Abstract
Silymarin contains a group of closely-related flavonolignan compounds including silibinin, and is extracted from Silybum marianum species, also called milk thistle. Silymarin has been shown to protect the liver in both experimental models and clinical studies. The chemopreventive activity of silymarin has shown some efficacy against cancer both in vitro and in vivo. Silymarin can modulate apoptosis in vitro and survival in vivo, by interfering with the expression of cell cycle regulators and apoptosis-associated proteins. In addition to its anti-metastatic activity, silymarin has also been reported to exhibit anti-inflammatory activity. The chemoprotective effects of silymarin and silibinin (its major constituent) suggest they could be applied to reduce the side effects and increase the anti-cancer effects of chemotherapy and radiotherapy in various cancer types, especially in gastrointestinal cancers. This review examines the recent studies and summarizes the mechanistic pathways and down-stream targets of silymarin in the therapy of gastrointestinal cancer.
Keywords: Silymarin, Silibinin, Milk thistle, Gastrointestinal cancer, Targeted therapy
1. Introduction
Many recent studies have scientifically investigated the use of natural-derived components or micronutrients, and their potent benefits on human health [1,2]. Plant-derived compounds have been reported to target various cellular processes at a molecular level [3]. Currently, there are more than 1600 flavonoid-related and 3000 polyphenol-related patents. The pharmaceutical benefits of phytomolecules have been recognized in many disorders, including cancers, inflammatory disorders like arthritis, cardiovascular disorders (CVDs), autoimmune diseases, ophthamological disorders, etc [4–8]. As a prominent class of micronutrients and natural products, plant-derived flavonolignans contain a flavonoid moiety linked to a lignan or phenylpropanoid moiety.
The concept of chemoprevention was first described by Michael Sporn in 1976 in an investigation evaluating the preventive effects of natural forms of vitamin A against epithelial carcinogenesis [9]. Currently, chemoprevention is being tested as a strategy, which uses natural or synthetic substances for the inhibition, reversal, or delay of multistage carcinogenesis-related processes, with minimum toxicity against normal cells or tissue.
In 2002, Nanjoo Suh and Michael Sporn, in a commentary in Nature Reviews Cancer, stated that “Elongation of the latency period of carcinogenesis is a very beneficial approach for managing cancer and increasing lifespan, even if complete treatment of malignancy is not achieved” [10]. If cancer could be made into a chronic disease, there could be a higher quality of life for patients before death from other causes in old age. In addition to chemoprevention, some molecules, especially those with a natural origin, have been reported to be able to reverse the problem of chemoresistance frequently encountered in cancer [11]. The concept of chemosensitization is based on using specific drugs to promote the pharmaceutical activities of other drugs by regulating specific chemoresistance-related mechanisms. A chemosensitizer compound may also exert intrinsic anti-cancer activity, or may be a component without any significant chemopreventive activity. In other words, various compounds may be used to minimize the toxicity of traditional chemotherapy to normal cells, to modulate the alterations that occur in drug metabolism, and to overcome mechanisms of intrinsic or extrinsic resistance in cancer patients. According to the FDA (Food and Drug Administration), the plant kingdom has been the most significant source of newly discovered anti-proliferative agents. Forty percent of the presently approved chemotherapy drugs are plant-derived, or have been inspired by natural products, of which > 70% are used in cancer therapy.
The use of natural products such as polyphenols, may be useful for modulating xenobiotic-metabolizing enzymes and metabolic pathways to induce death in cancer cells. In addition to their chemopreventive properties, many natural products have been shown to be chemosensitizers. Flavonoids and flavolignans are a class of polyphenols, typified by silymarin an extract from Silybum marianum, also called milk thistle [12]. The major biologically active flavonolignan compound is called silibinin. Emerging evidence has shown that silymarin may have both chemopreventive and chemosensitizing activity in various experimental cancer models.
1.1. Silymarin structure and chemistry
Pelter and Hansel were the first to establish the chemical structure of silybin in 1968 (Fig. 1). They elucidated the chemical structure using a laborious study of MS and 1 H NMR spectra (DMSO-d6, 100 MHz) [13]. The absolute configuration of silybin at the C-2 and C-3 positions was revealed by a degradative approach in 1975 [14]. Silybin (also known as flavobin, silybine, silliver, silymarin I) has a molecular weight of 482.441, a molecular formula of C25H22O10, and a CAS Number of 22888–70–6 (PubChem). Two main domains exist in the molecular structure of silybin. The first domain is the flavononol group, found in flavonoids such as taxifolin. The second domain is the phenylpropanoid group, found in coniferyl alcohol. These domains are connected by a 1, 4-dioxane ring [15,16].
Fig. 1.
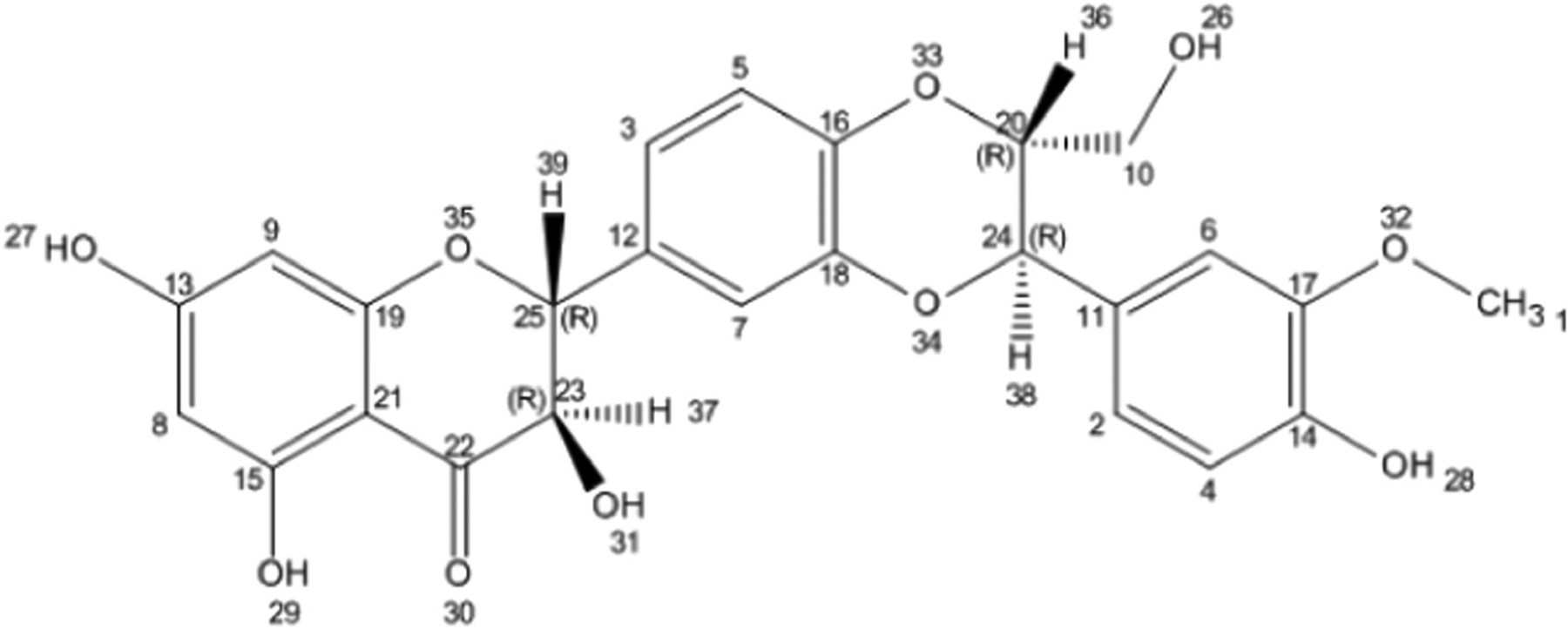
Chemical structure of silybin. Structure generated from InChI code from http://pubchem.ncbi.nlm.nih.gov/. Flavonolignans contain a flavonoid moiety linked to a lignan or phenylpropanoid moiety.
Silybin is a highly stable compound in Brønsted acidic media, but under basic conditions or in Lewis acids, it shows lower stability. Extended heating over 100 C results in skeleton disruption. This compound shows good resistance to reduction but is easily oxidized to 2,3-dehydrosilybin by reaction with two O2 molecules [17]. In aqueous media at neutral pH, silybin acts as a weak acid with, pKa = 6.63 for 5-OH group, pKa = 7.7–7.95 for 7-OH group, and pKa = 11.0 for 20-OH group [18,19]. Silybin contains five hydroxyl groups, which serve as the main targets for derivatization. The 5-OH, 7-OH, and 20-OH groups all show phenolic properties. The 5-OH group undergoes strong hydrogen bonding with the adjacent oxo group, which is conjugated to an aromatic ring and acts as a free electron donor to form a hydrogen bond with the 5-OH group. The 20-OH and 7-OH groups show the same properties, however the 7-OH group shows higher reactivity than the 20-OH group because of its lower steric hindrance and the presence of hydrogen bonding. The 23-OH group undergoes oxidation or esterification with carboxylic acids. The 3-OH group is easily oxidized to a ketone (even with atmospheric O2), resulting in the production of 2, 3-dehydrosilybin. Silybin is poorly soluble in polar protic solvents (MeOH and EtOH) and cannot be dissolved in non-polar solvents (chloroform and petroleum ether). However it is extensively soluble in polar aprotic solvents, including DMF, acetone, THF and DMSO [17]. Silybin exists as two trans diastereoisomers called A and B which are different due to the C-11 and C-10 asymmetric positions within the 1, 4-benzodioxane ring [15,20]. Both silybin A and silybin B show identical 13 C and 1 H NMR spectra, and the individual isomer detection is difficult [21]. High-performance liquid chromatography (HPLC) is the best separation method for these diastereoisomers, which have different retention times [22,23]. Silybin A has 11R, 10R, 3R and 2R isomers, with the IUPAC name of (2 R,3 R)–2-[(2R,3R)–2,3-dihydro-3-(4-hydroxy-3-methoxyphenyl)–2-(hydroxymethyl)–1,4-benzodioxin-6-yl]–2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyran-4-one. Silybin B has 11R, 10R, 3S, 2S configuration, with the IUPAC name of (2R,3R)–2-[(2S,3S)–2,3-dihydro3-(4-hydroxy-3-methoxyphenyl)–2-(hydroxymethyl)–1,4-benzodioxin-6-yl]–2,3-dihydro-3,5,7-trihydroxy4H-1-benzopyran-4-one (Fig. 1) [21,24,25]. These silybin diastereoisomers have different optical rotations: silybin A has [α] 23 D + 20.0 (c 0.21, acetone), while silybin B has [α] 23 D − 1.07 (c 0.28, acetone) [25]. Crystallization also leads to differences between silybin A and B. Silybin A crystallized from MeOH–H2O produces flat yellow crystals yellow with a melting point of 162–163 C, while silybin B forms grainy yellow crystals from MeOH–H2O with a melting point of 158–160 C [21].
1.2. Silymarin metabolism and absorption
There have been some studies on the metabolism and bioavailability of silymarin and silibinin [26–28]. Silibinin was reported to show low bioavailability after oral administration, with only 0.95% found in rats [29]. Following oral consumption of extract of milk thistle, the flavonolignans were analyzed. Legalon was absorbed and excreted rapidly [28], while silibinin showed a 6-h half-life [30–32]. Conjugation was shown to be the main biotransformation pathway of silybin and its derivatives [26]. Silibinin, like other flavonoids, is metabolized by phase II drug metabolism enzymes. Oral administration of silibinin leads to an increase in glutathione S-transferase (GST) and quinone reductase (QR) activity in the liver, stomach, lungs, small bowel, and skin, in a time- and dose-dependent manner [33]. A conjugated form of silibinin can frequently be detected in the systemic circulation [33,34]. After oral consumption of silymarin in healthy volunteers, only 10% [22] to 17% [35] of overall plasma silibinin was free and non-conjugated. The most common metabolites are mono-glucuronides, di-glucuronides, and sulpho-glucuronides, but in total 31 different compounds have been characterized [23]. Both conjugated and free forms of silibinin are rapidly excreted in rats and humans with a half-life of 6.32 h [30]. Thus, silibinin is characterized by rapid metabolism, comparatively low bioavailability, and its plasma concentration is commonly within the nanomolar range limits.
2. Silymarin and gastrointestinal cancer
2.1. Silymarin and gastric cancer
Apoptosis or programmed cell death is a key function of normal cells designed to maintain homeostasis through modulating cell numbers in specific locations, and removing damaged or dysfunctional cells that are unable to recover or be repaired by physiological processes [36]. Two pathways have been found to play critical roles in the induction of apoptosis: (1) death receptor-related extrinsic pathway; (2) mitochondrial-related intrinsic pathway (Fig. 2) [37]. In the extrinsic pathway, the binding of a death ligand to a death-related receptor located on the cell membrane activates the caspase pathway [38,39]. In the intrinsic pathway the release of apoptotic-associated mediators from the mitochondria triggers a signaling cascade [40]. Following the delivery of extracellular stimuli, a signaling cascade involving the nucleus, leads to activation of mitogen-activated protein kinases (MAPK) which regulate cell proliferation, growth, differentiation, and death [37]. MAPKs include, c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK), extracellular signal-regulated protein kinase 1/2 (ERK1/2), and p38 MAPK. ERK1/2 affects growth hormone-associated signal transduction, and regulates cell proliferation, viability, and differentiation [37]. Stimuli such as extracellular stress can activate p38 and JNK, and their downstream signaling to cause inflammation and cell death.
Fig. 2.
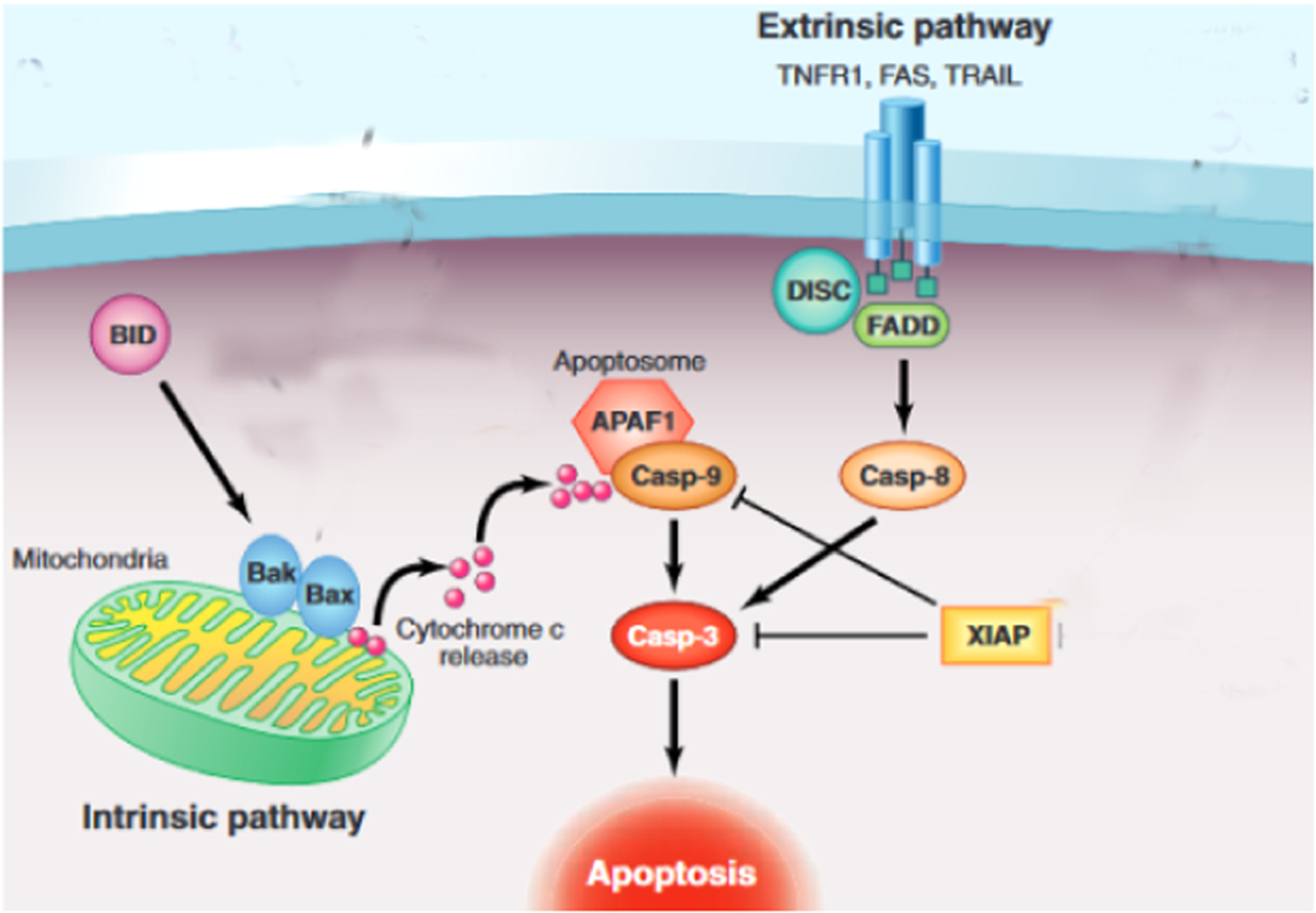
Apoptosis pathways.
Figure adapted from [66].
