors (p< 0.0001). Lastly, there was a characteristic decrease and increase (peak and valley) in CEC at fractions #45 and #57 among the extreme high efflux group without a discernable peak and valley pattern in the extreme low efflux group. There was no significant correlation between CEC from ApoB-depleted plasma and total fraction-specific CEC among the extreme high or low groups (coefficient of correlation: 0.16 and 0.15 for high and low groups respectively; P=NS) (Supplemental Figure VA–B).
Figure 3: Cholesterol efflux capacity in fractionated plasma after size exclusion chromatography.
Panel A. Efflux elicited by eluting fractions 30–69 from apoB-depleted plasma separated using Superose 6 increase SE column. High efflux participants are represented in red and low efflux group in blue. Data plotted are median values for each fraction with 95% confidence intervals. Efflux values were significantly different (p≤0.05) for every fraction except #31, #45, and #62–69.
Panel B. Statistical comparison of total area under the curve. Whiskers depict standard error of the area.
Characterization of Cholesterol, Phospholipid, Total Protein, and Apolipoprotein-I by SEC-FPLC
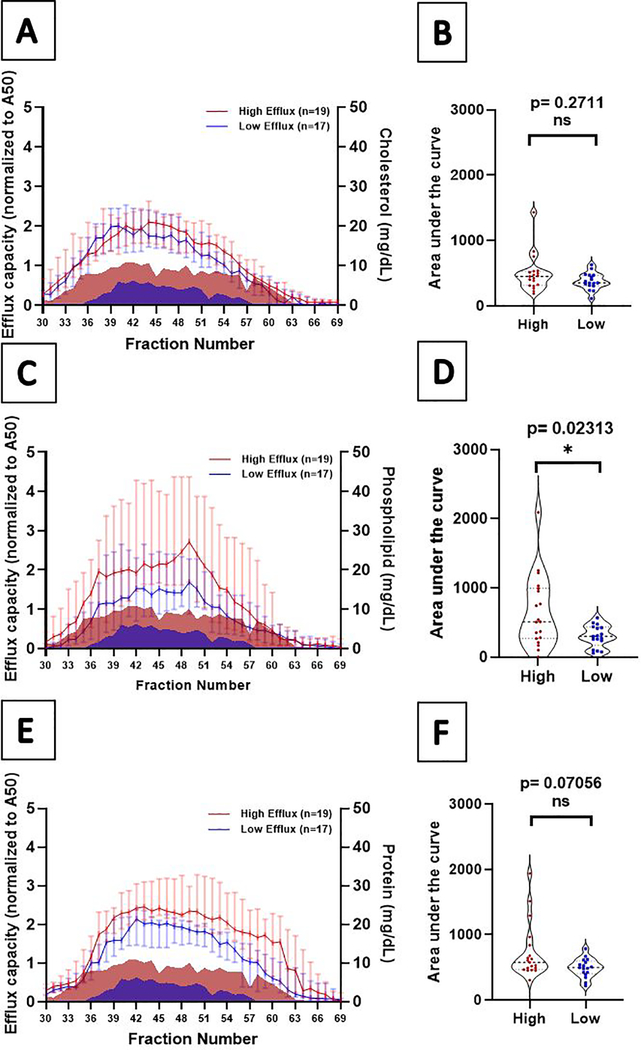
Comparing extreme high to extreme low efflux groups, total cholesterol levels were not different across fractions and overall (p>0.05) (Figure 4 A–B). Phospholipid levels were significantly lower in the extreme low efflux group in individual fractions #45 – 54 (p < 0.05) and overall (p = 0.02) (Figure 4 C–D). In models adjusted for demographics and risk factors, the significance was attenuated and borderline significant (p = 0.04). Total protein levels were not significantly different across fractions (p > 0.05) (Figure 4 E–F).
Figure 4: Fraction-specific Cholesterol, Phospholipid, and Total Protein in Persistent High and Low Cholesterol Efflux.
Panels A, C, and E: Total cholesterol, phospholipid, and protein by eluting fractions 30–69 from apoB-depleted plasma separated using Superose 6 increase SE column. Persistent high efflux participants are represented in red and persistent low efflux group are represented in blue. Median values for each fraction are displayed with 95% confidence intervals. Mountain plots represent cholesterol efflux for comparison. Panels B, D, and F: Total area under the curve for the fraction-specific values (whiskers depict standard error of the area).
ApoA-I levels identified in SEC-FPLC fractions
The major protein constituent found on HDL particles and a protein that is known to be capable of picking up excess cholesterol efficiently is ApoA-I. Fraction-specific ApoA-I levels were not significantly different in the extreme low efflux group compared with the extreme high efflux group (Figure 5 A–B).
