Abstract
Objective:
The central vein sign (CVS) and “paramagnetic rim lesions” (PRL) are emerging imaging biomarkers in multiple sclerosis (MS) reflecting perivenular demyelination and chronic, smoldering inflammation. The objective of this study was to assess relationships between cognitive impairment (CI) and the CVS and PRL in radiologically isolated syndrome (RIS).
Methods:
Twenty-seven adults with RIS underwent 3.0 T MRI of the brain and cervical spinal cord (SC) and cognitive assessment using the minimal assessment of cognitive function in MS battery. The CVS and PRL were assessed in white-matter lesions (WMLs) on T2*-weighted segmented echo-planar magnitude and phase images. Multivariable linear regression evaluated relationships between CI and MRI measures.
Results:
Global CI was present in 9 (33%) participants with processing speed and visual memory most frequently affected. Most participants (93%) had ⩾ 40% CVS + WML (a threshold distinguishing MS from other WM disorders); 63% demonstrated PRL. Linear regression revealed that CVS + WML predicted performance on verbal memory(β =-0.024, p = 0.03) while PRL predicted performance on verbal memory (β = -0.040, p = 0.04) and processing speed (β = -0.039, p = 0.03).
Conclusions:
CI is common in RIS and is associated with markers of perivenular demyelination and chronic inflammation in WML, such as CVS + WML and PRL. A prospective follow-up of this cohort will ascertain the importance of CI, CVS, and PRL as risk factors for conversion from RIS to MS.
Keywords: Radiologically isolated syndrome, central vein sign, paramagnetic rim lesions, multiple sclerosis, magnetic resonance imaging
Introduction
Radiologically isolated syndrome (RIS) refers to individuals with brain MRI lesions suggestive of multiple sclerosis (MS), but without typical clinical symptoms. Retrospective studies have identified putative factors that increase the likelihood of developing MS over ~5 years, including male sex, younger age, and the presence of a spinal cord (SC) lesion on MRI. 1 However, these have limited utility in guiding treatment decisions, particularly in the absence of established links with pathophysiologic processes underlying clinical dysfunction in MS. Furthermore, there are as yet no clinical trials demonstrating efficacy of existing disease-modifying treatments (DMTs) in RIS.
Several emerging MRI measures hold promise as diagnostic and prognostic biomarkers in MS, and potentially, RIS. Using susceptibility-weighted imaging techniques, veins located centrally within white-matter lesions (WMLs) can be visualized (the “central-vein sign” (CVS)). 2 Identifying a large proportion of WML positive for the CVS(CVS + WML) has significant utility in distinguishing MS from other WM disorders.3,4 Paramagnetic rim lesions (PRLs) are another emerging imaging finding that histopathologically correlates with a rim of activated microglia and macrophages which accompany chronic, ongoing demyelination around WML. 5 These chronic, smoldering lesions have been shown to be associated with greater physical and cognitive disability in MS and slowly expand over time, suggesting ongoing inflammatory disease activity.5,6 In two recent studies, we demonstrated that the majority of RIS subjects have CVS + WML > 40%, a threshold that distinguishes MS versus other WM disorders, suggesting that most WMLs in RIS develop due to perivenular inflammation and demyelination. 7 Furthermore, we found that the majority of RIS subjects have at least one PRL suggesting that these patients harbor chronic inflammation, raising the possibility that PRL may be a prognostic biomarker of RIS converting to MS. 8
Cognitive impairment (CI) affects 40%–90% of people with MS depending on disease subtype, 9 with those impaired less likely to remain employed, maintain relationships and pursue leisure activities. 10 CI is also present in 30% of people with a clinically isolated syndrome (CIS) and predicts early conversion to MS. 11 The cognitive picture with respect to radiologically isolated syndrome (RIS) is more equivocal with some studies noting a prevalence similar to CIS,12,13 but more subtle deficits have also been reported. 14 The presence of CI in RIS is of particular interest as these are individuals who came to medical attention often by chance without overt functional difficulties.
To date, the relationships between CI and the CVS and PRLs in RIS have not been evaluated. Given that positive associations have been reported in clinically definite MS, we hypothesized that a similar picture would be present in RIS. Accordingly, the objectives of this study were to evaluate the prevalence of CI in our cohort of RIS subjects, and to characterize the relationships between CI and a spectrum of MRI measures of clinical relevance in MS, including the CVS, PRLs, and brain and SC MRI measures. We hypothesized that there would be correlations between CI and the CVS, PRLs, and SC lesions in RIS.
Methods
Participants
Twenty-seven people diagnosed with RIS by a neurologist at a tertiary care MS clinic were recruited as a prospective cohort for clinical and MRI evaluation between July 2017 and August 2018. Inclusion criteria were: ⩾18 years of age and meeting published clinical and MRI criteria for RIS (the presence of asymptomatic WM abnormalities that are ovoid, well-circumscribed, ⩾ 3 mm in maximal diameter and fulfilling 3 out of 4 Barkhof criteria for dissemination in space.2,15 Brain MRIs were reviewed by two experienced neuroradiologists (S.S. and A.B.), and a neurologist (J.O.) confirmed the inclusion criteria. Individuals who were > 65 years of age or who had a history of vascular risk factors, neurological illness, major mental illness (the presence of psychosis), significant substance abuse or toxic exposure were excluded.
