Abstract
Partial sequencing of the hsp65 gene was used for the identification of rapidly growing mycobacteria (RGM). A 441-bp fragment (A. Telenti, F. Marchesi, M. Balz, F. Bally, E. Böttger, and T. Bodmer, J. Clin. Microbiol. 31:175–178, 1993) was amplified and sequenced by an automated fluorescence-based method involving capillary electrophoresis. Type strains of 10 RGM species were first studied. Each species had a unique nucleotide sequence, distinguishing it clearly from the other species. A panel of strains from the four main RGM species responsible for human infections, Mycobacterium abscessus, Mycobacterium chelonae, Mycobacterium fortuitum, and Mycobacterium peregrinum, was also studied. There were few sequence differences within each of these species (<2% of bases were different from the type strain sequence), and they had no effect on species assignment. hsp65 sequencing unambiguously differentiated M. chelonae and M. abscessus, two species difficult to identify by classical methods and 16S rRNA gene sequencing. The devised procedure is a rapid and reliable tool for the identification of RGM species.
Rapidly growing mycobacteria (RGM) are increasingly recognized as a cause of human infections (3, 25, 26). This group of mycobacteria is heterogeneous in terms of epidemiology, clinical disease spectrum, and drug susceptibility. It is therefore important to identify RGM to the species level. Identification of RGM by conventional biochemical methods is cumbersome and time-consuming. Phenotypic tests sometimes fail to discriminate between closely related species, such as Mycobacterium abscessus and Mycobacterium chelonae (14). Precise identification is not always possible, even with the powerful techniques used in reference laboratories, such as thin-layer chromatography of mycolic acids or mycobactin analysis.
Genotypic methods for the identification of mycobacteria have been developed in recent years. Approaches based on the polymorphism of the 16S rRNA gene have been of value for the identification of slowly growing mycobacterial species. They involve hybridization with species-specific nucleotide probes, PCR-restriction fragment length polymorphism analysis (PRA) (10, 24), or direct sequencing of PCR-amplified products (5, 6, 10, 17). However, there is little variability within the mycobacterial 16S rRNA gene sequence in RGM, making this target a poor discriminator for closely related species such as M. abscessus and M. chelonae (5, 7).
The hsp65 gene, which is present in all mycobacteria, is more variable than the 16S rRNA gene sequence and is therefore potentially useful for the identification of genetically related species. Sequence variations in the hsp65 gene can be exploited to identify both slowly growing mycobacteria and RGM to the species level (11, 18, 22, 23, 27). hsp65 PRA has been widely used for identification, and an algorithm based on this approach has recently been developed for differentiating 34 mycobacterial species, including members of the RGM group (1). hsp65 gene sequencing is an alternative approach that has been little used with RGM (4, 9, 18). hsp65 sequences from very few species of RGM have been published or deposited in databases. A sequence-based strategy has several potential advantages. It generates direct, unambiguous data and can distinguish medically relevant subspecific phylogenetic lineages. Recent advances in automated DNA sequencing have also made this approach much easier. The aim of this work was to evaluate the potential of partial hsp65 sequencing for the rapid identification of RGM.
Bacterial strains.
