Abstract
Background:
Although women with heart failure (HF) are potentially more likely to be physically frail compared with men with HF, the underlying contributors to this sex difference are poorly understood. The purpose of this study was to characterize sex differences in physical frailty phenotypes in HF.
Methods:
We prospectively enrolled adults with Class I-IV HF. Physical frailty was measured with the Frailty Phenotype Criteria. Symptoms of dyspnea, sleep-related impairment, pain interference, depression, and anxiety were assessed. Body composition was measured using dual energy x-ray absorptiometry. Simple comparative statistics and stepwise regression modeling were used.
Results:
The average age of the sample (n = 115) was 63.6±15.7 years, 49% were women, and 73% had non-ischemic etiology. 43% of the sample was physically frail. Women had a 4.6 times greater odds of being physically frail compared with men, adjusting for covariates (OR = 4.63, 95% CI [1.81, 11.84], p = 0.001). Both physically frail men and women were characterized by more type 2 diabetes, higher comorbidity burden, and worse dyspnea symptoms. Physically frail women had significantly worse symptoms compared with non-physically frail women but no difference in body composition characteristics. Physically frail men had significantly lower appendicular muscle mass, higher percent fat, lower hemoglobin, and more depressive symptoms compared with non-physically frail men.
Conclusions:
Women are significantly more likely to be physically frail compared with men in HF. Physical frailty in both women and men is characterized by comorbidities and worse symptoms; physical frailty in men is characterized by worse physiological characteristics.
Journal Subject Terms: dyspnea, frailty, heart failure, phenotype
Introduction
It has been recognized that while women live longer lives, they suffer from worse health compared with men, termed the male-female health-survival paradox.1 Specifically, it has been shown that frailty, a geriatric syndrome of decreased physiological reserves and increased vulnerability to stressors,2 is more prevalent among older women compared with older men.3 Within heart failure (HF), the intersection of frailty and HF has become an area of intense interest given that about one out of every two HF patients is considered frail4 and the strong association between frailty and poor clinical outcomes in HF.5 Sex differences in frailty in HF, however, have not been fully examined even though half of adults with HF are women.6 A few studies have noted significantly more frail women compared with frail men with HF;7–9 however, a focused examination of the relationship between sex and physical frailty in HF, including underlying clinical and physiological drivers of physical frailty in women and men, has not been performed to date.
There are a number of well-known sex differences in HF that might contribute to sex differences in physical frailty phenotypes. Women with HF are often older at diagnosis10 and more commonly have non-ischemic HF etiologies as a result of comorbidities (e.g. hypertension, diabetes, and obesity) compared with men.11 Women also have different ventricular geometrical dimensions and function,12 including differences in the effect of obesity on ventricular geometry,13 and they are more likely to have HF with preserved ejection fraction versus HF with reduced ejection fraction.14 Additionally, there are known sex differences in HF symptom presentations.15 We lack an understanding of how these notable sex differences may contribute to poor health and physical frailty among women with HF. Specifically, identifying the characteristics underlying physical frailty phenotypes among women and men with HF would be helpful in identifying appropriately tailored interventions to mitigate physical frailty. The purpose of this study was to 1) characterize sex differences in physical frailty phenotypes in HF and 2) identify distinguishing clinical, symptom, and physiological characteristics of physical frailty among women and men with HF.
Methods
The corresponding author will make the data and methods used in the analysis available to any qualified researcher upon reasonable request for purposes of reproducing the results.
Study Design and Sample
This article addresses a primary aim of a National Institutes of Health-funded study that was designed to identify sex differences in physical frailty phenotypes in HF. A key feature of this study was the enrollment of approximately equal numbers of women and men with HF. Inclusion criteria were age 21 years or older, ability to read and comprehend 5th grade English, and New York Heart Association (NYHA) functional classification I-IV. Exclusion criteria were documented major cognitive impairment (e.g. Alzheimer’s disease) or active psychosis that would preclude study participation, prior heart transplantation or durable mechanical circulatory support, major and uncorrected hearing dysfunction, or were otherwise unable to complete the requirements of the study (e.g. life-threatening illness). We used convenience sampling to recruit eligible participants. After initial screening and approval by the attending cardiologists, participants were recruited through HF and general cardiology clinics at a single center in the Pacific Northwest of the United States between May 2018 and February 2020. Figure 1 shows the enrollment flow diagram, including the sex balance across each phase of screening, consent, and analysis. The Institutional Review Board at Oregon Health & Science University approved this study, and written informed consent was obtained from all participants.
Figure 1: Enrollment flow diagram for the Sex-Associated Differences in Physical Frailty Phenotypes in Heart Failure study.
