Abstract
B7-H3 (also known as CD276) is associated with aggressive characteristics in various cancers. Meanwhile, in alveolar rhabdomyosarcoma (ARMS), PAX3-FOXO1 fusion protein is associated with increased aggressiveness and poor prognosis. In the present study, we explored the relationship between PAX3-FOXO1 and B7-H3 and the biological roles of B7-H3 in ARMS. Quantitative real time PCR and flow cytometry revealed that PAX3-FOXO1 knockdown downregulated B7-H3 expression in all the selected cell lines (Rh-30, Rh-41, and Rh-28), suggesting that PAX3-FOXO1 positively regulates B7-H3 expression. Gene expression analysis revealed that various genes and pathways involved in chemotaxis, INF-γ production, and myogenic differentiation were commonly affected by the knockdown of PAX3-FOXO1 and B7-H3. Wound healing and transwell migration assays revealed that both PAX3-FOXO1 and B7-H3 were associated with cell migration. Furthermore, knockdown of PAX3-FOXO1 or B7-H3 induced myogenin expression in all cell lines, although myosin heavy chain induction varied depending on the cellular context. Our results indicate that PAX3-FOXO1 regulates B7-H3 expression and that PAX3-FOXO1 and B7-H3 are commonly associated with multiple pathways related to an aggressive phenotype in ARMS, such as cell migration and myogenic differentiation block.
Subject terms: Cancer, Oncology
Background
B7-H3 (also known as CD276), a member of the B7 family of type I transmembrane proteins, is known to modulate T-cell function in a costimulatory1,2 or coinhibitory3,4 manner. Although B7-H3 protein has limited expression in normal human tissues, it is broadly overexpressed in various human cancers 5–9, including rhabdomyosarcoma 10. Therefore, B7-H3 has been highlighted in recent years as an attractive surface antigen for molecular targeted therapy, such as those using monoclonal antibody 11 and chimeric antigen receptor (CAR) T-cells10,12. In addition to its immune regulatory roles in the tumor microenvironment, B7-H3 is known to be associated with tumor cell proliferation, migration, invasion, metabolism, and angiogenesis; therefore, it is related to a poor prognosis13–19.
Rhabdomyosarcoma (RMS) is the most common soft tissue malignancy in children20. It consists of two major subtypes, namely the alveolar RMS (ARMS) and the embryonal RMS (ERMS). The majority of ARMS are associated with specific fusion proteins, PAX3-FOXO1 or PAX7-FOXO121–23. The expression of PAX3-FOXO1 is associated with increased aggressiveness and a poor prognosis24. PAX3-FOXO1 may function as a more potent transcriptional activator than PAX3, and induces the expression of a number of transcriptional targets that lead to tumorigenesis, cell proliferation, migration, invasion, and differentiation block25–27.
Although PAX3-FOXO1 contributes to aggressive characteristics in ARMS, and B7-H3 does the same in other cancers, limited information is available regarding the function of B7-H3 in ARMS and about the relationship between PAX3-FOXO1 and B7-H3. In this context, we hypothesized that PAX3-FOXO1 regulates B7-H3 expression and contributes to aggressive characteristics in ARMS. In this study, we analyzed the association of PAX3-FOXO1 and B7-H3 by knocking down the expression of PAX3-FOXO1 and performing gene expression analysis. We demonstrate that PAX3-FOXO1 knockdown downregulates the expression of B7-H3 in ARMS and that PAX3-FOXO1 and B7-H3 share common gene expression profiles, which contribute to the aggressive phenotype of ARMS.
Results
B7-H3 expression is downregulated by PAX3-FOXO1 knockdown in alveolar rhabdomyosarcoma
To investigate the effect of PAX3-FOXO1 on the expression of B7-H3, knockdown of PAX3-FOXO1 transcripts was performed by siRNA targeting PAX3-FOXO1 (siPF). In each PAX3-FOXO1 fusion gene-positive ARMS cell line (RH-30, RH-41, and RH-28), a high knockdown efficiency of PAX3-FOXO1 was confirmed by quantitative real time polymerase chain reaction (qRT-PCR), although PAX3-FOXO1 was not detected in PAX3-FOXO1-negative RD cells (Fig. 1a). Next, qRT-PCR for B7-H3 revealed that PAX3-FOXO1 knockdown decreased the expression of B7-H3 in the PAX3-FOXO1-positive cell line, but not in the PAX3-FOXO1-negative RD cells (Fig. 1b). In addition, flow cytometry analysis demonstrated that PAX3-FOXO1 knockdown decreased the expression of B7-H3 in the PAX3-FOXO1-positive cell lines, but not in the PAX3-FOXO1-negative RD cells (Fig. 1c,d). These results suggest that PAX3-FOXO1 positively regulates the expression of B7-H3 at both the mRNA and protein levels.
