Abstract
Aims:
The Fibrosis-4 Index (FIB-4) and NAFLD fibrosis score (NFS) are non-invasive and accessible methods for assessing advanced liver fibrosis risk in primary care. We evaluated the distribution of FIB-4 and NFS scores in primary care patients with clinical signals for non-alcoholic fatty liver disease (NAFLD).
Materials and Methods:
This retrospective cohort study of electronic record data between 2007–2018 included adults with at least one abnormal aminotransferase and no known (non-NAFLD) liver disease. We calculated patient-level FIB-4 and NFS scores, the proportion of patients with mean values exceeding advanced fibrosis thresholds (indeterminate risk: FIB-4>1.3, NFS>−1.455; high-risk: FIB-4>2.67, NFS>0.676), and the proportion of patients with a NAFLD ICD-9/10 code. Logistic regression models evaluated the associations of metabolic syndrome components with elevated FIB-4 and NFS scores.
Results:
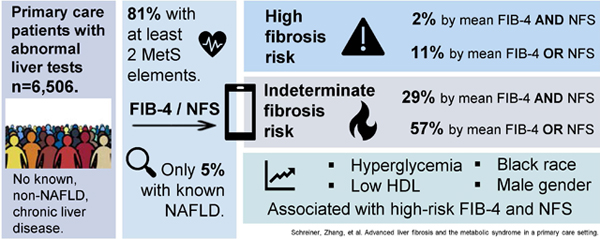
The cohort included 6,506 patients with a median of 6 (IQR: 3–13) FIB-4 and NFS scores per patient. Of these patients, 81% had at least 2 components of metabolic syndrome, 29% had mean FIB-4 and NFS scores for indeterminate fibrosis risk, and 11% had either mean FIB-4 or NFS scores exceeding the high advanced fibrosis risk thresholds. Regression models identified associations of low HDL, hyperglycemia, Black race, and male gender with high-risk FIB-4 and NFS values. Only 5% of patients had existing diagnoses for NAFLD identified.
Conclusions:
Many primary care patients have FIB-4 and NFS scores concerning for advanced fibrosis, but rarely a diagnosis of NAFLD. Elevated FIB-4 and NFS scores may provide signals for further clinical evaluation of liver disease in primary care settings.
Keywords: non-alcoholic fatty liver disease, non-invasive testing, fibrosis, cirrhosis, hepatology, FIB-4, NFS
Graphical Abstract
Introduction
Non-alcoholic fatty liver disease (NAFLD) affects an estimated 25% of the global population, and is a leading cause of cirrhosis, hepatocellular carcinoma, and liver transplantation.1–5 NAFLD is associated with obesity, diabetes mellitus, hypertension, and dyslipidemia.1,5–7 More than just a manifestation metabolic syndrome (MetS), recent evidence suggests NAFLD increases the incident risk of MetS, diabetes, and cardiovascular disease.8-10Despite the close relationship to these primary care conditions, NAFLD is underdiagnosed in primary care.11–16 Broad screening recommendations for at-risk patients are conflicting, but multiple guidelines address the importance of NAFLD detection in patients with abnormal liver tests (with NAFLD the most common cause of aminotransferase abnormality) and those with diabetes.1,5,17–22
Fibrosis is the result of advanced NAFLD liver injury and accurate identification is critical, because fibrosis itself predicts adverse outcomes.16,23,24 The prevalence of NAFLD-associated fibrosis has surged, with a more than 2-fold increase over the past 2 decades, and is associated with increased mortality, liver cancer, and liver transplantation.25,26 While liver biopsy is the best method for detecting fibrosis, its cost and invasiveness limit its utility for such a large population.27 Measurement of liver stiffness (i.e. with transient elastography) has proven useful in this regard, but the familiarity and accessibility of the technology currently limit its use in the primary care setting.28
Non-invasive blood testing addresses the need for accessible NAFLD fibrosis risk stratification.29–31 The Fibrosis 4 index (FIB-4) and the NAFLD fibrosis score (NFS), non-invasive tests that combine age and commonly available biochemical lab results, stand out for their ability to exclude advanced fibrosis (Metavir stage 3 [F3] and stage 4 [F4]) in NAFLD patients.32–35 In a meta-analysis of non-invasive fibrosis assessment tools, FIB-4 and NFS exceeded all other non-imaging tests with summary areas under the ROC curve (AUROC) of 0.84 (for each) for detecting advanced fibrosis.30 In one study comparing FIB-4 to other non-invasive fibrosis markers, FIB-4 most effectively “ruled out” advanced fibrosis with values < 1.3 (negative predictive value 90%), and correctly identified F3-F4 fibrosis with values > 2.67 (positive predictive value 80%) in biopsy-proven NAFLD patients. Using these values as cutoffs, the absence (<1.3) or presence (>2.67) of advanced fibrosis was identified with 89% accuracy.34 In a meta-analysis of 13 studies and 3,064 patients, the NFS had an AUROC of 0.85 for detecting F3 or greater fibrosis. Specifically, an NFS value of −1·455 had a sensitivity of 90% and a negative likelihood ratio (LR) of 0.17 for advanced fibrosis, and an NFS of 0.676 had a specificity of 97% and a positive LR of 20.3 for advanced fibrosis.36–38
Applying FIB-4 and NFS to primary care patients with NAFLD can reliably exclude advanced fibrosis (FIB-4 < 1.3, NFS < −1.455), and reduce unnecessary further testing and hepatology referrals.39–43 Conversely, non-invasive risk scores above these limits can identify patients in need of confirmatory testing (e.g. elastography) and consultation with a hepatology specialist. Unfortunately, many primary care patients with NAFLD remain undiagnosed.11,16 FIB-4 and NFS values concerning for advanced fibrosis in patients with elevated aminotransferases and no known liver disease may represent undiagnosed NAFLD, and elevated non-invasive fibrosis risk scores could provide a signal to improve the primary care diagnosis of NAFLD and other chronic liver diseases.