Figure 5: Fraction-specific Apolipoprotein A-I levels in Persistent High and Low Cholesterol Efflux.
Panel A: Apolipoprotein A-I (ApoA-I) levels by eluting fractions 30–69 from apoB-depleted plasma separated using Superose 6 increase SE column. High efflux participants are represented in red and low efflux group in blue. Data plotted are median values for each fraction with 95% confidence intervals. Mountain plots represent cholesterol efflux for comparison. Panel B: Statistical comparison of total area under the curve. Whiskers depict standard error of the area.
Correlation of total phospholipid, cholesterol, protein, and ApoA-I with cholesterol efflux in eluting fractions
Although there is a statistically significant difference in total levels of fraction-specific phospholipid between persistent high and low efflux groups, these entities were not correlated with total fraction-specific efflux (Supplemental Figure VI A–C). Among the persistent high efflux group, there was a trend toward a direct correlation between total fraction-specific phospholipid levels and total fraction-specific CEC (Spearman R = 0.45, p=0.057; Supplemental Figure VI B). Among the persistent low efflux group, after removing one extreme outlier, there was a significant direct correlation between total fraction-specific phospholipid levels and total fraction-specific CEC (Spearman R = 0.53, p=0.03*; Supplemental Figure VI B). Total fraction-specific ApoA-I levels did not significantly correlate with total fraction-specific efflux in either our high or low efflux groups. (Spearman R = 0.35 and −0. 14 for high and low groups respectively; p=NS for both) (Supplemental Figure VI D). Heat map analysis highlighting specific fractions with correlation coefficients that were 0.40 or greater demonstrated that, for phospholipid and Apo A-I levels, more than twice as many fractions had at least moderate correlation with efflux in the extreme high efflux group vs. the extreme low efflux group (Supplemental Figure VII). In the extreme high efflux group, phospholipid levels were the only class that correlated with efflux at the largest size distribution (fractions #30–34), and total cholesterol was the only class that correlated with efflux at the smallest size distribution (fractions #58–69) (Supplemental Figure VII).
Effect Modification: Cholesterol Efflux Patterns by Sex, Race/Ethnicity, Diabetes, and Fasting Status
To evaluate whether sex, race, diabetes, or fasting status influenced the differences seen in our high and low efflux groups; we analyzed each group separately. Within each group, the differences in total fraction-specific efflux between the extreme high and low groups persisted (Supplemental Figure VIII A–H) with no significant statistical interactions (all P = NS). Qualitatively, the magnitude of these differences was reduced in White participants and in men.
Discussion
Our prospective study revealed that, among those with extreme high or low cholesterol efflux, two-thirds persist with this phenotype after over 15 years of follow up. We also found that those with persistent high and low cholesterol efflux displayed distinctive efflux profiles in fractionated plasma. In addition, we identified heterogeneity in patterns of cholesterol, phospholipid, and proteins that correlated with efflux at the largest size range versus patterns that correlated with efflux at the smallest size range. These findings suggest that understanding the determinants of variation in the cholesterol efflux trait may require nuanced analysis across the size distribution rather than simple measurements in whole plasma.
With respect to atherosclerotic cardiovascular disease (ASCVD), cholesterol efflux has been established as a biologically and clinically relevant trait in the population 11, 12. As part of the reverse cholesterol transport pathway, cholesterol efflux from macrophages to extracellular acceptors is the first key step in the movement of cholesterol out of the arterial wall. In animal models, macrophage-specific cholesterol efflux is causally associated with atherosclerosis, more so than HDL-C or Apo A-I levels 13, 14. In multiple epidemiologic studies in large human cohorts, cholesterol efflux capacity (CEC) has been inversely associated with prevalent coronary atherosclerosis and incident ASCVD events in most but not all studies 2–8. Importantly, results have been mostly consistent across laboratories, differing human populations, and slightly different methods, as well as independent of HDL-C levels. In addition, clinical relevance was demonstrated by showing in one large study that CEC predicted ASCVD independent of coronary calcium levels, family history, and C-reactive protein, all clinically used risk factors to refine ASCVD risk estimation 15. However, major factors that determine variation in cholesterol efflux remain unidentified. In most human studies, levels of HDL-C and Apo A-I are correlated with CEC, but these correlations are modest at best. Other traditional risk factors and lipids do not explain any substantive variation in CEC (less than 5% variation in one study) 2. None of these factors attenuate the associations with ASCVD, further suggesting little contribution to variation in the trait. By elucidating determinants of cholesterol efflux, the reverse cholesterol transport pathway can then be manipulated to prevent and treat ASCVD. Moreover, cholesterol efflux is relevant in other disease processes such as insulin resistance 16, kidney disease17, macular degeneration 18, vascular dementia 19, infection 20 and immunity as well as oncologic processes 21; therefore, elucidating factors that regulate cholesterol efflux in humans may likely have broad implications across multiple pathobiologies.