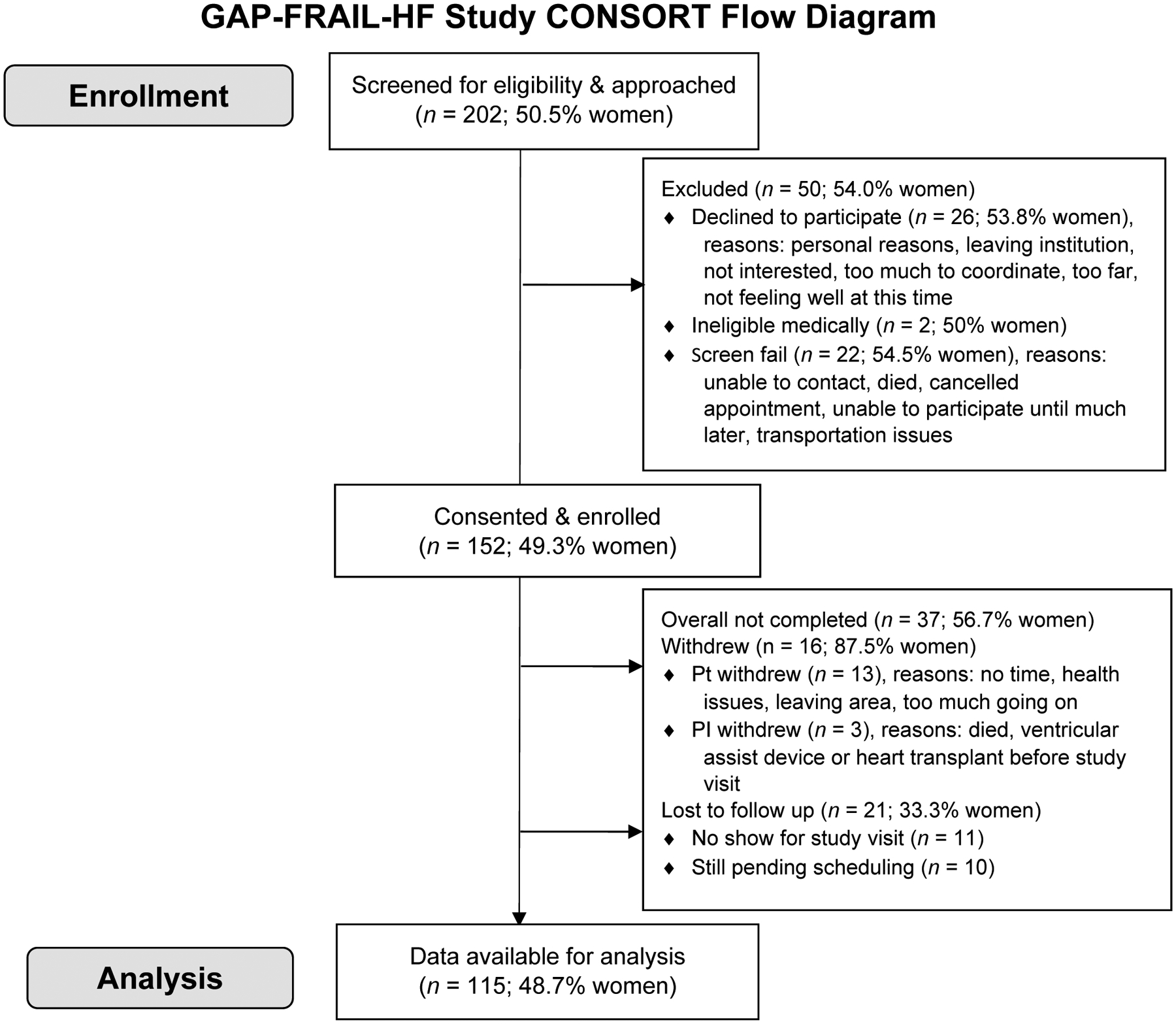
We screened and approached 202 adults for our study, 152 adults were enrolled, and 115 adults completed the baseline study visit and were analyzed. Overall, the balance between women and men remained about the same (~1:1); however, more women than men withdrew from the study after consent, but fewer women were lost to follow-up.
Measurement
Sociodemographic and clinical data.
A sociodemographic questionnaire was used to collect data on age, marital status, race, and education. We asked participants to self-report sex. Baseline history, etiology, NYHA functional class, clinical and laboratory data, and treatment of HF, including current medications, were collected from a review of medical records. For medications, we focused on those that are used in the care of adults with HF16 and could be potentially relevant to frailty (e.g. beta blockers7 and angiotensin converting enzyme inhibitors or angiotensin II receptor blockers17). Participants were categorized as HF with reduced (left ventricular ejection fraction < 40%) or preserved ejection fraction (left ventricular ejection fraction > 50%) based on their initial presentation at diagnosis, even if their ejection fraction improved as a result of HF therapies. Attending cardiologists adjudicated any participant with midrange ejection fraction (40–50%) to determine the most appropriate phenotype based on clinical characteristics.
The Charlson Comorbidity Index18 was used to summarize the impact of comorbid conditions. In addition to the overall summary score, we examined specific items from the index: previous myocardial infarction, peripheral vascular disease, and history of asthma, emphysema, chronic bronchitis or chronic obstructive pulmonary disease. In addition, we collected data on the presence of the following comorbidities: type 2 diabetes, stage 3 chronic kidney disease, atrial fibrillation, hypertension, pulmonary hypertension, and obstructive sleep apnea. All comorbidities were ascertained from a review of the medical records.
Physical frailty.
Physical frailty was measured using the Frailty Phenotype Criteria2 as we have adapted and used specifically for the HF population7, 19 based on the 5 criteria: unintentional weight loss, weakness, physical exhaustion, slowness, and low physical activity. The details of our approach are described in Denfeld et al.7 Briefly, we asked participants about unintentional weight loss of > 10 pounds over the last year. We measured weakness of the lower extremities using 5-repeat chair stands; a cut point of > 12 seconds or inability to rise 5 times indicated weakness.20 We measured slowness with gait speed assessed over 4 meters; a cut point of < 0.9 meters per second indicated slowness.21 We measured physical exhaustion using the 13-item Functional Assessment of Chronic Illness Therapy Fatigue Scale (FACIT-F; v.4).22 The 13 items are rated from 0 (not at all) to 4 (very much), and cumulative scores range from 0 to 52 with lower scores indicating more fatigue; we used a cut point of < 17 to identify those with severe physical exhaustion.23 Cronbach’s α of the FACIT-F in this sample was 0.94. We measured level of physical activity by asking how much time was spent exercising over the past week; those who reported less than 1 hour per week (to approximate expending ~300 kcal/week in physical activity) were classified as having low physical activity. After completing the measures for each of the 5 criteria, the scores were totaled (range 0 to 5). Each participant was then classified as either “non-frail” (0 criteria met), “pre-frail” (1–2 criteria met), or “physically frail” (≥3 criteria met). Similar to our previous work,7 and because of the small numbers in the non-frail group (n = 10), we combined the non-frail group with the pre-frail group (i.e. “non-physically frail”; n = 56), as compared with the “physically frail” group (n = 49).
Symptoms.
We assessed symptoms of dyspnea, sleep-related impairment, pain intensity and interference, depression, and anxiety. In addition to HF specific measures, we also used universal symptom measures developed within the NIH-sponsored PROMIS Initiative,24 which was designed to develop, validate, and standardize item banks and short forms to measure patient-reported outcomes relevant across common medical conditions. For PROMIS measures, the raw score is translated into a T-score, resulting in a standardized score with a mean of 50 and a standard deviation of 10. A higher PROMIS T-score represents more of the concept being measured.
The Heart Failure Somatic Perception Scale (HFSPS; v.3) was used to measure dyspnea.25 The HFSPS asks how much the participant was bothered by physical symptoms and provides 6 response options ranging from 0 (I did not have this symptom) to 5 (extremely bothersome). The 6-item HFSPS-Dyspnea (HFSPS-D) subscale scores range from 0–30 with higher scores indicating worse dyspnea. The reliability and validity of the HFSPS-D have been established previously.25 Cronbach’s α on the HFSPS-D was 0.92 in this sample.
Sleep-related impairment was measured with the 8-item PROMIS Sleep-Related Impairment Short Form.24 The Sleep-Related Impairment Short Form focuses on self-reported perceptions of alertness, sleepiness, and tiredness during usual waking hours, including the perceived functional impairments associated with sleep problems or impaired alertness. There are 5 response options ranging from 1 (not at all) to 5 (very much); a T score > 50 indicates more sleep-related impairment. The reliability and validity of the Sleep-Related Impairment Short Form have been established.26 Cronbach’s α on the PROMIS Sleep-Related Impairment Short Form was 0.92 in this sample.