We hypothesized that in a sample of primary care patients with elevated aminotransferases and a high burden of MetS, many would have elevated FIB-4 (> 1.3) and NFS (> −1.455) values, likely reflecting unrecognized advanced NAFLD diagnoses. Therefore, we calculated FIB-4 and NFS scores in primary care patients with abnormal serum aminotransferases and no other known liver disease diagnoses. We analyzed the relationship between objective measures of MetS and elevated mean FIB-4 and NFS scores in this sample.
Materials and Methods
This retrospective study of electronic health record (EHR) data from a large patient-centered medical home (PCMH) between 2007 and 2018 analyzed a sample of patients with elevated aminotransferases for: (1) the distribution of patient-level FIB-4 and NFS scores and the proportion of patients with values greater than the indeterminate and high-risk thresholds for advanced fibrosis; and (2) demographic and clinical factors associated with non-invasive tests scores above these thresholds.34 The Institutional Review Board at the Medical University of South Carolina (MUSC) approved this study.
Study Population
All patients seen in the Internal Medicine PCMH at MUSC between January 1, 2007 and December 31, 2018 were evaluated. The practice conducts 32,000 patient visits yearly and delivers care to a diverse (39% non-white) adult (mean age 59 years) population with chronic and complex medical problems. The PCMH is a primary care clinic that utilizes an EHR fully integrated with the tertiary care academic medical center, allowing for primary care access to patient data from the emergency room and inpatient settings. Key patient-level variables included demographics, vital signs, laboratory test results, and International Classification of Diseases (ICD)-9 and −10 codes.
We constructed a sample of patients with at least one aminotransferase abnormality defined as: aspartate aminotransferase (AST) > 34 U/L, or alanine aminotransferase (ALT) > 45 U/L, or both. The ALT threshold, though too high to adequately detect all patients with NAFLD or advanced fibrosis, was chosen because it is the value designated as “abnormal” in the EHR at MUSC.44 We calculated FIB-4 and NFS scores for each liver test panel using the equations:45,46
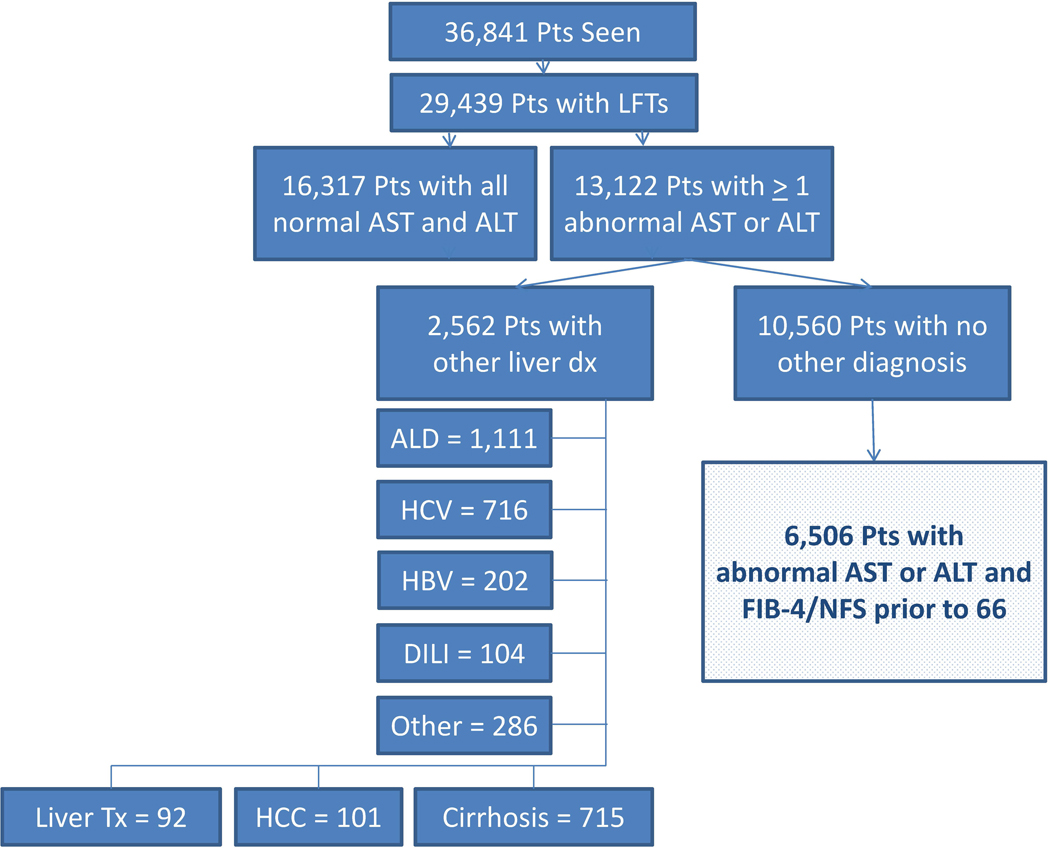
Both calculations used platelet count assessments from the same day as the liver tests when available, and the most recent prior platelet value otherwise. Each unique patient possessed a FIB-4 and NFS score for each liver test panel completed (normal or abnormal), provided there was a simultaneous or previous platelet assessment. For the NFS calculations, the most recently recorded BMI was used, and diabetes was assigned to those patients with hemoglobin A1c values equal to or exceeding 6.5% (measures of fasting glucose impairment were not included). Due to concerns of confounding and loss of specificity for FIB-4 and NFS in patients of advanced age, only those assessments gathered in patients prior to the age of 66 years were included (Figure 1).47–49
Figure 1.