Clinical assessment
Study participants underwent a neurological examination with a neurologist within 30 days of the MRI scan. None of the participants experienced neurological symptoms suggestive of MS between recruitment and the study visit.
Brain and cervical spinal cord MRI
MRIs were performed on a 3 T scanner (Siemens Skyra, Erlangen, Germany) with a 20-channel head-neck coil and a 16-channel spine-array coil. Brain sequence parameters consisted of:3D T1-weighted magnetization-prepared rapid acquisition with gradient echo (MPRAGE): repetition time (TR) = 1900 ms, echo time (TE) = 2.52 ms, flip angle (FA) = 9°, number of averages = 1, slice thickness = 1 mm, in-plane resolution = 1 x 1 mm2, and number of slices = 176. 3D T2-weighted fluid-attenuated inversion recovery (T2-FLAIR): TR = 4800 ms, TE = 353 ms, FA = 120°, number of averages = 1, slice thickness = 1 mm, in-plane resolution = 1 x 1 mm2, and number of slices = 176. 3D T2*-weighted segmented echo-planar imaging (EPI) providing magnitude and phase images: TR = 64 ms, TE = 35 ms, FA = 10°, number of averages = 1, slice thickness = 0.65 mm, number of slices = 265; in-plane resolution = 0.65 x 0.65 mm2. 16 Phase images were prepared as previously described. 17
Cervical SC sequence parameters included: sagittal 2D T1-weighted phase-sensitive inversion recovery (PSIR): TR = 2400 ms, TE = 9.4 ms, inversion time (TI) = 400 ms, number of averages = 2, slice thickness = 3 mm, in plane resolution = 0.7 x 0.7 mm2, and number of slices = 15.
Image analysis
All reviewers are experienced with evaluating for the CVS and PRLs and have completed training in CVS assessment according to the consensus criteria of the North America Imaging in Multiple Sclerosis (NAIMS) Cooperative. 2 CVS and PRL assessments were performed blinded to clinical information and the presence of SC lesions.
CVS assessment
3D T2-FLAIR and 3D T2* EPI images of each MRI study were analyzed independently by two reviewers (S.S. and P.S.) to assess WMLs for the CVS using recently published NAIMS Cooperative guidelines. 2 Central veins identified had to: have a small apparent diameter of less than 2 mm, appear as a small hypointense dot or thin hypointense line or, be visible in a minimum of two perpendicular planes and appear as a thin hypointense line in one plane, run partially or through the entire lesion, and be positioned in the approximate center of the WML. WMLs that were excluded from this analysis were: less than 3 mm in maximum diameter, confluent or contiguous, contained more than 1 vein, or poorly visible.
If there was disagreement between the two reviewers, a third reviewer (J.O.) assessed the lesion, and consensus agreement was obtained.
The proportion of CVS + WMLs in each RIS case was expressed as a percentage of the total number of WMLs within each RIS subject. Previously published criteria that reportedly distinguishes MS from other WM disorders—the “40% rule”—was applied. 18
PRL assessment
Three reviewers (S.S., P.S., and M.A.) independently assessed each WML for the PRL. 3D T2-FLAIR and phase images were reviewed by each reviewer. WMLs were defined as being a PRL when either a complete or incomplete rim of hypointense signal (“paramagnetic rim”) was identified; signal in the center of the lesion was either hyperintense or isointense to the extralesional white matter.19,20 WMLs were excluded from the PRL analysis if they were too small (<3 mm in greatest dimension) or poorly visible on phase images. If there was disagreement, all three reviewers re-evaluated the WML and reached a consensus.
Brain volumetric analysis
Brain volumetric segmentation was performed on the T1-MPRAGE and T2-FLAIR image data set using Multi-atlas Cortical Reconstruction Using Implicit Surface Evolution (MaCRUISE) to extract whole-brain volume, intracranial volume, thalamic volume, and gray-matter volume. 21 Cerebral volume fraction (CVF) was calculated according to the formula: CVF = brain volume / total intra-cranial volume. Thalamic fraction and gray-matter fraction were calculated by dividing each volume by total intra-cranial volume.
Cervical spinal cord lesion count
Sagittal 2D PSIR images of the cervical SC were reviewed by a neuroradiologist (S.S.) to count the total number of cervical SC lesions per case, while blinded to clinical information and the CVS and PRL analysis.