Pain intensity was measured with the 1-item PROMIS Pain Intensity Short Form, which asks how much a participant would rate pain on average over the past 7 days (range 0–10).24 Pain interference was measured with the 4-item PROMIS Pain Interference Short Form.24 The Pain Interference Short Form measures the self-reported consequences of pain on relevant aspects of one’s life over the past 7 days. The scale ranges from 1 (not at all) to 5 (very much); a T score > 50 indicates more pain interference. Reliability and validity of the Pain Interference Short Form have been evaluated across diverse clinical populations.27 Cronbach’s α on the PROMIS Pain Interference Short Form was 0.96 in this sample.
The 9-Item Patient Health Questionnaire (PHQ9)28 was used to measure depressive symptoms. The PHQ9 scores each of the 9 related DSM-IV criteria for depression. Scores on the PHQ9 range from 0 to 27 with higher scores indicating worse depressive symptoms. The PHQ9 is a valid and reliable measure of depressive symptoms in HF.29 Cronbach’s α on the PHQ-9 was 0.88 in this sample.
Anxiety was measured with the 8-item PROMIS Emotional Distress-Anxiety Short Form.24 The Anxiety Short Form measures self-reported fear, anxious misery, hyperarousal, and somatic symptoms related to arousal over the past 7 days. There are 5 response options ranging from 0 (never) to 4 (always); a T score > 50 indicates more anxiety. The reliability and validity of the PROMIS Anxiety items have been evaluated across diverse clinical populations.30 Cronbach’s α on the PROMIS Emotional Distress-Anxiety Short Form was 0.96 in this sample.
Body Composition.
Whole body composition was assessed using dual energy x-ray absorptiometry (DXA) (Hologic-QDR Discovery Wi; APEX software, v.4.02). We used DXA over other modalities (e.g. computed tomography scans) because of the low level of radiation exposure and the availability and ease of use with this patient population. We collected data on whole body bone-free lean mass (an approximation of muscle mass) and fat mass, as well as percent fat for the whole body and for the appendicular (i.e. extremity) and trunk regions. We also collected data on visceral adipose tissue. Based on the estimated values, we indexed whole body lean mass and fat mass to height (m2). We calculated the appendicular muscle mass index by summing the bone-free lean mass for all 4 extremities indexed to body mass index. The appendicular muscle mass index is used as a measure of muscle mass and one indicator to confirm the presence of sarcopenia, which is defined as a progressive and generalized skeletal muscle disorder associated with adverse outcomes.31
Statistical Analysis
Characteristics of the sample are presented using standard descriptive statistics, including measures of central tendency and dispersion. Comparative statistics (Student’s t-, Mann-Whitney U, Fisher exact, or the Pearson χ2 tests) were used to determine significant differences in characteristics between physically frail and non-physically frail adults with HF overall and then stratified by sex. Effect sizes were calculated and reported for the comparison of characteristics of physical frailty overall using Hedge’s g, Spearman’s rho, or odds ratios, with corresponding 95% confidence intervals (CI). Stepwise backward logistic regression was used to identify significant predictors of physical frailty in a parsimonious model that was not oversaturated with non-significant factors. Candidate patient and clinical characteristic variables significant at p < 0.20 in bivariate comparisons were entered into the stepwise backward logistic regression model. Then after adjustment in a full model, variables were removed at p > 0.10 to generate a final model. We performed interaction testing to quantify the influence of sex on the relationship between characteristics and physical frailty. Significance was set at α < 0.05. There were very little missing data on the patient, clinical, and symptom characteristics (at most missing data on n = 8/115 (7%) of clinical NT-proBNP lab values). We were missing DXA data on 7 participants (1 declined the DXA, 1 was over the weight limit, and 5 scans were unusable). There were no significant differences in sex, age, or physical frailty status between those with and without completed DXA scans. All analyses were performed using Stata/MP version 15MP (StataCorp, College Station, TX).
Results
Characteristics of the sample (n = 115) are presented in Table 1. The sample was 64 years of age on average, and most participants had HF of non-ischemic etiology. Physical frailty was identified in almost half (43%) of the sample overall, but more women than men were physically frail (OR 3.86 [95% CI 1.76, 8.44], p = 0.001). Other significant factors associated with physical frailty were more comorbidity burden (specific comorbidities and overall comorbid burden), higher left ventricular ejection fraction, HF with preserved ejection phenotype, lower hemoglobin, and not being on an angiotensin converting enzyme inhibitor or angiotensin II receptor blocker. Symptoms of dyspnea, sleep-related impairment, pain intensity and interference, and depression were also worse comparing physically frail with non-physically frail adults with HF. Comparison of characteristics between women and men are presented in the Supplementary Table.
Table 1.