Consort diagram of included patients
We excluded patients with diagnoses of chronic liver disease, hepatocellular carcinoma, history of liver transplantation, and cirrhosis by ICD-9/10 codes (Table S1).50,51 Patients with elevated aminotransferases and an ICD code for alcohol abuse were also excluded. Formal diagnoses of NAFLD included patients with at least one ICD-9 code of 571.8 or an ICD-10 code of K75.81 or K76.0.11
Outcomes
The primary outcomes of interest were the FIB-4 and NFS scores. We calculated functions (mean, median, maximum, and minimum) of patient-level scores and evaluated the proportion exceeding the thresholds for indeterminate (FIB-4 > 1.3, NFS > −1.455) and high-risk (FIB-4 > 2.67, NFS > 0.676) of advanced fibrosis. Patient-level mean FIB-4 and NFS scores surpassing the advanced fibrosis risk cutoffs served as the binary dependent variables in the logistic regression models.
Primary Independent Variables
The independent variables of greatest interest were those associated with obesity and MetS. Variables associated with MetS deviated from the classical metabolic syndrome criteria on account of administrative data availability, with us choosing to use body mass index (BMI) in place of waist circumference and glycosylated hemoglobin (A1c) in place of fasting glucose.The population was characterized by BMI as a binary variable (BMI > 30 kg/m2), and BMI was evaluated as a continuous variable in the logistic regression model. BMI values were the mean patient-level values during the study period. MetS elements were categorical variables defined by recordings of the following on at least one occasion during the study period: hyperglycemia (hemoglobin A1C ≥ 6.5%), low high-density lipoprotein (HDL < 40 mg/dL in men, HDL < 50 mg/dL in women), and hypertriglyceridemia (TG > 150 mg/dL). Elevated blood pressure required at least two readings greater than 130/85 mm Hg.1,5
Other Independent Variables
Gender was coded as Male / Female.
Race was categorically coded as Black / non-Black, owing to a small number of non-Black, non-White patients in the sample (n=281). The presence of alcohol, tobacco, and drug use were identified by an Elixhauser ICD-9/10 coding algorithm.52
Data Sources
All data came from Medical University Hospital Authority Enterprise and EPIC© (EPIC Systems Corporation, WI) Clarity databases. Clinical, laboratory, and demographic data were obtained in the ambulatory, emergency room, and inpatient settings at MUSC during the study period.
Analysis
The proportion of patients in the sample with an elevated mean FIB-4, mean NFS, or both were calculated. We also performed univariate analyses of patient-level mean FIB-4 and NFS scores by the components of the MetS, the proportion of patients with mean scores exceeding the fibrosis risk thresholds, and the proportion of patients receiving diagnostic codes for NAFLD. Multivariable logistic regression models for four dependent variables (mean FIB-4 > 1.3, mean FIB-4 > 2.67, mean FIB-4 > 1.3 and mean NFS > −1.455, and mean FIB-4 > 2.67 and mean NFS > 0.676) were developed. A model with NFS scores as the sole dependent variable was not utilized given the endogeneity of the primary variables of interest (including BMI and the presence of diabetes) and NFS calculation. The MetS variables were forced into the model based on clinical relevance.1 Interactions between obesity and elevated blood pressure and hyperglycemia were assessed at a significance level of 0.20. Sensitivity analyses, including development of regression models for the outcome of median summary scores were performed.
Statistical analyses were performed using SAS version 9.4 (Cary, NC).
Post-Hoc Analysis
After identifying patients with a mean FIB-4 and a mean NFS exceeding the threshold for high-risk of advanced fibrosis, we performed a chart review for a convenience sample of 25 randomly selected patients for evidence of prior abdominal imaging and the presence of steatosis or cirrhosis in the radiology report. We also sought alternative diagnoses (i.e. malignancy resulting in thrombocytopenia) that may have resulted in elevated FIB-4 and NFS scores.
Results
The 6,506 unique patients included had 73,067 liver test panel results, with a median of 6 (IQR: 3–13) values per patient during the study period (Figure 1, Table 1). Of these liver test panels, 27,019 had at least one abnormal aminotransferase value and the median number of abnormal liver panels per patient was 2 (IQR: 1–4). Platelet counts for non-invasive risk calculations were from the same day for 84% of scores and within the preceding 3 months for 90%. Of the sample, 97.6% had at least one component of metabolic syndrome, 81.1% had at least 2 elements, and 9.6% had all five components. Only 313 (4.8%) patients carried a NAFLD ICD-9/10 code.
Table 1:
Characteristics of patient sample (N=6,506)
| N (%) | |
|---|---|
| Demographics | |
| Age (years)* | |
| Mean | 53.8 (13.9) |
| Gender | |
| Female | 3,791 (58.3%) |
| Male | 2,715 (41.7%) |
| Race | |
| Black | 2,657 (40.8%) |
| Non-Black | 3,849 (59.2%) |
| Liver Tests | |
| LFT Panel Results** | 73,067 |
| Mean per patient (SD) | 11.2 (16.0) |
| Median per patient (IQR) | 6 (3–13) |
| LFT Panels with Abnormal | 27,019 |
| Mean per patient (SD) | 4.5 (8.2) |
| Median per patient (IQR) | 2 (1–4) |
| NAFLD Diagnoses † | 313 (4.8%) |
| MetS Components | |
| BP > 130/85 | 5,820 (89.5%) |
| A1c > 6.5% | 1,693 (26.0%) |
| Low HDL | 4,504 (69.2%) |
| Triglyceride > 150 | 2,729 (42.0%) |
| BMI > 30 | 3,025 (46.5%) |
| # of MetS Components | |
| 0 | 159 (2.4%) |
| 1 | 1,072 (16.5%) |
| 2 | 1,654 (25.4%) |
| 3 | 1,719 (26.4%) |
| 4 | 1,276 (19.6%) |
| 5 | 626 (9.6%) |
| Comorbidities‡ | |
| Smoking history | 2,813 (43.2%) |
| Drug Use | 530 (8.2%) |
Age is calculated by the patient’s last data point in the study period
total number of liver function test panel results, not the individual components (transaminases)
Pattern assigned on the first liver test abnormality.
NAFLD and NASH diagnosis codes (571.8, K75.81, or K76.0.