Figure 1.
Knockdown of PAX3-FOXO1 downregulates the expression of B7-H3. (a) Quantitative real-time PCR (qRT-PCR) analysis of PAX3-FOXO1. Knockdown efficacy of PAX3-FOXO1 was confirmed in three PAX3-FOXO1 positive ARMS cell lines. RD, a PAX3-FOXO1-negative cell line, was used as a negative control. (b) qRT-PCR analysis of B7-H3. (c) Representative histogram of flow cytometry analysis of B7-H3. (d) Mean fluorescence index of B7-H3 in flow cytometry analysis. Results shown are means ± SD. *p < 0.05, **p < 0.01, n.s. p > 0.05, by two-tailed unpaired t test.
PAX3-FOXO1 and B7-H3 are associated with multiple pathways related to an aggressive rhabdomyosarcoma phenotype
To unravel the pathways affected by PAX3-FOXO1 and B7-H3 in ARMS, gene expression analysis was performed using microarray and gene set enrichment analysis (GSEA). The gene expression signature of siPF or siRNA targeting B7-H3 (siB7-H3) transfected Rh-30 cells (Rh-30 siPF or Rh-30 siB7-H3, respectively) was compared with that of control siRNA (siCont) transfected Rh-30 cells (Rh-30 siCont). Gene expression analysis revealed that in Rh-30 siPF and Rh-30 siB7-H3, the expression levels of 636 and 565 genes were decreased, respectively (log 2 ratio, ≤ 1.0), and those of 700 and 549 genes were increased, respectively (log 2 ratio, ≥ 1.0), compared with the respective levels in Rh-30 siCont (Fig. 2a,b). Among them, the expression levels of 130 genes were downregulated and those of 159 genes were upregulated in both the Rh-30 siPF and Rh-30 siB7-H3 compared to the respective levels in the Rh-30 siCont (Fig. 2a,b). A heat map of top 50 shared downregulated or upregulated genes is shown in Fig. 2c.
Figure 2.
Gene expression analysis. Knockdown of PAX3-FOXO1 and B7-H3 reveal shared down/upregulated genes. (a) Venn diagram of genes that were downregulated by the knockdown of PAX3-FOXO1 or B7-H3. (b) Venn diagram of genes that were upregulated by the knockdown of PAX3-FOXO1 or B7-H3. (c) Heat map of the top 50 genes that were down/up-regulated by the knockdown of PAX3-FOXO1 or B7-H3. The left line shows Rh-30 siCont, the middle Rh-30 siPF, and the right siB7-H3.
GSEA revealed that several pathways related to chemotaxis, INF-γ production, and regulation of T cell immunity were inactivated in both Rh-30 siPF and Rh-30 siB7-H3 unlike in Rh30 siCont, suggesting that PAX3-FOXO1 and B7-H3 may contribute to tumor cell metastasis and immune evasion (Fig. 3a,b). In contrast, several pathways related to muscle cell differentiation were activated in both Rh-30 siPF and Rh-30 siB7-H3 but not in Rh30 siCont, suggesting that PAX3-FOXO1 and B7-H3 may block myoblast differentiation (Fig. 3c,d).
Figure 3.
Gene set enrichment analysis (GSEA). (a) Representative list of impoverished gene sets upon knockdown of PAX3-FOXO1 and B7-H3. (b) Enrichment plot from the GSEA. Interferon gamma production (left panel) and chemotaxis (right panel). (c) Representative list of enriched gene sets upon knockdown of PAX3-FOXO1 and B7-H3. (d) Enrichment plot from the GSEA. Actin filament polymerization (left panel) and actin binding (right panel).
