Abstract
Conidia are used as inocula for the in vitro susceptibility testing of Aspergillus fumigatus. Since the MIC is defined on the basis of visible mycelial growth, conidia should germinate and produce sporelings (germinated conidia) for monitoring of the growth inhibition and fungicidal activity of a drug. If a compound is capable of inhibiting germination of conidia while affecting or not affecting the growth of the organism, the MIC obtained will be the concentration of the drug required for the inhibition of conidial germination but not necessarily that required for inhibition of the growth of the organism. We investigated the susceptibility of germinated and ungerminated conidia to amphotericin B, itraconazole, voriconazole, and SCH56592. The MICs of various antifungal agents for germinated conidia were almost identical to those obtained for ungerminated conidia. In addition, both the germinated and ungerminated conidia were killed with almost equal efficiency by all of the compounds tested when exposed to the drugs for 24 h. These results suggest that either germinated or ungerminated conidia could be used as inocula for in vitro susceptibility studies of A. fumigatus with identical results.
The availability of a standardized method for the in vitro susceptibility testing of filamentous fungi is essential for inter- and intralaboratory comparisons of MICs of various antifungal agents. MIC comparisons are often useful for the identification of clinical isolates with reduced susceptibility to antifungal agents, as well as for examining the correlation between treatment failure and in vitro resistance to antifungal agents. Efforts have been recently made to standardize a technique of MIC determination for filamentous fungi (2, 3, 9, 10) based on the method recommended by the National Committee for Clinical Laboratory Standards for the susceptibility testing of pathogenic yeasts (7). As a matter of convenience and for reproducibility, conidial suspensions have been used as the source of inocula for MIC determination for various Aspergillus species. Overall, use of conidia has resulted in excellent reproducibility of the results obtained in different laboratories (1, 5, 8, 11). However, the suitability of using ungerminated conidia as inocula for MIC determination for Aspergillus species has been a matter of concern. The lack of growth obtained in the presence of an antifungal agent may be due to inhibition of conidial germination or inhibition of growth. Are the growth inhibition and the fungicidal activity of an antifungal agent the same for germinated and ungerminated conidia? To answer this question, we examined the growth inhibition and fungicidal activities of various antifungal agents against germinated and ungerminated conidia of Aspergillus fumigatus.
Ten clinical isolates (W73355, F55064, W27023, T53454, W43719, H55622, H70853, W63928, H38167, and W33299) of A. fumigatus obtained from the Microbiology Laboratory of the Detroit Medical Center were used in this study. The original cultures obtained on Sabouraud dextrose agar slants were subcultured on the same medium to check the purity and viability of the culture. For long-term storage, cultures were kept as conidial suspensions in 25% glycerol at −70°C. Cultures were grown on Sabourand dextrose agar for 6 days at 35°C for the production of conidia. The conidia were collected, and the density of the conidial suspension was determined as described previously (5).
For the germination assay, fresh conidia were resuspended in peptone-yeast extract-glucose (PYG; 1 g of peptone, 1 g of yeast extract, and 3 g of glucose per liter of distilled water) medium (106 conidia/ml) and incubated at 35°C with gentle agitation (160 rpm) on a gyratory shaker. At various time intervals, 10-μl aliquots were removed and the numbers of germinated conidia were assessed by hemocytometer counting. Percent germination was calculated and graphed against time of incubation in PYG broth. Conidia were counted at a magnification of ×400, and a total of 200 conidia per field were counted. The counting was done three times, and the mean value of these three independent counts was used to obtain the percent germination.
The MICs of various antifungal agents for germinated and ungerminated conidia were determined by broth macrodilution. Briefly, fresh conidia were resuspended in 50 ml of PYG broth at a density of 106/ml and allowed to germinate for 8 h as described above. One-milliliter aliquots of the germinated and ungerminated conidial suspensions were diluted 50-fold to obtain a cell density of 2 × 104 conidia/ml, and the MICs of various antifungal agents were determined in PYG broth as previously described (5). The MIC was defined as the drug concentration that inhibited visible growth completely. It was important to keep the conidial density at 106 conidia/ml for optimum germination. Higher conidial densities produced lower germination frequencies (presumably due to a population effect). A density lower than 106 conidia/ml posed problems for accurate counting, kill curve experiments, and MIC studies.
For the kill curve experiments, 106 germinated (8 h) and ungerminated conidia were incubated in 1 ml of PYG broth at 35°C for 24 h in the presence of various antifungal agents (5 μg/ml). At the end of the incubation period, the cell suspension was diluted 10- to 1,000-fold and 0.1-ml aliquots were spread on PYG agar plates. The plates were incubated at 35°C for 48 h, and the numbers of CFU were determined. Each treatment was done in duplicate, and the experiment was repeated six times by using three different clinical isolates.