Comorbidities according to ICD-9/10 Elixhauser coding algorithm (38). LFT=liver function test; NAFLD=nonalcoholic fatty liver disease; MetS=metabolic syndrome; BP=blood pressure; HDL=high-density lipoprotein; BMI=body mass index (kg/m2)
Based on the patient-level mean FIB-4 scores, 37.3% patients had values exceeding 1.3, and 5.4% patients surpassed 2.67 (Table 2). Using the maximum scores, 38.1% of patients had no FIB-4 scores > 1.3. When applying the NFS, 48.5% of patients had mean values above −1.455, and 8.0% had mean values greater than 0.676. With the maximum NFS scores, 31.2% of patients had no values greater than −1.455. When combining the fibrosis risk assessment tools, 56.9% of patients had either a mean FIB-4 or mean NFS above the indeterminate risk threshold, and 28.9% had both a mean FIB-4 and mean NFS exceeding indeterminate risk. Using maximum values, 24.0% never had an elevated FIB-4 or NFS score.
Table 2:
The proportion of patients with non-invasive prognostic tests exceeding threshold for fibrosis by patient-level summary statistic (N=6,506).
| Clinical Thresholds | ||
|---|---|---|
| FIB-4 | % > 1.3 (n) | % > 2.67 (n) |
|
| ||
| Mean | 37.3% (2,424) | 5.4% (354) |
| Median | 32.7% (2,125) | 3.5% (229) |
| Maximum | 61.9% (4,029) | 20.3% (1,320) |
| Minimum | 14.9% (970) | 1.3% (87) |
|
| ||
| NFS | % > −1.455 (n) | % > 0.676 (n) |
|
| ||
| Mean | 48.5% (3,157) | 8.0% (519) |
| Median | 48.4% (3,149) | 7.9% (516) |
| Maximum | 68.8% (4,475) | 23.2% (1,510) |
| Minimum | 27.3% (1,774) | 2.9% (190) |
|
| ||
| FIB-4 OR NFS | % > 1.3 OR −1.455 (n) | % > 2.67 OR 0.676 (n) |
|
| ||
| Mean | 56.9% (3,703) | 11.2% (731) |
| Median | 55.3% (3,598) | 9.8% (638) |
| Maximum | 76.0% (4,947) | 30.6% (1,990) |
| Minimum | 31.5% (2,052) | 3.7% (240) |
|
| ||
| FIB-4 AND NFS | % > 1.3 AND −1.455 (n) | % > 2.67 AND 0.676 (n) |
|
| ||
| Mean | 28.9% (1,878) | 2.2% (142) |
| Median | 25.8% (1,676) | 1.6% (107) |
| Maximum | 54.7% (3,557) | 12.9% (840) |
| Minimum | 10.6% (692) | 0.6% (37) |
In the patient sample, 731 (11.2%) patients had either a mean FIB-4 or NFS above the threshold for high-risk of advanced fibrosis, and 142 (2.2%) patients had both a mean FIB-4 and a mean NFS score in the high-risk range. In-depth analysis of 25 randomly selected patients from this latter group revealed that 56% (14/25) had imaging reports documenting liver steatosis and/or the appearance of cirrhosis with or without portal hypertension. Two patients had a non-liver malignancy, and one was diagnosed with thrombotic thrombocytopenic purpura.
In the univariate analysis (Table 3), there were no statistically significant differences in average mean FIB-4 scores by the presence of elevated blood pressure, low HDL, or hypertriglyceridemia. Patients with a mean BMI > 30 kg/m2 had a lower average mean FIB-4 score (1.25 [SD 1.05]) compared to those with a BMI ≤ 30 kg/m2 (1.59 [SD 2.61], p < 0.01), and patients with an A1c > 6.5% had higher mean FIB-4 scores (1.50 to 1.34, p=0.03). Mean of mean NFS scores were significantly higher for patients with elevated blood pressure, hyperglycemia, low HDL, hypertriglyceridemia, and obesity. Patients with triglycerides ≤ 150 mg/dL and BMI ≤ 30 kg/m2 had a higher proportion of high-risk mean FIB-4 scores, whereas patients with triglycerides > 150 mg/dL and BMI > 30 kg/m2 had a higher proportion of high-risk mean NFS scores. Patients with hypertension, hyperglycemia, and low HDL also had a higher proportion of high-risk mean NFS scores. Patients with hyperglycemia had the highest proportion of NAFLD ICD-9/10 assignment (8.2%), followed by 7.6% of patients with hypertriglyceridemia, and 6.0% of patients with a BMI exceeding 30 kg/m2.
Table 3:
Patient-level mean FIB-4 and NFS scores by component of metabolic syndrome, the proportion of patients by metabolic syndrome component with mean FIB-4 and NFS values exceeding the thresholds for advanced fibrosis, and the proportion of patients receiving a NAFLD ICD-9/10 code.
