Abstract
Objective
There is increasing evidence that the high-sensitivity modified Glasgow prognostic scores are inflammatory indices that can predict survival for many cancer types. However, there is limited information regarding their prognostic values in cases of head and neck cancer. This study aimed to evaluate whether the high-sensitivity modified Glasgow prognostic scores could predict outcomes among patients with oropharyngeal squamous cell carcinoma (OPC).
Study Design
Retrospective study.
Setting
University hospital.
Methods
We reviewed the records of 106 patients with histologically confirmed OPC between March 2009 and June 2020. The high-sensitivity modified Glasgow prognostic scores were calculated as 0 (C-reactive protein [CRP] concentration: ≤0.3 mg/dL), 1 (CRP concentration >0.3 mg/dL and albumin concentration ≥3.5 mg/dL), or 2 (CRP concentration >0.3 mg/dL and albumin concentration <3.5 mg/dL). Univariate and multivariable Cox proportional hazard analyses were performed for overall survival (OS) and disease-free survival (DFS).
Results
Forty-four of these patients had human papillomavirus (HPV)–positive OPC, and 62 had HPV-negative OPC, and these populations were analyzed separately. The high-sensitivity modified Glasgow prognostic score was significantly associated with age, performance status, and HPV. On univariate analysis, high-sensitivity modified Glasgow prognostic score showed associations with OS and DFS in both subpopulations. Moreover, on multivariable analysis, the high-sensitivity modified Glasgow prognostic score showed associations with OS and DFS in both subpopulations. Poor performance status predicted OS in both subpopulations.
Conclusion
We conclude that the high-sensitivity modified Glasgow prognostic score is useful as an independent prognostic factor in OPC.
Keywords: high-sensitivity modified Glasgow prognostic score, head and neck cancer, C-reactive protein, survival, oropharyngeal squamous cell carcinoma
To date, there is limited molecular characterization of the driver mutations of the various subtypes of head and neck squamous cell carcinoma (HNSCC), with human papillomavirus (HPV), smoking, and alcohol the only identified causative agents. Therefore, understanding the biological mechanisms that lead to cancer progression and identification of prognostic factors are essential to improve the clinical management of HNSCC.
There is a 28% reduced risk of death and a 49% reduced risk of disease recurrence for patients who have HPV-positive oropharyngeal squamous cell carcinoma (OPC) vs those with HPV-negative OPC.1 Patients with HPV-positive cancer are generally younger and healthier than their HPV-negative counterparts, with better performance status, no smoking history, and fewer comorbidities.1 HPV-OPC may be detected earlier, because 51% of patients initially present with a neck mass vs 18% of patients with HPV-negative OPC, which is less likely to metastasize to cervical lymph nodes.2 HPV-negative OPC demonstrates greater locoregional progression than HPV-positive OPC, thus making HPV-negative OPC more refractory to treatment.3
Ang et al4 retrospectively analyzed the 0129 Radiation Therapy Oncology Group trial and found that 3-year overall survival (OS) was 82.4% for patients with HPV-positive disease and 57.1% for those with HPV-negative disease. In HPV-positive patients with no smoking history and a low nodal status (N0, N1, N2a), the 3-year OS could reach up to 93%. However, a significant minority of HPV-positive patients (20%) have poor OS. Identification of this high-risk group is important in an era of potential treatment deescalation and introduction of molecularly targeted therapies.
Several inflammation-based scoring systems have been devised, and they are strongly associated with prognoses among patients with various neoplasms. The modified Glasgow prognostic score (mGPS) can be used to evaluate systemic inflammation and nutritional status in patients with cancer based on serum concentrations of C-reactive protein (CRP) and albumin. The mGPS has been validated as an independent prognostic factor in various malignancies, including hypopharyngeal cancer,5 and Iuchi et al5 reported that combining the mGPS with conventional TNM staging provided more accurate prognostic predictions. Recent research has also established that the high-sensitivity mGPS (HS-mGPS) is an even more sensitive prognostic marker for several cancers.6,7 The mGPS is calculated as a score of 0 (CRP concentration ≤1.0 mg/dL), 1 (CRP concentration >1.0 mg/dL), or 2 (CRP concentration >1.0 mg/dL and albumin concentration <3.5 mg/dL), while the CRP threshold level is set to 0.3 mg/dL for the HS-mGPS.6
This retrospective cohort study aimed to evaluate whether HS-mGPS could predict outcomes among patients with OPC and to compare other risk factors, including HPV.
Materials and Methods
Patient Selection
This retrospective study evaluated 117 consecutive patients who underwent initial treatment for OPC at the Department of Otolaryngology, Head and Neck Surgery at Kagoshima University between March 2009 and June 2020. The exclusion criteria were (1) 2 duplicated cancer cases, (2) 3 cases with recurrence, (3) 2 cases with distant metastasis, and (4) 4 cases in which the CRP and albumin concentrations were not measured at the initial diagnosis. Based on these criteria, the study ultimately included 106 patients with OPC. The pathological classification of the primary tumor, degree of lymph node involvement, and presence of organ metastasis were determined according to the eighth edition of the Union for International Cancer Control TNM classification system.8 This retrospective study was approved by the institutional review board of Kagoshima University (180238).