Amphotericin B (batch 20-914-29670) was obtained from the Squibb Institute for Medical Research, Princeton, N.J. Itraconazole (R51 211; batch STAN-9304-005-1) was from Janssen Pharmaceutica, Beerse, Belgium. Voriconazole was from Pfizer Pharmaceuticals (New York, N.Y.), and SCH56592 was obtained from the Schering-Plough Research Institute (Kenilworth, N.J.). Compounds were dissolved in dimethyl sulfoxide at a concentration of 1 mg/ml and stored as 0.25-ml aliquots at −20°C. The frozen stock was thawed at room temperature and vortexed gently several times to ensure that any crystals present were completely dissolved before use. Comparable concentrations of dimethyl sulfoxide were used to examine its effect on the growth of A. fumigatus. No detectable inhibition of growth occurred at the concentrations used. Since amphotericin B is light sensitive, the stock solutions and the MIC tubes were covered with aluminum foil to prevent light exposure. For all antifungal agents, a concentration range of 0.125 to 16 μg/ml was used for MIC determination.
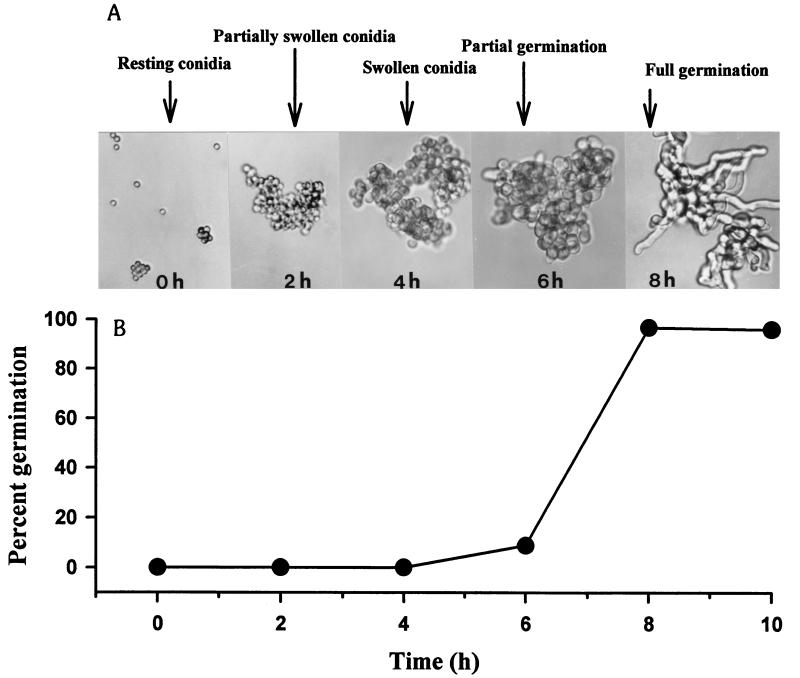
As shown in Fig. 1, the germination of A. fumigatus conidia at 35°C in PYG broth was a multistep process. During the initial 4 h of incubation, the conidia underwent a significant increase in volume by swelling, and the diameter of the conidia increased approximately twofold compared to that of the resting conidia. The swelling of the conidia was followed by the appearance of a small protuberance on the conidial cell wall, and the conidium was now primed for polarized growth. The appearance of the protuberance was followed by the formation of a germ tube, and 10.67% ± 2.16% of the conidia germinated and produced sporelings (germinated conidia with the length of the germ tube equal to or greater than the diameter of swollen conidia) within 6 h after incubation. The emergence of germ tubes occurred rapidly after 6 h of incubation, and 96.5% ± 2.47% of the conidia were germinated by 8 h of incubation in PYG broth at 35°C.
FIG. 1.
Photomicrographs (original magnification, ×400) (A) and kinetics (B) of germinating conidia of A. fumigatus W73355 in PYG broth at 35°C. The experiment was repeated six times by using three different clinical isolates; similar results were obtained for all isolates. The data shown were obtained in a typical experiment with isolate W73355. Each point on the graph represents the mean of triplicate determinations, and the standard deviation was <5% of the mean value.