| FIB-4 | NFS | NAFLD Dx | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean (SD) | %>1.3 (n) | %>2.67 (n) | Mean (SD) | %>−1.455 (n) | %>0.676 (n) | %(n) | ||
|
Metabolic syndrome component
| ||||||||
| BP > 130/85 | Yes (n=5,820) | 1.38 (1.98) | 38.0% (2,209) | 5.5% (322) | −1.36 (1.49) | 51.3% (2,986) | 8.6% (499) | 5.2% (301) |
| No (n=686) | 1.35 (2.80) | 31.3% (215) | 4.7% (32) | −2.33 (1.48) | 24.9% (171) | 2.9% (20) | 1.8% (12) | |
| p-value | 0.78* | <0.01** | 0.34** | <0.01* | <0.01** | <0.01** | <0.01** | |
|
| ||||||||
| A1c > 6.5% | Yes (n=1,693) | 1.50 (3.09) | 41.1% (696) | 5.6% (95) | −0.35 (1.39) | 80.5% (1,362) | 21.4% (362) | 8.2% (139) |
| No (n=4,813) | 1.34 (1.58) | 35.9% (1,728) | 5.4% (259) | −1.85 (1.37) | 37.3% (1,795) | 3.3% (157) | 3.6% (174) | |
| p-value | 0.03* | <0.01** | 0.72** | <0.01* | <0.01** | <0.01** | <0.01** | |
|
| ||||||||
| Low HDL | Yes (n=4,504) | 1.36 (1.50) | 35.6% (1,603) | 5.8% (260) | −1.37 (1.60) | 51.6% (2,323) | 9.6% (433) | 5.4% (243) |
| No (n=2,002) | 1.42 (3.00) | 41.0% (821) | 4.7% (94) | −1.66 (1.31) | 41.7% (834) | 4.3% (86) | 3.5% (70) | |
| p-value | 0.39* | <0.01** | 0.08** | <0.01* | <0.01** | <0.01** | <0.01** | |
|
| ||||||||
| Trig > 150 | Yes (n=2,729) | 1.39 (1.42) | 38.1% (1,040) | 4.6% (125) | −1.19 (1.45) | 56.0% (1,528) | 9.8% (266) | 7.6% (206) |
| No (n=3,777) | 1.37 (2.45) | 36.6% (1,384) | 6.1% (229) | −1.66 (1.54) | 43.1% (1,629) | 6.7% (253) | 2.8% (107) | |
| p-value | 0.74* | 0.23** | <0.01** | <0.01* | <0.01** | <0.01** | <0.01** | |
|
| ||||||||
| BMI > 30 | Yes (n=3,025) | 1.24 (1.06) | 31.9% (966) | 3.9% (117) | −0.85 (1.46) | 66.1% (1,998) | 14.0% (423) | 6.0% (182) |
| No (n=3,481) | 1.50 (2.66) | 41.9% (1,458) | 6.8% (237) | −1.99 (1.38) | 33.3% (1,159) | 2.8% (96) | 3.8% (131) | |
| p-value | <0.01* | <0.01** | <0.01** | <0.01* | <0.01** | <0.01** | <0.01** | |
Dx=diagnosis. BP=blood pressure (mm Hg). A1c=hemoglobin a1c. HDL=high-density lipoprotein (mg/dL).Trig= Triglycerides (mg/dL). BMI=body mass index (kg/m2).
Comparison using 2 sample t-test.
Comparison using Chi square test.
In the logistic regression models (Table 4), male gender (OR 1.52, 95% CI 1.22–1.89), Black race (OR 1.75, 95% CI 1.40–2.19), and low HDL (OR 1.42, 95% CI 1.11–1.82) were associated with a mean FIB-4 score greater than 2.67. Patients with higher BMI values (OR 0.96, 95% CI 0.94–0.98) and triglycerides > 150 mg/dL had lower odds of having mean FIB-4 scores exceeding 2.67. No significant interactions appeared between obesity and hyperglycemia or elevated blood pressure. Male gender (OR 1.52, 95% CI 1.08–2.14), Black race (OR 1.88, 95% CI 1.31–2.70), hyperglycemia (OR 2.30, 95% CI 1.60–3.32), and low HDL (OR 2.26, 95% CI 1.40–3.65) were associated with having mean FIB-4 and mean NFS scores exceeding the high-risk thresholds for advanced fibrosis.
Table 4:
Logistic regression models in patients with abnormal liver tests for the outcomes of 1) mean FIB-4 > 1.30; 2) mean FIB-4 > 2.67; 3) mean FIB-4 >1.3 and mean NFS > −1.455; and 4) mean FIB-4 > 2.67 and mean NFS > 0.676.
| Sample | Patient Sample (N=6,506) | |||||
|---|---|---|---|---|---|---|
| Dependent variable | Mean FIB-4 > 1.30 | Mean FIB-4 > 2.67 | ||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Male | 1.52 | 1.37 – 1.69 | <0.0001 | 1.52 | 1.22 – 1.89 | 0.0002 |
| Black | 1.28 | 1.15 – 1.42 | <.00001 | 1.75 | 1.40 – 2.19 | <0.0001 |
| BP > 130/85 | 1.43 | 1.20 – 1.71 | <0.0001 | 1.39 | 0.94 – 2.04 | 0.0969 |
| A1C > 6.5% | 1.36 | 1.20 – 1.54 | <0.0001 | 1.10 | 0.84 – 1.43 | 0.4914 |
| Low HDL | 0.83 | 0.74 – 0.93 | 0.0013 | 1.42 | 1.11 – 1.82 | 0.0060 |
| Triglycerides > 150 | 1.07 | 0.95 – 1.19 | 0.2675 | 0.76 | 0.59 – 0.96 | 0.0229 |
| BMI | 0.97 | 0.96 – 0.97 | <0.0001 | 0.96 | 0.94 – 0.98 | <0.0001 |
| Dependent variable | Mean FIB-4 > 1.30 AND Mean NFS > −1.455 | Mean FIB-4 > 2.67 AND Mean NFS > 0.676 | ||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Male | 1.68 | 1.50 – 1.88 | <0.0001 | 1.52 | 1.08 – 2.14 | 0.0173 |
| Black | 1.41 | 1.26 – 1.58 | <0.0001 | 1.88 | 1.31 – 2.70 | 0.0006 |
| BP > 130/85 | 1.53 | 1.24 – 1.88 | <0.0001 | 1.33 | 0.63 – 2.78 | 0.4537 |
| A1C > 6.5% | 1.76 | 1.55 – 2.01 | <0.0001 | 2.30 | 1.60 – 3.32 | <0.0001 |
| Low HDL | 1.04 | 0.92 – 1.18 | 0.5274 | 2.26 | 1.40 – 3.65 | 0.0008 |
| Triglycerides > 150 | 1.10 | 0.98 – 1.24 | 0.1054 | 0.75 | 0.52 – 1.07 | 0.1133 |
| BMI | 1.00 | 0.99 – 1.01 | 0.7929 | 1.02 | 1.00 – 1.04 | 0.0704 |
BP=blood pressure (mm Hg). A1c=hemoglobin a1c. HDL=high-density lipoprotein (mg/dL).Trig= Triglycerides (mg/dL). BMI=body mass index (kg/m2).