The following reference strains were used in this study (other designations are shown in parentheses): M. abscessus IP140420023 (ATCC 19977T) and IP140420009 (ATCC 14472); Mycobacterium brumae IP144010001 (CIP 103465T); M. chelonae IP140420003 (ATCC 35752T), IP140420006 (ATCC 19236), IP140420005 (JS 133), and IP140420019; Mycobacterium chitae IP141150001 (ATCC 19627T); Mycobacterium confluentis IP141540001 (DSM 44017T); Mycobacterium fortuitum IP140410001 (ATCC 06841T), biovariant IP980015 (ATCC 4903), biovariant IP980016 (ATCC 4904); Mycobacterium mucogenicum IP140430001 (ATCC 49650T); Mycobacterium peregrinum IP140410020 (ATCC 14467T); Mycobacterium senegalense IP141350002 (ATCC 35796T); and Mycobacterium smegmatis IP141330001 (ATCC 19420T). M. abscessus ATCC 14472, M. chelonae ATCC 19236, and JS 133 were used in a previous DNA relatedness study of the M. fortuitum-M. chelonae complex (8). Fifty-seven clinical and environmental isolates were also studied; these were 16 M. abscessus isolates (IP970515, IP971181, N94130020, N96118308, N96125674, N96127994, N97119238, N94050100, N96117130, N96147159, IP970272, IP970453, IP140420009, N92129889, N94117403, and N95120337), 13 M. chelonae isolates (IP970212, IP970640, IP970663, IP970691, IP970838, IP140420005, IP140420006, N93107077, IP140420019, IP970474, IP970475, IP970476, and IP970570), 14 M. fortuitum isolates (IP970186, IP970196, IP970218, IP970323, IP970331, IP970376, IP970396, IP970420, IP970436, IP971263, L97117576, L97124472, N96113001, and IP970522), and 14 M. peregrinum isolates (IP970300, IP970301, IP970432, IP970477, N97100967, L97109695, IP970227, IP970674, IP971262, IP971265, L97105494, IP970455, IP970407, and IP970408). These isolates were recovered from samples of water (n = 7), sputum (n = 30), abscess (n = 6), bronchial aspirate (n = 4), gastric aspirate (n = 3), urine (n = 3), lymph node (n = 2), pleural fluid (n = 1), and spleen (n = 1). Strains were obtained from the Centre National de Référence des Mycobactéries (Institut Pasteur [IP], Paris, France), Laennec Hospital (L), and Necker-Enfants Malades Hospital (N). Clinical and environmental isolates were identified by using conventional phenotypic tests and PRA with the enzymes BstEII and HaeIII (23).
PCR amplification and sequencing.
For hsp65 sequencing, RGM strains were cultivated at 30°C on Löwenstein-Jensen medium. DNA was extracted from a loopful of bacterial cells by using acid-washed glass beads (Sigma, St. Louis, Mo.), as previously described (6). The −21M13 forward primer Tb11 (5′ - TGTAAAACGACGGCCAGTACCAACGATGGTGTGTCCAT-3′) and M13 reverse primer Tb12 (5′-CAGGAAACAGCTATGACCCTTGTCGAACCGCATACCCT-3′) were used to amplify a 441-bp portion of the hsp65 gene as previously described (positions 396 to 836 of the published sequence from Mycobacterium tuberculosis) (13). The primers were designed to contain either the −21M13 forward primer or the M13 reverse primer (underlined nucleotides) to facilitate sequencing. Lysate (10 μl of a 1/100 dilution) was subjected to amplification in a final volume of 100 μl containing 0.25 μM each oligonucleotide primer, 200 μM (each) dATP, dCTP, dGTP, and dTTP (Pharmacia Biotech, Uppsala, Sweden), 10 mM Tris-HCl (pH 9), 5 mM KCl, 0.01% (wt/vol) gelatin, 1.5 mM MgCl2, 5% dimethyl sulfoxide (DMSO), and 0.5 U of DNA Taq polymerase (ATGC; Biotechnologies, Noisy-le-Grand, France). PCR was performed for 35 cycles of 20 s at 94°C, 20 s at 60°C, and 45 s at 72°C in a 9600 thermal cycler (Perkin-Elmer). Amplified product (10 μl) was subjected to electrophoresis in an agarose gel, and the gel was viewed under UV to check for DNA amplification. The PCR product (50 μl) was purified by filtration through a Pharmacia S400 HR purification column (Pharmacia Biotech). The DNA was sequenced with the Taq Dye Primer Cycle Sequencing kit (Applied Biosystems, Inc., Foster City, Calif.) with fluorescent primers (Genset, Paris, France). The labeled extension products were precipitated, washed, and dried before being loaded on the sequencing gel. The samples were suspended in 20 μl of Template Suppression reagent (Applied Biosystems), denatured by heating for 2 min at 95°C, and passed through POP6 capillary columns (Applied Biosystems, Inc.) on an ABI PRISM 310 Genetic Analyzer (Perkin-Elmer). The sequences of both strands were determined.
Analysis of sequence data.
Sequences were edited with the Sequence Navigator (Applied Biosystems) program and aligned by using the Clustal X program (Higgins, Heidelberg, Germany). Amino acid sequences were deduced from nucleotide sequences and restriction enzyme sites identified by using DNA strider 1.2 (CEA, Gif-sur-Yvette, France). Phylogenetic relationships were reconstructed by using PHYLIP software (DNADIST) to estimate the matrix of pairwise sequence distances, and then phylogenetic trees were constructed by the neighbor-joining method by using NEIGHBOR software. This software was part of the Genetic Data Environment package. The degree of confidence in phylogenetic branching was assessed by using 1,000 bootstrap resamplings.
