Abstract
Tuberculosis (TB) remains the leading infectious killer worldwide. The World Health Organization’s (WHO) End-TB strategy is falling short of the 2020 mileposts in several domains. Undernutrition remains the leading population-level risk factor for TB. Studies have consistently found that undernutrition is associated with increased TB incidence, increased severity, worse treatment outcomes, and increased mortality. Modeling studies support implementing nutritional interventions for persons living with TB (PLWTB) and those at risk of TB disease to ensure the success of the End-TB strategy. In this Personal Views essay, we highlight nutrition-related immunocompromise, implications of undernutrition for TB treatment and prevention, the role of nutritional supplementation, pharmacokinetics and pharmacodynamics of antimycobacterial medications in undernourished persons living with TB (PLWTB), and the role of social protection interventions in addressing undernutrition as a TB risk factor. To catalyze action on this insufficiently-addressed accelerant of the global TB epidemic, we must prioritize research to understand the immunological pathways impaired by nutrient deficiencies, develop tools to diagnose clinical and subclinical TB in undernourished persons, understand how nutritional status affects the efficacy of TB vaccine and therapy. Through further primary research, modeling and implementation research, we must also catalyze policy change, particularly in countries with high TB burdens.
Introduction:
With 10 million cases and 1.5 million deaths in 2018, tuberculosis (TB) remains the leading infectious killer worldwide.1 The World Health Organization’s (WHO) End-TB strategy aims to cut TB deaths by 95% and TB incidence by 90% by 2035, and to eliminate catastrophic costs incurred by persons living with TB (PLWTB) and their families by 2020.2 However, we are falling short of the 2020 mileposts in several domains. For instance, the TB incidence rate only decreased by 6.3% between 2015 and 2018, a fraction of the targeted rate of 20%.3 One reason for the slow progress may be that investments in diagnostics, therapeutics, and vaccine development have not been complemented by investments to eliminate socio-economic risk factors that fuel TB worldwide. Undernutrition, in particular, deserves urgent attention.
Undernutrition is the most common secondary immunodeficiency which has been termed nutritional acquired immunodeficiency syndrome (N-AIDS).5,6 There is considerable geographic overlap between TB and undernutrition (Figure 1).4 Indeed, undernutrition was the leading risk factor for TB worldwide with a population-attributable fraction (PAF) of 2.3 million cases.1 By comparison, the PAF for TB from HIV was 1.2 million. The PAF addresses increased incidence, but undernutrition also increases the risk of treatment failure, relapse, and mortality.7 Climate change threatens food production and distribution systems in high TB burden regions such as South Asia and sub-Saharan Africa.8 Undernutrition and food insecurity are likely to become even more prevalent in the wake of the coronavirus disease-19 pandemic and the accompanying economic devastation worldwide.9 This heightens the urgency to understand and ameliorate the biological and social factors by which undernutrition contributes to TB incidence and poor treatment outcomes.
Figure 1: Geographic overlap between tuberculosis and undernutrition across the globe.
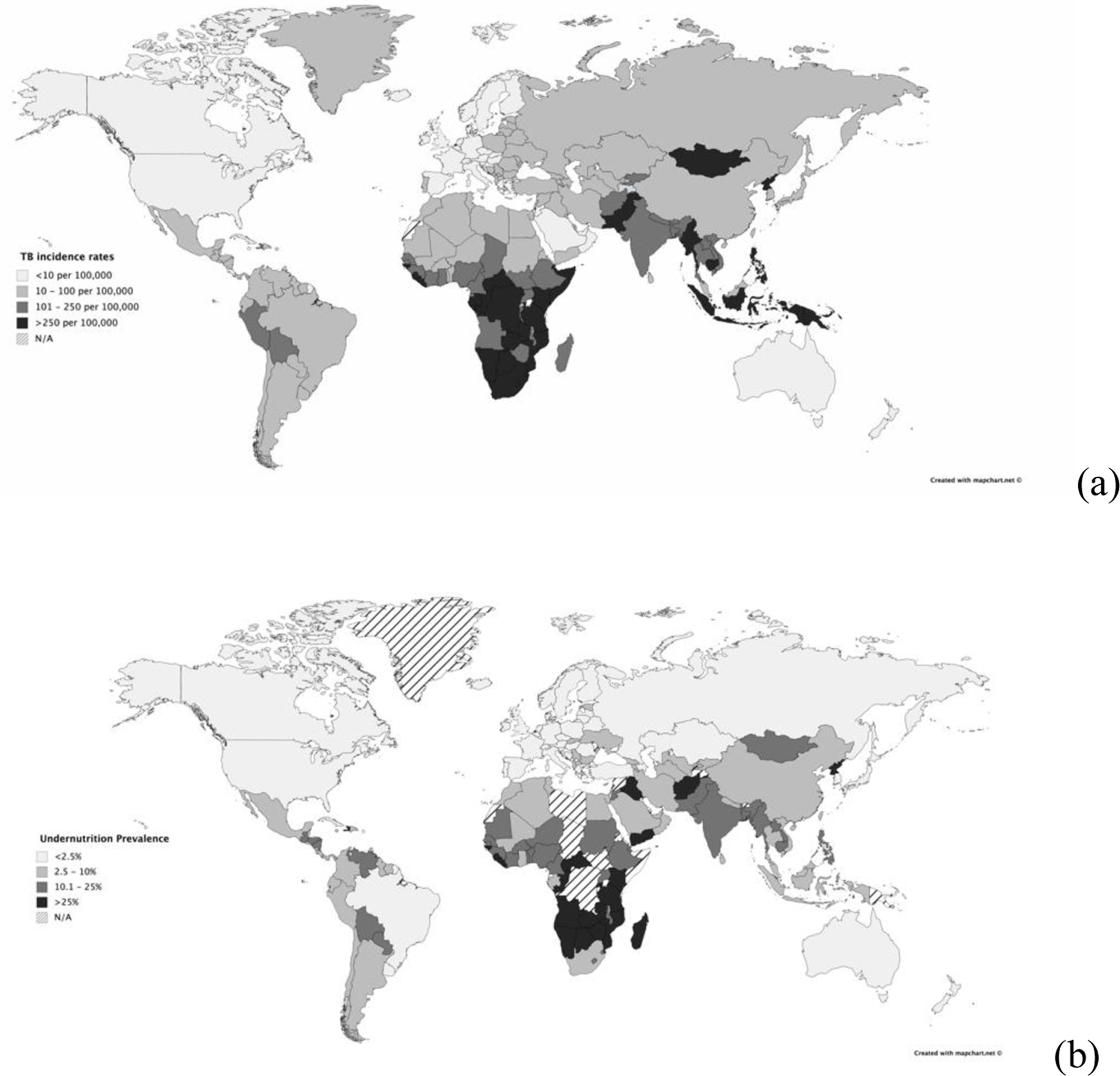
Representation of (a) Tuberculosis (TB) incidence rates and (b) Undernutrition prevalence globally which shows geographic overlap.1,4