A comparison of the MICs of amphotericin B, itraconazole, voriconazole, and SCH56592 obtained for germinated and ungerminated conidia is shown in Table 1. No significant change in MICs was obtained with the germinated conidia. The maximum change obtained was ±1 dilution. However, the conidia should be germinated more or less synchronously, within a period of 8 h of incubation, to obtain reproducible results. Longer incubation resulted in rapid growth, and the mycelial mass increased markedly. This increase in mycelial mass resulted in a decrease in the antibiotic-to-fungal-mass ratio which, in turn, resulted in lower effective concentrations of the drug. It has been shown that the MICs of antifungal agents for filamentous fungi are dependent on the nature (4) and size (5) of the inoculum. Thus, the occurrence of a higher MIC for hyphae is due not necessarily to their reduced susceptibility to a drug but rather to an increase in mycelial mass.
TABLE 1.
Susceptibilities of germinated and ungerminated conidia of A. fumigatus to various antifungal agents
| Conidial form used | MIC (μg/ml; geometric mean ± SD)a of:
|
|||
|---|---|---|---|---|
| AMB | ITZ | VCZ | SCH | |
| Ungerminated | 0.406 ± 0.229 | 0.406 ± 0.229 | 0.307 ± 0.120 | 0.267 ± 0.118 |
| Germinated | 0.466 ± 0.079 | 0.287 ± 0.105 | 0.329 ± 0.129 | 0.267 ± 0.079 |
Pairwise comparisons of the geometric mean MICs of the same antifungal agent obtained for germinated and ungerminated conidia (n = 10) by two-tailed t-test analysis showed no significant differences. The P values obtained for amphotericin B (AMB), itraconazole (ITZ), voriconazole (VCZ), and SCH56592 (SCH) were 0.7486, 0.0768, 0.6600, and 0.7846, respectively.
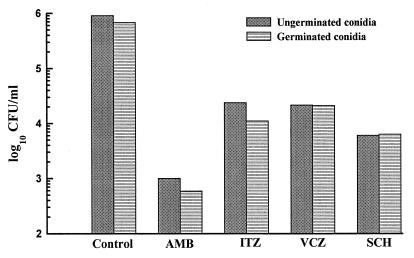
The fungicidal activities of various antifungal agents against germinated and ungerminated conidia of A. fumigatus are shown in Fig. 2. We used approximately 10 times the MIC of the compounds for kill curve experiments. Amphotericin B, itraconazole, voriconazole, and SCH56592 at 5 μg/ml killed 99.36% ± 0.74%, 96.79% ± 0.83%, 97.77% ± 0.19%, and 98.74% ± 0.84%, respectively, of the ungerminated conidia after 24 h of exposure. No significant difference in the killing ability of these antifungal agents (amphotericin B, 99.88% ± 0.056%; itraconazole, 97.27% ± 1.55%; voriconazole, 97.38% ± 0.69%; SCH56592, 94.86% ± 5.95%) against germinated conidia was obtained. In addition to A. fumigatus W73355, we examined the susceptibility of two additional clinical isolates (F55064 and W27023) to various antifungal agents. Both germinated and ungerminated conidia from these two isolates also provided similar results, suggesting that the observed results were not a strain-dependent phenomenon. As opposed to the common notion that azoles are generally fungistatic agents and not fungicidal, we previously demonstrated (6) that triazoles such as itraconazole and voriconazole are fungicidal for Aspergillus species, including A. fumigatus. Our present data confirm the previous finding and further show that the observed killing was not restricted to resting conidia but extended to germinated conidia as well.
FIG. 2.
Fungicidal activities of amphotericin B (AMB) and various triazoles on germinated and ungerminated conidia of A. fumigatus. The experiment was repeated three times with isolate W73355 and twice with isolates F55064 and W27023. Similar results were obtained each time with all three isolates. The data shown were obtained in a typical experiment with isolate W73355. Each histogram represents the mean of duplicate determinations, and the standard deviations were ≤50% of the mean value in all cases. ITZ, itraconazole; VCZ, voriconazole; SCH, SCH56592.
One of the concerns in the development of a standard method for the in vitro susceptibility testing of filamentous fungi is the nature of the inoculum. Vegetative mycelia have several disadvantages. One, it is important to dispense the inoculum uniformly throughout the test samples, since the MIC will be greatly dependent on the inoculum size. Second, Guarro et al. (4) noted that the use of vegetative mycelia produced consistently higher MICs than did the use of conidia. Third, since filamentous fungi grow by apical elongation, major portions of the hyphae away from the tips are metabolically inactive. Consequently, if mycelia are used as inocula, the compounds are tested against relatively nongrowing parts of the organism. A fourth consideration is the difficulty of dealing rapidly with large numbers of samples for susceptibility testing in clinical laboratories. Susceptibility testing will be time consuming and cumbersome if performed with vegetative mycelia.