Kim et al. evaluated the effects of silymarin on proliferation and apoptosis in human gastric cancer cells (AGS) [37]. Viability and migration of AGS cells were significantly decreased in a dose-dependent manner after administration of silymarin. Silymarin increased the apoptosis rate and the number of apoptotic bodies in a concentration-dependent manner. Silymarin significantly up-regulated the levels of phosphorylated (p)-JNK, Bax, and p-p38, and cleaved poly-ADP ribose polymerase (PARP), while it down-regulated Bcl-2 and p-ERK1/2 expression, in a dose-dependent manner. The inhibitory effect of silymarin against in vivo tumor growth was also investigated. Silymarin (100 mg/kg) decreased the tumor volume in an AGS xenograft mouse model and increased apoptosis in the tumors. These effects were attributed to inhibition of p-ERK and p-p38, and activation of p-JNK. Silymarin may have a role in cancer treatment due to its ability to induce apoptosis and inhibit tumor growth [37].
Several plant-derived phytochemicals, such as resveratrol, lycopene, sulforaphane, or silybinin have been shown to have anti-tumor activity, along with relatively low-toxicity to normal cells. Therefore they could be used in combination with conventional anti-tumor drugs, and may show synergistic effects as a cancer treatment [41–44].
Paclitaxel (PTX, brand name Taxol) is a naturally occurring tricyclic diterpenoid compound isolated from the needles and bark of Taxus brevifolia, the Pacific yew tree [45]. This widely used naturally-derived drug has shown a unique anti-cancer mechanism [46]. Tubulin-binding anticancer compounds prevent tubulin assembly within microtubules. In contrast, PTX induces the accumulation of tubulin in the microtubules and thus blocks the disassembly of microtubules, and prevents mitosis, cell cycle progression and cancer cell proliferation [47]. The synergistic therapeutic effect of silibinin combined with PTX has been evaluated [48]. Silibinin alone, paclitaxel alone, or the mixture of silibinin plus PTX triggered apoptosis, and cell cycle arrest at G2/M stage. Furthermore, the combination of silibinin plus PTX increased the Bcl-2/Bax ratio, and the expression of TNF receptor superfamily member 6 (TNFRSF6)/Fas ligand, to trigger the extrinsic apoptosis pathway of SGC-7901 human gastric cancer cells. This study suggested that silibinin could be used to promote the therapeutic effect of PTX against SGC-7901 cells [48].
Several different kinds of epithelium-derived cancers (prostate, colorectal, bladder, and lung cancer) have been shown to be strongly inhibited by silibinin [49,50]. Silibinin inhibited tumor growth, invasiveness, and the metastasis of various tumor cells in vitro [51].
Different doses of silibinin were used to treat BGC-823 gastric cancer cells [52]. Silibinin produced a time- and dose-dependent inhibitory effect on cancer cell proliferation. The higher silibinin doses induced both late and early apoptosis. Silibinin up-regulated the production of mitochondrial-mediated apoptosis-associated signaling mediators [52]. The signal transducer and activator of transcription (STAT)-3 can be constitutively activated in several malignancies and tumor cell lines, including prostate, breast, pancreatic ductal adenocarcinoma (PDAC), colon carcinoma, and gastric cancer. It is known that the increased activation and expression of STAT3 promotes cancer cell survival, proliferation, and angiogenesis, and is strongly associated with tumor invasion, metastasis, and poor prognosis in patients [53–59]. Moreover, inhibition of STAT3 activation can impede growth and invasiveness, while inducing apoptosis [60–63]. STAT3 can be targeted using low molecular weight chemical inhibitors, or other natural products and plant extracts [61,63,64].
Wang and colleagues investigated the effects of different concentrations of silibinin against human gastric cancer cells MGC803 [65]. MGC803 cell growth was inhibited in a silibinin concentration-dependent and time-dependent manner. Silibinin induced apoptosis and cell cycle arrest in G2/M phase in MGC803 cells. Silybinin down-regulated p-STAT3 protein expression and also its downstream genes (such as Mcl-1, survivin, Bcl-xL, and STAT3). Silibinin increased caspase-3 and caspase-9 mRNA and protein expression levels. Furthermore, silibinin significantly reduced the expression level of cyclin B1 and CDK1 at the protein and mRNA levels. These findings suggested that silibinin could inhibit cell proliferation, and triggered apoptosis and cell cycle arrest by inhibiting survivin, cyclinB1, Bcl-xL, CDK1, and Mcl-1, as well as promoting caspase-3 and caspase-9 activity [65].
Table 1 lists some studies reporting anti-gastric cancer effects of Silymarin.
Table 1.
Effects of silymarin in gastric cancer.
| Agents | Dose | Model (in vivo/in vitro) | Cell line | Results | Ref |
|---|---|---|---|---|---|
| Silymarin | 100 mg/kg | In vivo, in vitro | AGS (ER-positive human gastric cancer cell line) | Inhibited tumor growth, induced apoptosis. | [67] |
| Silibinin, paclitaxel | 25 or 50 μM | In vitro | SGC-7901 | Enhanced the effects of paclitaxel against human GC cells. | [48] |
| Silibinin | 0, 25, 50, 100, 150, 200 μM | In vitro | BGC-823 | Promoted apoptosis and inhibited proliferation. | [50] |
| Silibinin | 0, 50, 100, 200 μM | In vitro | MGC803 | Decreased protein expression of cyclin B1 and CDK1, increased mRNA and protein levels of caspase-3 and -9. | [65] |
| Silibinin | 50, 100, 200, 400 μM | In vitro | SGC-7901 | Regulated cell cycle and induced apoptosis. | [51] |
| Silibinin | 50 or 100 μM | In vitro | SNU216 & SNU668 | Down-regulated TNF-α, increased MMP-9 expression, inhibited MEK/ERK signaling pathway. | [68] |
2.2. Silymarin and pancreatic cancer
Amylin (islet amyloid polypeptide) increased apoptosis in INS-1 rat islet β cells, by increasing the TUNEL-positive ratio and decreasing the expression level of PKA and glucagon-like peptide-1 receptor (GLP-1R) [69]. Antagonists of GLP-1R or PKA inhibitors strongly up-regulated apoptosis-related proteins and increased the TUNEL-positive ratio. Addition of silibinin produced an anti-apoptotic effect by up-regulating GLP-1R and PKA expression levels. They concluded that silibinin protected INS-1 cells against amylin-triggered apoptosis by activating GLP-1R/PKA signaling. Silibinin also inhibited the toxic effects of Aβ1–42 peptide, but this was reported to be independent of GLP-1R/PKA signaling [69].
Various studies have shown that there are low expression levels of concentrative nucleoside transporter (CNT) family members in human pancreatic adenocarcinoma cells such as capan-2 cells [70–74]. Therefore the bi-directional human equilibrative nucleoside transporter (hENT1) is involved in gemcitabine uptake, but can also act as an efflux protein. Although hENT1 is abundantly expressed, its expression was altered during the induction of chemoresistance to gemcitabine [75]. Furthermore, other efflux proteins such as, MRP5 (ABCC5), MRP1 (ABCC1), and P-gp (ABCB1) are expressed in pancreatic cancer cell lines, and govern the chemosensitivity to gemcitabine [70,75–77]. Indeed, some drugs with the ability to modulate the expression level of influx and efflux proteins (i.e. hENT1, ABCC5, ABCC1, and ABCB1) have been shown to be potential therapeutic agents in combination with chemotherapy. Butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) are two important synthetic phenolic antioxidants, which have found widespread use in medicine and the food industry. They have been extensively used in the daily human diet, particularly for protecting fat containing foods against air oxidation [78,79]. BHT is also used as a synthetic antioxidant in the cosmetics and pharmaceutical industries, while BHA is also employed as a food preservative and in stabilizing some medications [79–81]. Both these chemicals has been tested in animals to assess their carcinogenicity, while a report from the International Agency for Research on Cancer suggested that there was limited evidence for BHT-induced carcinogenicity in both experimental animals and in humans [82]. Calcitriol (1,25-dihydroxycholecalciferol) is an active vitamin D metabolite that regulates bone mineral metabolism, and intestinal absorption of calcium [83,84]. Calcitriol has been shown to have promising anti-proliferative activity by affecting different cell processes, including apoptosis and cell cycle arrest [83–86]. Both in vivo and in vitro models have been used to show that the activity of chemotherapy drugs, such as gemcitabine and paclitaxel, could be enhanced by calcitriol [87,88].
In one study, Capan-2 pancreatic cancer cells were pretreated with BHT, BHA, calcitriol, or silibinin for 24 h, and then treated with gemcitabine [89]. BHT, BHA and silibinin all up-regulated the expression levels of efflux proteins and decreased gemcitabine uptake, while calcitriol strongly suppressed the expression of efflux proteins, and increased gemcitabine uptake. Pre-treatment with calcitriol protected the cells against gemcitabine toxicity, while BHA increased the cell death rate. Neither BHT nor silibinin had any significant effect on the cell death rate [89].
Silibinin inhibited hypoxia-inducible factor 1α (HIF1α) and mTOR signaling, which are involved in cancer progression [90,91]. The anti-cancer and anti-cachectic effects of silibinin in pancreatic cancer were assessed using in vivo and in vitro experiments [92]. Silibinin significantly suppressed the growth rate and glycolytic activity of pancreatic cancer cells in a dose-dependent manner, and triggered global metabolic reprogramming as shown by metabolomics data based on LC-MS/MS. Silibinin decreased the expression of c-MYC, and inhibited STAT3 signaling in the tumor cells. Over-expression of active STAT3 reversed the silibinin-mediated down-regulation of the metabolic phenotype and c-MYC inhibition. An in vivo model of pancreatic cancer demonstrated that silibinin caused significant reduction in tumor growth and proliferation, and prevented loss of muscle and body weight. The physical activity of the mice was improved after silibinin administration in orthotopic tumor-bearing mice [92].
Histone deactylase (HDAC) inhibitors have emerged as a major class of anti-cancer compounds, and have produced meaningful anti-proliferative effects in various cancers, including pancreatic adenocarcinoma. By altering the acetylation of both non-histone and histone proteins, HDACs can modify the chromatin structure [93]. An imbalance between the acetylation and deacetylation of histones has been found to affect the aberrant transcription of target genes, and has detrimental effects on multiple cellular processes [94–96]. Studies have reported that the cytotoxicity of anti-cancer drugs, including gemcitabine [97], proteasome inhibitors [73], and the smoothened antagonist SANT-1, could be enhanced by treatment with the HDAC inhibitor TSA (trichostatin A) [98]. Therefore, the anti-cancer activity of silibinin might be enhanced by HDAC inhibitors [93]. The synergistic effects of HDAC inhibitors combined with silibinin have been evaluated in colon cancer cells and non-small lung cancer cells in previous investigations [93,99, 100].
Feng et al. evaluated the cytotoxicity of a combination of TSA and silibinin in Panc1 and Capan2 pancreatic cancer cell lines [93]. Results indicated that combination of the HDAC inhibitor TSA and silibinin caused synergistic effects on inhibiting pancreatic cancer cell growth. Besides, the combination treatment significantly triggered cell cycle arrest (G2/M phase) and apoptosis in Panc1 and Capan2 cells. Moreover, the TSA and silibinin mixture significantly decreased the expression levels of cyclinB1-Cdk1 complex, cyclinA2, and survivin [93].
Table 2 summarizes some studies on the anti-pancreatic cancer effects of silymarin and silibinin.
Table 2.
Anti-pancreatic cancer effects of silymarin.
| Agents | Dose | Target gene (s) | Model (In vivo, In vitro) | Cell line | Effects | Ref |
|---|---|---|---|---|---|---|
| Silibinin | 20 μmol/L | GLP-1R | In vitro | INS-1 | Activated GLP-1R/PKA signaling pathway. | [69] |
| Silibinin | 1 μM | MRP1, MRP5 | In vitro | Capan-2 | Increased efflux proteins, reduced overall uptake of gemcitabine. | [89] |
| Silibinin | 10 μM to 250 μM | c-MYC | In vitro, in vivo | S2–013, MIA PaCa-2, T3M4, AsPC-1, BxPC-3, PANC-1 | Down-regulated c-MYC, inhibited tumor cell growth. | [92] |
| Silibinin; Trichostatin A | 50 μM | CyclinA2, CyclinB1-Cdk1 | In vivo, in vitro | Patu8988, Panc1, Capan2 | Reduced cyclin A2, cyclin B1/Cdk1, and survivin expression levels; activated G2/M cell cycle arrest; induced apoptosis. | [93] |
| Silymarin | 120 mg/kg | In vivo | Protected cardiomyocytes against apoptosis. | [101] | ||
| Silibinin | 25–100 μM | In vitro, in vivo | PANC-1, BxPC-3 | Inhibited cell proliferation and angiogenesis; induced apoptosis. | [102] | |
| Silibinin | 12.5, 25, 50, 100, 200, 400, 800 μM | caspase-3, -8, and -9 | BxPC-3, Panc-1, AsPC-1 | Induced G1 phase cell cycle arrest in AsPC-1 cells; triggered apoptosis. | [103] |
2.3. Silymarin and colorectal cancer
The molecular mechanisms underlying the initiation and progression colitis-associated colorectal cancer and sporadic colorectal cancer have been suggested to be different [104]. However, these two types of colorectal cancer often show some common genetic alterations, such as tumor suppressor gene silencing, dysregulation of oncogene expression, and genetic instability. The classical sequence of progression from normal mucosa-to-adenoma-to-carcinoma, which has been established in sporadic colorectal cancer progression has not been established in colitis-associated cancer. Colitis-associated cancer originates in chronically inflamed mucosa, but the typical sequence of inflammation-to-dysplasia-to-carcinoma has not been confirmed in sporadic colorectal cancer [105,106]. The IL-6/STAT3 signaling pathway is a critical tumor promoter in colitis-associated cancer [107,108]. T cells within the regions of chronic inflammation, as well as monocytes and macrophages within the region of acute inflammation are involved in the production of IL-6 that binds to the membrane-bound IL-6 receptor (mIL-6R) and the soluble IL-6 receptor (sIL-6R) [104]. The complex of IL-6 and its receptor has been demonstrated to interact with glycoprotein130 (gp130) to activate its down-stream targets [107]. STAT3 is one of the most important gp130-associated targets [104], and is involved in the regulation of cell proliferation and cell cycle [104]. Chemotherapeutic drugs designed to target the IL-6/STAT3 signaling pathway may be a candidate to prevent or delay colitis-related cancer [104].
Azoxymethane (AOM) is one of the most commonly used carcinogens employed as a model for sporadic colorectal cancer (CRC) to study the underlying mechanisms and possible treatments [109]. AOM is a potent carcinogen that strongly induces colon cancer in rodent models [110]. The carcinogenesis process accurately reflects the pattern observed in humans. Rodents, especially susceptible mice, subjected to frequent intraperitoneal AOM treatment, reliably and easily develop distal colon-specific tumors, which make them good models of colon cancer. Epithelial cells exposed to AOM develop aberrant crypt foci (ACF) that progress to adenomas and malignant adenocarcinoma [111,112]. Under in-vivo conditions, the AOM metabolites induce mutations in DNA molecules and shifts the nucleotides from G:C to A:T. The time of colon cancer induction in rats or mice is 14 weeks [112].
A study by Velmurugan et al., showed that silibinin significantly decreased the formation of aberrant crypt foci induced by AOM in vivo [113]. Moreover, silibinin also decreased the formation of polyps in another mouse model [114]. Zheng and colleagues investigated the chemopreventive effects of silibinin in mice with colitis-associated cancer, and the effects of silibinin on the IL-6/STAT3 signaling pathway [104]. Various concentrations of silibinin were also used to treat IMCE and HCT-116 intestinal tumor cells. AOM and dextran sulfate sodium salt was administered to mice at 750 mg/kg/day for 10 weeks to induce the formation of polyps. Silibinin significantly suppressed the production of inflammatory cytokines, phosphorylation of STAT3, and intestinal tumor cell viability. Furthermore, the number and size of AOM/DSS-induced polyps were significantly decreased after silibinin administration in the mice. Silibinin also decreased colitis and tumor scores, inhibited cell proliferation of the colonic tumor, and promoted cellular apoptosis. Additionally, silibinin can be used for promoting the function of the colonic mucosal barrier. STAT3 phosphorylation was inhibited after silibinin treatment, suggesting that by inhibiting IL-6/STAT3 signaling in mice, silibinin could act as a chemopreventive agent against colitis-associated cancer [104].
The poor bioavailability of silymarin has been suggested to be a crucial reason for explaining its limited therapeutic efficacy. Therefore, the encapsulation of silymarin into nanovehicles, such as liposomes, micelles, polymeric nanoparticles, or solid lipid nanoparticles could be a route to improving its solubility and bioavailability [115–119]. Generally speaking, both small hydrophilic and lipophilic molecules, or large proteins and nucleic acids can be delivered using liposomes [120]. Liposomes are vesicles enclosed by a spherical lipid bilayer, which have an aqueous core for carrying water soluble molecules, and a lipophilic membrane region for dissolving hydrophobic molecules. Liposomes are widely used to increase the safety and efficacy of chemotherapeutic drugs [116,121–126]. Micelles possess a lipophilic core, and are formed from detergents with a polar head group and a non polar tail [127]. With a size range between 5 and 20 nm, micelles are generally considered to be smaller than liposomes [128].
Silymarin-loaded liposomes significantly increased the cellular uptake of silymarin compared to its free form [129]. In a recent investigation, the absorption of silymarin-loaded micelles was found to be much higher than free silymarin in different sections of the intestine in an in vivo model [130].