PAX3-FOXO1 and B7-H3 are associated with cell migration
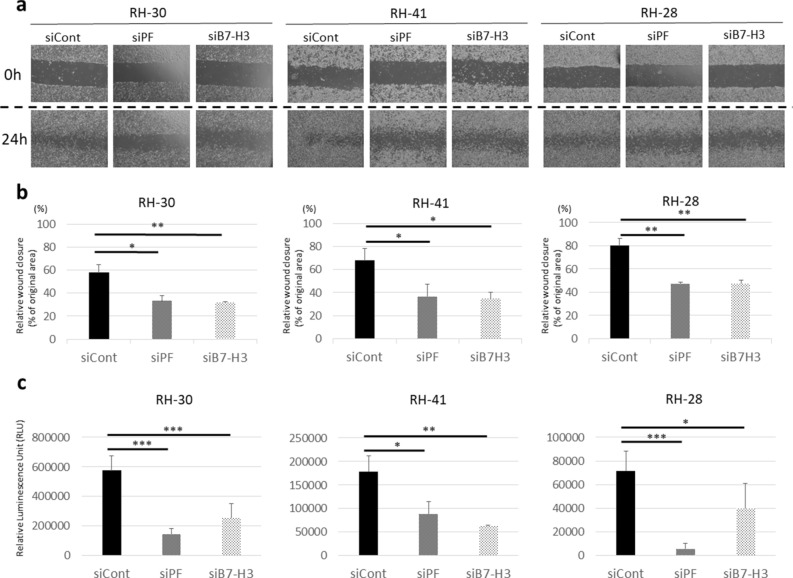
To confirm that PAX3-FOXO1 and B7-H3 contribute to metastasis in ARMS, as suggested by GSEA, we performed wound healing and transwell assays. Wound healing assays revealed that cell migration in ARMS was significantly attenuated by the knockdown of PAX3-FOXO1 or B7-H3 in all the three cell lines (Fig. 4a,b). The transwell assays similarly demonstrated that cell migration in ARMS was significantly attenuated by the knockdown of PAX3-FOXO1 or B7-H3 in all the three cell lines (Fig. 4c).
Figure 4.
Knockdown of PAX3-FOXO1 or B7-H3 attenuates cell migration. (a) Representative figure of a wound healing assay. (b) Relative healed wound area (% of original wound area) in a wound healing assay. Knockdown of PAX3-FOXO1 or B7-H3 attenuates cell migration. (c) Transwell assay. Relative luminescence units are shown as the quantification of migratory cells. Results shown are means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, by two-tailed unpaired t test.
To determine the pathways contributing to cell migration that are affected by PAX3-FOXO1 and B7-H3, we performed qRT-PCR for CXCR4 and STYK1, as gene expression analysis indicated that both these genes were downregulated in Rh-30 siPF and Rh-30 siB7-H3. As expected, CXCR4 was downregulated by transfection of all the three cell lines with siPF or siB7-H3 (Fig. 5a). In addition, STYK1 was downregulated in Rh-30 and Rh-41 by transfection with siPF or siB7-H3, although in Rh28, STYK1 was downregulated by transfection with siPAX3-FOXO1, but not siB7-H3 (Fig. 5b).
Figure 5.
CXCR4 and STYK1 expression levels are regulated by PAX3-FOXO1 and B7-H3. (a) Quantitative real-time PCR (qRT-PCR) analysis of CXCR4. (b) qRT-PCR analysis of STYK1. Results shown represent means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, n.s. p > 0.05, by two-tailed unpaired t test.
PAX3-FOXO1 and B7-H3 negatively regulate myogenin expression and interfere with myogenic differentiation
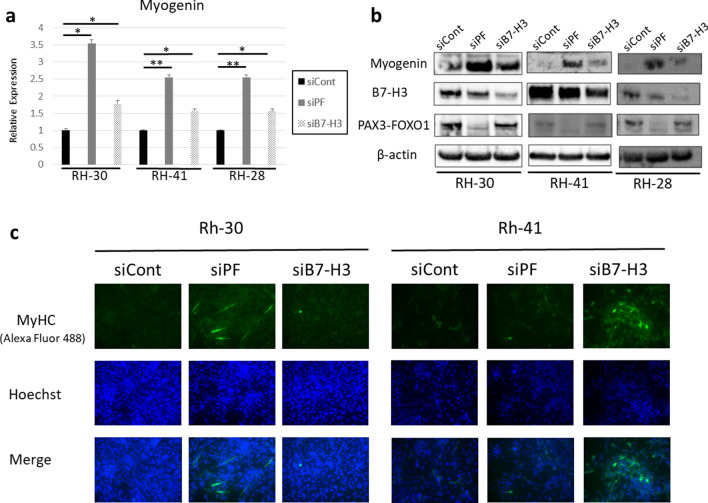
Because the results of GSEA suggested that PAX3-FOXO1 and B7-H3 may block myoblast differentiation, we investigated the expression level of myogenin, a transcription factor required for myoblast differentiation, following knockdown of PAX3-FOXO1 or B7-H3. Both qRT-PCR and western blot analyses revealed that myogenin was upregulated in all the three cell lines by transfection with siPF or siB7-H3 (Fig. 6a,b). To investigate whether upregulation of myogenin led to differentiation, we examined the expression level of myosin heavy chain (MyHC) in the ARMS cell lines. Immunofluorescence of MyHC revealed that in Rh-30 cells, transfection with siPF induced MyHC more strongly than the transfection with siB7-H3, whereas in Rh-41 cells, transfection with siB7-H3 induced MyHC more strongly than did siPF (Fig. 6c).