Cognitive assessment
All participants underwent a detailed neuropsychological assessment with the Minimal Assessment of Cognitive Function in MS (MACFIMS), 22 which comprises the following cognitive domains: (1) information processing speed: Paced Auditory Serial Addition test (PASAT) 2 s and 3 s versions 10 and the Symbol Digit Modalities Test (SDMT); (2) verbal and visual memory: California Verbal Learning Test–II (CVLT-II) and the Brief Visuospatial Memory Test (BVMT); (3) executive function: Delis-Kaplan Executive Function System Sorting Test; (4) visuospatial processing: Judgment of Line Orientation; and (5) Verbal Fluency (Controlled Oral Word Association Test). The cognitive data were converted to Z scores. Failure on each test was defined as a score of 1.5 standard deviations (SD) below the normative mean, based on the Canadian normative data for MACFIMS. 23 By convention, global impairment was defined as impairment on two or more cognitive domains. Composite failure scores were obtained for the domains of memory (CVLT, BVMT) and processing speed (SDMT, PASAT-2, PASAT-3)
Statistical analysis
Statistical calculations were performed using STATA 15.0 (College Station, Texas). Linear regression analyses were used to detect imaging predictors of cognitive performance. The sample size allowed for three imaging predictors for each cognitive variable. The imaging variables of greatest interest were the proportion of CVS + WML and PRLs. Thalamic fraction and SC lesion count were included in each model as independent covariates of interest as normalized thalamic volume is a known correlate of cognitive dysfunction in people with established MS, and the presence of SC lesions is a known risk factor for RIS developing MS thereby making it likely that the presence of SC lesions would be relevant to cognitive dysfunction in RIS, and therefore a variable that should be controlled for as a confounding covariate. CVS + WML and PRLs were evaluated in separate models, as they were highly correlated (ρ = 0.79, p < 0.01; Spearman’s rank). Statistical significance was set at p < 0.05. There was no missing data with regards to the variables of interest from the n = 27 RIS cases.
Standard protocol approvals, registrations, and patient consents
This study was approved by the Institutional Review Board of St Michael’s Hospital. All participants provided written informed consent.
Data availability statement
Anonymized data will be shared by request from any qualified investigator.
Results
We report cross-sectional results of this prospective cohort of RIS cases. Demographic data are shown in Table 1. The reasons for the initial MRI are displayed in Table e-1. None of the 27 RIS cases had any current or prior neurological symptoms characteristic of demyelinating disease. Nine of the cases had incidental neurological findings, including mildly impaired vibration sensation (n = 7), brisk reflexes (n = 1), and horizontal gaze-evoked nystagmus without any associated visual deficits (n = 1). One participant was involuntarily unemployed due to mood and cognitive concerns. The remaining participants were either employed (n = 22), retired (n = 1), on disability related to a motor vehicle accident (n = 1) or homemakers (n = 2).
Table 1.
Clinical and MRI characteristics.
| Clinical characteristics | |
| Participants, n | 27 |
| Age, mean (range), years | 45.0 (11.3) |
| Female, n (%) | 21 (78) |
| MRI characteristics | |
| Whole brain volume, mL | 1183.8 (111.5) |
| Brain lesion volume, mL | 1.0 (4.7) |
| Brain lesions per case, median (range) | 34 (9–165) |
| CVF (SD) | 0.887 (0.017) |
| Thalamic volume, mean (SD), mL | 146.6 (18.2) |
| Thalamic fraction (SD) | 0.011 (0.0016) |
| Cervical spinal cord lesion count, median (range) | 1 (0–4) |
| Number (%) of RIS cases with at least one cervical spinal cord lesion | 18 (67%) |
| CVS lesions | |
| CVS + WML count, n (%) | 480 (76) |
| Percentage of CVS + WML per case, median (range) | 86 (30–100) |
| Cases with > 40% CVS + lesions, n (%) | 25 (93) |
| PRL | |
| PRL count, n (%) | 129 (12) |
| Cases with PRLs, n (%) | 17 (63) |
| PRLs per case, median (range) | 1 (0–23) |
MRI: magnetic resonance imaging; CVF: cerebral volume fraction; SD: standard deviation; RIS: radiologically isolated syndrome; CVS: central vein sign; WML: white-matter lesions; PRL: paramagnetic rims lesion.
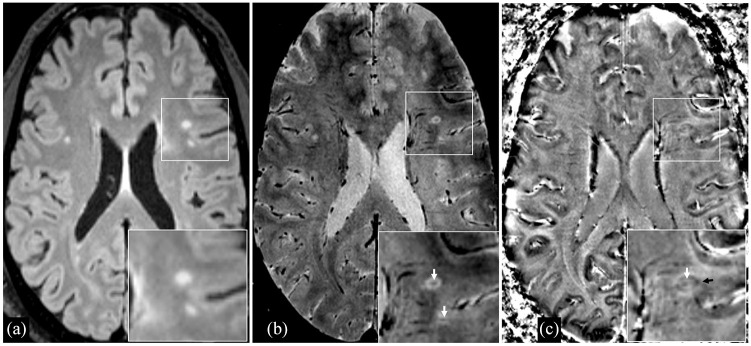
Ninety-three percent of participants had a proportion of CVS + WML that was ⩾ 40% (the proposed threshold that distinguishes MS from other WM disorders 3 ), 63% demonstrated PRLs, and the mean proportion of PRLs per subject was 12% (see Table 1 and Figure 1).
Figure 1.
A 55-year-old man with radiologically isolated syndrome and a relatively high proportion of lesions with the central-vein sign (87%) and lesions with paramagnetic rims (14%), as well as global CI. (a) 3D Axial FLAIR sequence demonstrating T2-hyperintense white-matter lesions. (b) 3D Axial T2*-weighted segmented echo-planar magnitude image demonstrating central veins within white-matter lesions (white arrows). (c) Phase image (corresponding to the magnitude image in B) demonstrating a paramagnetic rim (black arrow) in a white-matter lesion with a visible central vein (white arrow).