The bioavailability and solubility of silymarin can be improved by its encapsulation in solid lipid nanoparticles, micelles, liposomes, or polymer nanoparticles [118,131,132]. Liposomes are often used for delivering nucleic acids, large-structured proteins, and delicate materials with lipophilic or hydrophilic properties. The liposomal structure consists of a closed spherical lipid bilayer with an internal aqueous region capable of being loaded with therapeutic drugs to enhance their activity and alter the immune response [133,134]. The micelle structure is composed of a cluster of amphiphlic lipid molecules with a lipophilic core containing fatty acids [135]. Micelles vary in size between 5 and 20 nm and smaller than liposomes [128]. Nanosilymarin showed a more potent cytotoxic effect against HT-29 cells compared to free silymarin (p < 0.01). Colony numbers were remarkably decreased in the nanosilymarin-treated cells compared to free silymarin-treated cells. Nanosilymarin-treated HT-29 cells also showed more apoptosis and necrosis in comparison with free silymarin-treated cells. Nanosilymarin or free silymarin caused no significant alteration in the proliferation, viability, or apoptosis of NIH-3T3 normal control cells. Hence, nanosilymarin could be an improved formulation of silymarin to control colon cancer in humans [120]. Fig. 3 shows some mechanisms such as inflammatory pathways that could be affected by silibinin and silymarin.
Fig. 3.
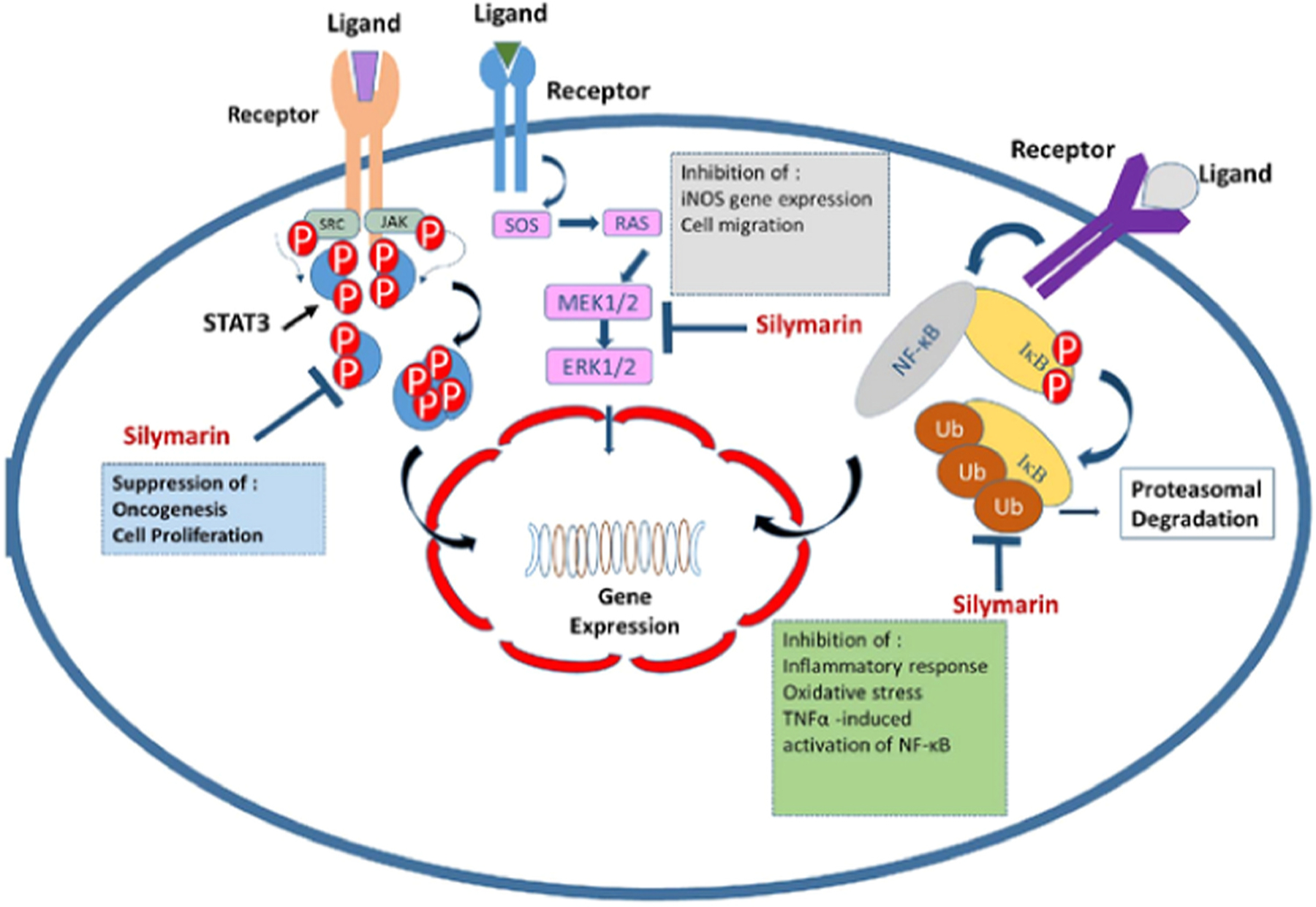
The anti-cancer and anti-inflammatory effects of silymarin. Silymarin can inhibit NF-B by preventing the degradation of inhibitor kappa B (IB), and thus decreases oxidative stress and inflammatory responses. Silymarin inhibits STAT3 and ERK1/2 signaling pathways that can lead to suppression of oncogenesis, cell proliferation, cell migration, and iNOS gene expression.
Figure adapted from [147].
Nafees et al. showed that a combination of two different flavonoid compounds (rutin and silibinin) produced synergistic effects against HT29 human colorectal cancer cells [136]. The combination produced more apoptosis, and regulated the expression of apoptosis-associated genes, modulated inflammation- and MAPK pathway-related proteins compared to either compound used alone.
CD44 is a membrane adhesion molecule that specifically binds to hyaluronic acid. CD44 has been found to regulate various biological mechanisms in cancer, including cell growth, cell adhesion, epithelial-mesenchymal transition (EMT), and tumor progression [137,138]. Transcripts of CD44 can undergo complex alternative splicing, which leads to the production of functionally different isoforms in various epithelial cells [139]. Although the CD44 variant isoform (CD44v) plays a role in the development and progression of cancer, the standard isoform (CD44s) has been more comprehensively investigated [139,140]. Amongst the variant isoforms, CD44v6 has been reported to act as a functional biomarker, which is strongly associated with cancer cell growth, tumor metastasis, tumor recurrence, poor prognosis, and significantly worse 5-year survival in patients with colon cancer. Indeed, this marker of cancer stem cells (CSCs) needs to be further investigated to evaluate the effectiveness of agents that could affect CD44v6, as a potential therapeutic target [140–142]. Therefore, it is necessary to discover potential lead compounds which are active against CD44v6, and to investigate the molecular mechanisms and various signaling pathways involved with CD44v6 in CSCs [143]. Targeting CD44v6 could overcome chemoresistance, and reduce cancer relapse and tumor metastasis, and improve the survival rate in patients with colon cancer [144–146].
Although CD44v6 could be a viable therapeutic target, the lack of a holistic model is hindering the discovery and development of potential lead compounds [143]. Patel et al. carried out a study to predict the protein structure of human CD44v6, screen different anti-CD44v6 lead compounds, using different modeling strategies, including molecular docking, homology modeling, and a molecular dynamic simulation approach [143]. HCT116-derived cancer stem-like CD44 + cells were used to investigate the effect of candidate compounds on the mechanistic and functional properties, including anchorage-independent growth, invasion, migration, expression of EMT markers, regulation of cell cycle, induction of apoptosis, autophagy, and various down-stream pathways [143]. A thorough analysis of CD44v6 and these compounds could help to discover novel CSC-targeted drugs in the future [143].
Patel et al. carried out the screening and prediction of 1674 potential lead compounds against CD44v6 [143]. HCT116-derived CD44 + cells were used to evaluate the cytotoxicity of silibinin, 5FU (5-fluorouracil), and 5FU + silibinin. The proliferation of CD44 + subpopulation cells was inhibited at lower concentrations of 5FU + silibinin compared with silibinin alone. Furthermore, 5FU + silibinin reduced the expression of CD44v6, CDKN2A, CTNNB1, and Nanog, while the expression of E-cadherin was enhanced in the HCT116-derived CD44 + subpopulation. The synergistic effects of these two compounds significantly inhibited the formation of cell spheroids and cell migration, while also inducing PARP cleavage, mitochondrial membrane potential perturbation, resulting in the activation of intrinsic apoptosis and autophagic cell death. Notably, 5FU + silibinin suppressed activation of PI3K/MAPK and triggered G0/G1 cell cycle arrest. Thus, synergistic CD44v6 inhibition could attenuate the stemness of colon cancer stem cells [143].
Table 3 lists some studies on anti-colorectal cancer effects of silymarin and silibinin.
Table 3.
Anti-colorectal cancer effects of silymarin and silibinin.
| Agent | Dose | Model (in vivo, in vitro, Human) | Cell line | Effects | Ref |
|---|---|---|---|---|---|
| Silymarin | 22.4 mg/100 g | In vivo | Showed significant anti-inflammatory and chemopreventive effects | [148] | |
| Silymarin | 0, 50, 100 μM | In vitro | HCT116, SW480 | Down-regulated β-catenin and TCF4; inhibited Wnt signaling. | [149] |
| Silymarin | 50, 100, 200 μM | In vitro | HCT116, SW480 | Down-regulated cyclin D1. | [150] |
| Silibinin | 50–800 μM | In vivo, in vitro | IMCE, HCT-116 | Significantly inhibited IL-6/STAT3 signaling. | [104] |
| Nano-silymarin | 25 μM/mL | In vitro | HT-29 | Cytotoxicity against HT-29 cells. | [120] |
| Silibinin | In vitro | HT-29 | Induced apoptosis | [136] | |
| Silibinin | Human | Suppressed the progression of pre-neoplastic adenomatous polyps. | [151] | ||
| Silymarin + Curcumin | 1.56, 3.125, 6.25, 12.5, 25, 50, 100 μM | In vitro | LoVo, DLD-1, HCT116 | Inhibited cell proliferation; induced apoptosis. | [152] |
| Free silymarin, silymarin-loaded micelles, silymarin-loaded silica NPs | In vivo, in vitro | CaCo-2 | Significantly reduced viability of CaCo-2 cells. | [153] | |
| Silibinin + metformin | 100 μmol/L | In vitro | COLO 205 | Induced apoptosis. | [154] |
| Silibinin-oxidovanadium (IV) complex | 150 μM | In vitro | HT-29 | Inhibited NF-kB; promoted apoptosis. | [155] |
| Silibinin + 1, 25-vitamin D3 | In vitro | SW480-R, HT-29 | Decreased migration & proliferation | [156] | |
| Silibinin | 25–100 μM | In vitro | HT29, SW480, LoVo CRC | Suppressed the IL-4/-6 tumor promoter signaling pathway. | [146] |
| 5FU + Silibinin | 10–1000 μM | In vitro | HCT116 | Inhibited activation of PI3K/MAPK; induced G0/G1 cell cycle arrest | [143] |
| Silibinin + α-tocopherol | 10 μM | In vivo, in vitro | HT29, SH-SY5Y | Decreased oxidative damage. | [157] |
| Silibinin | 0, 0.1, 1, 10, 100 μM | In vivo, in vitro | HT-29 | Affected Cdk4 signaling pathway; inhibited cell growth; induced apoptosis. | [158] |
| Silibinin | 50–100 μM | In vivo, in vitro | SW480, HT29, LoVo | Inhibited PI3K/AKT/mTOR axis; activated MAP2K1/2 & MAPK1/3 signaling pathways. | [159] |
| Silibinin | In vitro | LoVo | Suppressed tumor cell invasion; reduced MMP-2 expression; attenuated binding activity of AP-1. | [160] | |
| Silibinin | 300 mg/kg | In vivo | Down-regulation of MMP-7 and Bcl-2 expression; reduced the number of pre-neoplastic lesions; up-regulated the expression of Bax protein. | [161] | |
| Silibinin | 100, 200, 300 μM | In vitro | SW480, SW620 | Up-regulated DR4 & DR5 expression; down-regulated Mcl-1 & XIA.P expression | [162] |
| Silibinin | 5–50 mg/mL | In vivo, in vitro | CCS, HT-29 | Inhibited PP2Ac/AKT Ser473/mTOR signaling pathway. | [163] |
| Silibinin | 20 mg/kg | In vivo | Regulated Wnt/β-catenin signaling pathway. | [164] | |
| Silibinin | 50–200 μM | In vitro | SW480, LoVo, HT29 | Suppressed CRC growth; inhibited activation of NF-κB. | [165] |
| Silymarin | 100 & 500 p.p.m. | In vivo | Significantly reversed DMH/DSS-induced inflammation. | [166] | |
| Silibinin | 300 μM | In vitro | SW480, SW620 | Induced apoptosis, autophagy, DNA fragmentation, & caspase-3 activation. | [167] |
| Silibinin | 250, 750 mg/kg | In vivo | Modulated IGF-1Rβ & β-catenin signaling pathways; decreased PCNA & cyclin D1 expression levels, while up-regulated Cip1/p21 expression levels. | [168] | |
| Silibinin | 200 mg/kg | In vivo, in vitro | SW480, SW620 | Decreased β-catenin & phospho-GSK3β expression; inhibited cyclin D1, c-Myc, survivin, VEGF, & iNOS. | [169] |
| Silibinin | In vivo | Regulated Wnt/β-catenin signaling | [170] | ||
| Silibinin | 100, 200 mg/kg | In vivo, in vitro | SW480, HCT116 | Triggered apoptosis; inhibited cell growth; decreased cyclin D1, c-Myc, β-catenin, & CDK8 expression | [171] |
| Silymarin | 1–100 μg/mL | In vitro | LoVo, LoVo/DX | Showed promising anti-proliferative effects. | [172] |
| Silibinin | 50 mg/kg | In vivo | Modulated xenobiotic metabolizing enzymes; suppressed 1, 2-dimethylhydrazine-mediated colon carcinogenesis. | [173] | |
| Silibinin | 100, 200 mg/kg/d | In vivo, in vitro | LoVo | Inhibited cell proliferation; inhibited retinoblastoma phosphorylation; promoted apoptosis. | [174] |
| Silibinin | 50 mg/kg | In vivo | Suppressed oxidative stress-induced formation of aberrant crypt foci; inhibited lipid peroxidation. | [175] | |
| Silibinin | 0.033%, 0.1%, 0.33%, 1%, w/w | In vivo | Reduced AOM-triggered expression of inducible nitric oxide synthase; inhibited PCNA, & cyclin D1. | [113] | |
| Silibinin | 200 mg/kg/d | In vivo, in vitro | HT29 | Down-regulated NOS, NOS3, COX-1, HIF-1α, & VEGF expression | [176] |
| Silibinin | 40 μg/mL | In vitro | Fet, Geo, HCT116 | Suppressed cyclin-CDK promoter activity; increased expression of p21 & p27. | [177] |
| Silibinin | 0–5000 ng/mL | Human | Chemopreventative properties. | [178] | |
| Silibinin | 360, 720, 1440 mg per person | Human | Increased expression of IGFBP-3; decreased expression of IGF-I. | [179] | |
| Silibinin formulated with phosphatidylcholine (silipide) | 20–141 nmol/g | Human | No effect on circulating levels of IGF-I, IGFBP-3, M1dG (DNA oxidative damage marker) | [179] | |
| Silibinin + silymarin | 30 μg/mL | In vivo, in vitro | LoVo, EA.hy 926 | Up-regulated VEGFR-1 expression | [180] |
| Silymarin | 5000 ppm | In vivo | Reduced aberrant crypt foci & precancerous lesions; promoted apoptosis. | [181] | |
| Silibinin + silymarin + thalidomide | In vitro | LoVo, EA. hy 926 | Inhibited migration & differentiation of cancer cells. | [182] | |
| Silymarin | 100, 500, 1000 ppm | In vivo | Increased apoptosis; decreased PCNA expression. | [183] |
2.4. Silymarin and hepatocellular carcinoma
Carvedilol is a non-selective anti-oxidant β-blocker used for the reduction of portal blood pressure, prevention of esophageal varices, and reducing the risk of bleeding in chronic liver disease [184]. Carvedilol possesses potent anti-fibrotic, anti-inflammatory, anti-proliferative, and anti-carcinogenesis properties. Balaha and colleagues assessed the possible preventative effects of carvedilol in rats with hepatic cirrhosis who were at risk of progressing to hepatocarcinogenesis [184]. Carvedilol (10 mg/kg/day) silymarin (50 mg/kg/day), or both were administered to manage hepatic cirrhosis for 69 days. Their results indicated that the increased serum concentrations of ALT, ALP, AST, BIL, and IL-6, and the hepatic levels of IL-6, MDA, STAT-3 and hydroxyproline were significantly decreased by both silymarin and carvedilol used individually, and given in combination. Besides, treatment restored the reduced serum level of ALB, the activity of hepatic CAT, and GSH concentration. Treatment strongly restored the normal architecture of the hepatic tissue, the distribution of collagen, and the expression levels of E-and N-cadherin. Interestingly, the effect of carvedilol on the improvement of MDA levels was more pronounced compared with silymarin. Moreover, the combination treatment showed significantly better effects against CCL4-induced hepatotoxicity in comparison with either treatment alone [184].
Silymarin extracts were administered to chronic hepatitis C patients without cirrhosis at doses of 2.1 g/d [185]. To increase the absorption and bioavailability of the silymarin extract, it was combined with phosphatidylcholine (silybin/phosphatidylcholine ratio of 1:2) was introduced as a commercially available formulation called “Siliphos” (Indena S.p.A, Milan, Italy). Ladas et al. administered 320 mg of silybin phosphatidylcholine per day, in a randomized, controlled trial with 50 children suffering from acute lymphoblastic leukemia (ALL), who were receiving chemotherapy with vincristine, 6-mercaptopurine, or methotrexate [186]. The goal was to test if Siliphos could reduce dose-limiting hepatic toxicity. The results showed that Siliphos a trend toward significant reductions in liver toxicity, but did not antagonize the effects of chemotherapy agents used for the treatment of ALL. The results from another phase I trial [187] showed that a maximal dose of silybin phosphatidylcholine (13 g per day) was tolerated in subjects with prostate cancer.