Figure 6.
Knockdown of PAX3-FOXO1 and B7-H3 induces the expression of myogenin and may induce myoblast differentiation. (a) Quantitative real-time PCR analysis of myogenin. (b) Western blot analysis of myogenin. Knockdown of PAX3-FOXO1 (detected by anti-PAX3 antibody) and B7-H3 induces the expression of myogenin. (c) Immunofluorescence of myosin heavy chain. Results shown represent means ± SD. *p < 0.05, **p < 0.01, by two-tailed unpaired t test.
Discussion
In this study, we investigated the regulatory mechanism and function of B7-H3 in PAX3-FOXO1-positive ARMS. PAX3-FOXO1 regulates the expression of many target genes25, and we determined that PAX3-FOXO1 also regulates B7-H3 expression in ARMS. Our data indicate that although regulated by PAX3-FOXO1, B7-H3 regulates the expression of numerous genes, some of which are also regulated by PAX3-FOXO1. Therefore, B7-H3 is considered to be an important protein downstream of PAX3-FOXO1 that contributes to aggressive characteristics of PAX3-FOXO1-positive ARMS.
Based on the GSEA results, we demonstrate that B7-H3 is associated with cell migration in ARMS, similar to that in various other cancers. However, the mechanisms by which B7-H3 regulates migration differ according to the type of cancer. For example, B7-H3 regulates CXCR4 in gastric cancer16, PI3K signaling in bladder and colorectal cancers14,15, JAK2-STAT3 signaling in hepatocellular carcinoma28, and MMP-2 in melanoma29. Our data show that B7-H3 regulates CXCR4 in ARMS. Notably, CXCR4 is also regulated by PAX3-FOXO130. Previous report have shown that antibody to CXCR4 inhibit metastasis in ARMS 31. In addition, we found that PAX3-FOXO1 regulates the expression of STYK1, which is also associated with tumor invasion and metastasis32,33, although molecular targeted therapy against STYK1 is not developed. B7-H3 also regulates STYK1 expression in Rh-30 and Rh-41 cells, but not in Rh-28 cells, indicating that the effect of B7-H3 on STYK1 varies depending on the ARMS cell line.
Furthermore, we demonstrate that knockdown of B7-H3, as well as of PAX3-FOXO1, upregulates the expression of myogenin, a transcription factor needed for myogenic differentiation34. Although PAX3-FOXO1 inhibits myogenic differentiation in ARMS26,35, the effect of B7-H3 on myogenic differentiation has not been determined. Among the various functions of B7-H3 in cancer, inhibition of differentiation (not limited to myogenic differentiation) has not been reported. However, despite the observation regarding the induction of myogenin by B7-H3 knockdown in all three cell lines, the induction of further differentiation as detected by the expression of MyHC was found to vary with the cell line. Knockdown of PAX3-FOXO1 induced the expression of MyHC more strongly than did the knockdown of B7-H3 in Rh-30 cells, whereas B7-H3 knockdown induced the expression of MyHC to a greater extent than did PAX3-FOXO1 in Rh-41 cells, indicating that myogenin expression, which leads directly to increased expression of MyHC, may be dependent on the cellular context.
These results suggest that B7-H3 might be a potential therapeutic target in ARMS with PAX3-FOXO1, which itself is not yet targetable in the present clinical settings. In various cancers, B7-H3 is an attractive surface antigen for molecular targeted therapy36. Enoblituzumab (MGA271) is a monoclonal antibody for B7-H3 and causes antibody-dependent cell-mediated cytotoxicity (ADCC)11. Enoblituzumab was evaluated in a phase 1 clinical study and demonstrated acceptable tolerability in the treated patients with solid cancer (trial NCT01391143). Additionally, antibody drug conjugate (ADC) therapies and CAR T cells for B7-H3 also demonstrated outstanding results in preclinical studies and in a phase 1 clinical trial for childhood cancer10,12,37,38. These targeted therapies for B7-H3 might be used for treating PAX3-FOXO1 positive ARMS that exhibits a poor prognosis with conventional intensive therapy. A phase 1 clinical trial of enoblituzumab in children and young adults with B7-H3-expressing relapsed or refractory malignant solid tumors, including ARMS, is currently being conducted (trial NCT02982941).