The majority of RIS cases (n = 18, 67%) had at least one cervical SC lesion. The median number of cervical SC lesions was 1 (range: 0–4). The number of SC lesions correlated significantly with proportions of CVS + WML (ρ = 0.42, p = 0.02) and PRLs (ρ = 0.37, p = 0.04) respectively. However, SC lesions did not demonstrate consistent relationships with any cognitive tests. (Tables 3 and 4).
Table 3.
Relationships between tests of memory and information processing speed and CVS + WML and other MRI measures in multivariable regression models (p-values displayed).
| CVLT-total | CVLT-delayed | BVMT-total | BVMT-delayed | PASAT3 | PASAT2 | SDMT | |
|---|---|---|---|---|---|---|---|
| CVS + WML (proportion) | 0.04 | 0.29 | 0.53 | 0.16 | 0.08 | 0.19 | 0.05 |
| Thalamic fraction | 0.72 | 0.70 | 0.37 | 0.40 | 0.51 | 0.31 | 0.32 |
| SC lesion count | 0.90 | 0.68 | 0.97 | 0.75 | 0.85 | 0.48 | 0.50 |
| r 2 | 0.20 | 0.06 | 0.05 | 0.11 | 0.27 | 0.17 | 0.18 |
CVS: central vein sign; WML: white-matter lesions; MRI: magnetic resonance imaging; CVLT: California Verbal Learning Test; BVMT: Brief Visuospatial Memory Test; PASAT: Paced Auditory Serial Addition test; SDMT: Symbol Digit Modalities Test; SC: spinal cord; r2: regression model coefficient of determination.
Significant/trend toward significant p = values bolded.
Table 4.
Relationships between tests of memory and information processing speed and PRLs and other MRI measures in multivariable regression models (p-values displayed).
| CVLT-total | CVLT-delayed | BVMT-total | BVMT-delayed | PASAT3 | PASAT2 | SDMT | |
|---|---|---|---|---|---|---|---|
| PRLs (proportion) | 0.04 | 0.25 | 0.47 | 0.86 | 0.03 | 0.46 | 0.04 |
| Thalamic fraction | 0.29 | 0.97 | 0.55 | 0.41 | 0.16 | 0.20 | 0.11 |
| SC lesion count | 0.54 | 0.82 | 0.67 | 0.78 | 0.89 | 0.93 | 0.79 |
| r 2 | 0.21 | 0.06 | 0.06 | 0.03 | 0.21 | 0.11 | 0.19 |
PRL: paramagnetic rim lesion; MRI: magnetic resonance imaging; CVLT: California Verbal Learning Test; BVMT: Brief Visuospatial Memory Test; PASAT: Paced Auditory Serial Addition test; SDMT: Symbol Digit Modalities Test; SC: spinal cord; r2: regression model coefficient of determination.
Significant/trend toward significant p = values bolded.
Global CI was present in 33% of participants. The most frequently impaired domains were processing speed (41%) and memory (33%). The individual test impairment rates on the MACFIMS are shown in Table 2.
Table 2.
Cognitive impairment in RIS subjects.
| Visual-spatial function | n of subjects (%) |
|---|---|
| JOLO a | 3 (11.5) |
| Processing speed | |
| Impaired | 11 (40.7) |
| SDMT | 5 (18.5) |
| PASAT3 | 5 (18.5) |
| PASAT2 b | 6 (22.2) |
| Executive function | |
| Impaired | 4 (14.8) |
| COWAT | 4 (14.8) |
| DKEFS—correct sort | 2 (7.4) |
| DKEFS—description | 1 (3.7) |
| Memory | |
| Impaired | 9 (33.3) |
| CVLT—total | 2 (7.4) |
| CVLT—delayed | 1 (3.7) |
| BVMT—total | 6 (22.2) |
| BVMT—delayed | 7 (25.9) |
| Global impairment | 9 (33.3) |
COWAT: Controlled Oral Word Association Test; DKEFS: Delis-Kaplan Executive Function System Sorting Test; JOLO: Judgment of Line Orientation Test; RIS: radiologically isolated syndrome; SDMT: Symbol Digit Modalities Test; PASAT: Paced Auditory Serial Addition test; CVLT: California Verbal Learning Test; BVMT: Brief Visuospatial Memory Test.
One subject did not complete this test; total N = 26 tested.
Six subjects skipped PASAT-2; total N = 21 tested.
A summary of CVS + WML, PRL, SC lesions, and global CI by RIS case is presented in Table e-2.