In one trial on advanced HCC patients, the authors evaluated Siliphos in doses of 2, 4, 8, and 12 g per day for 12 weeks [188]. Advanced HCC patients who were not eligible for conventional therapy were included in the phase I clinical trial of silybin phosphatidylcholine [188]. The trial was planned to include four cohorts receiving different doses (2, 4, 8, and 12 g/day) over 12 weeks, but only three patients were recruited. The 3 patients received 2 g of Siliphos in divided doses, daily. Results indicated that serum levels of silibinin glucuronide and silibinin were increased during the first to third weeks of intervention. In all three subjects, abnormalities of hepatic α-fetoprotein and hepatic function worsened in the first 3 weeks, but after 56th day, liver function abnormalities and biomarkers of inflammation were improved in one patient. All 3 subjects died between the 23th to 69th days of the study, possibly from hepatic failure. Taken together, silibinin and silibinin glucuronide were reported to be increased after short-term silybin phosphatidylcholine intervention in subjects with advanced HCC [188].
Roundabout receptor (Robo) and Slit glycoprotein (Slit) are axonal guide molecules, which are involved in the modulation of numerous physiological processes [189]. These proteins play pivotal functions in the development of different organs, including liver, kidney, lungs, eyes, breast, and reproductive organs [189]. The slits family in humans is composed of three members, Slit-1, -2, and -3 all containing a single polypeptide with approximately 1500 amino acids [190]. Slit family proteins have been shown to be structurally similar in all vertebrates [189]. Slit proteins contain four N-terminal regions (D1–D4) with six EGF-like sequences (EGF), leucine-rich repeats (LRR), a laminin-G region, and a C-terminal domain with a cysteine-rich node [189]. The LRR D2 region is conserved and has a critical function in binding to Robo proteins [191]. The high molecular weight Robo proteins are composed of a conserved cytoplasmic region, and a transmembrane receptor protein containing 1000–1600 amino acids [191,192]. All Robo proteins have five fibronectin III regions (Fn III) and immunoglobulin regions (Ig) in the extracellular region, but Robo-4 Fn III motifs possess just two Ig regions [189]. Robos and Slits are large proteins responsible for different cellular signaling pathways, such as cell proliferation and motility, axonal guidance, and angiogenesis [193]. Recent investigations have suggested that Robos and Slits can play crucial functions in tumor initiation, tumor progression, and metastasis. Studies have shown that the Slit/Robo signaling pathway can modulate many oncogenic pathways.
Chemokines have a critical role in tumor cell growth and tumor progression and can also induce angiogenesis [189]. Chemokines and chemokine receptors are involved in the pathogenesis of hepatocellular carcinoma and its metastasis mechanisms [194]. CXCR4 is a common chemokine receptor, which has been reported to be over-expressed in different cancers, and has important roles in tumorigenesis [189]. Taken together, the expression level of CXCR4 could be considered as a prognostic biomarker for the progression and survival rate of cancer patients [189].
Silymarin significantly induced apoptosis and decreased the expression level of CXCR-4 in HepG2 cells in a concentration-dependent manner. Silymarin also reduced the protein level of Slit-2/Robo-1 at a low dose, but increased it at a high dose. Through modulating the Slit-2/Robo-1 signaling pathway in HepG2 cells, silymarin showed proapoptotic, anti-carcinogenic, and anti-metastatic properties, in a dose-dependent manner [189].
To overcome the decreased oral bioavailability of silymarin, and protect it from chemical, physical, or environmental degradation, some novel strategies including nano-based methods have been developed [195]. Nanomaterials with nanoscale dimensions, have various advantages compared with conventional biomaterials, including improved bioavailability and enhanced cellular interactions [64,130]. Nano-structured biomaterials can be solutions to various challenges in tissue engineering and drug delivery systems [196]. Nano-based vehicles have been used to deliver conventionally undeliverable compounds, including molecules with low solubility, and gene therapy associated molecules [197,198]. These approaches include the incorporation of active compounds inside inert lipid carrier vehicles [199]. These can be made from oils [200], surfactants [201–203], self-emulsifying complexes [204,205], emulsions [206–209], micro-emulsions or nano-emulsions [210–212], or liposomes [213]. Nano-emulsions offer some benefits compared with conventional drug delivery systems, such as higher solubility, earlier onset of action, decreased inter-subject variability by protection from gastrointestinal fluid (GIF), an extended shelf life [214], and greater safety and non-toxicity [215].
Ahmad et al. characterized and evaluated a prepared nano-emulsion loaded with silymarin, based on the conductivity, refractive index, volume, viscosity, surface morphology, and in vitro drug release [195]. They also tested the effects of an optimized silymarin nano-emulsion against human hepatic carcinoma cells [195].
A nano-emulsion was developed for silymarin delivery, using the aqueous titration method [195]. Polyethylene glycol 400 (Smix; 2/1; 28.99% v/v, Kolliphor RH40, Sefsol 218 (5.8% v/v) were used as the oil phase, surfactant and co-surfactant, in distilled water (65.22% v/v). The mean particle size (21.24 nm) with the optimized formulation (NE9) showed the maximum drug release (97.75%), minimum viscosity (9.59 cps) during 24 h. Besides, the NE9 complex had a higher Cmax (p < 0.01) and AUC (p < 0.01) as well as lower Tmax (p < 0.05) compared to standard conventional silymarin suspensions. The complexes were very stable when stored in a refrigerator compared to room temperature (p > 0.05). In vitro investigations with cancer cells showed that the silymarin-loaded nano-emulsion significantly decreased viability, while it promoted chromatin condensation and ROS generation, suggesting that silymarin- nano-emulsion could improve the oral administration and bioavailability of silymarin, and could be used to treat human hepatocarcinoma with relative non-toxicity to normal cells [195].
Table 4 lists some studies on the anti-hepatocarcinoma cancer effects of silymarin and silybinin.
Table 4.
Anti-hepatocellular carcinoma effects of silymarin and silibinin.
| Agent | Dose | Model (in vivo, in vitro, Human) | Cell line | Effects | Ref |
|---|---|---|---|---|---|
| Silymarin | In vivo, in vitro | Decreased cell viability; increased ROS | [195] | ||
| Silymarin | In vivo, in vitro | HepG2 | Decreased CXCR-4 protein; induced apoptosis. | [189] | |
| Silymarin + carvedilol | 50 mg/kg/day + 10 mg/kg/day | In vivo | Decreased CCl4 liver toxicity, elevated serum concentrations of BIL, ALP, AST, ALT & IL-6, | [184] | |
| Silybin phosphatidylcholine (Siliphos) | 2, 4, 8, 12 g per day | Improved liver enzyme abnormalities & inflammatory biomarkers. | [188] | ||
| Silymarin | 420 mg/day | No promising effects were observed. | [216] |
2.5. Silymarin and esophageal cancer
Stress can reportedly activate adenosine monophosphate-activated protein kinase (AMPK) which functions as a cellular “fuel gauge” [217]. Phosphorylated AMP-activated protein kinase (p-AMPK) can be thought of as an active form of AMPK, which is associated with decreased energy-consumption and increased generation of adenosine triphosphate [218,219].
Metformin is an oral biguanide drug used as an antihyperglycemic medication to treat diabetes, that was first isolated from Galega officinalis, also known as French lilac, Italian fitch or goat’s rue [9–15]. Biguanide drugs, including metformin, buformin, and phenformin were usually adminstered over short-term treatment plans. Later, phenformin and buformin were excluded due to toxicity and lactic acid formation [220,221]. Metformin can be added to chemotherapy and radiotherapy regimens, and to improve the response to androgen deprivation therapy (ADT) for prostate cancer, and in chemoprevention of different types of cancer [222]. Metformin can reduce the harmful effects of ADT, as well as the possibility of relapse, cancer mortality, and cancer incidence. It can improve the response in cancer cells during chemotherapy and radiotherapy. Therefore, metformin can be included in complementary therapies for prevention and treatment of cancer [222].
In one investigation, the authors evaluated p-AMPK expression levels in human ESCC, and found that the p-AMPK expression level in ESCC tumor tissues were significantly lower than in paired contiguous normal tissue samples [217]. The exact function of p-AMPK in the pathogenesis of ESCC is still obscure, but it has been demonstrated that metformin-treated acute myeloid leukemia, melanoma, and hepatocellular carcinoma cells showed remarkable AMPK activation, and the proliferation and invasion of these cells was decreased [223–225]. However, metformin is generally recognized to induce lactic acidosis, which could be considered an adverse effect.
Silibinin significantly reversed the chemoresistance of ESCC cells to conventional chemotherapeutic drugs (5-FU and cisplatin) [217]. In addition, there is limited evidence for silibinin-mediated effects on the AMPK signaling pathway [217]. Silibinin-mediated anticancer activity against ESCC using both in vivo and in vitro experiments, suggested the beneficial effects of silibinin on ESCC significantly depended on AMPK induction [217].
A comparison between human esophageal normal tissue and ESCC samples showed that AMPK was inactivated (69.4%) in ESCC tissues. Silibinin triggered apoptosis, suppressed in vitro ESCC cell proliferation, and inhibited in vivo tumor growth with no sign of any adverse effects. Silibinin also significantly inhibited the invasion of ESCC cells in vitro, and suppressed ESCC lung metastasis in vivo. Delivery of a shRNA targeted against AMPK abrogated the silibinin-mediated anti-cancer effects. Besides, silibinin significantly reversed the chemoresistance of ESCC cells and tumors to both 5-FU and cisplatin. They suggested that AMPK could be a promising therapeutic target in ESCC, and silibinin could have substantial therapeutic benefits [217].
2.6. Limitations of silymarin and silibinin and some possible solutions
As mentioned earlier, silymarin shows relative low toxicity and has generally been reported to be safe. However, because silymarin can induce an increase in bile secretion and bile flow, some instances of laxative effects at high doses have been reported as a rare adverse effect [226].
GI tract-associated adverse effects, including diarrhea, nausea, bloating, and dyspepsia were reported in 2–10% of subjects in a clinical trial study [227]. However, serious rare side effects such as gastroenteritis–induced collapse or serious allergies have been only occasionally observed in patients [228].
Silymarin can be regarded as a potential natural immunomodulatory therapy, but its poor bioavailability has limited its wider application. Considering this issue, higher concentrations of silymarin are needed to achieve sufficient plasma levels to have a therapeutic effect. Therefore several literature studies have suggested a varity of approaches to improve silymarin bioavailability.
Blumenthal et al. [229] showed that the levels of absorption of silymarin varied from 20% to 50%. Different reasons, including poor enteral absorption [230,231], gastric acid-mediated degradation of silymarin [229], and its poor solubility [229,232–234] have all been proposed to explain the poor bioavailability of silymarin. Therefore higher doses of silymarin are often to increase its absorption and overcome the problems.
As mentioned above, pharmaceutical formulation approaches have successfully been tested to improve the delivery and bioavailability of silymarin. These formulations have included a combination with cyclodextrin [235,236], incorporation as a fine solid dispersion [237], providing silymarin in a salt form with polyhydroxyphenylchromanones [232], preparation of various more soluble derivatives [238], and complex formation with phospholipids [233,239,240].
Soo Woo et al. formulated silymarin in a self-micro-emulsifying drug delivery system (SMEDDS) to improve the solubility [241]. A diagram of the pseudo-ternary phases led to the optimal composition, namely 15% silymarin, 37.5% polysorbate 20, 10% glyceryl monooleate (oil phase), 37.5% transcutol (cosurfactant), and HCO-50 (1:1, surfactant), at a surfactant/cosurfactant ratio of 1:1. The average oil phase droplet size was 67 nm within the microemulsion. The release profile of silybin from the SMEDDS capsules showed significantly higher drug release compared to Legalonβ as a reference capsule formulation. The percentage release of silybin from SMEDDS after six hours was 2.5 times greater than the reference. The pharmacokinetics demonstrated about a two-fold reduction in tmax with SMEDDS, an eight-fold increase in Cmax, and a 3.6 fold increase in AUC. A marked improvement was seen in silymarin bioavailability using SMEDDS, compared to the standard reference dosage.
Abrol et al. investigated a combination of silymarin and phospholipids, which were encapsulated in microspheres to demonstrate its hepatoprotective effects, and compared the mentioned formulation with free silymarin [242]. Different silymarin-loaded emulsions were formulated using soybean oil, lecithin, Tween 80 and propylene glycol as the internal oily phase and surfactant, respectively. The in vitro release profile when stirred at 50 rpm indicated that the release approximately was increased 4-fold after 36 h, suggesting that the sub-micron sized lipid emulsion could promote the drug bioavailability. Furthermore, an experiment was conducted in an animal model of hepatoprotective activity in vivo. This involved measuring the length of sleep time induced by administration of phenobarbitone in rats. The silymarin loaded emulsion significantly reduced the phenobarbitone-induced sleep time, and also reduced the elevation of liver enzymes (SGOT and SGPT) compared with rats who received silymarin solution or controls. The silymarin lipid emulsion induced hepatoprotective effects against carbon tetrachloride-mediated liver toxicity, which were more pronounced compared to silymarin solution or plain lipid emulsion as judged by histopathological examination at necroscopy.
Arcari et al. developed another formulation containing silybin and β-cyclodextrin to overcome the poor bioavailability of silymarin. This complex was compared with silybin, silymarin, and a conventional silybin-based formulation in dissolution rate testing in vitro, and in a rat bile elimination test in vivo. The findings indicated that the drug complex dissolution rate (>90% in 5 min) was dramatically faster than silybin (< 5%). The in vivo investigation confirmed the result of the dissolution rate; oral administration of the silybin complex increased the rat bile silybin concentration approximately 20-fold compated to free silybin or the conventional formulation. These findings revealed that the complexation with β-cyclodextrin increased the silybin bioavailability [236].
Yanyu et al. investigated phospholipid and silybin formulations for increasing poor silymarin bioavailability in rats, and comparing the bioavailability and pharmacokinetics of silybin-N-methylglucamine and silybin-phospholipid complexes [243]. The phospholipid and silybin were dissolved in an organic solvent (ethanol), and then evaporated under vacuum, resulting in the complex formation in a thin film. The solubility of the silybin complex in water and octanol was increased 1000 fold compared to a simple physical mixture. The solubility of the silybin at pH 6.8 was much higher than at pH 1.2. At the end of 1 h, 158.4 mg of silybin was dissolved at pH 6.8 in comparison with only 6.3 mg at pH 1.2. Therefore, the silybin solubility was pH dependent. Rat bioavailability studies showed that the bioavailability of the silymarin phospholipid complex was up to 5 times greater than silybin-N-methylglucamine.
Yanyu and colleagues studied oral silymarin bioavailability in beagle dogs by preparing silymarin proliposomes [244]. Proliposomes are a free flowing dried preparation that can easily be rehydrated to form liposomes. They used a round-bottom flask with a dry film formed from a silymarin-phospholipid mixture and a mannitol vector. Proliposomes solubility at either pH 1.2 or 6.8 showed that the silymarin was completely dissolved after 20 min, regardless of the pH value. A silymarin-loaded mannitol powder as a control was also prepared by the same approach but without the phospholipids. Findings also showed that the proliposomes-mediated dissolution of silymarin was greater than the control. The formulation encapsulation efficacy was reported to be > 90% with a 196.4 nm mean particle size, which at 40 °C was stable for 3 months. The pharmacokinetics indicated a tmax of 30 min for either silymarin proliposomes or silymarin, aCmax of 472.62 ng/mL and 89.78 ng/mL, and AUC values equal to 2606.21 ng/mL and 697 ng/mL for silymarin proliposomes and free silymarin, respectively. Results showed that the proliposomes promoted silymarin absorption in the GI tract.
El-Samaligy and colleagues prepared hybrid liposomes loaded with silymarin, using a reverse evaporation technique with lecithin, stearyl amine, cholesterol, and Tween 20 [245]. The encapsulation efficiency of the hybrid liposomes was 69.22%. A 1:1 mixture of silymarin-loaded liposomes and unloaded liposomes was found to be the most effective to prevent aggregation. The formulation was stable after three months at a temperature of 4 °C as judged by drug loading and particle size. Differential scanning calorimetry and Fourier transform infrared spectroscopy provided evidence of phospholipid and silymarin interactions. The goal was to increase permeation and thus enhance the bioavailability of silymarin. According to in vivo experiments, the liposomal silymarin formulation decreased the elevated serum levels of SGPT which were due to acute carbon tetrachloride-induced liver toxicty, better than a silymarin suspension. Histopathological examination showed that carbon tetrachloride-mediated liver necrosis was improved by silymarin liposomes.
As mentioned previously, lipid-based SMEDDS were prepared using a dialysis bag technique from ethanol, Tween 80 and ethyl linoleate as cosurfactant, surfactant and oil phase, respectively [246]. In vitro studies revealed that the release of silymarin from SMEDDS was incomplete, as expected from a sustained release system. The silymarin SMEDDS relative bioavailability was 1.88-fold higher than silymarin PEG 400 suspension, and 48.82-fold higher than silymarin solution. The high bioavailability of the SMEDDS silymarin was attributed to its effects on the lymphatic transport system [247], and the presence of long-chain fatty acids, which promote lipoprotein biosynthesis and lymphatic absorption.
2.7. Clinical trials
The effectiveness of silymarin and silymarin-related preparations in several cancer types has been evaluated in a limited number of phase 1, 2, and 3 clinical trials. When silymarin is administered at therapeutic doses it seems to be relatively safe in humans. It has been shown to be tolerated by patients at a large dose (700 mg) thrice per day over six months [248]. Interestingly, two clinical studies reported that silymarin has promising protective effects in patients undergoing chemotherapy. Based on a phase I clinical trial, daily administration of silymarin (800 mg) for three months in patients receiving chemotherapy with 6-mercaptopurine or methotrexate showed normal levels of hepatic transaminase [249]. Silymarin was used in combination with lycopene, soy and anti-oxidants in one phase III clinical trial to delay the progression of prostate specific antigen following radiotherapy and prostatectomy in subjects with prostate cancer, [250]. Results showed that silymarin supplementation produced a 2-log reduction in PSA compared with the placebo control.