Our results demonstrate that B7-H3 affects cell migration and myogenic differentiation and is one of the cancer driver genes in PAX3-FOXO1 positive ARMS. As cancer antigen escape seems less likely to occur when the cancer driver gene is a target antigen, B7-H3-targeting immunotherapy might be an attractive treatment option for PAX3-FOXO1 positive ARMS, although we did not examine the anti-B7-H3 targeted therapy for ARMS in this study. The analysis of B7-H3 targeted therapy is warranted in the future.
This study has several limitations. First, we did not investigate the association between B7-H3 and tumor immunity in ARMS, although the GSEA indicated that gene sets related to INF-γ production and T cell-mediated cytotoxicity were inactivated by knockdown of PAX3-FOXO1 or B7-H3. As B7-H3 is thought to be involved in tumor evasion from host immunity in other cancers, the relationship between B7-H3 and tumor evasion should be a focus of future studies. Second, we used only one siRNA for each gene. Therefore, we could not exclude the possibility of off-target effect by siRNA. Third, we did not investigate the function of B7-H3 in ARMS by in vivo experiments. In vivo experiments using the siRNA-treated cells would provide more evidence that strengthen our study results.
In conclusion, our findings demonstrate that PAX3-FOXO1 regulates B7-H3 expression, and PAX3-FOXO1 and B7-H3 are commonly associated with multiple pathways related to an aggressive phenotype in ARMS. In particular, PAX3-FOXO1 and B7-H3 contribute to cell migration and inhibit myogenic differentiation. This study provides evidence that B7-H3 contributes to the malignant characteristics of PAX3-FOXO1 positive ARMS.
Materials and methods
Cell lines and cell culture
PAX3-FOXO1 positive human ARMS cell lines, Rh-30, Rh-41, and Rh-28 that were kindly provided by Peter J. Houghton M (The Greehey Children's Cancer Research Institute, San Antonio, TX), as well as PAX3-FOXO1 negative human ERMS cell line, RD that was obtained from JCRB (Japanese Collection of Research Bioresources) Cell Bank, were used in this study. The cells were maintained in RPMI 1640 medium (Nakarai Tesque, Kyoto, Japan), supplemented with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA), penicillin (100 U/mL), and streptomycin (100 µg/mL) at 37 °C in a humidified atmosphere of 5% CO2.
siRNA and knockdown
siCont and an siB7-H3 were purchased from Invitrogen (Carlsbad, CA; catalog numbers AM4611 and AM16708-s37290, respectively). siPF was custom-synthesized as described previously26. Briefly, siPF targets the fusion sites between exon 7 of PAX3 and exon 2 of FOXO1. The sense and antisense sequences for siPF were 5′-CCUCUCACCUCAGAAUUCAtt-3′ and 5′-UGAAUUCUGAGGUGAGAGGtt-3′, respectively. Transfection of cells with the siRNAs was carried out using Lipofectamine RNAiMAX (Invitrogen) according to the procedures recommended by the manufacturer. The final concentration of siRNAs was 5 nM. Six hours after transfection, the medium containing the siRNAs and Lipofectamine RNAiMAX was replaced with fresh RPMI1640 medium containing 10% FBS.
RNA extraction, reverse transcription, and qRT-PCR
Total RNA was extracted from rhabdomyosarcoma cell lines using an RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. cDNA was synthesized using a SuperScript VILO cDNA synthesis kit (Invitrogen) according to the manufacturer’s instructions. qRT-PCR was carried out in a 7300 Real time PCR System (Applied Biosystems, Carlsbad, CA) with TB Green Premix Ex Taq II (Clontech Laboratories, Madison, WI) according to the manufacturer’s protocol. The PCR primers used in the study were as follows: for GAPDH: 5′-GCACCGTCAAGGCTGAGAAC-3′ (forward) and 5′-ATGGTGGTGAAGACGCCAGT-3′ (reverse); for PAX3-FOXO1: 5′-TCCAACCCCATGAACCCC-3′ (forward) and 5′-GCCATTTGGAAAACTGTGATCC-3′ (reverse); for B7-H3: 5′-CAAGGCAATGCATCCCTGAG-3′ (forward) and 5′-CTTCGAGTAGGGAGCGGC-3′ (reverse); for MYOG: 5′-GGACGGAGCTCACCCTGA-3′ (forward) and 5′-TTACACACCTTACACGCCCA-3′ (reverse). The levels of target mRNAs were determined using the delta delta CT method and were normalized to the expression level of GAPDH. All experiments were performed in triplicate.