In all the human studies demonstrating a link to ASCVD, macrophages were used as the cholesterol donors and apolipoprotein B-depleted plasma as the accepting medium 11. Thus, we focused our investigation on this system used consistently in epidemiologic studies. In the Dallas Heart Study, CEC was normally distributed in a multi-ethnic population-based sample of young participants free of ASCVD (median age 44; 50% Black) 2. Our strategy to establish the major factors determining variation in cholesterol efflux involved focusing on the extremes of the distribution and those that remained persistently extreme in follow up. Participants below the 10th or above the 90th percentile of CEC were recruited for blood collection over 15 years later. Two-thirds remained persistently high or low with respect to cholesterol efflux, as defined by a two-fold difference in CEC using apo B-depleted plasma. Prior reports have also suggested that the cholesterol efflux trait is persistent over several years 9. We extend those findings by focusing on those at the extremes ends of CEC and follow up over 15 years. Thus, cholesterol efflux from standardized macrophages to Apo B-depleted plasma is a robust clinical characteristic in humans.
Having established persistence of the cholesterol efflux trait, we sought to leverage the extreme distribution to investigate classes of molecular species linked to either high or low cholesterol efflux. Measurement of cholesterol efflux in Apo B-depleted plasma fractionated by size-exclusion chromatography (SEC) accentuated the differences seen in unfractionated plasma, revealing an overall three-fold difference in cholesterol efflux between high and low participants. In addition, those with low efflux elicited efflux over a much narrower size range than those with high efflux, suggesting pattern differences beyond total abundance. Prior studies have also suggested that fractionated plasma can elucidate new insights into HDL metabolism based on composition and function. For example, recent studies in diabetes, Alzheimer’s dementia, fatty liver, and CKD revealed differences in the proteomic composition and functionality of HDL isolated by SEC 22–26. These studies have focused on specific disease states and on HDL, whereas our study focused on cholesterol efflux, a functional measure, as the primary trait. A recent investigation in mice more closely aligns with our approach by focusing on cholesterol efflux in fractionated plasma 27. In this study, ABCA1-specific CEC demonstrated two peaks in fractionated plasma, one associated with HDL and the other not associated with HDL. The size separation allowed for the identification of a novel protein, plasminogen, to be implicated in regulating cholesterol efflux. While specifics regarding the methodology in fractionating plasma by size vary across studies, the overall approach clearly provides new insights into the determinants of HDL metabolism.
With respect to our study, we noted that despite persistent high and low efflux groups having a 3-fold difference in fractionated CEC, total cholesterol and protein levels aggregated across fractions were not different. Importantly, ApoA-I levels were not different in persistent high and low efflux groups. In addition, within the persistent high and low efflux groups, neither fraction-specific total cholesterol nor fraction-specific total protein correlated with fraction-specific CEC. These data suggest that overall cholesterol and protein levels as well as ApoA-I abundance do not distinguish this phenotype. In contrast, total phospholipid (PL) aggregated across fractions were significantly lower in those with persistent low cholesterol efflux. Fraction-specific phospholipid and Apo A-I levels moderately correlated with fraction-specific CEC in those with persistent high cholesterol efflux. In the persistent low group, fraction-specific phospholipids were the only class that correlated with fraction-specific CEC. The patterns of correlation also varied at the highest size range compared to the lowest size range. These data suggest that phospholipids and specific proteins other than apo A-I may be the distinguishing feature and ultimately the driving force behind extreme variation in CEC. Lipoprotein particles containing ApoA-I efficiently pick-up cholesterol from cell membranes, especially via ABCA1 transporters when lipid-poor. Phospholipids are well known to impart cholesterol efflux functionality as well 28, 29. Phospholipid content is reduced in individuals with very high HDL-C levels and coronary artery disease as evidenced by previous studies 29. What is not well known, however, is whether modification of ApoA-I or levels of other proteins or combinations of proteins and lipids may explain variation in the cholesterol efflux trait in humans. Candidates such as apo A-II and apo C-III are attractive based on prior literature as well as unconventional proteins such as plasminogen and serpinA1. Lipid classes such as sphingomyelins and ceramides may also contribute. Establishing this cohort with extreme and persistent cholesterol efflux will pave the way for future studies to identify the molecular determinants of cholesterol efflux including both candidate and untargeted –omics approaches.