3. Conclusion
Recent advances in epidemiology, molecular biology, and biochemistry have extended our knowledge of the use food as pharmaceuticals. Our dietary habits have crucial roles in health maintenance, and several health problems can be caused by a misbalanced diet. Anti-oxidants have been reported to be one of the main modulators of many physiological pathways, and the anti-oxidant/pro-oxidant balance (redox balance) in our diet, can affect the gastrointestinal organs, blood circulation, and tissues. There are thousands of phenolic compounds found in natural products, which are consumed by humans and other animals. Furthermore, different types of phytochemicals like flavonoids, are an indispensable part of our diet, and are involved with the maintenance of the optimal status of anti-oxidant defenses. Flavonoids in general are not well absorbed in the intestines, therefore their serum and tissue levels are relatively low. However even this low concentration seems to be sufficient for the activation of nuclear factor erythroid 2 (NF-E2)-related factor 2 (Nrf2), and the suppression of NF-κB. Indeed, the activation of the Keap1/Nrf2/ARE signaling pathway and the inhibition of the NF-κB pathway, could be considered to be the most important mechanisms behind the phytochemical-induced health benefits, rather than direct scavenging of free radicals.
The redox-sensitive Nrf2 belongs to the transcription factor family called “Cap’n’collar” (CNC), which is involved in adaptation to cellular stress. Nrf2 is trapped in the cytoplasm by Keap1 (kelch-like ECH associated protein 1) under normal homeostatic conditions, and is targeted for degradation and ubiquitination via 6S proteoromes [251]. The Keap1/CUL3/RBX1 E3-ubiquitin ligase complex is deactivated by redox modification and/or phosphorylation of the cysteine residues in Keap1 during stress conditions [252]. In the next step, nuclear translocation of Nrf2 occurs, and it is dimerized via musculoaponeurotic fibrosarcoma (Maf) proteins. Then, it interacts with an antioxidant response element (ARE, also known as electrophile response element, EpRE), which is an enhancer sequence or a cis-regulatory element present in the promoter region of some genes that encode cytoprotective proteins and detoxification enzymes, such as glutathione synthetase (GSS), heme oxygenase-1 (HO-1), and NAD(P)H quinone dehydrogenase 1(NQO1) [252]. Different endogenous or exogenous molecules are able to activate the Nrf2/Keap1/ARE signaling system. This pathway can regulate expression of several genes related to cell survival and proliferation [253]. Hence, consumption of phytochemicals, including silymarin, may improve the human anti-oxidant systems in general. Silymarin, and its main component silibinin, can be consumed as dietary phytochemicals, and could play a crucial role in the modulation of anti-oxidant defenses in the intestines and other parts of the body. Only a few studies have investigated the clinical effectiveness of silybin in cancer in general [254,255]. Hence, more studies are required to establish the utility of silymarin for treating GI cancers in humans.
Footnotes
Conflict of interest statement
None
References
- [1].Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT, Natural products in drug discovery: advances and opportunities, Nat. Rev. Drug Discov 20 (2021) 200–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Newman DJ, Cragg GM, Natural products as sources of new drugs from 1981 to 2014, J. Nat. Prod 79 (2016) 629–661. [DOI] [PubMed] [Google Scholar]
- [3].Tewari D, Nabavi SF, Nabavi SM, Sureda A, Farooqi AA, Atanasov AG, Vacca RA, Sethi G, Bishayee A, Targeting activator protein 1 signaling pathway by bioactive natural agents: possible therapeutic strategy for cancer prevention and intervention, Pharmacol. Res 128 (2018) 366–375. [DOI] [PubMed] [Google Scholar]
- [4].Kashyap D, Tuli HS, Yerer MB, Sharma A, Sak K, Srivastava S, Pandey A, Garg VK, Sethi G, Bishayee A, Natural product-based nanoformulations for cancer therapy: opportunities and challenges, Semin. Cancer Biol 69 (2021) 5–23. [DOI] [PubMed] [Google Scholar]
- [5].Mardani R, Hamblin MR, Taghizadeh M, Banafshe HR, Nejati M, Mokhtari M, Borran S, Davoodvandi A, Khan H, Jaafari MR, Mirzaei H, Nanomicellar-curcumin exerts its therapeutic effects via affecting angiogenesis, apoptosis, and T cells in a mouse model of melanoma lung metastasis, Pathol., Res. Pract 216 (2020), 153082. [DOI] [PubMed] [Google Scholar]
- [6].Bagherian A, Mardani R, Roudi B, Taghizadeh M, Banfshe HR, Ghaderi A, Davoodvandi A, Shamollaghamsari S, Hamblin MR, Mirzaei H, Combination therapy with nanomicellar-curcumin and temozolomide for in vitro therapy of glioblastoma multiforme via wnt signaling pathways, J. Mol. Neurosci.: MN 70 (2020) 1471–1483. [DOI] [PubMed] [Google Scholar]
- [7].Davoodvandi A, Darvish M, Borran S, Nejati M, Mazaheri S, Reza Tamtaji O, Hamblin MR, Masoudian N, Mirzaei H, The therapeutic potential of resveratrol in a mouse model of melanoma lung metastasis, Int. Immunopharmacol 88 (2020), 106905. [DOI] [PubMed] [Google Scholar]
- [8].Shafabakhsh R, Pourhanifeh MH, Mirzaei HR, Sahebkar A, Asemi Z, Mirzaei H, Targeting regulatory T cells by curcumin: a potential for cancer immunotherapy, Pharmacol. Res 147 (2019), 104353. [DOI] [PubMed] [Google Scholar]
- [9].Sporn MB, Dunlop NM, Newton DL, Smith JM, Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids), Fed. Proc 35 (1976) 1332–1338. [PubMed] [Google Scholar]
- [10].Sporn MB, Suh N, Chemoprevention: an essential approach to controlling cancer, Nat. Rev. Cancer 2 (2002) 537–543. [DOI] [PubMed] [Google Scholar]
- [11].Gupta SC, Kannappan R, Reuter S, Kim JH, Aggarwal BB, Chemosensitization of tumors by resveratrol, Ann. N.Y. Acad. Sci 1215 (2011) 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mastron JK, Siveen KS, Sethi G, Bishayee A, Silymarin and hepatocellular carcinoma: a systematic, comprehensive, and critical review, Anti Cancer Drugs 26 (2015) 475–486. [DOI] [PubMed] [Google Scholar]
- [13].Pelter A, Hänsel R, The structure of silybin (silybum substance E6), the first flavonolignan, Tetrahedron Lett. 9 (1968) 2911–2916. [Google Scholar]
- [14].Pelter A, Hansel R, Structure of silybin. 1. Degradative experiments, Chem. Ber. Recl 108 (1975) 790–802. [Google Scholar]
- [15].Althagafy HS, Meza-Aviña ME, Oberlies NH, Croatt MP, Mechanistic study of the biomimetic synthesis of flavonolignan diastereoisomers in milk thistle, J. Org. Chem 78 (2013) 7594–7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kurkin V, Phenylpropanoids from medicinal plants: distribution, classification, structural analysis, and biological activity, Chem. Nat. Compd 39 (2003) 123–153. [Google Scholar]
- [17].Biedermann D, Vavříková E, Cvak L, Křen V, Chemistry of silybin, Nat. Prod. Rep 31 (2014) 1138–1157. [DOI] [PubMed] [Google Scholar]
- [18].Bai T-C, Zhu J-J, Hu J, Zhang H-L, Huang C-G, Solubility of silybin in aqueous hydrochloric acid solution, Fluid Phase Equilibr. 254 (2007) 204–210. [Google Scholar]
- [19].van Wenum E, Jurczakowski R, Litwinienko G, Media effects on the mechanism of antioxidant action of silybin and 2,3-dehydrosilybin: role of the enol group, J. Org. Chem 78 (2013) 9102–9112. [DOI] [PubMed] [Google Scholar]
- [20].Napolitano JG, Lankin DC, Graf TN, Friesen JB, Chen SN, McAlpine JB, Oberlies NH, Pauli GF, HiFSA fingerprinting applied to isomers with near-identical NMR spectra: the silybin/isosilybin case, J. Org. Chem 78 (2013) 2827–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lee DY, Liu Y, Molecular structure and stereochemistry of silybin A, silybin B, isosilybin A, and isosilybin B, Isolated from Silybum marianum (milk thistle), J. Nat. Prod 66 (2003) 1171–1174. [DOI] [PubMed] [Google Scholar]
- [22].Weyhenmeyer R, Mascher H, Birkmayer J, Study on dose-linearity of the pharmacokinetics of silibinin diastereomers using a new stereospecific assay, Int. J. Clin. Pharmacol. Ther. Toxicol 30 (1992) 134–138. [PubMed] [Google Scholar]
- [23].Rickling B, Hans B, Kramarczyk R, Krumbiegel G, Weyhenmeyer R, Two high-performance liquid chromatographic assays for the determination of free and total silibinin diastereomers in plasma using column switching with electrochemical detection and reversed-phase chromatography with ultraviolet detection, Journal of chromatography B, Biomed. Appl 670 (1995) 267–277. [DOI] [PubMed] [Google Scholar]
- [24].Han YH, Lou HX, Ren DM, Sun LR, Ma B, Ji M, Stereoselective metabolism of silybin diastereoisomers in the glucuronidation process, J. Pharm. Biomed. Anal 34 (2004) 1071–1078. [DOI] [PubMed] [Google Scholar]
- [25].Monti D, Gazák R, Marhol P, Biedermann D, Purchartová K, Fedrigo M, Riva S, Kren V, Enzymatic kinetic resolution of silybin diastereoisomers, J. Nat. Prod 73 (2010) 613–619. [DOI] [PubMed] [Google Scholar]
- [26].Křen V, Marhol P, Purchartová K, Gabrielová E, Modrianský M, Biotransformation of silybin and its congeners, Curr. Drug Metab 14 (2013) 1009–1021. [DOI] [PubMed] [Google Scholar]
- [27].Wen Z, Dumas TE, Schrieber SJ, Hawke RL, Fried MW, Smith PC, Pharmacokinetics and metabolic profile of free, conjugated, and total silymarin flavonolignans in human plasma after oral administration of milk thistle extract, Drug Metab. Dispos. Biol. Fate Chem 36 (2008) 65–72. [DOI] [PubMed] [Google Scholar]
- [28].Zhu HJ, Brinda BJ, Chavin KD, Bernstein HJ, Patrick KS, Markowitz JS, An assessment of pharmacokinetics and antioxidant activity of free silymarin flavonolignans in healthy volunteers: a dose escalation study, Drug Metab. Dispos. Biol. Fate Chem 41 (2013) 1679–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wu JW, Lin LC, Hung SC, Chi CW, Tsai TH, Analysis of silibinin in rat plasma and bile for hepatobiliary excretion and oral bioavailability application, J. Pharm. Biomed. Anal 45 (2007) 635–641. [DOI] [PubMed] [Google Scholar]
- [30].Lorenz D, Lücker PW, Mennicke WH, Wetzelsberger N, Pharmacokinetic studies with silymarin in human serum and bile, Methods Find. Exp. Clin. Pharmacol 6 (1984) 655–661. [PubMed] [Google Scholar]
- [31].Barzaghi N, Crema F, Gatti G, Pifferi G, Perucca E, Pharmacokinetic studies on IdB 1016, a silybin- phosphatidylcholine complex, in healthy human subjects, Eur. J. Drug Metab. Pharmacokinet 15 (1990) 333–338. [DOI] [PubMed] [Google Scholar]
- [32].Flory PJ, Krug G, Lorenz D, Mennicke WH, Studies on elimination of silymarin in cholecystectomized patients. I. Biliary and renal elimination after a single oral dose, Planta Med. 38 (1980) 227–237. [DOI] [PubMed] [Google Scholar]
- [33].Zhao J, Agarwal R, Tissue distribution of silibinin, the major active constituent of silymarin, in mice and its association with enhancement of phase II enzymes: implications in cancer chemoprevention, Carcinogenesis 20 (1999) 2101–2108. [DOI] [PubMed] [Google Scholar]
- [34].D’Andrea V, Pérez LM, Sánchez EJ, Pozzi, Inhibition of rat liver UDP-glucuronosyltransferase by silymarin and the metabolite silibinin-glucuronide, Life Sci. 77 (2005) 683–692. [DOI] [PubMed] [Google Scholar]
- [35].Calani L, Brighenti F, Bruni R, Del Rio D, Absorption and metabolism of milk thistle flavanolignans in humans, Phytomed. Int. J. Phytother. Phytopharm 20 (2012) 40–46. [DOI] [PubMed] [Google Scholar]
- [36].Hsu H-F, Houng J-Y, Kuo C-F, Tsao N, Wu Y-C, Glossogin, a novel phenylpropanoid from Glossogyne tenuifolia, induced apoptosis in A549 lung cancer cells, Food Chem. Toxicol 46 (2008) 3785–3791. [DOI] [PubMed] [Google Scholar]
- [37].Kim SH, Choo GS, Yoo ES, Woo JS, Han SH, Lee JH, Jung JY, Silymarin induces inhibition of growth and apoptosis through modulation of the MAPK signaling pathway in AGS human gastric cancer cells, Oncol. Rep 42 (2019) 1904–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kaufmann T, Strasser A, Jost PJ, Fas death receptor signalling: roles of Bid and XIAP, Cell Death Differ. 19 (2012) 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tummers B, Green DR, Caspase-8: regulating life and death, Immunol. Rev 277 (2017) 76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kim D, Chung J, Akt: versatile mediator of cell survival and beyond, J. Biochem. Mol. Biol 35 (2002) 106–115. [DOI] [PubMed] [Google Scholar]
- [41].Agarwal R, Cell signaling and regulators of cell cycle as molecular targets for prostate cancer prevention by dietary agents, Biochem. Pharmacol 60 (2000) 1051–1059. [DOI] [PubMed] [Google Scholar]
- [42].van Die MD, Bone KM, Emery J, Williams SG, Pirotta MV, Paller CJ, Phytotherapeutic interventions in the management of biochemically recurrent prostate cancer: a systematic review of randomised trials, BJU Int. 117 (2016) 17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wattenberg LW, Chemoprevention of cancer, Cancer Res. 45 (1985) 1–8. [PubMed] [Google Scholar]
- [44].Kelloff GJ, Perspectives on Cancer Chemoprevention Research and Drug Development, Advances in Cancer Research, Elsevier, 1999, pp. 199–334. [DOI] [PubMed] [Google Scholar]
- [45].Zhu L, Chen L, Progress in research on paclitaxel and tumor immunotherapy, Cell. Mol. Biol. Lett 24 (2019) 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT, Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia, J. Am. Chem. Soc 93 (1971) 2325–2327. [DOI] [PubMed] [Google Scholar]
- [47].Weaver BA, How taxol/paclitaxel kills cancer cells, Mol. Biol. Cell 25 (2014) 2677–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhang Y, Ge Y, Ping X, Yu M, Lou D, Shi W, Synergistic apoptotic effects of silibinin in enhancing paclitaxel toxicity in human gastric cancer cell lines, Mol. Med. Rep 18 (2018) 1835–1841. [DOI] [PubMed] [Google Scholar]
- [49].Singh RP, Sharma G, Dhanalakshmi S, Agarwal C, Agarwal R, Suppression of advanced human prostate tumor growth in athymic mice by silibinin feeding is associated with reduced cell proliferation, increased apoptosis, and inhibition of angiogenesis, Cancer Epidemiol. Prev. Biomark 12 (2003) 933–939. [PubMed] [Google Scholar]
- [50].Li R, Yu J, Wang C, Silibinin promotes the apoptosis of gastric cancer BGC823 cells through caspase pathway, J. BUON 22 (2017) 1148–1153. [PubMed] [Google Scholar]
- [51].Zhang Y, Li Q, Ge Y, Chen Y, Chen J, Dong Y, Shi W, Silibinin triggers apoptosis and cell-cycle arrest of SGC7901 cells, Phytother. Res 27 (2013) 397–403. [DOI] [PubMed] [Google Scholar]
- [52].Gong Q, Xie J, Liu Y, Li Y, Su G, Differentially expressed MicroRNAs in the development of early diabetic retinopathy, J. Diabetes Res 2017 (2017), 4727942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yakata Y, Nakayama T, Yoshizaki A, Kusaba T, Inoue K, Sekine I, Expression of p-STAT3 in human gastric carcinoma: significant correlation in tumour invasion and prognosis, Int. J. Oncol 30 (2007) 437–442. [PubMed] [Google Scholar]
- [54].Sato T, Neilson LM, Peck AR, Liu C, Tran TH, Witkiewicz A, Hyslop T, Nevalainen MT, Sauter G, Rui H, Signal transducer and activator of transcription-3 and breast cancer prognosis, Am. J. Cancer Res 1 (2011) 347–355. [PMC free article] [PubMed] [Google Scholar]
- [55].Singh N, Hussain S, Bharadwaj M, Kakkar N, Singh S, Sobti RC, Overexpression of signal transducer and activator of transcription (STAT-3 and STAT-5) transcription factors and alteration of suppressor of cytokine signaling (SOCS-1) protein in prostate cancer, J. Recept. Signal Transduct 32 (2012) 321–327. [DOI] [PubMed] [Google Scholar]