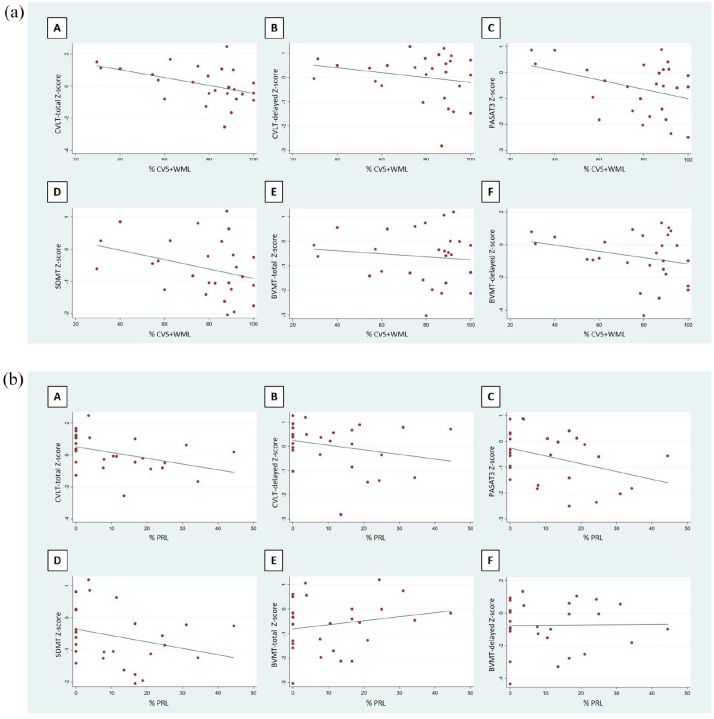
Scatterplots demonstrating univariable correlations between cognitive tests and the CVS + WML and PRLs are displayed in Figure 2(a) and (b). The results of the multivariable linear regression analyses are shown in Tables 3 and 4. The proportion of CVS + WML predicted performance on the CVLT (regression coefficient β = -0.024, p = 0.04). The proportion of PRLs predicted performance on the CVLT, PASAT-3, and SDMT (regression coefficient β = -0.040 and p = 0.04; β = -0.039 and p = 0.03; β = -0.031 and p = 0.04, respectively) (Tables 3 and 4). Thalamic fraction and SC lesion count did not predict performance on any cognitive index. There were no imaging predictors of performance on the BVMT, PASAT-2, JLO or COWAT. When we assessed multivariable linear regression models including either CVF or gray-matter fraction rather than thalamic fraction as independent covariates, none of these measures predicted performance on any cognitive tests (p > 0.50 for all models), similar to thalamic fraction.
Figure 2.
(a) Scatterplots of correlations between cognitive tests and the proportion of white-matter lesions demonstrating the central-vein sign. (b) Scatterplots of correlations between cognitive tests and the proportion of white-matter lesions that are paramagnetic-rim lesions.
Discussion
In our cohort of 27 adults with RIS, one third were cognitively globally impaired with the domains of processing speed and memory most frequently affected. There were high proportions of CVS + WML and PRLs, emerging MRI biomarkers indicative of perivenous demyelination and chronic inflammation. The proportion of PRLs was the most robust predictor of processing speed and memory impairment.
Prior studies have demonstrated that the CVS is related to perivenous inflammation and demyelination in WML.18,24 In recent years, there has been substantial interest in the utility of the CVS as a diagnostic biomarker of MS, both to facilitate the diagnosis of MS and to avoid misdiagnosis.25,26 The utility of the CVS in both of these clinical scenarios has been demonstrated in numerous studies evaluating MS patients and individuals with other WM disorders.3,4,27 There has also been increasing interest in the ability of susceptibility-weighted imaging techniques, such as 3D T2*-weighted segmented echo-planar phase images, to detect chronic, active lesions in MS by assessing for PRLs.5,19,28 Chronic, active lesions are thought to be an important pathological substrate of disease progression in MS, and the ability to identify these lesions on MRI may enable more rapid identification and monitoring of disease progression in MS, which is a significant unmet clinical need. 29 Recent studies have demonstrated that PRLs are histopathologically related to chronic lesions with an expanding border of microglia and macrophages, and that the presence of larger numbers of PRLs are related to higher levels of physical and cognitive disability, as well as progressive subtypes of MS.5,6 In this study, we found that the majority of RIS subjects (93%) have a proportion of CVS + WML that meets the threshold that reportedly differentiates MS versus other WM disorders, and that notably, there are correlations between CVS + WML and CI. Furthermore, we found that the majority of RIS subjects (63%) have at least one PRL, and that PRLs also correlate with CI. Of note, the high proportion of CVS + WML that we observe in our RIS patients is likely due to our stringent application of published RIS criteria, which require the presence of WML in 3 out of 4 characteristic locations (periventricular, juxtacortical, infratentorial, or SC). These observations, together with the lack of a correlation of CI in RIS with whole-brain atrophy and thalamic fraction, suggest that the CVS and PRL are sensitive at capturing subtle cognitive deficits in RIS and raise the possibility that those lesions are the basis of the cognitive deficits. In addition, when taking into account recent literature on PRLs and clinical deficits in MS, 6 our findings suggest that RIS subjects who demonstrate high proportions of the CVS and PRLs may progress clinically, and therefore have a worse clinical prognosis than those who do not. The observed correlations between the CVS, PRLs, and SC lesions, which is an identified risk factor associated with conversion of RIS to MS, 1 further supports the prognostic value of the CVS and PRLs in RIS.
Our finding of global CI in one third of participants is in keeping with some earlier reports,12,13 but exceeds that noted by others. 14 The most common deficits detected, namely those of processing speed followed by learning and memory, matches that seen in people with clinically definite MS. 30 Our data, however, differ from the MS literature when it comes to brain correlates of cognitive dysfunction. A well replicated finding in this regard has been the robust association between thalamic fraction, either measured directly 31 or via third ventricular width, 32 and numerous indices of CI, most prominently the SDMT. In those two studies, thalamic atrophy trumped other brain metrics including T1 and T2 lesion volume, brain parenchymal fraction and bi-caudate width as the most significant imaging correlate of cognitive dysfunction. In the only previous RIS study that included an imaging component and detailed cognitive inquiry, thalamic volume was not measured. 13 However, two other markers of atrophy, namely low cortical volume and T1 hypointense lesion volume, were significantly associated with worsening cognitive performance. The reason for our failure to find any marker of brain atrophy (thalamic or whole-brain atrophy) linked to deficits in processing speed and memory is unclear, but may reflect normal brain volume, or the presence of a reduction in brain volume as yet too subtle to significantly influence cognition. This negative finding was however offset by the emergence of PRLs and CVS + WML proportions as predictors of CI. While similar results have been reported in people with confirmed MS, it has not previously been noted in individuals with RIS.