Characteristics of the sample and by level of physical frailty*
| Total (n = 115) | Non-Physically Frail (n = 66)† | Physically Frail (n = 49) | p value‡ | Effect Size [95% CI]** | |
|---|---|---|---|---|---|
| Age (years) | 63.6±15.7 | 61.5±15.9 | 66.3±15.1 | 0.10 | −0.31 [−0.67, 0.06] |
| Sex | 0.001 | 3.86 [1.76, 8.44] | |||
| Men | 59 (51%) | 43 (65%) | 16 (33%) | ||
| Women | 56 (49%) | 23 (35%) | 33 (67%) | ||
| Non-Hispanic Caucasian | 97 (84%) | 55 (83%) | 42 (86%) | 0.73 | 1.20 [0.43, 3.36] |
| Married/Partnered | 61 (53%) | 39 (59%) | 22 (45%) | 0.13 | 0.56 [0.27, 1.19] |
| Education | 0.42 | ||||
| < High school | 26 (23%) | 15 (23%) | 11 (22%) | (referent) | |
| > High school but < College | 49 (43%) | 25 (38%) | 24 (49%) | 1.31 [0.50, 3.41] | |
| College degree | 40 (35%) | 26 (39%) | 14 (29%) | 0.73 [0.27, 2.02] | |
| Clinical Characteristics | |||||
| Body mass index (kg/m2) | 31.3±8.3 | 29.4±7.1 | 33.8±9.1 | 0.007 | −0.54 [−0.91, −0.16] |
| Charlson Comorbidity Index (weighted) | 3.2±2.0 | 2.8±1.7 | 3.7±2.1 | 0.015 | −0.48 [−0.85, −0.11] |
| Previous myocardial infarction | 38 (33%) | 19 (29%) | 19 (39%) | 0.26 | 1.57 [0.72, 3.43] |
| Peripheral vascular disease | 4 (3%) | 2 (3%) | 2 (4%) | 1.00 | 1.36 [0.19, 10.02] |
| Asthma, emphysema, chronic bronchitis, or COPD | 35 (30%) | 15 (23%) | 20 (41%) | 0.037 | 2.34 [1.04, 5.27] |
| Atrial fibrillation | 55 (48%) | 29 (45%) | 26 (53%) | 0.37 | 1.40 [0.67, 2.95] |
| Stage 3 chronic kidney disease | 31 (27%) | 13 (20%) | 18 (37%) | 0.044 | 2.37 [1.02, 5.48] |
| Type 2 diabetes | 46 (40%) | 18 (27%) | 28 (57%) | 0.001 | 3.56 [1.62, 7.78] |
| Heart Failure Characteristics | |||||
| Time with heart failure (years) | 3.6 [1.4–7.2] | 3.8 [1.8–8.4] | 3.1 [1.3–5.4] | 0.18 | −0.13 [−0.30, 0.06] |
| ≥ 1 heart failure hospitalization in past year | 33 (29%) | 19 (29%) | 14 (29%) | 0.94 | 0.97 [0.43, 2.20] |
| New York Heart Association functional class | 0.08 | 1.97 [0.93, 4.17] | |||
| Class I/II | 58 (50%) | 38 (58%) | 20 (41%) | ||
| Class III/IV | 57 (50%) | 28 (42%) | 29 (59%) | ||
| Non-ischemic etiology | 84 (73%) | 48 (73%) | 36 (73%) | 0.93 | 1.04 [0.45, 2.39] |
| Heart failure phenotype | 0.004 | 3.38 [1.45, 7.84] | |||
| HF with reduced ejection fraction | 82 (71%) | 54 (82%) | 28 (57%) | ||
| HF with preserved ejection fraction | 33 (29%) | 12 (18%) | 21 (43%) | ||
| Left ventricular end-diastolic diameter (cm) | 5.4±1.0 | 5.4±1.0 | 5.2±1.0 | 0.30 | 0.20 [−0.18, 0.57] |
| Left ventricular ejection fraction (%) | 42.9±15.8 | 39.9±15.2 | 46.9±15.9 | 0.019 | −0.45 [−0.82, −0.08] |
| Serum sodium (mEq/L) | 138.6±3.0 | 138.7±3.3 | 138.4±2.5 | 0.54 | 0.11 [−0.26, 0.48] |
| Serum hemoglobin (g/dL) | 13.0±2.0 | 13.3±1.9 | 12.5±2.1 | 0.041 | 0.40 [0.02, 0.77] |
| Serum BUN:Creatinine ratio | 21.5±7.7 | 21.4±8.2 | 21.6±7.0 | 0.87 | −0.03 [−0.40, 0.34] |
| Serum N-terminal pro-B-type natriuretic peptide (pg/mL) | 739 [190–1715] | 674 [172–1696] | 896 [324–2089] | 0.33 | 0.10 [−0.10, 0.28] |
| Prescribed a β-blocker | 99 (86%) | 60 (91%) | 39 (80%) | 0.08 | 0.39 [0.13, 1.16] |
| Prescribed an angiotensin-converting enzyme-inhibitor or angiotensin II receptor blocker | 83 (72%) | 55 (83%) | 28 (57%) | 0.002 | 0.27 [0.11, 0.63] |
| Prescribed an aldosterone antagonist | 52 (45%) | 33 (50%) | 19 (39%) | 0.23 | 0.63 [0.30, 1.34] |
| ICD or Biventricular ICD | 49 (43%) | 30 (45%) | 19 (39%) | 0.47 | 0.76 [0.36, 1.61] |
| Symptom Characteristics | |||||
| HFSPS-Dyspnea score | 3 [0–10] | 1 [0–6] | 5 [1–15] | <0.001 | 0.32 [0.15, 0.48] |
| PROMIS Sleep-Related Impairment T-score | 51.7±9.9 | 49.7±9.2 | 54.3±10.4 | 0.016 | −0.47 [−0.84, −0.10] |
| PROMIS Pain Intensity score | 2 [0–5] | 1 [0–4] | 4 [2–6] | <0.001 | 0.38 [0.21, 0.52] |
| PROMIS Pain Interference T-score | 56.8±8.2 | 54.4±6.8 | 59.3±8.9 | 0.005 | −0.62 [−1.05, −0.19] |
| Patient Health Questionnaire-9 score | 3 [1–7] | 2 [1–5] | 5 [3–8] | 0.003 | 0.28 [0.10, 0.44] |
| PROMIS Anxiety T-score | 47.4±9.4 | 46.2±8.5 | 49.1±10.3 | 0.10 | −0.32 [−0.68, 0.05] |
Data presented as mean±standard deviation, median [interquartile range], or N(%)
Non-physically frail includes both non-frail (n = 10) and pre-frail (n = 56)
p values comparing physically frail versus non-physically frail
Presented as Hedge’s g or Spearman’s rho for continuous variables and odds ratios for categorical variables with 95% CI
Abbreviations: BUN, blood urea nitrogen; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HFSPS, Heart Failure Somatic Perception Scale; ICD, internal cardioverter defibrillator; PROMIS, Patient-Reported Outcomes Measurement Information System.
In the backward stepwise regression model (Table 2), women had a 4.6 times greater odds of being physically frail compared with men (model χ2 = 39.45, p < 0.001). Type 2 diabetes and not being on an angiotensin converting enzyme inhibitor or angiotensin II receptor blocker were also significant predictors of physical frailty. In examining the individual physical frailty criteria (Figure 2), women were more likely than men to meet the criteria for weakness (OR 2.43 [95% CI 1.05, 5.66], p = 0.039), slowness (OR 4.69 [95% CI 2.03, 10.85], p < 0.001), physical exhaustion (OR 5.46 [95% CI 1.12, 26.50], p = 0.035), and low physical activity (OR 2.34 [95% CI 1.09, 4.99], p = 0.028).
Table 2.
| OR [95%CI] | p value | |
|---|---|---|
| 4.63 [1.81, 11.84] | 0.001 | |
| Body mass index (kg/m2) | 1.06 [0.99, 1.13] | 0.07 |
| History of asthma, emphysema, chronic bronchitis, or chronic obstructive pulmonary disease | 2.71 [0.98, 7.50] | 0.06 |
| Type 2 diabetes | 3.34 [1.26, 8.86] | 0.016 |
| Prescribed an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker | 0.21 [0.07, 0.61] | 0.004 |
| Model Pseudo R2 / Area Under the ROC Curve | 25.2% / 0.83 | |
Reference group is non-physically frail
Results reported were factors retained in backward stepwise regression model (p < 0.10 retention) that included: age; sex; married/partnered; body mass index; Charlson Comorbidity Index total score; history of asthma, emphysema, chronic bronchitis, or COPD; Stage 3 chronic kidney disease; type 2 diabetes; time with heart failure; New York Heart Association functional class; type of heart failure (heart failure with reduced vs. preserved ejection fraction); left ventricular ejection fraction; serum hemoglobin; prescribed a beta blocker; and prescribed an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker.