- [56].Morikawa T, Baba Y, Yamauchi M, Kuchiba A, Nosho K, Shima K, Tanaka N, Huttenhower C, Frank DA, Fuchs CS, STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers, Clin. Cancer Res 17 (2011) 1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Huang C, Huang R, Chang W, Jiang T, Huang K, Cao J, Sun X, Qiu Z, The expression and clinical significance of pSTAT3, VEGF and VEGF-C in pancreatic adenocarcinoma, Neoplasma 59 (2012) 52–61. [DOI] [PubMed] [Google Scholar]
- [58].Lee SW, Ahn YY, Kim YS, Kang SB, Nam SW, Lee DS, Jeong HY, Kim JM, The immunohistochemical expression of STAT3, Bcl-xL, and MMP-2 proteins in colon adenoma and adenocarcinoma, Gut Liver 6 (2012) 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Xiong H, Du W, Wang J-L, Wang Y-C, Tang J-T, Hong J, Fang J-Y, Constitutive activation of STAT3 is predictive of poor prognosis in human gastric cancer, J. Mol. Med 90 (2012) 1037–1046. [DOI] [PubMed] [Google Scholar]
- [60].Ahmad A, Sarkar SH, Aboukameel A, Ali S, Biersack B, Seibt S, Li Y, Bao B, Kong D, Banerjee S, Anticancer action of garcinol in vitro and in vivo is in part mediated through inhibition of STAT-3 signaling, Carcinogenesis 33 (2012) 2450–2456. [DOI] [PubMed] [Google Scholar]
- [61].Sun M, Liu C, Nadiminty N, Lou W, Zhu Y, Yang J, Evans CP, Zhou Q, Gao AC, Inhibition of Stat3 activation by sanguinarine suppresses prostate cancer cell growth and invasion, Prostate 72 (2012) 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wang J, Chen S, Xu S, Yu X, Ma D, Hu X, Cao X, In vivo induction of apoptosis by fucoxanthin, a marine carotenoid, associated with down-regulating STAT3/EGFR signaling in sarcoma 180 (S180) xenografts-bearing mice, Mar. Drugs 10 (2012) 2055–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhang X, Yue P, Page BD, Li T, Zhao W, Namanja AT, Paladino D, Zhao J, Chen Y, Gunnings PT, Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts, Proc. Nat. Acad. Sci. U.S.A 109 (2012) 9623–9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Shi Z-B, Zhao D, Huang Y-Y, Du Y, Cao X-R, Gong Z-N, Zhao R, Li J-X, Discovery, synthesis, and evaluation of small-molecule signal transducer and activator of transcription 3 inhibitors, Chem. Pharm. Bull 60 (2012) 1574–1580. [DOI] [PubMed] [Google Scholar]
- [65].Wang Y-X, Cai H, Jiang G, Zhou T-B, Wu H, Silibinin inhibits proliferation, induces apoptosis and causes cell cycle arrest in human gastric cancer MGC803 cells via STAT3 pathway inhibition, Asian Pac. J. Cancer Prev 15 (2014) 6791–6798. [DOI] [PubMed] [Google Scholar]
- [66].Denicourt C, Dowdy SF, Targeting apoptotic pathway in cancer cells, Science 305 (2004) 1411–1413. [DOI] [PubMed] [Google Scholar]
- [67].Kim SH, Choo GS, Yoo ES, Woo JS, Han SH, Lee JH, Jung JY, Silymarin induces inhibition of growth and apoptosis through modulation of the MAPK signaling pathway in AGS human gastric cancer cells, Oncol. Rep 42 (2019) 1904–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kim S, Choi MG, Lee HS, Lee SK, Kim SH, Kim WW, Hur SM, Kim J-H, Choe J-H, Nam SJ, Silibinin suppresses TNF-α-induced MMP-9 expression in gastric cancer cells through inhibition of the MAPK pathway, Molecules 14 (2009) 4300–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Yang J, Sun Y, Xu F, Liu W, Mai Y, Hayashi T, Hattori S, Ushiki-Kaku Y, Onodera S, i Tashiro S, Silibinin ameliorates amylin-induced pancreatic β-cell apoptosis partly via upregulation of GLP-1R/PKA pathway, Mol. Cell. Biochem 452 (2019) 83–94. [DOI] [PubMed] [Google Scholar]
- [70].Hagmann W, Faissner R, Schnölzer M, Löhr M, Jesnowski R, Membrane drug transporters and chemoresistance in human pancreatic carcinoma, Cancers 3 (2011) 106–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Spratlin JL, Mackey JR, Human equilibrative nucleoside transporter 1 (hENT1) in pancreatic adenocarcinoma: towards individualized treatment decisions, Cancers 2 (2010) 2044–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].García-Manteiga J, Molina-Arcas M, Casado FJ, Mazo A, Pastor-Anglada M, Nucleoside transporter profiles in human pancreatic cancer cells: role of hCNT1 in 2′, 2′-difluorodeoxycytidine-induced cytotoxicity, Clin. Cancer Res 9 (2003) 5000–5008. [PubMed] [Google Scholar]
- [73].Paproski RJ, Young JD, Cass CE, Predicting gemcitabine transport and toxicity in human pancreatic cancer cell lines with the positron emission tomography tracer 3′-deoxy-3′-fluorothymidine, Biochem. Pharmacol 79 (2010) 587–595. [DOI] [PubMed] [Google Scholar]
- [74].Young J, Yao S, Sun L, Cass C, Baldwin S, Human equilibrative nucleoside transporter (ENT) family of nucleoside and nucleobase transporter proteins, Xenobiotica 38 (2008) 995–1021. [DOI] [PubMed] [Google Scholar]
- [75].Kohan HG, Boroujerdi M, Time and concentration dependency of P-gp, MRP1 and MRP5 induction in response to gemcitabine uptake in Capan-2 pancreatic cancer cells, Xenobiotica 45 (2015) 642–652. [DOI] [PubMed] [Google Scholar]
- [76].O’DRISCOLL L, Walsh N, Larkin A, Ballot J, Ooi W, Gullo G, O’CONNOR R, Clynes M, Crown J, Kennedy S, MDR1/P-glycoprotein and MRP-1 drug efflux pumps in pancreatic carcinoma, Anticancer Res. 27 (2007) 2115–2120. [PubMed] [Google Scholar]
- [77].König J, Hartel M, Nies AT, Martignoni ME, Guo J, Büchler MW, Friess H, Keppler D, Expression and localization of human multidrug resistance protein (ABCC) family members in pancreatic carcinoma, Int. J. Cancer 115 (2005) 359–367. [DOI] [PubMed] [Google Scholar]
- [78].Lobo V, Patil A, Phatak A, Chandra N, Free radicals, antioxidants and functional foods: Impact on human health, Pharmacogn. Rev 4 (2010) 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Williams G, Iatropoulos M, Whysner J, Safety assessment of butylated hydroxyanisole and butylated hydroxytoluene as antioxidant food additives, Food Chem. Toxicol 37 (1999) 1027–1038. [DOI] [PubMed] [Google Scholar]
- [80].Young I, Woodside J, Antioxidants in health and disease, J. Clin. Pathol 54 (2001) 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Papas AM, Antioxidant Status, Diet, Nutrition, and Health, Routledge Taylor & Fransis Group, 1999, pp. 89–106. [Google Scholar]
- [82].Botterweck A, Verhagen H, Goldbohm R, Kleinjans J, Van P, den Brandt, Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: results from analyses in the Netherlands cohort study, Food Chem. Toxicol 38 (2000) 599–605. [DOI] [PubMed] [Google Scholar]
- [83].Ahmed S, Johnson CS, Rueger RM, Trump DL, Calcitriol (1, 25-dihydroxycholecalciferol) potentiates activity of mitoxantrone/dexamethasone in an androgen independent prostate cancer model, J. Urol 168 (2002) 756–761. [PubMed] [Google Scholar]
- [84].Hasan SS, Rizvi A, Naseem I, Calcitriol-induced DNA damage: Toward a molecular mechanism of selective cell death, IUBMB Life 65 (2013) 787–792. [DOI] [PubMed] [Google Scholar]
- [85].Pintado C, Carracedo J, Rodríguez M, Perez-Calderon R, Ramírez R, 1α, 25-dihydroxyvitamin D3 (calcitriol) induces apoptosis in stimulated T cells through an IL-2 dependent mechanism, Cytokine 8 (1996) 342–345. [DOI] [PubMed] [Google Scholar]
- [86].Zhang A, Wang Y, Xie H, Zheng S, Calcitriol inhibits hepatocyte apoptosis in rat allograft by regulating apoptosis-associated genes, Int. Immunopharmacol 7 (2007) 1122–1128. [DOI] [PubMed] [Google Scholar]
- [87].Yu W-D, Ma Y, Flynn G, Muindi JR, Kong R-X, Trump DL, Johnson CS, Calcitriol enhances gemcitabine antitumor activity in vitro and in vivo by promoting apoptosis in a human pancreatic carcinoma model system, Cell Cycle 9 (2010) 3094–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hershberger PA, Yu W-D, Modzelewski RA, Rueger RM, Johnson CS, Trump DL, Calcitriol (1, 25-dihydroxycholecalciferol) enhances paclitaxel antitumor activity in vitro and in vivo and accelerates paclitaxel-induced apoptosis, Clin. Cancer Res 7 (2001) 1043–1051. [PubMed] [Google Scholar]
- [89].Gilzad-Kohan H, Sani S, Boroujerdi M, Calcitriol reverses induced expression of efflux proteins and potentiates cytotoxic activity of gemcitabine in capan-2 pancreatic cancer cells, J. Pharm. Pharm. Sci 20 (2017) 295–304. [DOI] [PubMed] [Google Scholar]
- [90].Singh PK, Brand RE, Mehla K, MicroRNAs in pancreatic cancer metabolism, Nat. Rev. Gastroenterol. Hepatol 9 (2012) 334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Garcia-Maceira P, Mateo J, Silibinin inhibits hypoxia-inducible factor-1α and mTOR/p70S6K/4E-BP1 signalling pathway in human cervical and hepatoma cancer cells: implications for anticancer therapy, Oncogene 28 (2009) 313–324. [DOI] [PubMed] [Google Scholar]
- [92].Shukla SK, Dasgupta A, Mehla K, Gunda V, Vernucci E, Souchek J, Goode G, King R, Mishra A, Rai I, Silibinin-mediated metabolic reprogramming attenuates pancreatic cancer-induced cachexia and tumor growth, Oncotarget 6 (2015) 41146–41161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Feng W, Cai D, Zhang B, Lou G, Zou X, Combination of HDAC inhibitor TSA and silibinin induces cell cycle arrest and apoptosis by targeting survivin and cyclinB1/Cdk1 in pancreatic cancer cells, Biomed. Pharmacother 74 (2015) 257–264. [DOI] [PubMed] [Google Scholar]
- [94].Kumagai T, Wakimoto N, Yin D, Gery S, Kawamata N, Takai N, Komatsu N, Chumakov A, Imai Y, Koeffler HP, Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (Vorinostat, SAHA) profoundly inhibits the growth of human pancreatic cancer cells, Int. J. Cancer 121 (2007) 656–665. [DOI] [PubMed] [Google Scholar]
- [95].Johnstone RW, Histone-deacetylase inhibitors: novel drugs for the treatment of cancer, Nat. Rev. Drug Discov 1 (2002) 287–299. [DOI] [PubMed] [Google Scholar]
- [96].Marks P, Xu WS, Histone deacetylase inhibitors: potential in cancer therapy, J. Cell. Biochem 107 (2009) 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Arnold NB, Arkus N, Gunn J, Korc M, The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces growth inhibition and enhances gemcitabine-induced cell death in pancreatic cancer, Clin. Cancer Res 13 (2007) 18–26. [DOI] [PubMed] [Google Scholar]
- [98].Chun SG, Zhou W, Yee NS, Combined targeting of histone deacetylases and hedgehog signaling enhances cytoxicity in pancreatic cancer, Cancer Biol. Ther 8 (2009) 1328–1339. [DOI] [PubMed] [Google Scholar]
- [99].Mateen S, Raina K, ain AK J, Agarwal C, Chan D, Agarwal R, Epigenetic modifications and p21-cyclin B1 nexus in anticancer effect of histone deacetylase inhibitors in combination with silibinin on non-small cell lung cancer cells, Epigenetics 7 (2012) 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kauntz H, Bousserouel S, Gosśe F, Raul F, Epigenetic effects of the natural flavonolignan silibinin on colon adenocarcinoma cells and their derived metastatic cells, Oncol. Lett 5 (2013) 1273–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Tuorkey MJ, El-Desouki NI, Kamel RA, Cytoprotective effect of silymarin against diabetes-induced cardiomyocyte apoptosis in diabetic rats, Biomed. Environ. Sci 28 (2015) 36–43. [DOI] [PubMed] [Google Scholar]
- [102].Nambiar D, Prajapati V, Agarwal R, Singh RP, In vitro and in vivo anticancer efficacy of silibinin against human pancreatic cancer BxPC-3 and PANC-1 cells, Cancer Lett. 334 (2013) 109–117. [DOI] [PubMed] [Google Scholar]
- [103].Ge Y, Zhang Y, Chen Y, Li Q, Chen J, Dong Y, Shi W, Silibinin causes apoptosis and cell cycle arrest in some human pancreatic cancer cells, Int. J. Mol. Sci 12 (2011) 4861–4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zheng R, Ma J, Wang D, Dong W, Wang S, Liu T, Xie R, Liu L, Wang B, Cao H, Chemopreventive effects of silibinin on colitis-associated tumorigenesis by inhibiting IL-6/STAT3 signaling pathway, Mediat. Inflamm 2018 (2018), 1562010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Luo C, Zhang H, The role of proinflammatory pathways in the pathogenesis of colitis-associated colorectal cancer, Mediat. Inflamm 2017 (2017), 5126048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Itzkowitz SH, Molecular biology of dysplasia and cancer in inflammatory bowel disease, Gastroenterol. Clin 35 (2006) 553–571. [DOI] [PubMed] [Google Scholar]
- [107].Waldner MJ, Foersch S, Neurath MF, Interleukin-6-a key regulator of colorectal cancer development, Int. J. Biol. Sci 8 (2012) 1248–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Grivennikov S, Karin E, Terzic J, Mucida D, Yu G-Y, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer, Cancer Cell 15 (2009) 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Chen J, Huang XF, The signal pathways in azoxymethane-induced colon cancer and preventive implications, Cancer Biol. Ther 8 (2009) 1313–1317. [DOI] [PubMed] [Google Scholar]
- [110].Lijinsky W, Saavedra JE, Reuber MD, Organ-specific carcinogenesis in rats by methyl- and ethylazoxyalkanes, Cancer Res. 45 (1985) 76–79. [PubMed] [Google Scholar]
- [111].Takahashi M, Wakabayashi K, Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents, Cancer Sci. 95 (2004) 475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].O’Toole SM, Pegg AE, Swenberg JA, Repair of O6-methylguanine and O4-methylthymidine in F344 rat liver following treatment with 1,2-dimethylhydrazine and O6-benzylguanine, Cancer Res. 53 (1993) 3895–3898. [PubMed] [Google Scholar]
- [113].Velmurugan B, Singh RP, Tyagi A, Agarwal R, Inhibition of azoxymethane-induced colonic aberrant crypt foci formation by silibinin in male Fisher 344 rats, Cancer Prev. Res 1 (2008) 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Rajamanickam S, Velmurugan B, Kaur M, Singh RP, Agarwal R, Chemoprevention of intestinal tumorigenesis in APCmin/+ mice by silibinin, Cancer Res. 70 (2010) 2368–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Snima K, Arunkumar P, Jayakumar R, Lakshmanan V-K, Silymarin encapsulated poly (D, L-lactic-co-glycolic acid) nanoparticles: a prospective candidate for prostate cancer therapy, J. Biomed. Nanotechnol 10 (2014) 559–570. [DOI] [PubMed] [Google Scholar]
- [116].El-Samaligy MS, Afifi NN, Mahmoud EA, Evaluation of hybrid liposomes-encapsulated silymarin regarding physical stability and in vivo performance, Int. J. Pharm 319 (2006) 121–129. [DOI] [PubMed] [Google Scholar]
- [117].Yang G, Zhao Y, Zhang Y, Dang B, Liu Y, Feng N, Enhanced oral bioavailability of silymarin using liposomes containing a bile salt: preparation by supercritical fluid technology and evaluation in vitro and in vivo, Int. J. Nanomed 10 (2015) 6633–6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Yang G, Zhao Y, Feng N, Zhang Y, Liu Y, Dang B, Improved dissolution and bioavailability of silymarin delivered by a solid dispersion prepared using supercritical fluids, Asian J. Pharm. Sci 10 (2015) 194–202. [Google Scholar]
- [119].Li X, Huang Y, Chen X, Zhou Y, Zhang Y, Li P, Liu Y, Sun Y, Zhao J, Wang F, Self-assembly and characterization of Pluronic P105 micelles for liver-targeted delivery of silybin, J. Drug Target 17 (2009) 739–750. [DOI] [PubMed] [Google Scholar]
- [120].Mombeini M, Saki G, Khorsandi L, Bavarsad N, Effects of silymarin-loaded nanoparticles on HT-29 human colon cancer cells, Medicina 54 (2018) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Xu X, Khan MA, Burgess DJ, A two-stage reverse dialysis in vitro dissolution testing method for passive targeted liposomes, Int. J. Pharm 426 (2012) 211–218. [DOI] [PubMed] [Google Scholar]
- [122].Pandey H, Rani R, Agarwal V, Liposome and their applications in cancer therapy, Braz. Arch. Biol. Technol 59 (2016). [Google Scholar]
- [123].Paolino D, Cosco D, Gaspari M, Celano M, Wolfram J, Voce P, Puxeddu E, Filetti S, Celia C, Ferrari M, Targeting the thyroid gland with thyroid-stimulating hormone (TSH)-nanoliposomes, Biomaterials 35 (2014) 7101–7109. [DOI] [PubMed] [Google Scholar]