In this Personal Views article, we discuss a diverse range of issues including nutrition-related immunocompromise, implications of undernutrition in PLWTB and vulnerable populations for TB treatment and prevention, nutritional supplementation, pharmacokinetics and pharmacodynamics (PK/PD) of anti-mycobacterial medications in undernourished PLWTB, and the role of social protection interventions in addressing undernutrition as a TB risk factor and develop a conceptual framework as well as a list of priority actions and recommendations.
TB Prevention
In India, the country with the highest number of PLWTB, the PAF for undernutrition in most states was more than 50%.10 For instance, in Puducherry, up to 61.5% of TB cases in women and 57.2% in men are attributable to undernutrition.11 High TB burden countries such as Vietnam, Ethiopia, Bangladesh, and Thailand also have PAFs for undernutrition that exceed 40%.10 A BMI less than 18.5 kg/m2 is considered to represent undernutrition although this anthropometric measure does not necessarily capture all nutrient deficiencies. A longitudinal study of adults in the United States from 1971–1992 found that a BMI<18.5 had an adjusted hazard ratio of 12.43 (CI: 95% CI: 5.75, 26.95) for developing TB disease.12 A systematic review of six cohort studies comprising approximately 2.6 million individuals found a consistent log-linear relationship between BMI and TB incidence with a 14% decrease in TB incidence with every unit increase of BMI.13 This would suggest that an improvement in BMI of 3.5 kg/m2 for an undernourished individual would almost halve the risk of incident TB. On an individual level, this would be similar to the effect of the M72/AS01E vaccine and could potentially be accompanied by other positive externalities such as prevention of stunting, decreased metabolic dysregulation, and improved economic productivity.14–16
Undoubtedly, weight loss is a cardinal symptom of TB and analyses must consider reverse causality. However, there is ample historical evidence that highlights the impact of undernutrition on TB. This is illustrated by a World War II study of prisoners of war in German concentration camps.17 The British prisoners received nutritional supplementation from the Red Cross and had twice the caloric intake of Soviet prisoners, including thirty extra grams of animal protein. Despite similar living conditions, Soviet soldiers had an almost sixteen-fold higher incidence of TB, were more likely to suffer cavitary disease, and had a higher six-month mortality. There is additional evidence from numerous countries, as previously reviewed, to support the conclusion that undernutrition is an important cause of TB, not merely an effect of the disease.18–20
The End-TB strategy is banking on the development of a TB vaccine by 2025 to accelerate the decline in TB incidence. In animal and human studies, undernutrition has blunted immune response to the bacillus Calmette-Guerin (BCG) vaccine and may also inform the success of novel TB vaccines by blunting the immune response.7 This suggests addressing undernutrition may be important for the success of novel candidate TB vaccines as well.
Basic and translational research is needed to understand the immunologic pathways by which macronutrient and micronutrient deficiency increase the risk and severity of TB disease. Clinical research is necessary to ascertain vaccine effectiveness and optimal vaccine dosing in undernourished individuals. Additionally, the value of nutritional supplementation in concert with vaccination for severely undernourished individuals should also be explored.