Western blot analysis
Twenty-four hours after transfection with siRNAs, the cells were lysed with RIPA buffer (Nakarai Tesque). Samples were boiled for 10 min in NuPAGE sample buffer (Invitrogen) and were separated by SDS–polyacrylamide gel electrophoresis. The proteins were subsequently transferred onto an Immobilon-P PVDF transfer membrane (Millipore, Bedford, MA). The membranes were blocked with 3% bovine albumin (Nakarai Tesque) prepared in phosphate-buffered saline with Tween 20 (PBS-T) and then incubated with one of the primary antibodies: β-actin (1:10,000, Sigma-Aldrich, St. Louis, MO), PAX3 (1:500, R&D Systems, Minneapolis, MN), Myogenin (1:500, Novus Biologicals, Centennial, CO), and B7-H3 (1:1000, Cell Signaling Technology, Danvers, MA). The membranes were then washed with PBS-T and incubated with anti-mouse or anti-rabbit secondary antibody (1:10,000, Santa Cruz Biotechnology, Santa Cruz, CA). Antibody binding was detected using an ECL prime detection system (GE Healthcare, Buckinghamshire, UK).
Flow cytometry
Cells were harvested, washed twice with PBS, and incubated for 30 min on ice with PE-conjugated anti-human B7-H3 antibody (Biolegend, San Diego, CA). Next, the cells were analyzed on a FACS Calibur (BD Biosciences, Franklin Lakes, NJ) with the FlowJo software (Treestar, San Carlos, CA).
Gene expression analysis
Total RNA was extracted from RH-30 cells transfected with siCont, siPF, or siB7-H3. The total RNA was used for cRNA target synthesis using a GeneChip 3′IVT PLUS Reagent Kit (Thermo Fisher Scientific, Waltham, CA). Biotin-labeled cRNA was hybridized to a GeneChip Human Genome U133 Plus 2.0 Array (Thermo Fisher Scientific). Hybridized arrays were stained and scanned with a Genechip 3000 7G Scanner (Thermo Fisher Scientific). Data were analyzed with the Affymetrix Expression Console Software 1.4.1. All microarray data are available at the Gene Expression Omnibus database under accession number GSE127703. GSEA was performed using GSEA 3.0 (Broad Institute, San Diego, CA). A group consisting of RH-30-siPF and RH-30-siB7-H3 was compared with RH-30-siCont, and gene sets at a nominal p value (NOM p-val) < 5% were considered to be significantly enriched.
Wound healing assay
After transfection of cells with siRNAs, the cell layers were scratched using a pipette tip. Immediately after scratching (0 h), the plates were photographed and the wound area was measured using the ImageJ software and defined as the original area (100%). After 24 h of scratching, the plates were photographed again. In addition, the area of the healed wound was measured and presented as a percentage of the original area. All assays were performed in triplicate.
Transwell migration assay
At 24 h of transfection of cells with the siRNAs, each cell line was resuspended in serum-free RPMI medium. Twenty-four well-sized transwell plates with an 8-µm pore size (Corning, Corning, NY) were used for this assay. First, 1.0 × 105 cells suspended in 100 µL of serum-free medium were seeded on each insert, and 650 µL of RPMI medium with 10% FBS was added to the receiver plates. The plates were incubated for 24 h, and cells migrating onto the receiver plates were quantified as relative light units (RLU) using the Cell Titer Glo luminescent cell viability assay (Promega, Heidelberg, Germany) according to the procedures recommended by the manufacturer. All the assays were performed in triplicate.
Immunofluorescence
At 24 h of transfection of cells with the siRNAs, Rh-30 and Rh-41 cells were resuspended in serum-free RPMI medium. After 7 days of resuspension, cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton-X100. Next, the cells were washed and incubated with anti-MyHC antibody (Sigma-Aldrich) overnight (1:350), rinsed with PBS, and incubated with Alexa Fluor 488 goat anti-mouse IgG (Life Technologies, Carlsbad, CA) for 1 h (1:200). Nucleic acids were stained with Hoechst, using the NucBlue Live Cell Stain Ready Probes Reagent (Invitrogen). A BZ-X800 confocal microscope (KEYENCE, Osaka, Japan) was used for observation.