In conclusion, by prospectively recruiting those at the extremes of the cholesterol efflux trait within a multi-ethnic population-based sample, our study demonstrated that extreme high and low efflux are persistent and robust phenotypes. Additionally, fractionation of plasma by size magnified differences and patterns in efflux as well as identified the role of phospholipids in determining variation in efflux. By establishing that extreme CEC is a persistent trait, our study opens the door to advance our understanding of the molecular drivers of CEC. Further studies will incorporate both targeted and untargeted genomic, proteomic, and lipidomic phenotyping strategies to elucidate the interplay between gene, protein, and lipids on extreme variation in CEC. Insights into the molecular drivers of CEC can potentially provide new possibilities for therapeutic targets in the prevention and treatment of ASCVD and other diseases involving lipid metabolism and cholesterol transport.
Supplementary Material
Highlights:
Extreme high and low cholesterol efflux capacity are persistent and robust phenotypes over 15 years of follow up.
Cholesterol efflux to fractionated plasma reveals insightful patterns of the extreme high and low efflux groups across the size distribution.
Phospholipid but not total protein or ApoA-I levels may explain some of the variation in cholesterol efflux.
Establishment of this extreme and persistent cholesterol efflux cohort with refined phenotyping will facilitate discovery of molecular species driving variation in cholesterol efflux, a marker linked to atherosclerotic disease risk.
Acknowledgments
We would like to thank Kathleen Wilkinson for her support in participant recruitment and database creation. Study data were collected and managed using REDCap electronic data capture tools hosted at UT Southwestern.1 1Paul A. Harris, Robert Taylor, Robert Thielke, Jonathon Payne, Nathaniel Gonzalez, Jose G. Conde, Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform. 2009 Apr;42(2):377-81.
Sources of Funding: This study was supported by the NIH/NHLBI R01HL136724 and NIH/NHLBI K24HL146838. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award Number UL1TR001105 and Clinical and Translational Science Awards Program of the National Institutes of Health under award number 1UL1TR003163-01A1.
Disclosures: Anand Rohatgi: Merck, research grant, significant; CSL Limited, consultant, modest.
Nonstandard Abbreviations and Acronyms
- RCT
Reverse cholesterol transport
- CEC
Cholesterol efflux capacity
- ASCVD
Atherosclerotic cardiovascular disease
- CV
Cardiovascular
- DHS
Dallas heart study
- SEC
Size exclusion chromatography
- FPLC
Fast performance liquid chromatography
- PEG
Polyethylene glycol
- ApoA-I
Apolipoprotein A-I
- ApoB
Apolipoprotein B
- PL
Phospholipid
References
- 1.Rohatgi A Reverse cholesterol transport and atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2019;39:2–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. Hdl cholesterol efflux capacity and incident cardiovascular events. The New England journal of medicine. 2014;371:2383–2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. The New England journal of medicine. 2011;364:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu C, Zhang Y, Ding D, Li X, Yang Y, Li Q, Zheng Y, Wang D, Ling W. Cholesterol efflux capacity is an independent predictor of all-cause and cardiovascular mortality in patients with coronary artery disease: A prospective cohort study. Atherosclerosis. 2016;249:116–124 [DOI] [PubMed] [Google Scholar]
- 5.Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, Kastelein JJ, Boekholdt SM, Khaw KT, Wareham N, Rader DJ. Association of hdl cholesterol efflux capacity with incident coronary heart disease events: A prospective case-control study. The lancet. Diabetes & endocrinology. 2015;3:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khera AV, Demler OV, Adelman SJ, Collins HL, Glynn RJ, Ridker PM, Rader DJ, Mora S. Cholesterol efflux capacity, high-density lipoprotein particle number, and incident cardiovascular events: An analysis from the jupiter trial (justification for the use of statins in prevention: An intervention trial evaluating rosuvastatin). Circulation. 2017;135:2494–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shea S, Stein JH, Jorgensen NW, McClelland RL, Tascau L, Shrager S, Heinecke JW, Yvan-Charvet L, Tall AR. Cholesterol mass efflux capacity, incident cardiovascular disease, and progression of carotid plaque. Arteriosclerosis, thrombosis, and vascular biology. 2019;39:89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebtehaj S, Gruppen EG, Bakker SJL, Dullaart RPF, Tietge UJF. Hdl (high-density lipoprotein) cholesterol efflux capacity is associated with incident cardiovascular disease in the general population. Arteriosclerosis, thrombosis, and vascular biology. 2019;39:1874–1883 [DOI] [PubMed] [Google Scholar]