Nutritional Supplementation
PLWTB tend to be among the most impoverished worldwide. TB therefore overlaps with food insecurity which also can increase the risk of adverse health outcomes.21 Undernourished PLWTB have more extensive disease, worse treatment outcomes, and higher relapse rates.22,23 Despite appropriate therapy, undernourished individuals are also at a higher risk of mortality.24 In India, which bears a quarter of the global TB burden, the median BMIs of men and women with TB are 16 and 15, respectively.24 Similarly, children in Cote d’Ivore with TB have been reported to have weight-for-age Z-score less than −3 which placed them in the bottom 0.27 percentile in terms of undernutrition.25
An unpublished study of 24 hour-dietary recall data from 173 sputum-positive PLWTB in Puducherry, India was presented at the symposium. The findings showed striking gaps in nutrition: 87% of PLWTB were deficient in calories, 77% had deficient protein intake, and 23% had diets deficient in iron.26 These findings mirror data from other high burden countries like China.27
A Cochrane review found insufficient evidence for earlier sputum clearance or decreased mortality with macronutrient supplementation.28 However, the included studies had heterogeneous interventions and were underpowered. Furthermore, it is unclear whether the nutritional supplementation was adequate. Only two of the six included studies confirmed that their intervention improved caloric intake, and in neither study did the daily caloric intake exceed 2500 kcal/day (the average recommended requirement for uninfected individuals).29–31 Currently, the Reducing Activation of Tuberculosis by Improvement Of Nutritional Status (RATIONS) study, a large cluster randomized control trial, is studying the impact of nutritional intervention on TB treatment outcomes as well as the risk of TB reactivation among household contacts in Indian communities with a high burden of undernutrition.32 To be sure, severe undernutrition as a common and serious comorbidity among PLWTB, which should be recognized and treated regardless of its impact on TB based on existing WHO guidleines.31
We need clinical studies to understand nutritional requirements of PLWTB and optimal trajectory of weight change during TB therapy, particularly in pregnant women and children. Further, given the paucity of existing evidence, we must quantify the benefits of nutritional interventions on tuberculosis rates, treatment adherence, treatment outcomes, and functional recovery. To avoid methodologic shortcomings of previous studies, these clinical studies must be adequately powered and closely monitor caloric intake of study participants. It is also important to understand off-target effects of nutritional interventions such as increased economic productivity, resumption of employment or education, need for distress financing, decreased stunting in children, and decreased risk of other bacterial infections. Operational research will be crucial to develop sustainable systems for providing locally-acceptable nutritional supplements and to integrate care for TB and undernutrition using models implemented for integrated HIV and TB care.
Diagnosis and Screening
Increased risk for TB disease and poor treatment outcomes are likely associated with the impact of undernutrition on macrophage activity and phagocytosis, antigen presentation, and induction of the Th1 immune response.7 Consequently, current diagnostic tests for TB infection such as interferon-gamma release assays (IGRA) and tuberculin skin testing (TST) may have reduced sensitivity in severely undernourished individuals, although conflicting data exist.33,34 Blood transcriptomic biomarkers are being evaluated as surrogate predictors of disease.35 Promisingly, based on unpublished data from the Regional Prospective Observational Research in TB (RePORT) India consortium, undernutrition does not alter most validated TB gene signatures in distinguishing TB disease from latent infection.36 Currently, the TB LION (Learning the Impact Of Nutrition) study, also based in India, is assessing the use of surrogate transcriptomic biomarkers to determine whether nutritional supplementation and deworming will reduce TB progression and ameliorate the immune response in household contacts.37 These studies have the potential of providing a predictive risk signature for targeted TB prophylaxis and to evaluate the impact of nutritional interventions on these signatures.
Clinical and translational research is necessary to calibrate novel diagnostics to identify latent, sub-clinical, and active TB in severely undernourished individuals. We also need prospective clinical data to understand how undernutrition may alter an individual’s transition through subclinical, incipient, and clinical phases of TB disease. Blood transcriptomic signatures should be validated in undernourished populations to allow for targeted TB prophylaxis. Epidemiological studies can help us understand the value of bi-directional screening for undernutrition and TB.
TB Treatment
In some settings, suboptimal pharmacokinetics and pharmacodynamics (PK/PD) of TB medications have been the most important contributors to poor TB treatment outcomes including death, delayed microbiological cure, and acquired drug resistance.38,39 Undernutrition is a significant risk factor for individual PK variability due to altered body composition. The intestinal absorptive area is decreased in PLWTB which has the potential to impact drug absorption.40 Conversely, since malnutrition decreases fat-free mass, it can also result in supratherapeutic doses of drugs like ethambutol and aminoglycosides and increase the risk of drug toxicity.41 PK variability may be particularly harmful in malnourished children. For example, in an Indian study of 84 children with HIV/TB, stunted and underweight children had significantly lower serum peak concentrations of rifampin and isoniazid, the most bactericidal drugs in a first-line regimen.42Similarly, in a Tanzanian study of 51 HIV-negative children, undernutrition was significantly associated with lower estimated peak concentrations of rifampin and isoniazid in multivariate analyses. Importantly, children who received newer WHO-recommended doses had increased concentrations, but the majority of children still had peak concentrations below target range.43
In a separate cohort from rural Tanzania, where the median BMI-for-age Z-score was −2.0 (IQR 2.3), full PK exposure was measured in 51 children and of those with a calculable total serum area under the concentration curve (AUC), rifampin was at or above target in 35%, and for isoniazid, in only 4%.44 PK variability was more frequent among the most undernourished children and despite aggressive treatment of malnutrition in all, mortality remained high, with 8 of 9 deaths (89%) occurring in children with pretreatment BMI-for-age Z-score of −2.0 or below (Figure 2). These findings highlight the challenges in weight-based drug dosing in undernourished individuals and the importance of understanding whether early nutritional supplementation can improve PK exposure.
Figure 2: Malnourished Tanzanian children with tuberculosis have altered rifampin dose-response and increased mortality.