The use of conidia is an attractive option for in vitro susceptibility testing. It is comparatively fast and efficient, and precise amounts of the inocula can be delivered rapidly for MIC testing. However, a serious concern is the appropriateness of using dormant conidia for MIC determinations. The conidia must first germinate for the antifungal agents to inhibit their visible growth, which is the criterion for the definition of the MIC. The whole premise is based on the assumption that the germination process of the conidia per se is not affected by the test compounds. If the germination process is affected while growth is or is not affected, then the use of dormant conidia will provide erroneous results. It is therefore important to establish that both germinated and ungerminated conidia are equally susceptible to the inhibitory action of the antifungal agents commonly used, in particular, azoles and polyenes. The MICs of other compounds which may have activity against macromolecular synthesis (e.g., nucleic acid and protein syntheses) will be affected by inhibiting spore germination, since the germination of spores involves the synthesis of RNA and proteins. Our studies show that both germinated and ungerminated conidia of A. fumigatus are equally susceptible to the inhibitory and fungicidal activities of polyene and azole compounds. The use of conidia as inocula may not result in MICs of azoles and polyenes due to inhibition of spore germination. However, when screening new compounds for activity against filamentous fungi by using conidia as inocula, it is useful to test their effect on germinated and ungerminated conidia to confirm that the antifungal activity is due to inhibition of growth and not to inhibition of spore germination.
Acknowledgments
We thank Pfizer Pharmaceuticals and the Schering-Plough Research Institute, respectively, for providing voriconazole and SCH56592. Also, we thank William Brown of the Microbiology Laboratory, Detroit Medical Center, for providing the clinical isolates of A. fumigatus used in this study.
REFERENCES
- 1.Denning D W, Venkateswarlu K, Oakley K L, Anderson M J, Manning N J, Stevens D A, Warnock D W, Kelly S L. Itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1364–1368. doi: 10.1128/aac.41.6.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Espinel-Ingroff A, Dawson K, Pfaller M, Anaissie E, Breslin B, Dixon D, Fothergill A, Peter J, Rinaldi M, Walsh T. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi: 10.1128/aac.39.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Espinel-Ingroff A, Rex J H, Bowden R, Menezes P, Bartlett M, McGinnis M, Cooper C, Odds F C, Pfaller M, Messer S A, Nelson P W, Rinaldi M G, Fothergill A, Shankland G, Walsh T J, Peter J, Weitzman I, Chin N X. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1996;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guarro J, Llop C, Aguilar C, Pujol I. Comparison of in vitro antifungal susceptibilities of conidia and hyphae of filamentous fungi. Antimicrob Agents Chemother. 1997;41:2760–2762. doi: 10.1128/aac.41.12.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manavathu E K, Alangaden G J, Lerner S A. A comparative study of the broth micro- and macro-dilution techniques for the determination of the in vitro susceptibility of Aspergillus fumigatus. Can J Microbiol. 1996;42:960–964. doi: 10.1139/m96-123. [DOI] [PubMed] [Google Scholar]
- 6.Manavathu E K, Cutright J L, Chandrasekar P H. Organism-dependent fungicidal activity of azoles. Antimicro Agents Chemother. 1998;42:3018–3021. doi: 10.1128/aac.42.11.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 8.Oakley K L, Morrissey G, Denning D W. Efficacy of SCH-56592 in a temporarily neutropenic murine model of invasive aspergillosis with an itraconazole-susceptible and an itraconazole-resistant isolate of Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1504–1507. doi: 10.1128/aac.41.7.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odds F C, Gerven F V, Espinel-Ingroff A, Bartlett M S, Ghannoum M A, Lancaster M V, Pfaller M A, Rex J H, Rinaldi M G, Walsh T J. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob Agents Chemother. 1998;42:282–288. doi: 10.1128/aac.42.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfaller M A, Rex J H, Rinaldi M G. Antifungal susceptibility testing: technical advances and potential clinical applications. Clin Infect Dis. 1997;24:776–784. doi: 10.1093/clinids/24.5.776. [DOI] [PubMed] [Google Scholar]
- 11.Pujol I, Guarro J, Llop C, Soler L, Fernandez-Ballart J. Comparison study of broth macrodilution and microdilution antifungal susceptibility tests for the filamentous fungi. Antimicrob Agents Chemother. 1996;40:2106–2110. doi: 10.1128/aac.40.9.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]