In linear regression models, we observed the most robust correlations between CVS + WML and PRLs with CVLT-total, SDMT, and PASAT3 Z-scores. As SDMT and PASAT3 were impaired in nearly 20% of our RIS cohort, the observed correlations are in keeping with what one would expect. On the other hand, CVLT-total also demonstrated correlations with CVS + WML and PRLs but was impaired in only 7% of subjects. Although puzzling at first pass, this finding is linked to the performance of three RIS subjects whose CVLT scores fell just short of the 1.5 SD threshold used to define impairment. However, the scatter plots demonstrate a clear linear correlation between CVLT-total Z-scores and the imaging measures, suggesting that CVLT performance is linked to CVS + WML and PRLs. From a mechanistic perspective, the observed correlations between cognitive tests and CVS + WML and PRLs solidifies the link between inflammatory processes in the brain and cognitive dysfunction albeit for the first time in a sample here of people with no neurological illness.
Predicting which individuals with RIS will develop MS is a key question. Five-year follow-up data revealed the development of subsequent clinical events in 34% of cases, with 9.6% meeting diagnostic criteria for primary progressive MS. 1 Risk factors identified were younger age, being male and a spinal cord lesion. By 10 years following first MRI, the number of symptomatic individuals had increased to 50% with age, positive oligoclonal banding on cerebrospinal fluid, and infratentorial and SC lesions identified as risk factors. 33 Whether cognition is another negative prognostic factor for RIS converting to MS is not yet known, but the data from people with CIS suggests it may well be. 11 Moreover, the relationships that we observe with CI and the CVS + WML and PRLs suggest that both of these imaging metrics and CI may have clinical utility as potential markers indicative of future MS. Specifically, administering a brief cognitive battery focusing on memory and processing speed may be an important “screening” tool in RIS that is indicative of a poor prognosis, and can guide clinical management. A brief cognitive battery has the benefit of ease of administration without the need for additional MRI scan time and specialized image processing and analysis tools, which are factors that are often prohibitive of widespread dissemination and use of advanced imaging measures. Longitudinal research will clarify these points.
Our data underscore the importance of a cognitive assessment in people with RIS, which is typically not done in clinical practice outside of large academic centers. Here one cannot rely on the neurological examination alone, for the ability of neurologists to diagnose impairment without recourse to neuropsychological testing is no better than chance. 34 Moreover, it is evident that employment cannot be used as a surrogate measure of cognition, as only a single individual in our RIS cohort was unemployed due to performance difficulties at work. Current consensus guidelines dictate that every person with MS should have a baseline cognitive battery, and if that is not possible because of limited resources and expertise, at least a SDMT followed by a yearly SDMT to monitor change. 35 In light of the emerging RIS cognitive findings, this advice seems equally applicable to this group as well.
Our study has a number of limitations, including a relatively small sample size that is nevertheless comparable to others in the literature,12–14 the absence of a control group and a cross-sectional design. Furthermore, there are no established guidelines regarding the identification of the PRLs, particularly using 3 T MRI platforms.
In conclusion, we found a substantial proportion of individuals with RIS demonstrate global CI, despite being seemingly neurologically asymptomatic and without functional limitations. Furthermore, there were observed correlations between CI and imaging measures that are known to be related to chronic inflammation and greater disability in established MS. If substantiated by future longitudinal studies, this study suggests that in addition to obtaining MRI scans sensitive to the CVS and PRLs, a screening cognitive test may be a useful and practical clinical tool that can be used widely to guide clinical management in RIS, currently an unmet clinical need.