- [124].Brown BS, Patanam T, Mobli K, Celia C, Zage PE, Bean AJ, Tasciotti E, Etoposide-loaded immunoliposomes as active targeting agents for GD2-positive malignancies, Cancer Biol. Ther 15 (2014) 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Paolino D, Cosco D, Racanicchi L, Trapasso E, Celia C, Iannone M, Puxeddu E, Costante G, Filetti S, Russo D, Gemcitabine-loaded PEGylated unilamellar liposomes vs GEMZAR®: biodistribution, pharmacokinetic features and in vivo antitumor activity, J. Control. Release 144 (2010) 144–150. [DOI] [PubMed] [Google Scholar]
- [126].Celia C, Ferrati S, Bansal S, van de Ven AL, Ruozi B, Zabre E, Hosali S, Paolino D, Sarpietro MG, Fine D, Sustained zero-order release of intact ultra-stable drug-loaded liposomes from an implantable nanochannel delivery system, Adv. Healthc. Mater 3 (2014) 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K, Liposome: classification, preparation, and applications, Nanoscale Res. Lett 8 (2013) 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Florence AT, Attwood D, Physicochemical Principles of Pharmacy: In Manufacture, Formulation and Clinical Use, Pharmaceutical Press, 2015. [Google Scholar]
- [129].Elmowafy M, Viitala T, Ibrahim HM, Abu-Elyazid SK, Samy A, Kassem A, Yliperttula M, Silymarin loaded liposomes for hepatic targeting: in vitro evaluation and HepG2 drug uptake, Eur. J. Pharm. Sci 50 (2013) 161–171. [DOI] [PubMed] [Google Scholar]
- [130].Javed S, Kohli K, Ali M, Reassessing bioavailability of silymarin, Altern. Med. Rev. J. Clin. Ther 16 (2011) 239–249. [PubMed] [Google Scholar]
- [131].Li X, Huang Y, Chen X, Zhou Y, Zhang Y, Li P, Liu Y, Sun Y, Zhao J, Wang F, Self-assembly and characterization of Pluronic P105 micelles for liver-targeted delivery of silybin, J. Drug Target 17 (2009) 739–750. [DOI] [PubMed] [Google Scholar]
- [132].Yang G, Zhao Y, Zhang Y, Dang B, Liu Y, Feng N, Enhanced oral bioavailability of silymarin using liposomes containing a bile salt: preparation by supercritical fluid technology and evaluation in vitro and in vivo, Int. J. Nanomed 10 (2015) 6633–6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Paolino D, Cosco D, Racanicchi L, Trapasso E, Celia C, Iannone M, Puxeddu E, Costante G, Filetti S, Russo D, Fresta M, Gemcitabine-loaded PEGylated unilamellar liposomes vs GEMZAR: biodistribution, pharmacokinetic features and in vivo antitumor activity, J. Control. Release Off. J. Control. Release Soc 144 (2010) 144–150. [DOI] [PubMed] [Google Scholar]
- [134].Celia C, Ferrati S, Bansal S, van de Ven AL, Ruozi B, Zabre E, Hosali S, Paolino D, Sarpietro MG, Fine D, Fresta M, Ferrari M, Grattoni A, Sustained zero-order release of intact ultra-stable drug-loaded liposomes from an implantable nanochannel delivery system, Adv. Healthc. Mater 3 (2014) 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K, Liposome: classification, preparation, and applications, Nanoscale Res. Lett 8 (2013) 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Nafees S, Mehdi SH, Zafaryab M, Zeya B, Sarwar T, Rizvi MA, Synergistic interaction of rutin and silibinin on human colon cancer cell line, Arch. Med. Res 49 (2018) 226–234. [DOI] [PubMed] [Google Scholar]
- [137].Orian-Rousseau V, CD44 acts as a signaling platform controlling tumor progression and metastasis, Front. Immunol 6 (2015) 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Mani SA, Guo W, Liao M-J, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, The epithelial-mesenchymal transition generates cells with properties of stem cells, Cell 133 (2008) 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Tölg C, Hofmann M, Herrlich P, Ponta H, Splicing choice from ten variant exons establishes CD44 variability, Nucleic Acids Res. 21 (1993) 1225–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M, Apuzzo T, Sperduti I, Volpe S, Cocorullo G, CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis, Cell Stem Cell 14 (2014) 342–356. [DOI] [PubMed] [Google Scholar]
- [141].Saito S, Okabe H, Watanabe M, Ishimoto T, Iwatsuki M, Baba Y, Tanaka Y, Kurashige J, Miyamoto Y, Baba H, CD44v6 expression is related to mesenchymal phenotype and poor prognosis in patients with colorectal cancer, Oncol. Rep 29 (2013) 1570–1578. [DOI] [PubMed] [Google Scholar]
- [142].Peng J, Lu JJ, Zhu J, Xu Y, Lu H, Lian P, Cai G, Cai S, Prediction of treatment outcome by CD44v6 after total mesorectal excision in locally advanced rectal cancer, Cancer J. 14 (2008) 54–61. [DOI] [PubMed] [Google Scholar]
- [143].Patel S, Waghela B, Shah K, Vaidya F, Mirza S, Patel S, Pathak C, Rawal R, Silibinin A natural blend in polytherapy formulation for targeting Cd44v6 expressing colon cancer stem cells, Sci. Rep 8 (2018) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Sánchez-García I, Vicente-Dueñas C, Cobaleda C, The theoretical basis of cancer-stem-cell-based therapeutics of cancer: can it be put into practice? Bioessays 29 (2007) 1269–1280. [DOI] [PubMed] [Google Scholar]
- [145].Ward RJ, Dirks PB, Cancer stem cells: at the headwaters of tumor development, Annu. Rev. Pathol. Mech. Dis 2 (2007) 175–189. [DOI] [PubMed] [Google Scholar]
- [146].Kumar S, Raina K, Agarwal C, Agarwal R, Silibinin strongly inhibits the growth kinetics of colon cancer stem cell-enriched spheroids by modulating interleukin 4/6-mediated survival signals, Oncotarget 5 (2014) 4972–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Esmaeil N, Anaraki SB, Gharagozloo M, Moayedi B, Silymarin impacts on immune system as an immunomodulator: one key for many locks, Int. Immunopharmacol 50 (2017) 194–201. [DOI] [PubMed] [Google Scholar]
- [148].Girardi B, Principi M, Pricci M, Giorgio F, Iannone A, Losurdo G, Ierardi E, Di Leo A, Barone M, Chemoprevention of inflammation-related colorectal cancer by silymarin-, acetyl-11-keto-beta-boswellic acid-, curcumin-and maltodextrin-enriched dietetic formulation in animal model, Carcinogenesis 39 (2018) 1274–1282. [DOI] [PubMed] [Google Scholar]
- [149].Eo HJ, Park GH, Jeong JB, Inhibition of Wnt signaling by silymarin in human colorectal cancer cells, Biomol. Ther 24 (2016) 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Eo HJ, Park GH, Song HM, Lee JW, Kim MK, Lee MH, Lee JR, Koo JS, Jeong JB, Silymarin induces cyclin D1 proteasomal degradation via its phosphorylation of threonine-286 in human colorectal cancer cells, Int. Immunopharmacol 24 (2015) 1–6. [DOI] [PubMed] [Google Scholar]
- [151].Alfonso-Moreno V, López-Serrano A, Moreno-Osset E, Chemoprevention of polyp recurrence with curcumin followed by silibinin in a case of multiple colorectal adenomas, Rev. Esp. Enferm. Dig. Organo Of. Soc. Esp. Patol. Dig 109 (2017) 875, 875–875. [DOI] [PubMed] [Google Scholar]
- [152].Montgomery A, Adeyeni T, San K, Heuertz RM, Ezekiel UR, Curcumin sensitizes silymarin to exert synergistic anticancer activity in colon cancer cells, J. Cancer 7 (2016) 1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Scavo MP, Gentile E, Wolfram J, Gu J, Barone M, Evangelopoulos M, Martinez JO, Liu X, Celia C, Tasciotti E, Multistage vector delivery of sulindac and silymarin for prevention of colon cancer, Colloids Surf. B Biointerfaces 136 (2015) 694–703. [DOI] [PubMed] [Google Scholar]
- [154].Tsai C-C, Chuang T-W, Chen L-J, Niu H-S, Chung K-M, Cheng J-T, Lin K-C, Increase in apoptosis by combination of metformin with silibinin in human colorectal cancer cells, World J. Gastroenterol 21 (2015) 4169–4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].León IE, Cadavid-Vargas JF, Tiscornia I, Porro V, Castelli S, Katkar P, Desideri A, Bollati-Fogolin M, Etcheverry SB, Oxidovanadium (IV) complexes with chrysin and silibinin: anticancer activity and mechanisms of action in a human colon adenocarcinoma model, JBIC J. Biol. Inorg. Chem 20 (2015) 1175–1191. [DOI] [PubMed] [Google Scholar]
- [156].Bhatia V, Falzon M, Restoration of the anti-proliferative and anti-migratory effects of 1, 25-dihydroxyvitamin D by silibinin in vitamin D-resistant colon cancer cells, Cancer Lett. 362 (2015) 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Mannelli LDC, Zanardelli M, Failli P, Ghelardini C, Oxaliplatin-induced oxidative stress in nervous system-derived cellular models: could it correlate with in vivo neuropathy? Free Radic. Biol. Med 61 (2013) 143–150. [DOI] [PubMed] [Google Scholar]
- [158].Karim BO, Rhee K-J, Liu G, Zheng D, Huso DL, Chemoprevention utility of silibinin and Cdk4 pathway inhibition in Apc(−/+) mice, BMC Cancer 13 (2013) 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Raina K, Agarwal C, Wadhwa R, Serkova NJ, Agarwal R, Energy deprivation by silibinin in colorectal cancer cells: a double-edged sword targeting both apoptotic and autophagic machineries, Autophagy 9 (2013) 697–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Lin C-M, Chen Y-H, Ma H-P, Wang B-W, Chiu J-H, Chua S-K, Ong J-R, Shyu K-G, Silibinin inhibits the invasion of IL-6-stimulated colon cancer cells via selective JNK/AP-1/MMP-2 modulation in vitro, J. Agric. Food Chem 60 (2012) 12451–12457. [DOI] [PubMed] [Google Scholar]
- [161].Kauntz H, Bousserouel S, Gosse F, Marescaux J, Raul F, Silibinin, a natural flavonoid, modulates the early expression of chemoprevention biomarkers in a preclinical model of colon carcinogenesis, Int. J. Oncol 41 (2012) 849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Kauntz H, Bousserouel S, Gosśe F, Raul F, The flavonolignan silibinin potentiates TRAIL-induced apoptosis in human colon adenocarcinoma and in derived TRAIL-resistant metastatic cells, Apoptosis 17 (2012) 797–809. [DOI] [PubMed] [Google Scholar]
- [163].Wang JY, Chang CC, Chiang CC, Chen WM, Hung SC, Silibinin suppresses the maintenance of colorectal cancer stem-like cells by inhibiting PP2A/AKT/mTOR pathways, J. Cell. Biochem 113 (2012) 1733–1743. [DOI] [PubMed] [Google Scholar]
- [164].Sangeetha N, Viswanathan P, Balasubramanian T, Nalini N, Colon cancer chemopreventive efficacy of silibinin through perturbation of xenobiotic metabolizing enzymes in experimental rats, Eur. J. Pharmacol 674 (2012) 430–438. [DOI] [PubMed] [Google Scholar]
- [165].Raina K, Agarwal C, Agarwal R, Effect of silibinin in human colorectal cancer cells: targeting the activation of NF-κB signaling, Mol. Carcinog 52 (2013) 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Toyoda-Hokaiwado N, Yasui Y, Muramatsu M, Masumura K, Takamune M, Yamada M, Ohta T, Tanaka T, Nohmi T, Chemopreventive effects of silymarin against 1, 2-dimethylhydrazine plus dextran sodium sulfate-induced inflammation-associated carcinogenicity and genotoxicity in the colon of gpt delta rats, Carcinogenesis 32 (2011) 1512–1517. [DOI] [PubMed] [Google Scholar]
- [167].Kauntz H, Bousserouel S, Gossé F, Raul F, Silibinin triggers apoptotic signaling pathways and autophagic survival response in human colon adenocarcinoma cells and their derived metastatic cells, Apoptosis 16 (2011) 1042–1053. [DOI] [PubMed] [Google Scholar]
- [168].Ravichandran K, Velmurugan B, Gu M, Singh RP, Agarwal R, Inhibitory effect of silibinin against azoxymethane-induced colon tumorigenesis in A/J mice, Clin. Cancer Res 16 (2010) 4595–4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Velmurugan B, Gangar SC, Kaur M, Tyagi A, Deep G, Agarwal R, Silibinin exerts sustained growth suppressive effect against human colon carcinoma SW480 xenograft by targeting multiple signaling molecules, Pharm. Res 27 (2010) 2085–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Sangeetha N, Aranganathan S, Panneerselvam J, Shanthi P, Rama G, Nalini N, Oral supplementation of silibinin prevents colon carcinogenesis in a long term preclinical model, Eur. J. Pharmacol 643 (2010) 93–100. [DOI] [PubMed] [Google Scholar]
- [171].Kaur M, Velmurugan B, Tyagi A, Agarwal C, Singh RP, Agarwal R, Silibinin suppresses growth of human colorectal carcinoma SW480 cells in culture and xenograft through down-regulation of beta-catenin-dependent signaling, Neoplasia 12 (2010) 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Colombo V, Lupi M, Falcetta F, Forestieri D, D’Incalci M, Ubezio P, Chemotherapeutic activity of silymarin combined with doxorubicin or paclitaxel in sensitive and multidrug-resistant colon cancer cells, Cancer Chemother. Pharmacol 67 (2011) 369–379. [DOI] [PubMed] [Google Scholar]
- [173].Sangeetha N, Felix AJW, Nalini N, Silibinin modulates biotransforming microbial enzymes and prevents 1, 2-dimethylhydrazine-induced preneoplastic changes in experimental colon cancer, Eur. J. Cancer Prev 18 (2009) 385–394. [DOI] [PubMed] [Google Scholar]
- [174].Kaur M, Velmurugan B, Tyagi A, Deep G, Katiyar S, Agarwal C, Agarwal R, Silibinin suppresses growth and induces apoptotic death of human colorectal carcinoma LoVo cells in culture and tumor xenograft, Mol. Cancer Ther 8 (2009) 2366–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Sangeetha N, Aranganathan S, Nalini N, Silibinin ameliorates oxidative stress induced aberrant crypt foci and lipid peroxidation in 1, 2 dimethylhydrazine induced rat colon cancer, Investig. New Drugs 28 (2010) 225–233. [DOI] [PubMed] [Google Scholar]
- [176].Singh RP, Gu M, Agarwal R, Silibinin inhibits colorectal cancer growth by inhibiting tumor cell proliferation and angiogenesis, Cancer Res. 68 (2008) 2043–2050. [DOI] [PubMed] [Google Scholar]
- [177].Hogan FS, Krishnegowda NK, Mikhailova M, Kahlenberg MS, Flavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancer, J. Surg. Res 143 (2007) 58–65. [DOI] [PubMed] [Google Scholar]
- [178].Hoh CS, Boocock DJ, Marczylo TH, Brown V, Cai H, Steward WP, Berry DP, Gescher AJ, Quantitation of silibinin, a putative cancer chemopreventive agent derived from milk thistle (Silybum marianum), in human plasma by high-performance liquid chromatography and identification of possible metabolites, J. Agric. Food Chem 55 (2007) 2532–2535. [DOI] [PubMed] [Google Scholar]
- [179].Hoh C, Boocock D, Marczylo T, Singh R, Berry DP, Dennison AR, Hemingway D, Miller A, West K, Euden S, Pilot study of oral silibinin, a putative chemopreventive agent, in colorectal cancer patients: silibinin levels in plasma, colorectum, and liver and their pharmacodynamic consequences, Clin. Cancer Res 12 (2006) 2944–2950. [DOI] [PubMed] [Google Scholar]
- [180].Yang S-H, Lin J-K, Huang C-J, Chen W-S, Li S-Y, Chiu J-H, Silibinin inhibits angiogenesis via Flt-1, but not KDR, receptor up-regulation1, J. Surg. Res 128 (2005) 140–146. [DOI] [PubMed] [Google Scholar]
- [181].Volate SR, Davenport DM, Muga SJ, Wargovich MJ, Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin), Carcinogenesis 26 (2005) 1450–1456. [DOI] [PubMed] [Google Scholar]
- [182].Yang S-H, Lin J-K, Chen W-S, Chiu J-H, Anti-angiogenic effect of silymarin on colon cancer LoVo cell line, J. Surg. Res 113 (2003) 133–138. [DOI] [PubMed] [Google Scholar]
- [183].Kohno H, Tanaka T, Kawabata K, Hirose Y, Sugie S, Tsuda H, Mori H, Silymarin, a naturally occurring polyphenolic antioxidant flavonoid, inhibits azoxymethane-induced colon carcinogenesis in male F344 rats, Int. J. Cancer 101 (2002) 461–468. [DOI] [PubMed] [Google Scholar]
- [184].Balaha M, Kandeel S, Barakat W, Carvedilol suppresses circulating and hepatic IL-6 responsible for hepatocarcinogenesis of chronically damaged liver in rats, Toxicol. Appl. Pharmacol 311 (2016) 1–11. [DOI] [PubMed] [Google Scholar]