Statistical analysis
The results are shown as mean ± SD of three independent experiments. Statistical analysis was performed using the two-tailed Student’s t test. A p value < 0.05 was considered statistically significant.
Supplementary Information
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Abbreviations
- ARMS
Alveolar rhabdomyosarcoma
- CAR
Chimeric antigen receptor
- ERMS
Embryonal rhabdomyosarcoma
- GSEA
Gene set enrichment analysis
- qRT-PCR
Quantitative real time polymerase chain reaction
- RMS
Rhabdomyosarcoma
- siB7-H3
SiRNA targeting B7-H3
- siCont
Control siRNA
- siPF
SiRNA targeting PAX3-FOXO1
Author contributions
M.M. designed the study. T.K. and Y.S. contributed to the data collection. All authors contributed to the analysis and interpretation of the data. T.K. and M.M. drafted the manuscript. All authors critically reviewed and approved the final version of the manuscript.
Funding
The present study was supported by JSPS KAKENHI Grant-in-Aid for Scientific Research (C) [grant number 16K10036].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-98322-z.
References
- 1.Chapoval AI, et al. B7-H3: A costimulatory molecule for T cell activation and IFN-gamma production. Nat. Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 2.Luo L, et al. B7-H3 enhances tumor immunity in vivo by costimulating rapid clonal expansion of antigen-specific CD8+ cytolytic T cells. J. Immunol. 2004;173:5445–5450. doi: 10.4049/jimmunol.173.9.5445. [DOI] [PubMed] [Google Scholar]
- 3.Prasad DV, et al. Murine B7-H3 is a negative regulator of T cells. J. Immunol. 2004;173:2500–2506. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 4.Suh WK, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat. Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 5.Castriconi R, et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc. Natl. Acad. Sci. U S A. 2004;101:12640–12645. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crispen PL, et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin. Cancer. Res. 2008;14:5150–5157. doi: 10.1158/1078-0432.CCR-08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inamura K, et al. Tumor B7-H3 (CD276) expression and smoking history in relation to lung adenocarcinoma prognosis. Lung Cancer. 2017;103:44–51. doi: 10.1016/j.lungcan.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Loos M, et al. Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer. BMC Cancer. 2009;9:463. doi: 10.1186/1471-2407-9-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, et al. B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS ONE. 2013;8:e70689. doi: 10.1371/journal.pone.0070689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majzner RG, et al. CAR T Cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin. Cancer. Res. 2019;25:2560–2574. doi: 10.1158/1078-0432.CCR-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loo D, et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin. Cancer. Res. 2012;18:3834–3845. doi: 10.1158/1078-0432.CCR-12-0715. [DOI] [PubMed] [Google Scholar]
- 12.Du H, et al. Antitumor responses in the absence of toxicity in solid tumors by targeting B7-H3 via chimeric antigen receptor T Cells. Cancer Cell. 2019;35:221–237. doi: 10.1016/j.ccell.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, et al. B7-H3 expression associates with tumor invasion and patient's poor survival in human esophageal cancer. Am. J. Transl. Res. 2015;7:2646–2660. [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang B, et al. The co-stimulatory molecule B7-H3 promotes the epithelial-mesenchymal transition in colorectal cancer. Oncotarget. 2016;7:31755–31771. doi: 10.18632/oncotarget.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, et al. B7-H3 promotes the migration and invasion of human bladder cancer cells via the PI3K/Akt/STAT3 signaling pathway. J. Cancer. 2017;8:816–824. doi: 10.7150/jca.17759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, et al. B7-H3 promotes gastric cancer cell migration and invasion. Oncotarget. 2017;8:71725–71735. doi: 10.18632/oncotarget.17847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim S, et al. Immunoregulatory protein B7-H3 reprograms glucose metabolism in cancer cells by ROS-mediated stabilization of HIF1alpha. Cancer Res. 2016;76:2231–2242. doi: 10.1158/0008-5472.CAN-15-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin L, et al. B7-H3 promotes multiple myeloma cell survival and proliferation by ROS-dependent activation of Src/STAT3 and c-Cbl-mediated degradation of SOCS3. Leukemia. 2018;33:1475–1486. doi: 10.1038/s41375-018-0331-6. [DOI] [PubMed] [Google Scholar]