Abbreviations: ROC, Receiver Operating Characteristic
Figure 2: Physical Frailty Phenotype Criteria by Sex in Heart Failure.

Significantly more women than men met the weakness, slowness, physical exhaustion, and low physical activity criteria. There was no significant difference for the weight loss criterion. * p < 0.05
Characteristics of physical frailty are stratified by sex in Table 3. Physically frail women had higher overall comorbidity burden, and more had type 2 diabetes compared with non-physically frail women. Physically frail women had significantly worse dyspnea symptoms, similar to men, but they also had significantly worse sleep-related impairment, and pain intensity and interference symptoms compared with non-physically frail women. There were no significant differences in body composition characteristics comparing physically frail women with non-physically frail women.
Table 3.
Clinical, symptom, and body composition characteristics of physical frailty by sex*
| Women | Men | ||||||
|---|---|---|---|---|---|---|---|
| Not Physically Frail (n = 23) | Physically Frail (n = 33) | p value | Not Physically Frail (n = 43) | Physically Frail (n = 16) | p value | Interaction p value | |
| Age (years) | 63.6±16.1 | 65.9±16.6 | 0.61 | 60.4±15.9 | 67.1±11.9 | 0.09 | 0.37 |
| Non-Hispanic Caucasian | 19 (83%) | 30 (91%) | 0.43 | 36 (84%) | 12 (75%) | 0.47 | 0.24 |
| In menopause or menopause transition | 17 (81%) | 24 (83%)** | 0.87 | -- | -- | -- | -- |
| Comorbidity Clinical Characteristics | |||||||
| Body mass index (kg/m2) | 30.8±7.5 | 34.0±9.6 | 0.17 | 28.7±6.9 | 33.4±8.3 | 0.06 | 0.48 |
| Charlson Comorbidity Index (weighted) | 2.4±1.5 | 3.5±2.2 | 0.038 | 3.0±1.9 | 4.3±2.0 | 0.040 | 0.98 |
| Type 2 diabetes | 5 (22%) | 16 (48%) | 0.042 | 13 (30%) | 12 (75%) | 0.002 | 0.43 |
| Stage 3 chronic kidney disease | 6 (26%) | 9 (27%) | 0.92 | 7 (16%) | 9 (56%) | 0.002 | 0.041 |
| Heart Failure Clinical Characteristics | |||||||
| Heart failure phenotype | 0.09 | 0.15 | 0.96 | ||||
| HF with reduced ejection fraction | 17 (74%) | 17 (52%) | 37 (86%) | 11 (69%) | |||
| HF with preserved ejection fraction | 6 (26%) | 16 (48%) | 6 (14%) | 5 (31%) | |||
| Left ventricular ejection fraction | 42.7±16.3 | 49.0±15.3 | 0.16 | 38.4±14.6 | 42.7±16.9 | 0.38 | 0.80 |
| Serum hemoglobin (g/dL) | 12.8±1.7 | 12.7±2.0 | 0.87 | 13.6±1.9 | 12.3±2.2 | 0.042 | 0.16 |
| Prescribed a β-blocker | 20 (87%) | 26 (79%) | 0.43 | 40 (93%) | 13 (81%) | 0.33 | 0.64 |
| Prescribed an angiotensin-converting enzyme-inhibitor or angiotensin II receptor blocker | 18 (78%) | 20 (61%) | 0.16 | 37 (86%) | 8 (50%) | 0.013 | 0.29 |
| Symptom Characteristics | |||||||
| HFSPS-Dyspnea score | 1 [0–5] | 5 [1–15] | 0.010 | 1 [0–6] | 8.5 [0–15.5] | 0.043 | 0.91 |
| PROMIS Sleep-Related Impairment T score | 49.5±9.3 | 55.5±10.2 | 0.028 | 49.9±9.3 | 52.0±10.6 | 0.49 | 0.36 |
| PROMIS Pain Intensity score | 1 [0–4] | 5 [2–7] | <0.001 | 1 [0–4] | 3 [1.5–6] | 0.06 | 0.32 |
| PROMIS Pain Interference T score | 55.6±3.8 | 60.6±9.2 | 0.017 | 53.7±7.9 | 56.7±7.9 | 0.28 | 0.61 |
| Patient Health Questionnaire-9 score | 2 [1–5] | 5 [3–8] | 0.051 | 2 [1–5] | 4 [3–8] | 0.042 | 0.39 |
| Body Composition Characteristics | |||||||
| Whole body lean mass index (kg/m2) | 16.4±2.1 | 17.5±2.8 | 0.11 | 19.0±3.1 | 19.7±2.9 | 0.45 | 0.51 |
| Appendicular muscle mass index (kg/BMI) | 56.8±9.1 | 59.3±10.4 | 0.36 | 92.6±15.9 | 84.2±11.4 | 0.046 | 0.07 |
| Whole body fat mass index (kg/m2) | 13.3±4.2 | 14.8±5.3 | 0.26 | 9.1±3.6 | 10.6±2.6 | 0.12 | 0.60 |
| % body fat | 43.8±6.3 | 44.4±7.4 | 0.75 | 31.4±6.5 | 34.6±3.9 | 0.034 | 0.23 |
| % trunk fat | 45.0±7.9 | 44.0±8.7 | 0.68 | 33.9±8.1 | 38.0±4.4 | 0.026 | 0.11 |
| Average % appendicular fat | 46.1±5.7 | 48.4±7.7 | 0.22 | 29.4±5.9 | 32.0±4.0 | 0.07 | 0.54 |
| Visceral adipose tissue (kg) | 0.99±0.44 | 0.98±0.45 | 0.97 | 0.92±0.43 | 1.02±0.32 | 0.37 | 0.53 |
Data presented as mean±standard deviation, median [interquartile range], or N(%)
Menopause status only available on n = 50 women
Abbreviations: BMI, body mass index; HF, heart failure; HFSPS, Heart Failure Somatic Perception Scale; PROMIS, Patient-Reported Outcomes Measurement Information System.