- [185].Hawke RL, Schrieber SJ, Soule TA, Wen Z, Smith PC, Reddy KR, Wahed AS, Belle SH, Afdhal NH, Navarro VJ, Silymarin ascending multiple oral dosing phase I study in noncirrhotic patients with chronic hepatitis C, J. Clin. Pharmacol 50 (2010) 434–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Ladas EJ, Kroll DJ, Oberlies NH, Cheng B, Ndao DH, Rheingold SR, Kelly KM, A randomized, controlled, double-blind, pilot study of milk thistle for the treatment of hepatotoxicity in childhood acute lymphoblastic leukemia (ALL), Cancer Interdiscip. Int. J. Am. Cancer Soc 116 (2010) 506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Flaig TW, Gustafson DL, Su L-J, Zirrolli JA, Crighton F, Harrison GS, Pierson AS, Agarwal R, Glodé LM, A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients, Investig. N. Drugs 25 (2007) 139–146. [DOI] [PubMed] [Google Scholar]
- [188].Siegel AB, Narayan R, Rodriguez R, Goyal A, Jacobson D, Judith S, Kelly K, Ladas E, Lunghofer PJ, Hansen RJ, Gustafson DL, A phase I dose-finding study of silybin phosphatidylcholine (milk thistle) in patients with advanced hepatocellular carcinoma, Integr. Cancer Ther 13 (2014) 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Aykanat NEB, Kacar S, Karakaya S, Sahinturk V, Silymarin suppresses HepG2 hepatocarcinoma cell progression through downregulation of Slit-2/Robo-1 pathway, Pharmacol. Rep (2020) 1–9. [DOI] [PubMed] [Google Scholar]
- [190].Fujiwara M, Ghazizadeh M, Kawanami O, Potential role of the Slit/Robo signal pathway in angiogenesis, Vasc. Med 11 (2006) 69–74. [DOI] [PubMed] [Google Scholar]
- [191].Dickinson R, Dallol A, Bieche I, Krex D, Morton D, Maher E, Latif F, Epigenetic inactivation of SLIT3 and SLIT1 genes in human cancers, Br. J. Cancer 91 (2004) 2071–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Domyan ET, Branchfield K, Gibson DA, Naiche L, Lewandoski M, Tessier-Lavigne M, Ma L, Sun X, Roundabout receptors are critical for foregut separation from the body wall, Dev. Cell 24 (2013) 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T, Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance, Cell 96 (1999) 795–806. [DOI] [PubMed] [Google Scholar]
- [194].Gassmann P, Haier J, Schlüter K, Domikowsky B, Wendel C, Wiesner U, Kubitza R, Engers R, Schneider SW, Homey B, CXCR4 regulates the early extravasation of metastatic tumor cells in vivo, Neoplasia 11 (2009) 651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Ahmad U, Akhtar J, Singh SP, Badruddeen, Ahmad FJ, Siddiqui S, Wahajuddin, Silymarin nanoemulsion against human hepatocellular carcinoma: development and optimization, Artificial cells, Nanomed. Biotechnol 46 (2018) 231–241. [DOI] [PubMed] [Google Scholar]
- [196].Wagner V, Dullaart A, Bock A-K, Zweck A, The emerging nanomedicine landscape, Nat. Biotechnol 24 (2006) 1211–1217. [DOI] [PubMed] [Google Scholar]
- [197].Zhang P, Ye H, Min T, Zhang C, Water soluble poly (ethylene glycol) prodrug of silybin: design, synthesis, and characterization, J. Appl. Polym. Sci 107 (2008) 3230–3235. [Google Scholar]
- [198].Jain KK, Jain KK, The Handbook of Nanomedicine, Springer, 2008. [Google Scholar]
- [199].Aungst BJ, Novel formulation strategies for improving oral bioavailability of drugs with poor membrane permeation or presystemic metabolism, J. Pharm. Sci 82 (1993) 979–987. [PubMed] [Google Scholar]
- [200].Burcham D, Maurin M, Hausner E, Huang SM, Improved oral bioavailability of the hypocholesterolemic DMP 565 in dogs following oral dosing in oil and glycol solutions, Biopharm. Drug Dispos 18 (1997) 737–742. [DOI] [PubMed] [Google Scholar]
- [201].Serajuddin AT, Sheen PC, Mufson D, Bernstein DF, Augustine MA, Effect of vehicle amphiphilicity on the dissolution and bioavailability of a poorly water-soluble drug from solid dispersions, J. Pharm. Sci 77 (1988) 414–417. [DOI] [PubMed] [Google Scholar]
- [202].Serajuddin AT, Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs, J. Pharm. Sci 88 (1999) 1058–1066. [DOI] [PubMed] [Google Scholar]
- [203].Aungst B Nguyen N Rogers N Rowe S Hussain M Shum L White S. Improved oral bioavailability of an HIV protease inhibitor using Gelucire 44/14 and Labrasol vehicles BT Gattefosse 87199449–54.. [Google Scholar]
- [204].TOGUCHI H, OGAwA Y, IGA K, SHIMAMOTO T, Effects of physiological factors on the bioavailability of ethyl 2-chloro-3-[4-(2-methyl-2-phenylpropyloxy) phenyl] propionate in an emulsion in rats, Chem. Pharm. Bull 38 (1990) 2801–2804. [DOI] [PubMed] [Google Scholar]
- [205].Wu W, Wang Y, Que L, Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system, Eur. J. Pharm. Biopharm 63 (2006) 288–294. [DOI] [PubMed] [Google Scholar]
- [206].Stella V, Haslam J, Yata N, Okada H, Lindenbaum S, Higuchi T, Enhancement of bioavailability of a hydrophobic amine antimalarial by formulation with oleic acid in a soft gelatin capsule, J. Pharm. Sci 67 (1978) 1375–1377. [DOI] [PubMed] [Google Scholar]
- [207].Kararli TT, Needham TE, Griffin M, Schoenhard G, Ferro LJ, Alcorn L, Oral delivery of a renin inhibitor compound using emulsion formulations, Pharm. Res 9 (1992) 888–893. [DOI] [PubMed] [Google Scholar]
- [208].Myers RA, Stella VJ, Systemic bioavailability of penclomedine (NSC-338720) from oil-in-water emulsions administered intraduodenally to rats, Int. J. Pharm 78 (1992) 217–226. [Google Scholar]
- [209].Palin K, Phillips A, Ning A, The oral absorption of cefoxitin from oil and emulsion vehicles in rats, Int. J. Pharm 33 (1986) 99–104. [Google Scholar]
- [210].Lawrence MJ, Rees GD, Microemulsion-based media as novel drug delivery systems, Adv. Drug Deliv. Rev 64 (2012) 175–193. [DOI] [PubMed] [Google Scholar]
- [211].Jadhav K, Shaikh I, Ambade K, Kadam V, Applications of microemulsion based drug delivery system, Curr. Drug Deliv 3 (2006) 267–273. [DOI] [PubMed] [Google Scholar]
- [212].Akhtar J, Siddiqui HH, Fareed S, Badruddeen, Khalid M, Aqil M, Nanoemulsion: for improved oral delivery of repaglinide, Drug Deliv. 23 (2016) 2026–2034. [DOI] [PubMed] [Google Scholar]
- [213].Schwendener R, Schott H, Lipophilic 1-β-D-arabinofuranosyl cytosine derivatives in liposomal formulations for oral and parenteral antileukemic therapy in the murine L1210 leukemia model, J. Cancer Res. Clin. Oncol 122 (1996) 723–726. [DOI] [PubMed] [Google Scholar]
- [214].Shafiq-un-Nabi S, Shakeel F, Talegaonkar S, Ali J, Baboota S, Ahuja A, Khar RK, Ali M, Formulation development and optimization using nanoemulsion technique: a technical note, AAPS PharmSciTech 8 (2007) E12–E17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Mehnert W, Mäder K, Solid lipid nanoparticles: production, characterization and applications, Adv. Drug Deliv. Rev 47 (2001) 165–196. [DOI] [PubMed] [Google Scholar]
- [216].Trinchet J, Coste T, Levy V, Vivet F, Duchatelle V, Legendre C, Gotheil C, Beaugrand M, Treatment of alcoholic hepatitis with silymarin. A double-blind comparative study in 116 patients, Gastroenterol. Clin. Biol 13 (1989) 120–124. [PubMed] [Google Scholar]
- [217].Li J, Li B, Xu WW, Chan KW, Guan XY, Qin YR, Lee NPY, Chan KT, Law S, Tsao SW, Role of AMPK signaling in mediating the anticancer effects of silibinin in esophageal squamous cell carcinoma, Expert Opin. Ther. Targets 20 (2016) 7–18. [DOI] [PubMed] [Google Scholar]
- [218].Hardie DG, AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy, Nat. Rev. Mol. Cell Biol 8 (2007) 774–785. [DOI] [PubMed] [Google Scholar]
- [219].Li W, Saud SM, Young MR, Chen G, Hua B, Targeting AMPK for cancer prevention and treatment, Oncotarget 6 (2015) 7365–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Malek M, Aghili R, Emami Z, Khamseh ME, Risk of cancer in diabetes: the effect of metformin, Int. Sch. Res. Not 2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Dowling RJ, Goodwin PJ, Stambolic V, Understanding the benefit of metformin use in cancer treatment, BMC Med. 9 (2011) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Saraei P, Asadi I, Kakar MA, Moradi-Kor N, The beneficial effects of metformin on cancer prevention and therapy: a comprehensive review of recent advances, Cancer Manag. Res 11 (2019) 3295–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Green AS, Chapuis N, Trovati Maciel T, Willems L, Lambert M, Arnoult C, Boyer O, Bardet V, Park S, Foretz M, The LKB1/AMPK signaling pathway has tumor suppressor activity in acute myeloid leukemia through the repression of mTOR-dependent oncogenic mRNA translation, Blood, J. Am. Soc. Hematol 116 (2010) 4262–4273. [DOI] [PubMed] [Google Scholar]
- [224].Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M, Metformin is an AMP kinase–dependent growth inhibitor for breast cancer cells, Cancer Res. 66 (2006) 10269–10273. [DOI] [PubMed] [Google Scholar]
- [225].Cerezo M, Tichet M, Abbe P, Ohanna M, Lehraiki A, Rouaud F, Allegra M, Giacchero D, Bahadoran P, Bertolotto C, Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner, Mol. Cancer Ther 12 (2013) 1605–1615. [DOI] [PubMed] [Google Scholar]
- [226].Silybum marianum (milk thistle), Alternative medicine review: a journal of clinical therapeutic, 4, 1999, 272–274. [PubMed] [Google Scholar]
- [227].Jacobs BP, Dennehy C, Ramirez G, Sapp J, Lawrence VA, Milk thistle for the treatment of liver disease: a systematic review and meta-analysis, Am. J. Med 113 (2002) 506–515. [DOI] [PubMed] [Google Scholar]
- [228].Tyler V, Phytomedicines in Western Europe: their potential impact on herbal medicine in the United States, Herbalgram, 30, 1994, 24–30. [Google Scholar]
- [229].Blumenthal M, Goldberg A, Brinckmann J, Herbal medicine-integrative medicine communications, Austin 8 (2000) 401. [Google Scholar]
- [230].Comoglio A, Tomasi A, Malandrino S, Poli G, Albano E, Scavenging effect of silipide, a new silybin-phospholipid complex, on ethanol-derived free radicals, Biochem Pharm. 50 (1995) 1313–1316. [DOI] [PubMed] [Google Scholar]
- [231].Giacomelli S, Gallo D, Apollonio P, Ferlini C, Distefano M, Morazzoni P, Riva A, Bombardelli E, Mancuso S, Scambia G, Silybin and its bioavailable phospholipid complex (IdB 1016) potentiate in vitro and in vivo the activity of cisplatin, Life Sci. 70 (2002) 1447–1459. [DOI] [PubMed] [Google Scholar]
- [232].Madaus RHH, Halbach G, Trost W, Salt of the silymarin group with aminopolyhydroxy alcohols, Google Patents, 1976. [Google Scholar]
- [233].Gabetta B, Bombardelli E, Pifferi G, Complexes of flavanolignans with phospholipids, preparation thereof and associated pharmaceutical compositions, Google Patents, 1988. [Google Scholar]
- [234].Wachter W, Zaeske H, Process for the manufacture of flavanolignan preparation with improved release and absorbability, compositions obtainable thereby and their use for the preparation of pharmaceuticals, Google Patents, 2000. [Google Scholar]
- [235].Valcavi U, Monterosso V, Caponi R, Bosone E, Wachter W, Szejtli J, Inclusion complexes with silybinin, their preparation and pharmaceutical compositions containing them, Google Patents, 1993. [Google Scholar]
- [236].Arcari M, Brambilla A, Brandt A, Caponi R, Corsi G, Di Rella M, Solinas F, Wachter WP, A new inclusion complex of silibinin and beta-cyclodextrins: in vitro dissolution kinetics and in vivo absorption in comparison with traditional formulations, Boll. Chim. Farm 131 (1992) 205–209. [PubMed] [Google Scholar]
- [237].Koo C, Choi D, Song I-Y, Compositions and preparations of silymarin complex with improved bioavailability, WO 2 (2002), 069962. [Google Scholar]
- [238].Giorgi R, Conti M, Pifferi G, Soluble derivatives of silybin, a method of preparing them, and pharmaceutical compositions containing them, Google Patents, 1989. [Google Scholar]
- [239].Skottova N, Krecman V, Vana P, Chmela Z, Ulrichová J, Simanek V, Effect of silymarin and silibinin-phosphatidylcholine complex on plasma and lipoprotein cholesterol, and oxidation of LDL in rats fed on high cholesterol diet supplemented with currant oil, Biomed. Pap 144 (2000) 55–58. [Google Scholar]
- [240].Vailati A, Aristia L, Sozze E, Milani F, Inglese V, Galenda P, Bossolo P, Ascari E, Lampertico M, Comis S, Randomized open study of the dose-effect relationship of a short course of IdB 1016 in patients with viral or alcoholic hepatitis, Fitoterapia 64 (1993) 219–228. [Google Scholar]
- [241].Woo JS, Kim TS, Park JH, Chi SC, Formulation and biopharmaceutical evaluation of silymarin using SMEDDS, Arch. Pharm. Res 30 (2007) 82–89. [DOI] [PubMed] [Google Scholar]
- [242].Abrol S, Trehan A, Katare OP, Comparative study of different silymarin formulations: formulation, characterisation and in vitro/in vivo evaluation, Curr. Drug Deliv 2 (2005) 45–51. [DOI] [PubMed] [Google Scholar]
- [243].Yanyu X, Yunmei S, Zhipeng C, Qineng P, The preparation of silybin-phospholipid complex and the study on its pharmacokinetics in rats, Int. J. Pharm 307 (2006) 77–82. [DOI] [PubMed] [Google Scholar]
- [244].Yan-yu X, Yun-mei S, Zhi-peng C, Qi-neng P, Preparation of silymarin proliposome: a new way to increase oral bioavailability of silymarin in beagle dogs, Int. J. Pharm 319 (2006) 162–168. [DOI] [PubMed] [Google Scholar]
- [245].El-Samaligy MS, Afifi NN, Mahmoud EA, Evaluation of hybrid liposomes-encapsulated silymarin regarding physical stability and in vivo performance, Int. J. Pharm 319 (2006) 121–129. [DOI] [PubMed] [Google Scholar]
- [246].Wu W, Wang Y, Que L, Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system, Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr 63 (2006) 288–294. [DOI] [PubMed] [Google Scholar]
- [247].Haus DJ, Mehta S, Radebaugh G, Targeting lymphatic transport and modified systemic distribution of CI-976, a lipophilic lipid-regulator drug, via a formulation approach, Int. J. Pharm 108 (1994) 85–93. [Google Scholar]
- [248].Soleimani V, Delghandi PS, Moallem SA, Karimi G, Safety and toxicity of silymarin, the major constituent of milk thistle extract: an updated review, Phytother. Res. PTR 33 (2019) 1627–1638. [DOI] [PubMed] [Google Scholar]
- [249].Invernizzi R, Bernuzzi S, Ciani D, Ascari E, Silymarine during maintenance therapy of acute promyelocytic leukemia, Haematologica 78 (1993) 340–341. [PubMed] [Google Scholar]
- [250].Schröder FH, Roobol MJ, Boevé ER, de Mutsert R, Zuijdgeest-van Leeuwen SD, Kersten I, Wildhagen MF, van Helvoort A, Randomized, double-blind, placebo-controlled crossover study in men with prostate cancer and rising PSA: effectiveness of a dietary supplement, Eur. Urol 48 (2005) 922–930, discussion 930–921. [DOI] [PubMed] [Google Scholar]
- [251].Niture SK, Khatri R, Jaiswal AK, Regulation of Nrf2-an update, Free Radic. Biol. Med 66 (2014) 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Tebay LE, Robertson H, Durant ST, Vitale SR, Penning TM, Dinkova-Kostova AT, Hayes JD, Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease, Free Radic. Biol. Med 88 (2015) 108–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Paunkov A, Chartoumpekis DV, Ziros PG, Sykiotis GP, A Bibliometric Review of the Keap1/Nrf2 Pathway and its Related Antioxidant Compounds, Antioxidants (Basel, Switzerland), 8, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Vidlar A, Vostalova J, Ulrichova J, Student V, Krajicek M, Vrbkova J, Simanek V, The safety and efficacy of a silymarin and selenium combination in men after radical prostatectomy-a six month placebo-controlled double-blind clinical trial, Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub 154 (2010) 239–244. [DOI] [PubMed] [Google Scholar]
- [255].Flaig TW, Glodé M, Gustafson D, van Bokhoven A, Tao Y, Wilson S, Su LJ, Li Y, Harrison G, Agarwal R, A study of high-dose oral silybin-phytosome followed by prostatectomy in patients with localized prostate cancer, Prostate 70 (2010) 848–855. [DOI] [PubMed] [Google Scholar]