Interpretation
Our data reveal a high prevalence of elevated (indeterminate and high-risk for advanced fibrosis) FIB-4 and NFS scores in a large primary care sample with a heavy burden of metabolic syndrome elements. These data are striking, with 57% of patients having either a mean FIB-4 or mean NFS above the indeterminate risk threshold and 29% having both mean FIB-4 and mean NFS values at this level. Additionally, 11% had either a mean FIB-4 or mean NFS in the high-risk range, and 142 patients had mean FIB-4 and NFS scores exceeding the high-risk threshold for advanced fibrosis, suggesting that this group may have cirrhosis and not know it. Only 5% of patients carried an ICD-9/10 code for NAFLD.
Optimistically, FIB-4 and NFS may effectively rule out advanced fibrosis in 24% of our sample (76% with either a maximum FIB-4 > 1.3 or NFS > −1.455). However, a large proportion likely requires further evaluation with additional blood testing (i.e. ELF™), imaging (transient elastography), or referral. FIB-4 and NFS in sequence may help to better select patients for more advanced evaluation, as only 29% of this sample had both mean FIB-4 and mean NFS scores above the indeterminate threshold.53,54 The proportion of elevated non-invasive fibrosis assessment scores is higher than other primary care samples, but similar to that found in a study of patients with biopsy-proven NAFLD.33,40 Our sample patients were older and had high burdens of diabetes, hypertension, and dyslipidemia, factors associated with advanced fibrosis which may explain the higher FIB-4 and NFS scores. Additionally, our study liberalized the liver test abnormality requirements to include AST (vs. only ALT elevation) due to mounting evidence that ALT abnormalities alone may not detect NAFLD with advanced fibrosis.33,55,56 While a high burden of comorbidities and a broader abnormal liver test definition may impact the proportion of patients with elevated FIB-4 and NFS scores, limiting this work to patients with high ALT (>45 IU/L) inclusion criteria and excluding those FIB-4 and NFS scores after age 65 neglects a large component of the primary care population at risk for advanced fibrosis.
Though FIB-4 and NFS scores may not carry the same clinical significance as a histological analysis of the liver, we attempted to overcome this concern by using a combination of the two non-invasive assessments to evaluate fibrosis risk. We believe this should provide reassurance in our findings. Additionally, the relationships observed between male gender, elevated blood pressure, and hyperglycemia with elevated risk scores in the regression models are consistent with previous work.1,5–7,54,57–59 However, the relationship with FIB-4 and BMI was surprising. We analyzed BMI continuously and as a 2-level (≤ 30, or > 30) categorical variable, each time finding similar associations.
The findings from this study underscore the pervasiveness of NAFLD (and perhaps all liver disease) under-diagnosis in primary care. Despite an overall population prevalence of 30% and high proportions of objective measures of obesity and diabetes, only 5% of our sample carried an ICD-9/10 code for NAFLD in the presence of known clinical signals and risk factors. Possible reasons for this diagnostic gap are complex. ICD-9/10 codes are suboptimal tools in identifying cases of chronic liver disease in administrative datasets, and this under-diagnosis may reflect these coding limitations.60 Also, PCPs may not feel this diagnosis alters their treatment plan in patients with components of the metabolic syndrome, as they already address cardiovascular risk factors and counsel on behavioral modifications. But knowledge of advanced fibrosis may spur more intensive weight loss efforts, which can lead to reductions in fibrosis and improved outcomes.61–63 Application of FIB-4 or NFS scoring in primary care, especially in patients with type 2 diabetes, could prompt further testing or diagnostic assignment in those without a known liver disease diagnosis.22
We were surprised by the number of patients with both mean FIB-4 and NFS scores above the high-risk fibrosis thresholds and no diagnosis of cirrhosis. More than half of the sub-sample (14/25) had radiographic evidence of steatosis or cirrhosis, consistent with the conclusion that when both of these tests are elevated, there is likely underlying liver disease. Only 3 of the 25 had another definitive diagnosis that might lead to raised FIB-4 and NFS values, driven largely by thrombocytopenia. These findings suggest possible utility in applying non-invasive tests to carefully selected subsets (i.e. patients with aminotransferase elevations) of PCMH patient registries to identify undiagnosed patients with advanced fibrosis.
We recognize the limitations of this study. First, we cannot be sure of the burden of NAFLD in this population. But, the selection of patients with abnormal aminotransferases, the exclusion of other known liver diseases, the burden of metabolic syndrome, the relationships observed in the univariate analyses, and the findings on the post hoc chart review would support the presence of undiagnosed NAFLD in this sample. Secondly, the outcome of interest (elevated FIB-4 and NFS scores) is a proxy for liver disease, and structural analysis was not possible in all patients. Nonetheless, this patient sample is a realistic representation of what primary care clinicians encounter in the U.S. Also, we used summary statistics. Maximum FIB-4 and NFS values may reflect acute situations unrelated to fibrosis, so mean statistics were used for the logistic regression models. Means were chosen because this summary measure captures data from every FIB-4 and NFS value (unlike the median), adding robustness to the analyses. Similar associations appeared when using median scores as the outcome in the logistic regression models. We intentionally used a high threshold for ALT inclusion to simulate the real-life signal delivered to PCPs, but this signal would miss patients with NAFLD and possible fibrosis. The predictor variables for MetS include BMI and A1c instead of waist circumference and fasting glucose, respectively, a departure from the most recent definitions of metabolic syndrome. We do not have reliable waist circumference data in our EHR, and we were unable to ascertain the fasting state of patients.64 However, we opted to use objective measures to define MetS components to optimize authenticity (compared to ICD-9/10 coding). ICD coding was used for exclusion criteria, which may have inadequately identified all patients in the sample with other chronic liver disease. Also, this study does not include imaging data (e.g. ultrasound) which currently resides in the EHR in an unstructured format and would contribute to diagnostic assignment. Unfortunately, medication data was not yet available for analysis. Additionally, this study relied upon measures not included in the classical definition of MetS (BMI and A1c) and omitted waist circumference and fasting glucose. This exchange was done due to data availability and could influence the MetS relationships observed. Finally, this is a single site study with patients located in a PCMH, which could limit generalizability.