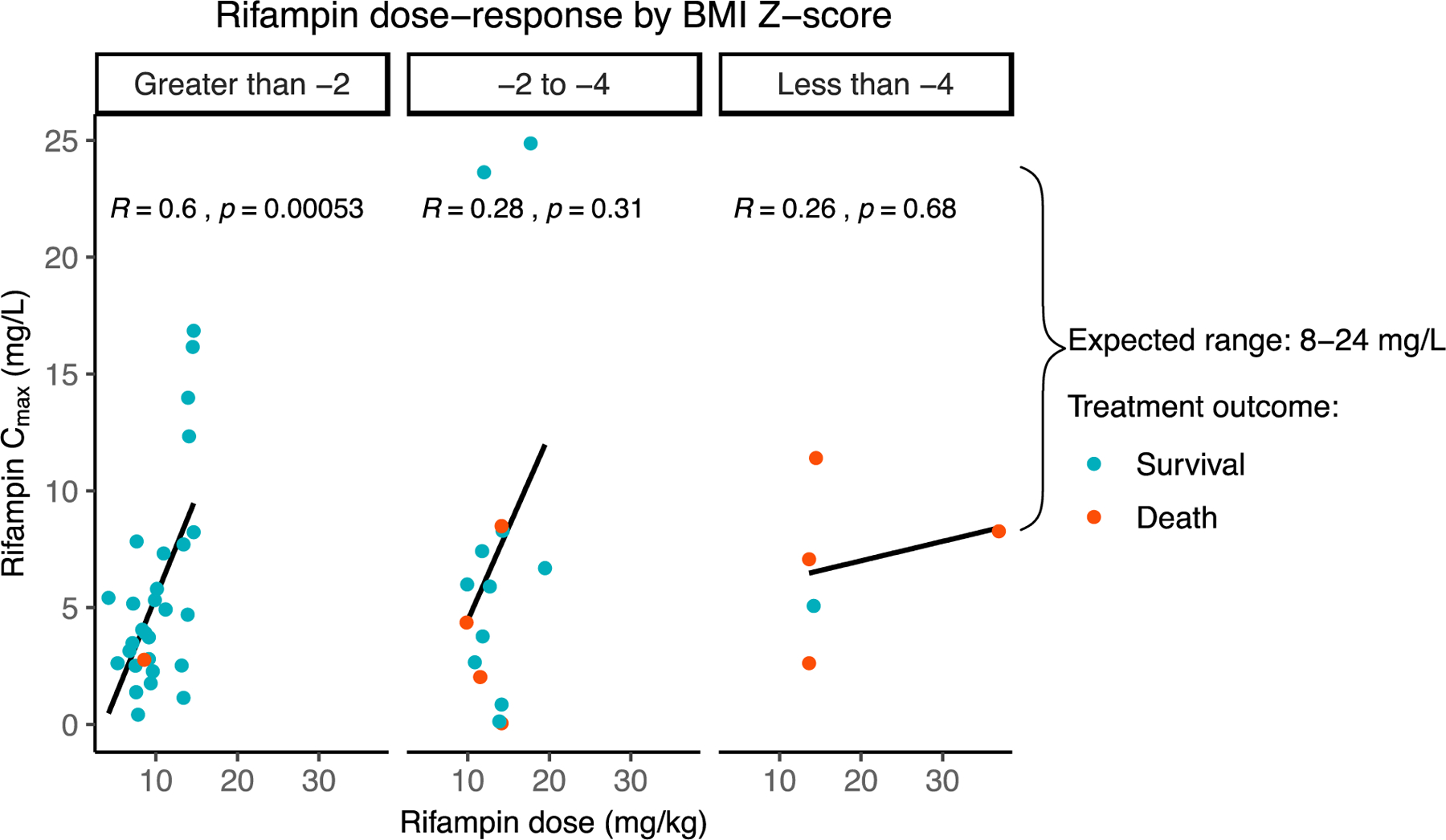
Scatterplot of peak rifampin concentration versus rifampin dose among 51 pediatric PLWTB in Haydom, Tanzania with body mass index BMI z-scores less than −2, −2 to −4, and less than −4, with greater pharmacokinetic variability in those with lower BMI z-scores. Mortality is indicated in red: 9/51 (17%) died during treatment, with 8/9 (89%) deaths occurring in PLWTB with baseline BMI Z-score less than −2.36 (Cmax: peak concentration; BMI: body mass index; PLWTB: persons living with TB)
Another way in which nutritional supplementation may impact drug exposure is through improved adherence. In Senegal, PLWTB who received deliveries of food baskets and ready-to-use therapeutic foods which cost less than a dollar a day achieved 95% adherence to both antiretroviral and antimycobacterial therapy as well as reduction in food insecurity and improvement in nutritional status.45 In India, PLWTB who received nutritional supplementation that cost $10 per month were less likely to disengage with care than those who did not receive supplementation. Only one person (1%) was lost to follow up in the supplementation arm as compared to 41 individuals (10%) in the no supplementation arm.46
Larger clinical studies are needed to understand the impact of undernutrition on the PK/PD of first and second-line antimycobacterial drugs in adults and children and to correlate PK/PD with clinical outcomes. Studies should also assess if therapeutic drug monitoring-- the measurement of serum drug concentrations -- with subsequent correction of suboptimal PK exposure through improved dosing can improve TB treatment outcomes and decrease toxicity in undernourished individuals. Strategies have been proposed for the operational research needed to scale up this form of personalized dosing including the use of novel drug concentration measurement assays that are more accessible in TB-endemic settings.47 Lastly, behavioral economic studies might help us weave nutritional supplementation into TB therapy to improve treatment adherence and completion.