Physically frail men had higher overall comorbidity burden, and more had type 2 diabetes and stage 3 chronic kidney disease compared with non-physically frail men (Table 3). Physically frail men also had lower hemoglobin and worse dyspnea and depressive symptoms compared with non-physically frail men. In examining the body composition characteristics, physically frail men had significantly lower appendicular muscle mass index, and higher percent body fat and percent trunk fat compared with non-physically frail men. Interaction testing between sex and each of the covariates for the outcome of physical frailty, however, was non-significant except for stage 3 chronic kidney disease.
Discussion
In this study of 56 women and 59 men with HF, we found that women were significantly more likely to be physically frail compared with men. Higher comorbidity burden, especially type 2 diabetes, was a common underlying factor of physical frailty among both women and men with HF. On the other hand, there were potential sex differences in other defining symptom and body composition characteristics. Our preliminary evidence shows that physical frailty among women with HF may be predominantly characterized by worse symptom characteristics; whereas physical frailty among men with HF may be primarily characterized by worse body composition characteristics. Identifying key similarities and differences in physical frailty phenotypes among women and men with HF will be critical for understanding the drivers of physical frailty and informing the design and implementation of interventions.
The primary goal of this prospective, sex-balanced study was to characterize sex differences in physical frailty phenotypes in HF and secondarily to explore defining characteristics of physical frailty phenotypes among women and men with HF. Sex differences in the prevalence of frailty have been understudied both generally among older adults and in chronic conditions such as HF. A meta-analysis showed that across five studies of older adults, all of which used a multidimensional frailty assessment (i.e. combined physical, emotional, and social frailty),32 women consistently had higher rates of frailty.3 In HF, studies have noted that female sex is associated with frailty status using either a multidimensional frailty assessment8 or a physical frailty assessment.9 Our recent meta-analysis of 29 HF studies demonstrated that women have a 26% higher relative risk of being frail compared with men.33 However, sex differences have not been examined further particularly in sex-balanced studies. Our study demonstrated that women are indeed significantly more likely to be physically frail in HF compared with men even after adjusting for other significant factors. In sum, consistent with previous research, being female is a strong determinant of physical frailty in HF.
More women than men met the majority of the physical frailty criteria, which included both objective (i.e. weakness and slowness) and self-reported (i.e. physical exhaustion and low physical activity) measures. The physical frailty phenotype, as initially described by Fried and colleagues,2 is designed to focus on the accumulation of physiological insults that results in the cycle of frailty; a key point being that the composite of the frailty criteria is more important than each individual criteria.34 The composite score has also been shown to be more predictive of poor HF outcomes than any individual criterion.35 Our findings indicate that there is no one criterion that stands out as the driving factor for physical frailty among women; rather, it is the aggregation of impairments that results in the physical frailty phenotype among women. Given these findings, it begs the question: why are women with HF more physically frail compared with men with HF?
Among women with HF, we found that a worse symptom profile was one of the dominant characteristics of physical frailty. Physically frail women had significantly worse dyspnea, sleep-related impairment, and pain intensity and interference symptoms compared with non-physically frail women. These findings are similar to our previous work in which we found that physical frailty was associated with considerably worse symptoms among both women and men with moderate to advanced HF.19 However, it is unclear why symptoms appear to be a larger contributor to the physical frailty phenotype in women but not men. Previous work has demonstrated sex differences in symptom presentations in HF,15 which could play a role in this relationship. Moreover, sex differences in other contextual factors not measured in this study (e.g. caregiving36) could also contribute to this finding. Future research should focus on understanding the association between symptoms and physical frailty among women with HF, including biological and/or behavioral factors.
Interestingly, none of the body composition characteristics distinguished physical frailty from non-physical frailty among women with HF, but the reasons for this are unclear. In comparing the estimated body composition values with the DXA references values from the National Health and Nutrition Examination Survey,37 both physically frail and non-physically frail women had just slightly higher average percent body fat (44.4% and 43.8%, respectively) compared with women at age 65 (43.0% to 43.6%, depending on race/ethnicity). Physically frail and non-physically frail women also had similar average whole body lean mass (17.5 kg/m2 and 16.4 kg/m2, respectively) compared with women at age 65 (16.1 to 17.8 kg/m2, depending on race/ethnicity). While not significant, physically frail women had numerically higher body mass index, whole body lean mass, and whole body fat mass compared with non-physically frail women; a large body habitus makes it more difficult to move around and participate in physical activity. Additionally, there may be more nuanced changes in skeletal muscle (e.g. intramuscular fat deposition, impaired metaboreceptors) not captured in this study that may contribute to physical frailty in women with HF, and in turn, contribute to worse symptoms.38, 39
Among men with HF, in contrast, we found that an unfavorable body composition phenotype was one of the major characteristics of physical frailty. Unfavorable body composition characteristics, including increased body mass index and adipose tissue40, 41 and decreased lean mass,42 have been associated with frailty among older adults. In our sample, physically frail men had increased percent body fat (overall and in the trunk region) and decreased appendicular muscle mass (i.e. less muscle mass in the extremities) compared with non-physically frail men. In comparing the estimated body composition values with the DXA references values from the National Health and Nutrition Examination Survey,37 physically frail men with HF had higher average percent body fat (34.6%) compared with men at age 65 (28.6% to 31.1%, depending on race/ethnicity). Physically frail men with HF had similar average whole body lean mass (19.7 kg/m2) compared with men at age 65 (19.6 to 20.0 kg/m2, depending on race/ethnicity). Thus, increased adiposity and proportions of fat to muscle may be a contributing factor of physical frailty among men with HF.
Lastly, there were a few other significant determinants of physical frailty in HF, with some sex similarities and differences. First, type 2 diabetes was a significant determinant of physical frailty, consistent in both women and men. There is a strong association between frailty and diabetes in general,43 and our findings demonstrate that this association persists in HF. Second, we found that sex moderates the relationship between having a comorbidity of stage 3 chronic kidney disease and physical frailty in HF. Similar to HF, frailty is highly prevalent among those with chronic kidney disease,44 but this particular comorbidity appears to be more of a driving factor for men than women with HF. Third, those prescribed an angiotensin converting enzyme inhibitor or angiotensin II receptor blocker were significantly less likely to be physically frail compared with those not on one of those medications. There is preliminary evidence that these medications may have a beneficial effect on skeletal muscle and thus attenuate the development of frailty,17 and perhaps this effect may extend to those with HF.
There are limitations to this study beyond the inherent limitations in cross-sectional studies. First, this was a racially homogenous and primarily HFrEF and non-ischemic sample of HF patients from a single academic medical center in the Pacific Northwest of the United States; as such, these findings have limited generalizability to the broader HF population, especially minority populations. Second, given our sample size, we were likely underpowered to detect some differences between those physically frail and non-physically frail, overall and stratified by sex. Third, we had limited numbers of non-frail participants who did not meet any of the criteria (n = 10), and we combined this group with pre-frail participants, which made the non-physically frail group more heterogeneous. Finally, all participants chose sex using our binary category system (male vs. female).