- 19.Mesri M, et al. Identification and characterization of angiogenesis targets through proteomic profiling of endothelial cells in human cancer tissues. PLoS ONE. 2013;8:e78885. doi: 10.1371/journal.pone.0078885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer S, Meadows AT, Jarrett P, Evans AE. Incidence of childhood cancer: Experience of a decade in a population-based registry. J. Natl. Cancer Inst. 1983;70:49–55. [PubMed] [Google Scholar]
- 21.Barr FG, et al. Rearrangement of the PAX3 paired box gene in the paediatric solid tumour alveolar rhabdomyosarcoma. Nat. Genet. 1993;3:113–117. doi: 10.1038/ng0293-113. [DOI] [PubMed] [Google Scholar]
- 22.Barr FG, et al. In vivo amplification of the PAX3-FKHR and PAX7-FKHR fusion genes in alveolar rhabdomyosarcoma. Hum. Mol. Genet. 1996;5:15–21. doi: 10.1093/hmg/5.1.15. [DOI] [PubMed] [Google Scholar]
- 23.Galili N, et al. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat. Genet. 1993;5:230–235. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- 24.Sorensen PH, et al. PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: A report from the children's oncology group. J. Clin. Oncol. 2002;20:2672–2679. doi: 10.1200/JCO.2002.03.137. [DOI] [PubMed] [Google Scholar]
- 25.Cao L, et al. Genome-wide identification of PAX3-FKHR binding sites in rhabdomyosarcoma reveals candidate target genes important for development and cancer. Cancer Res. 2010;70:6497–6508. doi: 10.1158/0008-5472.CAN-10-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kikuchi K, et al. Effects of PAX3-FKHR on malignant phenotypes in alveolar rhabdomyosarcoma. Biochem. Biophys. Res. Commun. 2008;365:568–574. doi: 10.1016/j.bbrc.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Wang C. Identification of a new class of PAX3-FKHR target promoters: A role of the Pax3 paired box DNA binding domain. Oncogene. 2007;26:1595–1605. doi: 10.1038/sj.onc.1209958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang FB, et al. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 2015;15:45. doi: 10.1186/s12935-015-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tekle C, et al. B7-H3 contributes to the metastatic capacity of melanoma cells by modulation of known metastasis-associated genes. Int. J. Cancer. 2012;130:2282–2290. doi: 10.1002/ijc.26238. [DOI] [PubMed] [Google Scholar]
- 30.Libura J, et al. CXCR4-SDF-1 signaling is active in rhabdomyosarcoma cells and regulates locomotion, chemotaxis, and adhesion. Blood. 2002;100:2597–2606. doi: 10.1182/blood-2002-01-0031. [DOI] [PubMed] [Google Scholar]
- 31.Kashima K, et al. Inhibition of metastasis of rhabdomyosarcoma by a novel neutralizing antibody to CXC chemokine receptor-4. Cancer Sci. 2014;105:1343–1350. doi: 10.1111/cas.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, et al. Aberrant expression of STYK1 and E-cadherin confer a poor prognosis for pancreatic cancer patients. Oncotarget. 2017;8:111333–111345. doi: 10.18632/oncotarget.22794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Y, Zhang J, Liu M, Huang Y, Yin L. SMAD3 inducing the transcription of STYK1 to promote the EMT process and improve the tolerance of ovarian carcinoma cells to paclitaxel. J. Cell. Biochem. 2019;120:10796–10811. doi: 10.1002/jcb.28371. [DOI] [PubMed] [Google Scholar]
- 34.Wright WE, Sassoon DA, Lin VK. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989;56:607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- 35.Walters ZS, et al. JARID2 is a direct target of the PAX3-FOXO1 fusion protein and inhibits myogenic differentiation of rhabdomyosarcoma cells. Oncogene. 2014;33:1148–1157. doi: 10.1038/onc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Picarda E, Ohaegbulam KC, Zang X. Molecular pathways: targeting B7-H3 (CD276) for human cancer immunotherapy. Clin. Cancer Res. 2016;22:3425–3431. doi: 10.1158/1078-0432.CCR-15-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seaman S, et al. Eradication of tumors through simultaneous ablation of CD276/B7-H3-positive tumor cells and tumor vasculature. Cancer Cell. 2017;31:501–515. doi: 10.1016/j.ccell.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Souweidane MM, et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: A single-centre, dose-escalation, phase 1 trial. Lancet. Oncol. 2018;19:1040–1050. doi: 10.1016/S1470-2045(18)30322-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.