Results and discussion.
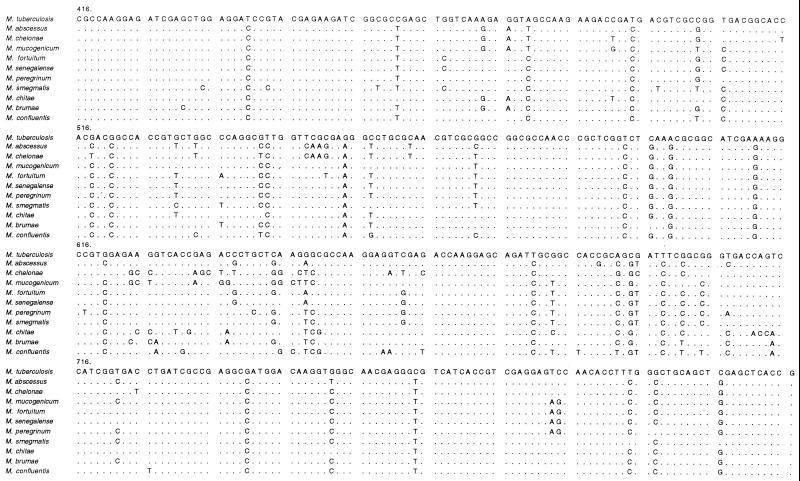
hsp65 sequences from type strains are shown in Fig. 1. Each species had a unique sequence, consistent with the results of hsp65 PRA. All species studied were readily discriminated from each other. M. fortuitum and M. senegalense, the two species with the highest degree of similarity, had sequences differing by three nucleotides. The M. chelonae and M. abscessus sequences differed by nearly 30 nucleotides, whereas their 16S rRNA genes differed by only four nucleotides. These two species were the only RGM studied with the sequence AAG at positions 549 to 551. This short sequence may thus constitute a signature for M. chelonae and M. abscessus within the RGM group. However, it is not unique to these species within the Mycobacterium genus, because it is also found in other species, such as Mycobacterium gordonae (2). Nucleotide differences were found along the length of the amplified hsp65 fragment but were particularly frequent in two regions (positions 624 to 664 and positions 683 to 725). These regions thus appear to be hypervariable regions of the hsp65 gene. Most nucleotide substitutions led to codon changes that either were conservative or encoded functionally similar amino acids (data not shown). This suggests that Hsp65 is functionally constrained by high selection pressure. This is consistent with the key role of this protein in the resistance of bacterial cells to environmental stresses (15).
FIG. 1.
Alignment of partial hsp65 sequences from RGM type strains. M. tuberculosis was used as a reference; nucleotides differing from those of the M. tuberculosis sequence are indicated; dots indicate identity. The first nucleotide shown corresponds to position 416 of the published sequence from M. tuberculosis (21).
A target gene must be sufficiently conserved among the strains of the species for use in genotypic identification. hsp65 allelic diversity among mycobacterial species has been reported in previous studies based on PRA and DNA sequencing (1, 4, 9, 19, 20, 23). We checked that allelic diversity within hsp65 was not detrimental to the identification of the main RGM species affecting humans by studying a representative panel of strains from the species M. abscessus, M. chelonae, M. fortuitum, and M. peregrinum. Some degree of intraspecific allelic diversity was found for RGM hsp65 (Table 1), consistent with the results of previous studies (1, 4, 9, 27) which developed the use of hsp65 as a target for routine identification. For the main species of the M. fortuitum complex, values of intraspecific divergence were <1.4% for M. chelonae, <1.4% for M. abscessus, 0% for 80% of the isolates within the M. fortuitum group (<2% when the sorbitol-negative biovariant was included), and <2% for the M. peregrinum species. This diversity is much lower than the interspecies divergence, even for the most closely related species such as M. abscessus and M. chelonae, and never affected species assignment. The 16S RNA sequencing that is now the reference sequencing method does not permit the differentiation of closely related RGM species. Even if the 16S RNA gene is highly conserved, some allelic intraspecific diversity (as occurs for Mycobacterium intracellulare, for example) that affects its use for identification is observed. The hsp65 sequencing method was developed for RGM species because it displays more polymorphism than does the 16S RNA gene sequence. The effects of allelic diversity on amino acid sequence were also studied (data not shown). Only a small proportion of variants would carry amino acid substitutions, and most amino acid substitutions would be isofunctional. This observation supports the notion that Hsp65 is highly conserved among mycobacteria.