Our data suggest that NAFLD is under-diagnosed in primary care and that a sizable subgroup of patients may have advanced fibrosis. As the burden of NAFLD and the complications from it grow, PCPs need strategies to improve the diagnosis of NAFLD, especially in patients with metabolic syndrome, and assess for the presence of advanced fibrosis. FIB-4 and NFS may prove to be useful tools in this setting, applied not only after a formal NAFLD diagnosis, but also used to identify those patients with elevated values in need of a more intensive liver evaluation.
Supplementary Material
Acknowledgments
Funding Source
The research leading to these results received funding from the National Institutes of Health and the National Institute of Diabetes and Digestive and Kidney Diseases under the grants NIH/NIDDK K23DK118200 (PI: Schreiner) and NIH/NIDDK P30DK123704 (PI: Rockey).
Abbreviations:
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUROC
area under the receiver operating characteristic
- BMI
body mass index
- EHR
electronic health record
- FIB-4
Fibrosis-4 Index
- HDL
high-density lipoprotein
- ICD
International Classification of Diseases
- IQR
inter-quartile range
- LR
likelihood ratio
- MetS
metabolic syndrome
- NAFLD
nonalcoholic fatty liver disease
- NFS
NAFLD fibrosis score
- PCMH
patient-centered medical home
- Plt
platelet
- ROC
receiver operating characteristic
Footnotes
IRB Approval
This study was approved by the Interval Review Board at the Medical University of South Carolina prior to data collection.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Conflict of Interests
All authors report no conflicts of interest with this work.
References
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md). 2018;67(1):328–357. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (Baltimore, Md). 2016;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 3.Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. Jama. 2015;313(22):2263–2273. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the L, European Association for the Study of D, European Association for the Study of O. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. [DOI] [PubMed] [Google Scholar]
- 6.Kanwal F, Kramer J, Li L, et al. Effect of Metabolic Traits on the Risk of Cirrhosis and Hepatocellular Cancer in Non-alcoholic Fatty Liver Disease. Hepatology (Baltimore, Md). 2019. [DOI] [PubMed] [Google Scholar]
- 7.Loomba R, Abraham M, Unalp A, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology (Baltimore, Md). 2012;56(3):943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballestri S, Capitelli M, Fontana MC, et al. Direct Oral Anticoagulants in Patients with Liver Disease in the Era of Non-Alcoholic Fatty Liver Disease Global Epidemic: A Narrative Review. Adv Ther. 2020;37(5):1910–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballestri S, Zona S, Targher G, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31(5):936–944. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut. 2020. [DOI] [PubMed] [Google Scholar]
- 11.Alexander M, Loomis AK, Fairburn-Beech J, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 2018;16(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polanco-Briceno S, Glass D, Stuntz M, Caze A. Awareness of nonalcoholic steatohepatitis and associated practice patterns of primary care physicians and specialists. BMC Res Notes. 2016;9:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright AP, Desai AP, Bajpai S, King LY, Sahani DV, Corey KE. Gaps in recognition and evaluation of incidentally identified hepatic steatosis. Dig Dis Sci. 2015;60(2):333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel PJ, Banh X, Horsfall LU, et al. Underappreciation of non-alcoholic fatty liver disease by primary care clinicians: limited awareness of surrogate markers of fibrosis. Internal medicine journal. 2018;48(2):144–151. [DOI] [PubMed] [Google Scholar]
- 15.Cleveland ER, Ning H, Vos MB, et al. Low Awareness of Nonalcoholic Fatty Liver Disease in a Population-Based Cohort Sample: the CARDIA Study. J Gen Intern Med. 2019;34(12):2772–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loomba R, Wong R, Fraysse J, et al. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: a real world analysis of Medicare data. Aliment Pharmacol Ther. 2020. [DOI] [PubMed] [Google Scholar]
- 17.Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol. 2017;112(1):18–35. [DOI] [PubMed] [Google Scholar]
- 18.Newsome PN, Cramb R, Davison SM, et al. Guidelines on the management of abnormal liver blood tests. Gut. 2018;67(1):6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes-2019. Diabetes care. 2019;42(Suppl 1):S34–s45. [DOI] [PubMed] [Google Scholar]
- 20.Tsochatzis EA, Newsome PN. Non-alcoholic fatty liver disease and the interface between primary and secondary care. Lancet Gastroenterol Hepatol. 2018;3(7):509–517. [DOI] [PubMed] [Google Scholar]
- 21.Budd J, Cusi K. Nonalcoholic Fatty Liver Disease: What Does the Primary Care Physician Need to Know? Am J Med. 2020;133(5):536–543. [DOI] [PubMed] [Google Scholar]
- 22.Cernea S, Raz I. NAFLD in type 2 diabetes mellitus: Still many challenging questions. Diabetes Metab Res Rev. 2020:e3386. [DOI] [PubMed] [Google Scholar]
- 23.Adler E, Brandman D. Treatment of Fatty Liver Disease-Time to Implement Common Sense Measures. JAMA internal medicine. 2019. [DOI] [PubMed] [Google Scholar]
- 24.Hagstrom H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265–1273. [DOI] [PubMed] [Google Scholar]
- 25.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149(2):389–397 e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabbany MN, Conjeevaram Selvakumar PK, Watt K, et al. Prevalence of Nonalcoholic Steatohepatitis-Associated Cirrhosis in the United States: An Analysis of National Health and Nutrition Examination Survey Data. Am J Gastroenterol. 2017;112(4):581–587. [DOI] [PubMed] [Google Scholar]
- 27.Tapper EB, Lok AS. Use of Liver Imaging and Biopsy in Clinical Practice. N Engl J Med. 2017;377(8):756–768. [DOI] [PubMed] [Google Scholar]