Given our findings, there is a need for future research. First, larger studies with equal numbers of women and men are needed to generate robust multivariate models that can further test sex interactions. Second, future research should explore other biological and physiologic metrics that could explain physical frailty phenotypes in women and men. While the biological mechanisms of physical frailty are still hypothetical, key pathophysiological pathways include inflammation, oxidative stress, and dysfunctional hormone regulation (e.g. anabolism).45 Sex differences in these processes could result in differential expression of physical frailty. Moreover, future research should further investigate defining body composition characteristics of physical frailty in HF using different modalities (e.g. bioelectrical impedance analysis,31 computed tomography of psoas muscle or pectoralis muscle46). Third, physical frailty is conceptualized as a continuum with potential for reversibility with HF therapies;47 future research should examine sex differences in the reversibility of physical frailty. Finally, future research should explore more in-depth the key drivers of physical frailty that emerged for women and men. Understanding the balance of biological vs. behavioral/social drivers of physical frailty may, in turn, open up possibilities for the design and implementation of interventions. Additionally, understanding sex differences in the impact of other potentially relevant non-cardiac (e.g. polypharmacy48) and cardiac (e.g. echocardiographic metrics49) characteristics on physical frailty may provide amenable clinical targets for intervention.
Conclusion
We found that physical frailty significantly affects more women compared with men with HF, even after adjusting for covariates. In exploring characteristics of physical frailty by sex, we provided preliminary evidence that physical frailty in women with HF may, in part, be characterized by worse symptom characteristics; whereas, physical frailty in men with HF may, in part, be characterized by worse physiological characteristics. Future research is needed to further identify distinguishing characteristics of physical frailty from the biological level to the behavioral level among women and men with HF.
Supplementary Material
What is New?
In our sex-balanced study of 115 adults with heart failure, being female was a significant predictor of physical frailty.
There were some similarities in defining characteristics of physical frailty among both women and men (i.e. comorbidities, dyspnea symptoms).
Differentiating factors of physical frailty among women were symptoms of sleep impairment and pain intensity and interference.
Differentiating factors of physical frailty among men were low lean mass, high percent body fat, low hemoglobin, and depressive symptoms.
What are the Clinical Implications?
In caring for adults with heart failure, we can expect that more women will be physically frail compared with men.
These high levels of physical frailty among women will have a significant impact on patient-reported and clinical outcomes.
Strategies to mitigate physical frailty may need to incorporate these sex differences.
Sources of Funding
This work was supported in part by the Office of Research on Women’s Health and the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the NIH (K12HD043488; Denfeld) and the Medical Research Foundation. The work reported in this paper was also supported by the National Center for Advancing Translational Sciences of the NIH (UL1TR002369). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Declaration of Conflicting Interests
None
References
- 1.Oksuzyan A, Juel K, Vaupel JW and Christensen K. Men: good health and high mortality. Sex differences in health and aging. Aging Clin Exp Res. 2008;20:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 3.Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE and Hubbard RE. Sex differences in frailty: A systematic review and meta-analysis. Exp Gerontol. 2017;89:30–40. [DOI] [PubMed] [Google Scholar]
- 4.Denfeld QE, Winters-Stone K, Mudd JO, Gelow JM, Kurdi S and Lee CS. The prevalence of frailty in heart failure: A systematic review and meta-analysis. Int J Cardiol. 2017;236:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uchmanowicz I, Lee CS, Vitale C, Manulik S, Denfeld QE, Uchmanowicz B, Rosinczuk J, Drozd M, Jaroch J and Jankowska EA. Frailty and the risk of all-cause mortality and hospitalization in chronic heart failure: a meta-analysis. ESC Heart Fail. 2020;7:3427–3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. , American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e66. [DOI] [PubMed] [Google Scholar]
- 7.Denfeld QE, Winters-Stone K, Mudd JO, Hiatt SO, Chien CV and Lee CS. Frequency of and significance of physical frailty in patients with heart failure. Am J Cardiol. 2017;119:1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gastelurrutia P, Lupon J, Altimir S, de Antonio M, Gonzalez B, Cabanes R, Rodriguez M, Urrutia A, Domingo M, Zamora E, et al. Fragility is a key determinant of survival in heart failure patients. Int J Cardiol. 2014;175:62–6. [DOI] [PubMed] [Google Scholar]
- 9.Vidan MT, Blaya-Novakova V, Sanchez E, Ortiz J, Serra-Rexach JA and Bueno H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail. 2016;18:869–75. [DOI] [PubMed] [Google Scholar]
- 10.Conde-Martel A, Arkuch ME, Formiga F, Manzano-Espinosa L, Aramburu-Bodas O, González-Franco Á, Dávila-Ramos MF, Suárez-Pedreira I, Herrero-Domingo A and Montero-Pérez-Barquero M. Gender related differences in clinical profile and outcome of patients with heart failure. Results of the RICA Registry. Rev Clin Espan. 2015;215:363–370. [DOI] [PubMed] [Google Scholar]
- 11.Azad N, Kathiravelu A, Minoosepeher S, Hebert P and Fergusson D. Gender differences in the etiology of heart failure: A systematic review. J Geriatr Cardiol. 2011;8:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayward CS, Kalnins WV and Kelly RP. Gender-related differences in left ventricular chamber function. Cardiovasc Res. 2001;49:340–350. [DOI] [PubMed] [Google Scholar]
- 13.De Simone G, Devereux RB, Chinali M, Roman MJ, Barac A, Panza JA, Lee ET and Howard BV. Sex differences in obesity-related changes in left ventricular morphology: The Strong Heart Study. J Hypertens. 2011;29:1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beale AL, Meyer P, Marwick TH, Lam CSP and Kaye DM. Sex differences in cardiovascular pathophysiology: Why women are overrepresented in heart failure with preserved ejection fraction. Circulation. 2018;138:198–205. [DOI] [PubMed] [Google Scholar]
- 15.Heo S, Moser DK and Widener J. Gender differences in the effects of physical and emotional symptoms on health-related quality of life in patients with heart failure. Eur J Cardiovasc Nurs. 2007;6:146–52. [DOI] [PubMed] [Google Scholar]