TABLE 1.
hsp65 sequence variations within M. abscessus, M. chelonae, M. fortuitum, and M. peregrinum
| Strain | Base substitutiona |
|---|---|
| M. abscessus IP140420023T | T (530) T (533) C (542) C (602) C (605) G (644) C (755) |
| IP970515, IP971181, N94130020, N96118308, N96125674, N96127994, N97119238 | • • • • • • • |
| N94050100, N96117130, N96147159, IP970272 | G C T • T • T |
| IP970453 | G C T • T C • |
| IP140420009, N92129889, N94117403, N95120337 | G C T T T C • |
| M. chelonae IP140420003T | C (548) T (572) C (626) A (646) T (662) C (752) |
| IP970212, IP970640, IP970663, IP970691, IP970838, IP140420005, IP140420006, N93107077 | • • • • C • |
| IP140420019 | • C T C C T |
| IP970474, IP970475, IP970476, IP970570 | G C T C C T |
| M. fortuitum IP140410001T | C (491) A (536) T (551) C (620) G (623) G (638) G (641) A (648) G (649) |
| IP970186, IP970196, IP970218, IP970323, IP970331, IP970376, IP970396, IP970420, IP970436, IP971263, L97117576, L97124472, N96113001 | • • • • • • • • • |
| IP970522 | • C • • • • • • • |
| Biovariant 3 | |
| IP980015, sorbitol + | • C C • • • • • • |
| IP980016, sorbitol − | T C C G A C C T C |
| M. peregrinum IP140410020T | T (461) C (491) G (503) T (506) C (542) T (617) C (638) C (641) T (648) C (649) A (707) G (797) G (800) |
| IP970300, IP970301, IP970432, IP970477, N97100967 | • • • • • • • • • • • • • |
| L97109695 | • • • • A • • • • • T • • |
| IP970227, IP970674, IP971262, IP971265, L97105494 | • • • • • C • • • • • • C |
| IP970455 | C T A • • • G G A G • • • |
| IP970407, IP970408 | • T • C • C G G A G • T C |
Bases differing between species are shown. Type strain sequence is used as a reference sequence, with nucleotide positions given in parentheses. Substituted bases are indicated; dots indicate identity.
A sequence-based strategy requires that the databases used are totally reliable. This is not always the case with RGM sequences. It is not uncommon to find sequences in databases that are obviously from misidentified strains. This is not particularly surprising because identification of RGM at the species level by means of phenotypic tests alone is difficult. There are reports of strains being initially identified as M. abscessus by conventional biochemical tests and later shown to be M. fortuitum by genotypic methods and reassessment of the biochemical test results (9). The commonest error we found in databases was the misidentification of M. abscessus as M. chelonae. Several strains described as M. chelonae had hsp65 sequences typical of M. abscessus (0 to 6 base differences from M. abscessus ATCC 19977T versus 30 to 32 base differences from M. chelonae ATCC 35752T). We overcame these problems by sequencing type strains or strains previously used in taxonomic studies based on DNA-DNA hybridization. Sequences from these strains may serve as “gold standards” for subsequent studies of RGM.
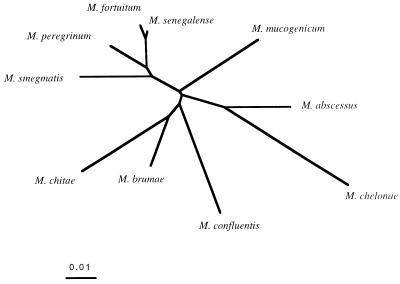
The taxonomy of RGM was not the main focus of this study, but phylogenetic trees constructed based on hsp65 and 16S rRNA sequences were compared. Figure 2 shows the hsp65 phylogenetic tree obtained with the 10 type strains studied in this work. M. fortuitum, M. senegalense, M. peregrinum, and M. smegmatis clustered together. This result is consistent with a phylogeny based on 16S rRNA sequences (17), as is the fact that M. abscessus and M. chelonae formed a distinct subgroup. However, unlike 16S rRNA sequencing, hsp65 sequencing clearly differentiated these species as distinct entities. We studied the positions of the M. abscessus, M. chelonae, M. fortuitum, and M. peregrinum strains of our panel in the hsp65-based tree constructed from type strain sequences (data not shown). Strains were located close to the type strain from the same species except for three M. peregrinum isolates (IP 970455, IP970407, and IP970408), which formed a distinct subgroup intermediate between M. peregrinum and M. fortuitum type strains. Each of the four species formed a tight group that was clearly separate from the others. The lack of overlap between hsp65 sequences from M. abscessus and M. chelonae is particularly valuable for the accurate identification of these species.