- 9.Koekemoer AL, Codd V, Masca NGD, Nelson CP, Musameh MD, Kaess BM, Hengstenberg C, Rader DJ, Samani NJ. Large-scale analysis of determinants, stability, and heritability of high-density lipoprotein cholesterol efflux capacity. Arteriosclerosis, thrombosis, and vascular biology. 2017;37:1956–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH. The dallas heart study: A population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. The American journal of cardiology. 2004;93:1473–1480 [DOI] [PubMed] [Google Scholar]
- 11.Rhainds D, Tardif JC. From hdl-cholesterol to hdl-function: Cholesterol efflux capacity determinants. Current opinion in lipidology. 2019;30:101–107 [DOI] [PubMed] [Google Scholar]
- 12.Rader DJ, Hovingh GK. Hdl and cardiovascular disease. Lancet (London, England). 2014;384:618–625 [DOI] [PubMed] [Google Scholar]
- 13.Rosenson RS, Brewer HB Jr., Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. Journal of lipid research. 2009;50Suppl:S189–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mody P, Joshi PH, Khera A, Ayers CR, Rohatgi A. Beyond coronary calcification, family history, and c-reactive protein: Cholesterol efflux capacity and cardiovascular risk prediction. Journal of the American College of Cardiology. 2016;67:2480–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manandhar B, Cochran BJ, Rye KA. Role of high-density lipoproteins in cholesterol homeostasis and glycemic control. Journal of the American Heart Association. 2020;9:e013531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsche G, Heine GH, Stadler JT, Holzer M. Current understanding of the relationship of hdl composition, structure and function to their cardioprotective properties in chronic kidney disease. Biomolecules. 2020;10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Betzler BK, Rim TH, Sabanayagam C, Cheung CMG, Cheng CY. High-density lipoprotein cholesterol in age-related ocular diseases. Biomolecules. 2020;10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chernick D, Zhong R, Li L. The role of hdl and hdl mimetic peptides as potential therapeutics for alzheimer’s disease. Biomolecules. 2020;10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meilhac O, Tanaka S, Couret D. High-density lipoproteins are bug scavengers. Biomolecules. 2020;10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Georgila K, Vyrla D, Drakos E. Apolipoprotein a-i (apoa-i), immunity, inflammation and cancer. Cancers. 2019;11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Y, Ronsein GE, Tang C, Jarvik GP, Davidson WS, Kothari V, Song HD, Segrest JP, Bornfeldt KE, Heinecke JW. Diabetes impairs cellular cholesterol efflux from abca1 to small hdl particles. Circulation research. 2020;127:1198–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gourgari E, Ma J, Playford MP, Mehta NN, Goldman R, Remaley AT, Gordon SM. Proteomic alterations of hdl in youth with type 1 diabetes and their associations with glycemic control: A case-control study. Cardiovascular diabetology. 2019;18:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson TJ, Sen A. Apolipoprotein e particle size is increased in alzheimer’s disease. Alzheimer’s & dementia (Amsterdam, Netherlands). 2019;11:10–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao PK, Merath K, Drigalenko E, Jadhav AYL, Komorowski RA, Goldblatt MI, Rohatgi A, Sarzynski MA, Gawrieh S, Olivier M. Proteomic characterization of high-density lipoprotein particles in patients with non-alcoholic fatty liver disease. Clinical proteomics. 2018;15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozlitina J, Zhou H, Brown PN, Rohm RJ, Pan Y, Ayanoglu G, Du X, Rimmer E, Reilly DF, Roddy TP, Cully DF, Vogt TF, Blom D, Hoek M. Plasma levels of risk-variant apol1 do not associate with renal disease in a population-based cohort. Journal of the American Society of Nephrology : JASN. 2016;27:3204–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pamir N, Hutchins PM, Ronsein GE, Wei H, Tang C, Das R, Vaisar T, Plow E, Schuster V, Koschinsky ML, Reardon CA, Weinberg R, Dichek DA, Marcovina S, Getz GS, Heinecke JW. Plasminogen promotes cholesterol efflux by the abca1 pathway. JCI insight. 2017;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swertfeger DK, Li H, Rebholz S, Zhu X, Shah AS, Davidson WS, Lu LJ. Mapping atheroprotective functions and related proteins/lipoproteins in size fractionated human plasma. Molecular & cellular proteomics : MCP. 2017;16:680–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwala AP, Rodrigues A, Risman M, et al. High-Density Lipoprotein (HDL) Phospholipid Content and Cholesterol Efflux Capacity Are Reduced in Patients With Very High HDL Cholesterol and Coronary Disease. Arterioscler Thromb Vasc Biol. 2015;35(6):1515–1519. doi: 10.1161/ATVBAHA.115.305504 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.