Supplemental Material
Supplemental material, sj-pdf-1-msj-10.1177_13524585211002097 for Cognitive impairment, the central vein sign, and paramagnetic rim lesions in RIS by Jiwon Oh, Suradech Suthiphosuwan, Pascal Sati, Martina Absinta, Blake Dewey, Melanie Guenette, Daniel Selchen, Aditya Bharatha, Emily Donaldson, Daniel S Reich and Anthony Feinstein in Multiple Sclerosis Journal
Supplemental material, sj-pdf-2-msj-10.1177_13524585211002097 for Cognitive impairment, the central vein sign, and paramagnetic rim lesions in RIS by Jiwon Oh, Suradech Suthiphosuwan, Pascal Sati, Martina Absinta, Blake Dewey, Melanie Guenette, Daniel Selchen, Aditya Bharatha, Emily Donaldson, Daniel S Reich and Anthony Feinstein in Multiple Sclerosis Journal
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. J.O. reports grants from MS Society of Canada, Barford and Love MS Fund of St. Michael’s Hospital Foundation, National MS Society, Brain Canada, Biogen-Idec, Roche, and EMD-Serono; and personal fees for consulting or speaking from Biogen-Idec, EMD-Serono, Roche, Sanofi-Genzyme, Novartis, and Celgene. Dr. S.S. has received fellowship educational support by unrestricted educational grant from Sanofi-Genzyme. Dr. P.S. reports no disclosures. Dr. M.A. reports grants from the National MS Society and the Conrad N. Hilton Foundation; and personal fees for consulting or speaking from Celgene. Mr. B.D. is partially supported by a research grant from Biogen. Ms. M.G. reports no disclosures. Dr. D.S. reports personal compensation for consulting or speaking from EMD-Serono, Celgene, Sanofi-Genzyme, Biogen-Idec, Novartis, Teva, and Roche. Dr. A.B. reports personal fees for consulting or speaking from EMD-Serono, Novartis, and Sanofi-Genzyme. Dr. E.D. reports no disclosures. Dr. D.S.R. has received research funding from Vertex Pharmaceuticals for collaborative studies. Dr. A.F. is on an Advisory Board for Akili Interactive and reports grants from the MS Society of Canada, book royalties from Johns Hopkins University Press, Cambridge University Press and Amadeus Press and speaker’s honoraria from Novartis, Biogen, Roche and Sanofi-Genzyme.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the MS Society of Canada and the St. Michael’s Hospital Foundation Barford and Love MS Fund. The study was partially supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health, USA.
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscripts; and decision to submit the manuscript for publication.
ORCID iDs: Jiwon Oh  https://orcid.org/0000-0001-5519-6088
https://orcid.org/0000-0001-5519-6088
Pascal Sati  https://orcid.org/0000-0002-6763-0125
https://orcid.org/0000-0002-6763-0125
Martina Absinta  https://orcid.org/0000-0003-0276-383X
https://orcid.org/0000-0003-0276-383X
Blake Dewey  https://orcid.org/0000-0003-4554-5058
https://orcid.org/0000-0003-4554-5058
Daniel S Reich  https://orcid.org/0000-0002-2628-4334
https://orcid.org/0000-0002-2628-4334
Anthony Feinstein  https://orcid.org/0000-0002-0132-0909
https://orcid.org/0000-0002-0132-0909
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Jiwon Oh, Division of Neurology, Department of Medicine, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada/Department of Neurology, Johns Hopkins University, Baltimore, MD, USA.
Suradech Suthiphosuwan, Division of Neurology, Department of Medicine, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada/Division of Neuroradiology, Department of Medical Imaging, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada.
Pascal Sati, Translational Neuroradiology Section, National Institute of Neurological Disorders and Stroke, NIH, Bethesda, MD, USA/Neuroimaging Unit, Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Martina Absinta, Department of Neurology, Johns Hopkins University, Baltimore, MD, USA/Translational Neuroradiology Section, National Institute of Neurological Disorders and Stroke, NIH, Bethesda, MD, USA.
Blake Dewey, Department of Neurology, Johns Hopkins University, Baltimore, MD, USA.
Melanie Guenette, Division of Neurology, Department of Medicine, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada.
Daniel Selchen, Division of Neurology, Department of Medicine, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada.
Aditya Bharatha, Division of Neuroradiology, Department of Medical Imaging, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada/Division of Neurosurgery, Department of Surgery, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada.
Emily Donaldson, Department of Psychiatry, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON, Canada.
Daniel S Reich, Department of Neurology, Johns Hopkins University, Baltimore, MD, USA/Translational Neuroradiology Section, National Institute of Neurological Disorders and Stroke, NIH, Bethesda, MD, USA.
Anthony Feinstein, Division of Neurology, Department of Medicine, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada/Department of Psychiatry, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON, Canada.
References
- 1. Okuda DT, Siva A, Kantarci O, et al. Radiologically isolated syndrome: 5-year risk for an initial clinical event. PLoS ONE 2014; 9(3): e90509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: A consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol 2016; 12(12): 714–722. [DOI] [PubMed] [Google Scholar]
- 3. Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol 2016; 3(2): 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maggi P, Absinta M, Grammatico M, et al. Central vein sign differentiates multiple sclerosis from central nervous system inflammatory vasculopathies. Ann Neurol 2018; 83(2): 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest 2016; 126: 2597–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Absinta M, Sati P, Masuzzo F, et al. Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol 2019; 76: 1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suthiphosuwan S, Sati P, Guenette M, et al. The central vein sign in radiologically isolated syndrome. Am J Neuroradiol 2019; 40(5): 776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suthiphosuwan S, Sati P, Absinta M, et al. Paramagnetic rim sign in radiologically isolated syndrome. JAMA Neurol 2020; 77: 653–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruano L, Portaccio E, Goretti B, et al. Age and disability drive cognitive impairment in multiple sclerosis across disease subtypes. Mult Scler 2017; 23(9): 1258–1267. [DOI] [PubMed] [Google Scholar]