FIG. 2.
Unrooted phylogenetic tree based upon hsp65 sequences from RGM type strains. The tree was constructed by using the neighbor-joining method. The bar indicates 1% estimated sequence divergence.
Although the results of hsp65 sequencing of type strains were similar to those for hsp65 PRA, there are several problems associated with hsp65 PRA. Major disadvantages of PRA are that fragments of similar sizes are not always well discriminated and that small fragments may be difficult to identify. Some authors have ignored restriction fragments shorter than 60 bp, as they may be primer or primer dimer bands (1, 23). Others take into account fragments up to 50 bp (18). Another difficulty with PRA is that a single base change may lead to the appearance or disappearance of a restriction site. Different alleles may therefore give different restriction patterns. For example, a C at position 542 in the M. abscessus hsp65 gene gives an HaeIII restriction site, whereas a T does not. The commonest PRA-based hsp65 alleles have been described for the main RGM species (1, 18). However, migration patterns have not been determined for all possible alleles for each of the RGM species.
Other target genes have been proposed for the identification of mycobacteria by PCR-based sequencing. They include the 32-kDa protein gene (16), the dnaJ gene (21), the superoxide dismutase (SOD) gene (28), and the internal transcribed spacer of the 16S-23S rRNA gene (12). These targets have been evaluated mostly for the identification of slowly growing mycobacteria. There are few available sequence data for RGM. Very few RGM species have been sequenced, and only one strain from each species was tested in most studies. The inter- and intraspecific variability of these targets is, therefore, difficult to assess. The few sequence data available suggest that the 32-kDa protein gene, the dnaJ gene, and the internal transcribed spacer of the 16S-23S rRNA gene are much more variable than the hsp65 gene in RGM. M. fortuitum and M. smegmatis type strains had 36 base differences within a 120-bp region of the gene encoding the 32-kDa protein (ca. 30% divergence versus ca. 4% for hsp65) (16). The M. fortuitum and M. chelonae dnaJ sequences were 29% different (versus ca. 10% difference for hsp65 sequences from type strains) (21). These targets may be too variable to be used for discrimination of RGM species. They may, however, be useful for delimiting subspecific groups. The SOD gene is slightly more variable than hsp65. For example, Zolg and Schulz found 9% nucleotide divergence between M. fortuitum and M. smegmatis SOD gene sequences and 11% divergence between M. fortuitum and M. chelonae (versus ca. 4% and ca. 10%, respectively, for hsp65 sequences from type strains) (28). The SOD gene may be as suitable as hsp65 for species assignment within the RGM group. However, more information is required to evaluate the intraspecific variability of this target.
The automated fluorescence-based sequencing method incorporating capillary electrophoresis gave rapid and reliable results. The entire process was performed within a few hours (from cell pellet preparation to nucleotide sequence determination). We used DyeDeoxy Primer Cycle Sequencing reactions because this system was found to give the best performance when this work was begun. Rhodamine DyeDeoxy Terminator Cycle Sequencing kits, which are more convenient and give better results, are now available. This new sequencing technology makes it possible to obtain unambiguous sequence data for both strands of the entire amplified 441-bp hsp65 fragment. We now use it routinely for the identification of RGM by hsp65 sequencing.
Thus, automated fluorescence-based hsp65 sequencing incorporating capillary electrophoresis is a rapid and reliable method for the identification of RGM at the species level. It unambiguously differentiates between genetically related species with restricted biochemical differences, such as M. chelonae and M. abscessus. Allelic diversity within hsp65 does not preclude the use of this target for the identification of RGM and may even serve as the basis for recognition of medically important subspecific strain groups, an area worthy of further study.
Acknowledgments
We thank P. Descamps, M. L. Chaix, C. Tinsley, and V. Escuyer for helpful discussions, C. Offredo for providing strains, and E. Abachin, G. Quesne, and J. L. Beretti for technical assistance.