Catalyzing Policy Change and Improving Implementation
Although guidelines for nutritional care of PLWTB exist, knowledge gaps persist about the specific dietary needs of PLWTB. 31,48 Further, it is unclear how many TB cases nutritional interventions can prevent in those with latent TB infection and undernutrition. Randomized-controlled studies are needed to understand the causal effects of nutritional intervention on TB-risk and treatment outcomes. Even in high-burden countries, TB disease is relatively rare in the general population. Consequently, very large studies of nutritional interventions are needed to avoid type II errors. Further RCTs are expensive, difficult, and sometimes ethically challenging given questionable equipoise and vulnerability of study participants.49
In the meantime, modeling studies have the capacity to draw on existing data to evaluate potential impacts of a chosen set of intervention scenarios that policymakers may be interested in exploring for their specific setting. For instance, a mathematical model projected that even modest reductions in undernutrition in the central-eastern states of India by implementing country level nutrition programs such as the prevention and treatment of moderate malnutrition program in pre-school age children could reduce TB incidence by approximately 4.8 million (95% uncertainty range, 0.5 million–17.1 million) over 20 years.50 TB models consistently suggest that the End-TB goals will not be achieved unless we focus on both prevention and treatment success. 51,52 Implementing broad nutritional policies both for the general population and for PLWTB is crucial to global TB elimination efforts, particularly in countries like India, China, and Indonesia with low HIV prevalence where undernutrition is the major driver of the TB epidemic.
The Social PROTection to Enhance the Control of Tuberculosis (S-PROTECT) group has developed a generalizable mathematical model to study the impact of social protection initiatives on national TB burden by improving nutrition.53 The S-PROTECT model uses published data to look at the impact of cash transfers on intermediary factors such as socioeconomic situation, health service access, and food security, and in turn the impact of these factors on proximal determinants of health including undernutrition. Finally, it accounts for the impact of improved nutrition on TB progression, diagnosis, and treatment success. Parametrized to the Brazilian setting, the S-PROTECT model showed that in the long term, cash transfers may reduce TB prevalence by 4% (95% uncertainty range, −1% to −24%) via the pathway of improved household income leading to reduced undernutrition.
The S-PROTECT investigators are now creating models that simultaneously estimate the effect of cash transfers via other distal factors such as food insecurity. This is expected to provide a more complete picture of the potential contribution of cash transfers to TB control. It will also enable the assessment of the interdependence of some of targets under Sustainable Development Goals (SDG) 1 (No poverty) and SDG2 (Zero hunger) and elucidate how they can synergistically support TB elimination globally.
Well-intentioned nutritional policies do not necessarily translate into tangible benefit. For instance, the Indian targeted public distribution system (TPDS), a government-run welfare program that provides food rations to impoverished Indian households, has been criticized for inefficiencies in procurement, storage, and distribution of food grains; diversion of food grains; and inadequate coverage of the most vulnerable households.54 Attempts to replace subsidized food rations through the TPDS direct cash transfers have been unsuccessful in parts of India due to delayed payments, technical glitches, food price volatility, and poor access to functional markets.55 Hurdles experienced by these programs highlight the need for implementation research to ensure that intended beneficiaries receive—either through cash or kind—adequate nutritious and locally acceptable supplements to curb the TB epidemic.
Mathematical modeling studies are needed to identify the most impactful and cost-effective nutritional interventions to prevent TB reactivation, increase treatment success rates, improve functional recovery, and decrease TB deaths as well as TB recurrence. Implementation research is crucial to help nutritional interventions policies attain their goals.
Conclusion
The connection between undernutrition and TB has been described for more than a century, but much remains to be learned and even more to be done. Here, we have covered key upstream and downstream factors that lead to undernutrition-related TB disease, death, and morbidity and created a list of priority actions (Table 1). We have also created a conceptual framework that identifies entry points for intervention (Figure 3).
Table 1:
Research priorities to address key knowledge gaps related to undernutrition and TB.
| Priorities |
|---|
| TB Prevention |
| • Identify major immunologic pathways impaired by macronutrient and micronutrient deficiencies. |
| • Evaluate effectiveness of TB vaccines and determine optimal dosing in severely undernourished individuals |
|
|
| Nutritional supplementation |
| • Determine nutritional requirements of PLWTB and people at-risk-for TB, particularly in pregnant women and children |
| • Quantify impact of nutritional interventions on tuberculosis incidence, treatment outcomes, and functional recovery among undernourished populations |
| • Define optimal trajectory of weight change during TB therapy |
| • Develop sustainable systems for providing locally-acceptable nutritional supplements |
| • Integrate assessment and care for undernutrition with TB care |
|
|
| Diagnosis and Screening |
| • Evaluate impact of undernutrition on novel diagnostics to identify latent, sub-clinical, and active TB |
| • Determine how undernutrition alters the subclinical, incipient, and clinical phases of TB disease |
| • Validate blood transcriptomic signatures of TB risk in undernutrition for targeted prophylaxis |
| • Assess value of bi-directional screening for undernutrition and active TB disease |
|
|
| TB treatment augmentation |
| • Understand impact of undernutrition on PK/PD of first and second-line TB drugs |
| • Determine value of therapeutic drug monitoring in improving TB treatment outcomes and reducing toxicity in severely undernourished PLWTB |
| • Leverage nutritional supplements to improve adherence to TB drugs |
|
|
| Catalyzing Policy Change and Improving Implementation |
| • Model impact of socio-economic nutritional interventions on tuberculosis incidence, treatment outcomes, functional recovery, and financial health of communities using available epidemiological and economic data |
| • Optimize social welfare and benefit programs through implementation research |
| • Understand the impact of nutritional supplementation on the financial health of PLWTB |
| • Compare value and cost-effectiveness of cash transfers versus nutritional supplementation for different communities as well as the operational implications |
(TB: tuberculosis; PK/PD: pharmacokinetics and pharmacodynamics; IGRAs: interferon-γ release assays; TST tuberculin skin test)
Figure 3: Conceptual framework of undernutrition as it relates to tuberculosis.