- 28.Cassinotto C, Boursier J, de Ledinghen V, et al. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology (Baltimore, Md). 2016;63(6):1817–1827. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqui MS, Patidar KR, Boyett S, Luketic VA, Puri P, Sanyal AJ. Performance of non-invasive models of fibrosis in predicting mild to moderate fibrosis in patients with non-alcoholic fatty liver disease. Liver international : official journal of the International Association for the Study of the Liver. 2016;36(4):572–579. [DOI] [PubMed] [Google Scholar]
- 30.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology (Baltimore, Md). 2017;66(5):1486–1501. [DOI] [PubMed] [Google Scholar]
- 31.Anstee QM, Lawitz EJ, Alkhouri N, et al. Noninvasive Tests Accurately Identify Advanced Fibrosis due to NASH: Baseline Data From the STELLAR Trials. Hepatology (Baltimore, Md). 2019;70(5):1521–1530. [DOI] [PubMed] [Google Scholar]
- 32.Patel YA, Gifford EJ, Glass LM, et al. Identifying Nonalcoholic Fatty Liver Disease Advanced Fibrosis in the Veterans Health Administration. Dig Dis Sci. 2018;63(9):2259–2266. [DOI] [PubMed] [Google Scholar]
- 33.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59(9):1265–1269. [DOI] [PubMed] [Google Scholar]
- 34.Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7(10):1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology (Baltimore, Md). 2007;46(1):32–36. [DOI] [PubMed] [Google Scholar]
- 36.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43(8):617–649. [DOI] [PubMed] [Google Scholar]
- 37.Kaswala DH, Lai M, Afdhal NH. Fibrosis Assessment in Nonalcoholic Fatty Liver Disease (NAFLD) in 2016. Dig Dis Sci. 2016;61(5):1356–1364. [DOI] [PubMed] [Google Scholar]
- 38.Imajo K, Kessoku T, Honda Y, et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology. 2016;150(3):626–637 e627. [DOI] [PubMed] [Google Scholar]
- 39.Srivastava A, Gailer R, Tanwar S, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol. 2019;71(2):371–378. [DOI] [PubMed] [Google Scholar]
- 40.Davyduke T, Tandon P, Al-Karaghouli M, Abraldes JG, Ma MM. Impact of Implementing a “FIB-4 First” Strategy on a Pathway for Patients With NAFLD Referred From Primary Care. Hepatol Commun. 2019;3(10):1322–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tapper EB, Loomba R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol. 2018;15(5):274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Libman H, Jiang ZG, Tapper EB, Reynolds EE. How Would You Manage This Patient With Nonalcoholic Fatty Liver Disease? Ann Intern Med. 2019;171(11):862. [DOI] [PubMed] [Google Scholar]
- 43.Paul S, Davis AM. Diagnosis and Management of Nonalcoholic Fatty Liver Disease. Jama. 2018;320(23):2474–2475. [DOI] [PubMed] [Google Scholar]
- 44.Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology (Baltimore, Md). 2003;37(6):1286–1292. [DOI] [PubMed] [Google Scholar]
- 45.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology (Baltimore, Md). 2006;43(6):1317–1325. [DOI] [PubMed] [Google Scholar]
- 46.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology (Baltimore, Md). 2007;45(4):846–854. [DOI] [PubMed] [Google Scholar]
- 47.McPherson S, Hardy T, Dufour JF, et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol. 2017;112(5):740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurosaki M, Izumi N. External validation of FIB-4: diagnostic accuracy is limited in elderly populations. Hepatology (Baltimore, Md). 2008;47(1):352; author reply 352–353. [DOI] [PubMed] [Google Scholar]
- 49.Pitisuttithum P, Chan WK, Piyachaturawat P, et al. Predictors of advanced fibrosis in elderly patients with biopsy-confirmed nonalcoholic fatty liver disease: the GOASIA study. BMC Gastroenterol. 2020;20(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. Journal of clinical gastroenterology. 2013;47(5):e50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mapakshi S, Kramer JR, Richardson P, El-Serag HB, Kanwal F. Positive Predictive Value of International Classification of Diseases, 10th Revision, Codes for Cirrhosis and Its Related Complications. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2018;16(10):1677–1678. [DOI] [PubMed] [Google Scholar]
- 52.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 53.Crossan C, Majumdar A, Srivastava A, et al. Referral pathways for patients with NAFLD based on non-invasive fibrosis tests: Diagnostic accuracy and cost analysis. Liver international : official journal of the International Association for the Study of the Liver. 2019;39(11):2052–2060. [DOI] [PubMed] [Google Scholar]
- 54.Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J Hepatol. 2018;68(2):305–315. [DOI] [PubMed] [Google Scholar]
- 55.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57(10):1441–1447. [DOI] [PubMed] [Google Scholar]
- 56.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology (Baltimore, Md). 2013;57(4):1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology (Baltimore, Md). 1999;30(6):1356–1362. [DOI] [PubMed] [Google Scholar]
- 58.Neuschwander-Tetri BA, Clark JM, Bass NM, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology (Baltimore, Md). 2010;52(3):913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kechagias S, Nasr P, Blomdahl J, Ekstedt M. Established and emerging factors affecting the progression of nonalcoholic fatty liver disease. Metabolism. 2020;111S:154183. [DOI] [PubMed] [Google Scholar]
- 60.Quan H, Li B, Saunders LD, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koutoukidis DA, Astbury NM, Tudor KE, et al. Association of Weight Loss Interventions With Changes in Biomarkers of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. JAMA internal medicine. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yeo SC, Ong WM, Cheng KSA, Tan CH. Weight Loss After Bariatric Surgery Predicts an Improvement in the Non-alcoholic Fatty Liver Disease (NAFLD) Fibrosis Score. Obes Surg. 2019;29(4):1295–1300. [DOI] [PubMed] [Google Scholar]
- 63.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149(2):367–378 e365; quiz e314–365. [DOI] [PubMed] [Google Scholar]
- 64.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.