REFERENCES
- 1.Devallois A, Goh K S, Rastogi N. Rapid identification of mycobacteria to species level by PCR-restriction fragment length polymorphism analysis of the hsp65 gene and proposition of an algorithm to differentiate 34 mycobacterial species. J Clin Microbiol. 1997;35:2969–2973. doi: 10.1128/jcm.35.11.2969-2973.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hance A J, Grandchamp B, Lévy-Frébault V, Lecossier D, Rauzier J, Bocart D, Gicquel B. Detection and identification of mycobacteria by amplification of mycobacterial DNA. Mol Microbiol. 1989;3:843–849. doi: 10.1111/j.1365-2958.1989.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 3.Ingram C W, Tanner D C, Durack D T, Kernodle G W, Jr, Corey G R. Disseminated infection with rapidly growing mycobacteria. Clin Infect Dis. 1993;16:463–471. doi: 10.1093/clind/16.4.463. [DOI] [PubMed] [Google Scholar]
- 4.Kapur V, Li L L, Hamrick M R, Plikaytis B B, Shinnick T M, Telenti A, Jacobs W R, Banerjee A, Cole S, Yuen K Y, Clarridge J E, Kreiswirth B N, Musser J M. Rapid Mycobacterium species assignment and unambiguous identification of mutations associated with antimicrobial resistance in Mycobacterium tuberculosis by automated DNA sequencing. Arch Pathol Lab Med. 1995;119:131–138. [PubMed] [Google Scholar]
- 5.Kirschner P, Kiekenbeck M, Meissner D, Wolters J, Böttger E C. Genetic heterogeneity within Mycobacterium fortuitum complex species: genotypic criteria for identification. J Clin Microbiol. 1992;30:2772–2775. doi: 10.1128/jcm.30.11.2772-2775.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirschner P, Springer B, Vogel U, Meier A, Wrede A, Kiekenbeck M, Bange F-C, Böttger E C. Genotypic identification of mycobacteria by nucleic acid sequence determination: report of a 2-year experience in a clinical laboratory. J Clin Microbiol. 1993;31:2882–2889. doi: 10.1128/jcm.31.11.2882-2889.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusunoki S, Ezaki T. Proposal of Mycobacterium peregrinum sp. nov., nom. rev., and elevation of Mycobacterium chelonae subsp. abscessus (Kubica et al.) to species status: Mycobacterium abscessus comb. nov. Int J Syst Bacteriol. 1992;42:240–245. doi: 10.1099/00207713-42-2-240. [DOI] [PubMed] [Google Scholar]
- 8.Lévy-Frébault V, Grimont F, Grimont P A D, David H L. Deoxyribonucleic acid relatedness study of the Mycobacterium fortuitum-Mycobacterium chelonae complex. Int J Syst Bacteriol. 1986;36:458–460. [Google Scholar]
- 9.Pai S, Esen N, Pan X, Musser J M. Routine rapid Mycobacterium species assignment based on species-specific allelic variation in the 65-kilodalton heat shock protein gene (hsp65) Arch Pathol Lab Med. 1997;121:859–864. [PubMed] [Google Scholar]
- 10.Patel S, Yates M, Saunders N A. PCR-enzyme-linked immunosorbent assay and partial rRNA gene sequencing: a rational approach to identifying mycobacteria. J Clin Microbiol. 1997;35:2375–2380. doi: 10.1128/jcm.35.9.2375-2380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plikaytis B B, Plikaytis B D, Yakrus A, Butler W R, Woodley C L, Silcox V A, Shinnick T M. Differentiation of slowly growing Mycobacterium species, including Mycobacterium tuberculosis, by gene amplification and restriction fragment length polymorphism analysis. J Clin Microbiol. 1992;30:1815–1822. doi: 10.1128/jcm.30.7.1815-1822.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth A, Fischer M, Hamid M E, Michalke S, Ludwig W, Mauch H. Differentiation of phylogenetically related slowly growing mycobacteria based on 16S-23S rRNA gene internal transcribed spacer sequences. J Clin Microbiol. 1998;36:139–147. doi: 10.1128/jcm.36.1.139-147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinnick T M. The 65-kilodalton of Mycobacterium tuberculosis. J Bacteriol. 1987;169:1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinnick T M, Good R C. Mycobacterial taxonomy. Eur J Clin Microbiol Infect Dis. 1994;13:884–901. doi: 10.1007/BF02111489. [DOI] [PubMed] [Google Scholar]