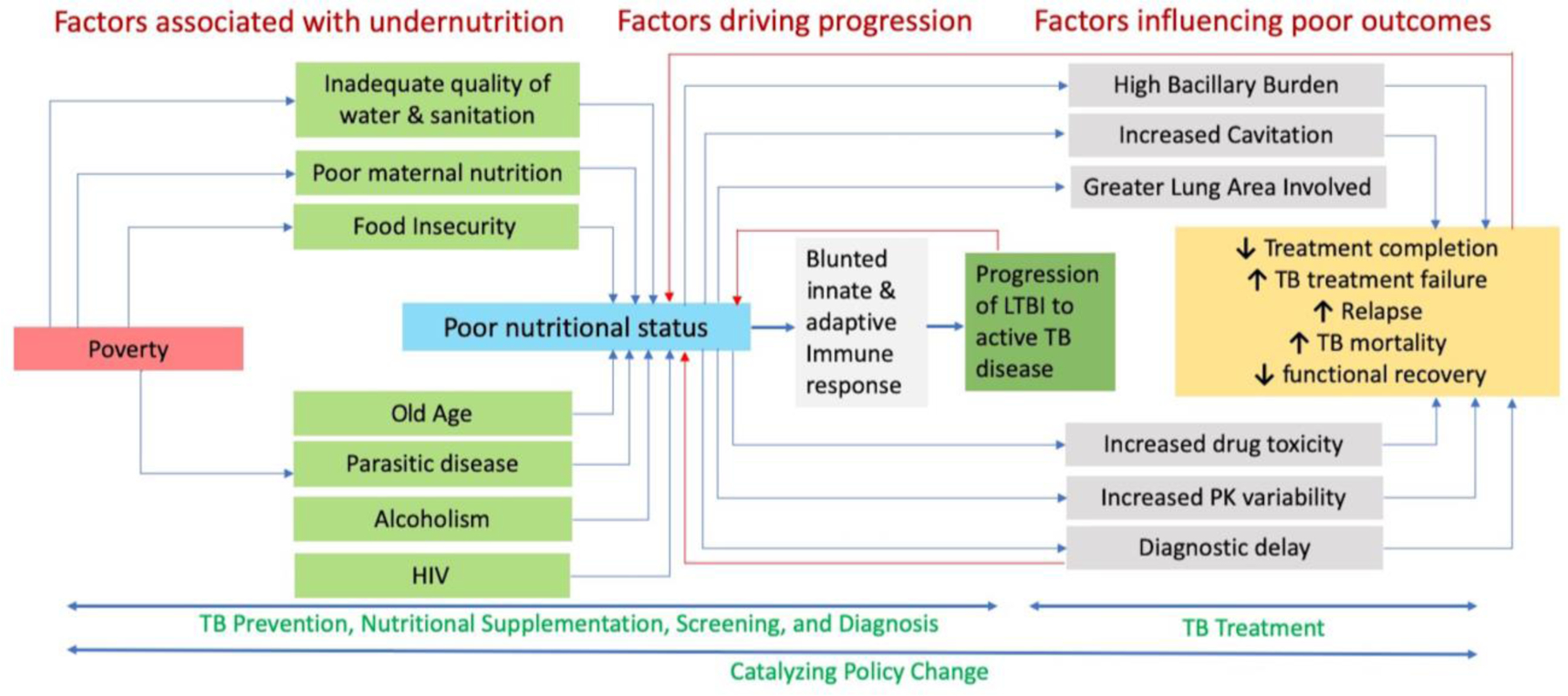
Numerous social determinants of health and diseases contribute to an undernourished state which can then increase the risk of TB disease, increase severity of TB disease, and increase risk of poor outcomes. Several factors included in the conceptual framework such as poverty, HIV, age, parasites, and alcoholism influence disease progression and treatment outcomes independently of nutrition. For clarity, these effects have not been depicted in the schematic diagram. (TB: tuberculosis; HIV: human immunodeficiency virus; PK: pharmacokinetics; LTBI; latent tuberculosis infection)
The magnitude of the problem is enormous. Left unaddressed, widespread undernutrition will thwart our efforts to end the TB epidemic. Although there are numerous important scientific questions that remain unaddressed with respect to undernutrition and its connection with TB, effective implementation remains a major challenge that we must surmount.
It is time for governments, non-governmental organizations, philanthropic foundations, and researchers to be invigorated by the challenge, not daunted by it. A rapid scale up in funding and research is needed. The same funding streams, scientific ingenuity, and international cooperation that brought us novel TB drugs and diagnostics must now be leveraged to mitigate this key accelerant of the TB epidemic. The payoff may be monumental. Investments in eradicating undernutrition will likely have long-reaching benefits far beyond their impact on TB and can be transformative, especially for the global South.
A Haitian saying provides us food for thought: “giving people medicine for TB and not giving them food is like washing your hands and drying them in the dirt.”56
Footnotes
Conflicts of Interest:
We have no conflicts of interest.