- 16.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Amer Coll Cardiol. 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 17.Keller K, Kane A, Heinze-Milne S, Grandy SA and Howlett SE. Chronic treatment with the ace inhibitor enalapril attenuates the development of frailty and differentially modifies pro- and anti-inflammatory cytokines in aging male and female c57bl/6 mice. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2019;74:1149–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL and MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 19.Denfeld QE, Winters-Stone K, Mudd JO, Hiatt SO and Lee CS. Identifying a relationship between physical frailty and heart failure symptoms. J Cardiovasc Nurs. 2018;33:E1–E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiedemann A, Shimada H, Sherrington C, Murray S and Lord S. The comparative ability of eight functional mobility tests for predicting falls in community-dwelling older people. Age Ageing. 2008;37:430–435. [DOI] [PubMed] [Google Scholar]
- 21.Pulignano G, Del Sindaco D, Di Lenarda A, Alunni G, Senni M, Tarantini L, Cioffi G, Tinti MD, Barbati G, Minardi G and Uguccioni M. Incremental value of gait speed in predicting prognosis of older adults with heart failure: Insights From the IMAGE-HF study. JACC Heart failure. 2016;4:289–98. [DOI] [PubMed] [Google Scholar]
- 22.Yellen SB, Cella DF, Webster K, Blendowski C and Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. [DOI] [PubMed] [Google Scholar]
- 23.Cella D, Lai JS, Chang CH, Peterman A and Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. [DOI] [PubMed] [Google Scholar]
- 24.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jurgens CY, Lee CS and Riegel B. Psychometric analysis of the Heart Failure Somatic Perception Scale as a measure of patient symptom perception. J Cardiovasc Nurs. 2017;32:140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu L, Buysse DJ, Germain A, Moul DE, Stover A, Dodds NE, Johnston KL and Pilkonis PA. Development of short forms from the PROMIS™ Sleep Disturbance and Sleep-Related Impairment item banks. Behav Sleep Med. 2011;10:6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Askew RL, Cook KF, Revicki DA, Cella D and Amtmann D. Evidence from diverse clinical populations supported clinical validity of PROMIS pain interference and pain behavior. J Clin Epidemiol. 2016;73:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroenke K, Spitzer RL and Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammash MH, Hall LA, Lennie TA, Heo S, Chung ML, Lee KS and Moser DK. Psychometrics of the PHQ-9 as a measure of depressive symptoms in patients with heart failure. Eur J Cardiovasc Nurs. 2013;12:446–453. [DOI] [PubMed] [Google Scholar]
- 30.Schalet BD, Pilkonis PA, Yu L, Dodds N, Johnston KL, Yount S, Riley W and Cella D. Clinical validity of PROMIS Depression, Anxiety, and Anger across diverse clinical samples. J Clin Epidemiol. 2016;73:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockwood K and Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Bio Sci Med Sci. 2007;62:722–727. [DOI] [PubMed] [Google Scholar]
- 33.Davis MR, Lee CS, Corcoran A, Gupta N, Uchmanowicz I and Denfeld QE. Gender differences in the prevalence of frailty in heart failure: A systematic review and meta-analysis. Int J Cardiol. 2021;333:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fried LP, Xue QL, Cappola AR, Ferrucci L, Chaves P, Varadhan R, Guralnik JM, Leng SX, Semba RD, Walston JD, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: Implications for etiology and treatment. J Gerontol A Bio Sci Med Sci. 2009;64:1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka S, Kamiya K, Hamazaki N, Matsuzawa R, Nozaki K, Maekawa E, Noda C, Yamaoka-Tojo M, Matsunaga A, Masuda T, et al. Incremental value of objective frailty assessment to predict mortality in elderly patients hospitalized for heart failure. J Card Fail. 2018;24:723–732. [DOI] [PubMed] [Google Scholar]
- 36.Pinquart M and Sorensen S. Gender differences in caregiver stressors, social resources, and health: an updated meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2006;61:P33–45. [DOI] [PubMed] [Google Scholar]
- 37.Kelly TL, Wilson KE and Heymsfield SB. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS ONE. 2009;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coats AJS, Clark AL, Piepoli M, Volterrani M and Poole-Wilson PA. Symptoms and quality of life in heart failure: The muscle hypothesis. Heart. 1994;72:S36–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Philippou A, Xanthis D, Chryssanthopοulos C, Maridaki M and Koutsilieris M. Heart Failure–Induced Skeletal Muscle Wasting. Curr Heart Fail Rep. 2020;17:299–308. [DOI] [PubMed] [Google Scholar]
- 40.Hubbard RE, Lang IA, Llewellyn DJ and Rockwood K. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Bio Sci Med Sci. 2010;65 A:377–381. [DOI] [PubMed] [Google Scholar]
- 41.Stout MB, Justice JN, Nicklas BJ and Kirkland JL. Physiological aging: Links among adipose tissue dysfunction, diabetes, and frailty. Physiology. 2017;32:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.RoyChoudhury A, Dam TTL, Xu C, Diah JH, Chaganty D, Solares J and Fried LP. Feed-forward loop between body composition, strength and performance in older adults. Mech Ageing Dev. 2019;183. [DOI] [PubMed] [Google Scholar]
- 43.Sinclair AJ, Sinclair H, Bellary S and Rodriguez-Manas L. The emergence of frailty and sarcopaenia in diabetes mellitus: Description of inter-relationships and clinical importance. Cardiovas Endocrinol. 2016;5:40–50. [Google Scholar]
- 44.Chowdhury R, Peel NM, Krosch M and Hubbard RE. Frailty and chronic kidney disease: A systematic review. Archives of Gerontology and Geriatrics. 2017;68:135–142. [DOI] [PubMed] [Google Scholar]
- 45.Bisset ES and Howlett SE. The biology of frailty in humans and animals: Understanding frailty and promoting translation. Aging Med. 2019;2:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teigen LM, John R, Kuchnia AJ, Nagel EM, Earthman CP, Kealhofer J, Martin C and Cogswell R. Preoperative Pectoralis Muscle Quantity and Attenuation by Computed Tomography Are Novel and Powerful Predictors of Mortality After Left Ventricular Assist Device Implantation. Circ Heart Fail. 2017;10:e004069. [DOI] [PubMed] [Google Scholar]
- 47.Flint KM, Matlock DD, Lindenfeld J and Allen LA. Frailty and the selection of patients for destination therapy left ventricular assist device. Circ Heart Fail. 2012;5:286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Unlu O, Levitan EB, Reshetnyak E, Kneifati-Hayek J, Diaz I, Archambault A, Chen L, Hanlon JT, Maurer MS, Safford MM, et al. Polypharmacy in Older Adults Hospitalized for Heart Failure. Circ Heart Fail. 2020;13:e006977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gharacholou SM, Tashiro T, Cha SS, Scott CG, Takahashi PY and Pellikka PA. Echocardiographic indices associated with frailty in adults >/=65 years. Am J Cardiol. 2015;116:1591–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