- 15.Shinnick T M. Mycobacterial heat shock proteins. In: Rom W N, Garay S M, editors. Tuberculosis. Boston, Mass: Little, Brown and Company; 1996. pp. 315–327. [Google Scholar]
- 16.Soini H, Viljanen M K. Diversity of the 32-kilodalton protein gene may form a basis for species determination of potentially pathogenic mycobacterial species. J Clin Microbiol. 1997;35:769–773. doi: 10.1128/jcm.35.3.769-773.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Springer B, Stockman L, Teschner K, Roberts G D, Böttger E C. Two-laboratory collaborative study on identification of mycobacteria: molecular versus phenotypic methods. J Clin Microbiol. 1996;34:296–303. doi: 10.1128/jcm.34.2.296-303.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steingrube V A, Gibson J L, Brown B A, Zhang Y, Wilson R W, Rajagonpalan M, Wallace R J., Jr PCR amplification and restriction endonuclease analysis of a 65-kilodalton heat shock protein gene sequence for taxonomic separation of rapidly growing mycobacteria. J Clin Microbiol. 1995;33:149–153. doi: 10.1128/jcm.33.1.149-153.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swanson D, Kapur V, Stockbauer K, Pan X, Frothingham R, Musser J M. Subspecific differentiation of Mycobacterium avium complex strains by automated sequencing of a region of the gene (hsp65) encoding a 65-kilodalton heat shock protein. Int J Syst Bacteriol. 1997;47:414–419. doi: 10.1099/00207713-47-2-414. [DOI] [PubMed] [Google Scholar]
- 20.Swanson D, Pan X, Musser J M. Identification and subspecific differentiation of Mycobacterium scrofulaceum by automated sequencing of a region of the gene (hsp65) encoding a 65-kilodalton heat shock protein. J Clin Microbiol. 1996;34:3151–3159. doi: 10.1128/jcm.34.12.3151-3159.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takewaki S I, Okuzumi K, Manabe I, Tanimura M, Miyamura K, Nakahara K I, Yazaki Y, Ohkubo A, Nagai R. Nucleotide sequence comparison of the mycobacterial dnaJ gene and PCR-restriction fragment length polymorphism analysis for identification of mycobacterial species. Int J Syst Bacteriol. 1994;44:159–166. doi: 10.1099/00207713-44-1-159. [DOI] [PubMed] [Google Scholar]
- 22.Taylor T B, Patterson C, Hale Y, Safranek W W. Routine use of PCR-restriction fragment length polymorphism analysis for identification of mycobacteria growing in liquid media. J Clin Microbiol. 1997;35:79–85. doi: 10.1128/jcm.35.1.79-85.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Telenti A, Marchesi F, Balz M, Bally F, Böttger E, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31:175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaneechoutte M, De Beenhouwer H, Claeys G, Verschraegen G, De Rouck A, Paepe N, Elaichouni A, Portaels F. Identification of Mycobacterium species by using amplified ribosomal DNA restriction analysis. J Clin Microbiol. 1993;31:2061–2065. doi: 10.1128/jcm.31.8.2061-2065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace R J, Jr, Zhang Y, Brown B A, Fraser V, Mazurek G H, Maloney S. DNA large restriction fragment patterns of sporadic and epidemic nosocomial strains of Mycobacterium chelonae and Mycobacterium abscessus. J Clin Microbiol. 1993;31:2697–2701. doi: 10.1128/jcm.31.10.2697-2701.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace R J., Jr Recent changes in taxonomy and disease manifestations of the rapidly growing mycobacteria. Eur J Clin Microbiol Infect Dis. 1994;13:953–960. doi: 10.1007/BF02111497. [DOI] [PubMed] [Google Scholar]
- 27.Wilson R W, Steingrube V A, Brown B, Wallace R J. Clinical application of PCR-restriction enzyme pattern analysis for rapid identification of aerobic actinomycete isolates. J Clin Microbiol. 1998;36:148–152. doi: 10.1128/jcm.36.1.148-152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zolg J W, Schulz S P. The superoxide dismutase gene, a target for detection and identification of mycobacteria by PCR. J Clin Microbiol. 1994;32:2801–2812. doi: 10.1128/jcm.32.11.2801-2812.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]