References:
- 1.WHO. Global Tuberculosis Report 2019 Geneva: WHO; 2019. [Google Scholar]
- 2.Lönnroth K, Raviglione M. The WHO’s new End TB Strategy in the post-2015 era of the Sustainable Development Goals. Trans R Soc Trop Med Hyg 2016; 110(3): 148–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harding E WHO global progress report on tuberculosis elimination. Lancet Respir Med 2020; 8(1): 19. [DOI] [PubMed] [Google Scholar]
- 4.Bhargava A. Undernutrition, nutritionally acquired immunodeficiency, and tuberculosis control. BMJ 2016; 355: i5407. [DOI] [PubMed] [Google Scholar]
- 5.Beisel WR. Nutrition and immune function: overview. The Journal of nutrition 1996; 126(10): 2611S–5S. [DOI] [PubMed] [Google Scholar]
- 6.FAO, IFAD, UNICEF, WFP and WHO. The state of food security and nutrition in the world Rome: FAO; 2019. [Google Scholar]
- 7.Sinha P, Davis J, Saag L, et al. Undernutrition and tuberculosis: public health implications. J Infect Dis 2019; 219(9): 1356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers SS, Smith MR, Guth S, et al. Climate change and global food systems: potential impacts on food security and undernutrition. Annu Rev Public Health 2017; 38: 259–77. [DOI] [PubMed] [Google Scholar]
- 9.Naja F, Hamadeh R. Nutrition amid the COVID-19 pandemic: a multi-level framework for action. European Journal of Clinical Nutrition 2020. 10.1038/s41430-020-0634-3 [DOI] [PMC free article] [PubMed]
- 10.Bhargava A, Benedetti A, Oxlade O, Pai M, Menzies D. Undernutrition and the incidence of tuberculosis in India: National and subnational estimates of the population attributable fraction related to undernutrition. Natl Med J India 2014; 27(3): 128–33. [PubMed] [Google Scholar]
- 11.Hochberg NS, Sarkar S, Horsburgh CR, Jr., et al. Comorbidities in pulmonary tuberculosis cases in Puducherry and Tamil Nadu, India: Opportunities for intervention. PLoS One 2017; 12(8): e0183195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cegielski JP, Arab L, Cornoni-Huntley J. Nutritional Risk Factors for Tuberculosis Among Adults in the United States, 1971–1992. Am J Epidemiol 2012; 176(5): 409–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lönnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol 2009; 39(1): 149–55. [DOI] [PubMed] [Google Scholar]
- 14.Tait DR, Hatherill M, Van Der Meeren O, et al. Final Analysis of a Trial of M72/AS01E Vaccine to Prevent Tuberculosis. N Engl J Med 2019; 381(25): 2429–39. [DOI] [PubMed] [Google Scholar]
- 15.Hoddinott J, Maluccio JA, Behrman JR, Flores R, Martorell R. Effect of a nutrition intervention during early childhood on economic productivity in Guatemalan adults. Lancet; 371(9610): 411–6. [DOI] [PubMed] [Google Scholar]
- 16.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AA. The impoverished gut—a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol 2013; 10(4): 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leyton G Effects of slow starvation. Lancet 1946; 2(6412): 73–9. [PubMed] [Google Scholar]
- 18.Cegielski J, McMurray D. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis 2004; 8(3): 286–98. [PubMed] [Google Scholar]
- 19.Faber K. Tuberculosis and nutrition. Acta Tuberc Scand 1938; 12: 306–9. [Google Scholar]
- 20.Leitch I, Westwater J, Keers R, Ritchie J, Chalmers C. Diet and Tuberculosis. Proc Nutr Soc 1945; 3: 155–94. [DOI] [PubMed] [Google Scholar]
- 21.Hargreaves JR, Boccia D, Evans CA, Adato M, Petticrew M, Porter JD. The social determinants of tuberculosis: from evidence to action. Am J Public Health 2011; 101(4): 654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoyt KJ, Sarkar S, White L, et al. Effect of malnutrition on radiographic findings and mycobacterial burden in pulmonary tuberculosis. PloS One 2019; 14(3): e0214011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan A, Sterling TR, Reves R, Vernon A, Horsburgh CR, Consortium TT. Lack of weight gain and relapse risk in a large tuberculosis treatment trial. Am J Respir Crit Care Med 2006; 174(3): 344–8. [DOI] [PubMed] [Google Scholar]
- 24.Bhargava A, Chatterjee M, Jain Y, et al. Nutritional status of adult patients with pulmonary tuberculosis in rural central India and its association with mortality. PloS One 2013; 8(10): e77979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auld AF, Tuho M, Ekra K, et al. Tuberculosis in human immunodeficiency virus-infected children starting antiretroviral therapy in Cote d’Ivoire. Int J Tuberc Lung Dis 2014; 18(4): 381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarkar S Dietary intake and nutritional status of TB patients in Puducherry, South India. Oral presentation at 50th World Conference on Lung Health; 2019 Oct 29-Nov 2; Hyderabad, India. [Google Scholar]
- 27.Ren Z, Zhao F, Chen H, et al. Nutritional intakes and associated factors among tuberculosis patients: a cross-sectional study in China. BMC Infect Dis 2019; 19(1): 907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grobler L, Nagpal S, Sudarsanam TD, Sinclair D. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst Rev 2016; (6). [DOI] [PMC free article] [PubMed]
- 29.Paton NI, Chua Y-K, Earnest A, Chee CB. Randomized controlled trial of nutritional supplementation in patients with newly diagnosed tuberculosis and wasting. Am J Clin Nutr 2004; 80(2): 460–5. [DOI] [PubMed] [Google Scholar]