- 10. Rao SM, Leo GJ, Ellington L, et al. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology 1991; 41(5): 692–696. [DOI] [PubMed] [Google Scholar]
- 11. Zipoli V, Goretti B, Hakiki B, et al. Cognitive impairment predicts conversion to multiple sclerosis in clinically isolated syndromes. Mult Scler 2010; 16(1): 62–67. [DOI] [PubMed] [Google Scholar]
- 12. Lebrun C, Blanc F, Brassat D, et al. Cognitive function in radiologically isolated syndrome. Mult Scler 2010; 16(8): 919–925. [DOI] [PubMed] [Google Scholar]
- 13. Amato MP, Hakiki B, Goretti B, et al. Association of MRI metrics and cognitive impairment in radiologically isolated syndromes. Neurology 2012; 78: 309–314. [DOI] [PubMed] [Google Scholar]
- 14. Menascu S, Stern M, Aloni R, et al. Assessing cognitive performance in radiologically isolated syndrome. Mult Scler Relat Disord 2019; 32: 70–73. [DOI] [PubMed] [Google Scholar]
- 15. Okuda DT, Mowry EM, Beheshtian A, et al. Incidental MRI anomalies suggestive of multiple sclerosis: The radiologically isolated syndrome. Neurology 2009; 72: 800–805. [DOI] [PubMed] [Google Scholar]
- 16. Sati P, Thomasson DM, Li N, et al. Rapid, high-resolution, whole-brain, susceptibility-based MRI of multiple sclerosis. Mult Scler 2014; 20(11): 1464–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li N, Wang WT, Sati P, et al. Quantitative assessment of susceptibility-weighted imaging processing methods. J Magn Reson Imaging 2014; 40(6): 1463–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology 2011; 76: 534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. Am J Neuroradiol 2018; 39(7): 1233–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Absinta M, Sati P, Reich DS. Advanced MRI and staging of multiple sclerosis lesions. Nat Rev Neurol 2016; 12(6): 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huo Y, Carass A, Resnick SM, et al. Combining multi-atlas segmentation with brain surface estimation. Proc SPIE Int Soc Opt Eng 2016; 9784: 97840E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benedict RH, Fischer JS, Archibald CJ, et al. Minimal neuropsychological assessment of MS patients: A consensus approach. Clin Neuropsychol 2002; 16(3): 381–397. [DOI] [PubMed] [Google Scholar]
- 23. Walker LAS, Marino D, Berard JA, et al. Canadian normative data for minimal assessment of cognitive function in multiple sclerosis. Can J Neurol Sci 2017; 44(5): 547–555. [DOI] [PubMed] [Google Scholar]
- 24. Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: A pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol 2013; 70(5): 623–628. [DOI] [PubMed] [Google Scholar]
- 25. Solomon AJ, Corboy JR. The tension between early diagnosis and misdiagnosis of multiple sclerosis. Nat Rev Neurol 2017; 13(9): 567–572. [DOI] [PubMed] [Google Scholar]
- 26. Oh J, Sati P. Detection of central vein should be part of MS diagnostic criteria: Commentary. Mult Scler 2020; 26(4): 409–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maggi P, Absinta M, Sati P, et al. The “central vein sign” in patients with diagnostic “red flags” for multiple sclerosis: A prospective multicenter 3T study. Mult Scler 2020; 26: 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaunzner UW, Kang Y, Zhang S, et al. Quantitative susceptibility mapping identifies inflammation in a subset of chronic multiple sclerosis lesions. Brain 2019; 142: 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oh J, Sicotte NL. New imaging approaches for precision diagnosis and disease staging of MS. Mult Scler 2020; 26(5): 568–575. [DOI] [PubMed] [Google Scholar]
- 30. Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol 2008; 7: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 31. Houtchens MK, Benedict RH, Killiany R, et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology 2007; 69: 1213–1223. [DOI] [PubMed] [Google Scholar]
- 32. Benedict RH, Weinstock-Guttman B, Fishman I, et al. Prediction of neuropsychological impairment in multiple sclerosis: Comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch Neurol 2004; 61(2): 226–230. [DOI] [PubMed] [Google Scholar]
- 33. Lebrun-Frenay C, Kantarci O, Siva A, et al. Radiologically isolated syndrome: 10-year risk estimate of a clinical event. Ann Neurol 2020; 88: 407–417. [DOI] [PubMed] [Google Scholar]
- 34. Romero K, Shammi P, Feinstein A. Neurologists accuracy in predicting cognitive impairment in multiple sclerosis. Mult Scler Relat Disord 2015; 4(4): 291–295. [DOI] [PubMed] [Google Scholar]
- 35. Kalb R, Beier M, Benedict RH, et al. Recommendations for cognitive screening and management in multiple sclerosis care. Mult Scler 2018; 24(13): 1665–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-msj-10.1177_13524585211002097 for Cognitive impairment, the central vein sign, and paramagnetic rim lesions in RIS by Jiwon Oh, Suradech Suthiphosuwan, Pascal Sati, Martina Absinta, Blake Dewey, Melanie Guenette, Daniel Selchen, Aditya Bharatha, Emily Donaldson, Daniel S Reich and Anthony Feinstein in Multiple Sclerosis Journal
Supplemental material, sj-pdf-2-msj-10.1177_13524585211002097 for Cognitive impairment, the central vein sign, and paramagnetic rim lesions in RIS by Jiwon Oh, Suradech Suthiphosuwan, Pascal Sati, Martina Absinta, Blake Dewey, Melanie Guenette, Daniel Selchen, Aditya Bharatha, Emily Donaldson, Daniel S Reich and Anthony Feinstein in Multiple Sclerosis Journal
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.