- 30.Sudarsanam T, John J, Kang G, et al. Pilot randomized trial of nutritional supplementation in patients with tuberculosis and HIV–tuberculosis coinfection receiving directly observed short-course chemotherapy for tuberculosis. Trop Med Int Health 2011; 16(6): 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO. Guideline: nutritional care and support for patients with tuberculosis Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 32.CTRI. The Reducing Activation of Tuberculosis by Improvement Of Nutritional Status study (RATIONS)[Internet] CTRI; 2019[cited2019 Dec 31]. http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=34822&EncHid=&userName=Anurag%20Bhargava [Google Scholar]
- 33.Rytter MJH, Kolte L, Briend A, Friis H, Christensen VB. The immune system in children with malnutrition—a systematic review. PloS One 2014; 9(8): e105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas TA, Mondal D, Noor Z, et al. Malnutrition and helminth infection affect performance of an interferon gamma-release assay. Pediatrics 2010; 126(6): e1522–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leong S, Zhao Y, Joseph NM, et al. Existing blood transcriptional classifiers accurately discriminate active tuberculosis from latent infection in individuals from south India. Tuberculosis 2018; 109: 41–51. [DOI] [PubMed] [Google Scholar]
- 36.Johnson WE. Optimising Parsimonious Gene Signatures Defining the Spectrum of Tuberculosis Infection. 50th Union World Conference on Lung Health; 2019; Hyderabad, India; 2019 Oct 29-Nov 2. [Google Scholar]
- 37.NIH. Tuberculosis-Learning the Impact of Nutrition (TB-LION)[Internet] Bethesda: NIH; 2018[updated2019 Aug 1; cited2019 Dec 21]. Available from: https://clinicaltrials.gov/ct2/show/NCT03598842 [Google Scholar]
- 38.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 2013; 208(9): 1464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alffenaar JC, Gumbo T, Dooley KE, et al. Integrating pharmacokinetics and pharmacodynamics in operational research to End TB. Clin Infect Dis 2019. 10.1093/cid/ciz942. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 40.Pinheiro VG, Ramos L, Monteiro HS, et al. Intestinal permeability and malabsorption of rifampin and isoniazid in active pulmonary tuberculosis. Braz J Infect Dis 2006; 10(6): 374–9. [DOI] [PubMed] [Google Scholar]
- 41.Ter Beek L, Alffenaar J-WC, Bolhuis MS, van der Werf TS, Akkerman OW. Tuberculosis-Related Malnutrition: Public Health Implications. J Infect Dis 2019; 220(2): 340–1. [DOI] [PubMed] [Google Scholar]
- 42.Ramachandran G, Hemanth Kumar A, Bhavani P, et al. Age, nutritional status and INH acetylator status affect pharmacokinetics of anti-tuberculosis drugs in children. Int J Tuberc Lung Dis 2013; 17(6): 800–6. [DOI] [PubMed] [Google Scholar]
- 43.Justine M, Yeconia A, Nicodemu I, et al. Pharmacokinetics of First-Line Drugs Among Children With Tuberculosis in Rural Tanzania. J Pediat Inf Dis Soc 2018. [DOI] [PMC free article] [PubMed]
- 44.NIH. Diagnostics and Pharmacotherapy for Severe Forms of TB (DMID 15–0100)[Internet] Bethesda: NIH;2018[updated2019 Jun 13; cited2019 Dec 21]. Available from: https://clinicaltrials.gov/ct2/show/NCT03559582 [Google Scholar]
- 45.Benzekri NA, Sambou JF, Tamba IT, et al. Nutrition support for HIV-TB co-infected adults in Senegal, West Africa: A randomized pilot implementation study. PLOS ONE 2019; 14(7): e0219118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samuel B, Volkmann T, Cornelius S, et al. Relationship between Nutritional Support and Tuberculosis Treatment Outcomes in West Bengal, India. Journal of tuberculosis research 2016; 4(4): 213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alffenaar JC, Heysell SK, Mpagama SG. Therapeutic Drug Monitoring: The Need for Practical Guidance. Clin Infect Dis 2019; 68(6): 1065–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Bank. Scaling Up Nutrition: A framework for action Washington, DC: World Bank; 2011. [Google Scholar]
- 49.Roth D An ethics-based approach to global child health research. Paediatr Child Health 2003; 8(2): 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oxlade O, Huang C-C, Murray M. Estimating the impact of reducing under-nutrition on the tuberculosis epidemic in the central eastern states of India: a dynamic modeling study. PloS One 2015; 10(6): e0128187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Houben RM, Menzies NA, Sumner T, et al. Feasibility of achieving the 2025 WHO global tuberculosis targets in South Africa, China, and India: a combined analysis of 11 mathematical models. Lancet Glob Health 2016; 4(11): e806–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vesga JF, Hallett TB, Reid MJ, et al. Assessing tuberculosis control priorities in high-burden settings: a modelling approach. The Lancet Global Health 2019; 7(5): e585–e95. [DOI] [PubMed] [Google Scholar]
- 53.Boccia D, Rudgard W, Shrestha S, et al. Modelling the impact of social protection on tuberculosis: the S-PROTECT project. BMC Public Health 2018; 18(1): 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhattacharya S, Falcao VL, Puri R. The public distribution system in India: policy evolution and program delivery trends. In: Alderman H, Gentilini U, Yemtsov R. The 1.5 Billion People Question: Food, Vouchers, or Cash Transfers? World Bank; 2017. p. 43–105. [Google Scholar]
- 55.Byatnal A India is Confronting a Cash Transfer Failure [Internet] Malnutrition Deeply; 2018. [cited2020 Jan 22] Available from: https://www.newsdeeply.com/malnutrition/articles/2018/05/10/india-is-confronting-a-cash-transfer-failure [Google Scholar]
- 56.Kidder T Mountains beyond mountains: The Quest of Dr. Paul Farmer, a Man Who Would Cure the World. New York. Random House Incorporated; 2003. p.30