HPV Status
The tumor HPV status was determined using p16 immunostaining and HPV DNA detection using polymerase chain reaction (PCR). All tests for the tumor HPV status were prospectively performed using untreated primary tumor tissue. Diffuse (at least more than 70%) and strong nuclear and cytoplasmic staining of p16 or the detection of high-risk HPV DNA (HPV 16, 18, 33, 35, 39, 45, 51, 52, 56, or 66) indicated positivity for HPV.
Treatment Protocol
After explaining the treatment options for surgery and chemoradiotherapy (CRT) or radiotherapy, informed consent for treatment was obtained from the patients. The general indications for surgery were as follows: T1 and T2 stage cancers for transoral surgery. Node-positive patients were also included if the tumors were resectable by neck dissection. Our procedure for transoral surgery was transoral videolaryngoscopic surgery. Postoperative adjuvant concurrent chemoradiotherapy was performed for high-risk patients based on positive surgical margins or extranodal/perineural invasion near the cervical lymph nodes. For definitive patients treated with chemoradiotherapy, the dose was 70 Gray (Gy) in 35 fractions with cisplatin (100 mg/m2 every 3 weeks). Postoperative patients received 60 Gy in 30 fractions. The radiotherapy was planned using computed tomography and administered as either 3-dimensional conformal or intensity-modulated radiotherapy.
Treatment Assessment and Follow-up
Clinical response was assessed according to the Response Evaluation Criteria in Solid Tumors (version 1.1) at 4 to 6 weeks after the completion of treatment. Follow-up evaluations included physical examination, blood tests, endoscopy, and enhanced computed tomography of the neck and chest. Patients were followed up every 3 months for the first year, every 6 months for the second year, and then annually thereafter.
Inflammation-Based Prognostic Scores
Laboratory testing had been performed on the day of admission, and the results were searched to determine the patients’ serum CRP and albumin concentrations. The HS-mGPS was calculated as previously reported6: a score of 2 was assigned to patients with an elevated CRP concentration (>0.3 mg/dL) and a reduced albumin concentration (<3.5 g/dL), a score of 1 was assigned to patients with an elevated CRP concentration (>0.3 mg/dL) and a nondecreased albumin concentration (≥3.5 g/dL), and a score of 0 was assigned to patients without an elevated CRP concentration (≤0.3 mg/dL), regardless of their albumin concentration.
Statistical Analyses
All statistical analyses were performed using SPSS for Windows software (version 22.0; SPSS, Inc). Overall survival (OS) was defined as the primary end point and was calculated as the interval between the first admission (the same time as the blood test) and the first instance of the date of death due to any cause or the last follow-up. Disease-free survival (DFS) was defined as the time to tumor recurrence or death. Survival outcomes were estimated using the Kaplan-Meier method and compared using the log-rank test. Subjects were treated as censored if they were lost to follow-up. The relationships between survival outcomes and the HS-mGPS were evaluated using multivariate Cox proportional hazards models, and the results were reported as the hazard ratio (HR) and 95% CI. All tests were 2-sided, and differences were considered significant at P < .05.
Results
Patient Characteristics
Table 1 shows the baseline characteristics of the 106 included patients. The average follow-up period was 42 months (range, 3-132 months). The median age at diagnosis was 66 years (range, 40-85 years), and most patients were male (88.7%). Most patients had a performance status of 0 (78.3%) and stage IV disease (44.4%). There were 44 (41.5%) patients who were positive for HPV and 62 (58.5%) patients who were negative or unknown. The primary tumors were located at the palatine tonsils (68.0%), base of the tongue (24.4%), posterior pharyngeal wall (3.8%), and soft palate (3.8%). The HS-mGPS scores were 0 for 57 (53.8%) patients, 1 for 37 (35.0%) patients, and 2 for 12 (11.2%) patients. The HS-mGPS was significantly associated with age (P = .037), Eastern Cooperative Oncology Group performance status (P = .007), and HPV (P = .013). Median radiotherapy dose was 70 Gy (range, 58-70 Gy) for definitive radiotherapy.
Table 1.
Patient Demographics (n = 106).
| Characteristic | No. (%) | HS-mGPS, No. | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | P valuea | ||
| Age | |||||
| <66 years | 58 (54.7) | 36 | 14 | 8 | .037 |
| ≥66 years | 48 (45.3) | 21 | 23 | 4 | |
| Sex | |||||
| Male | 94 (88.7) | 49 | 34 | 11 | .636 |
| Female | 12 (11.3) | 8 | 3 | 1 | |
| ECOG PS | |||||
| 0 | 83 (78.3) | 45 | 25 | 5 | .007 |
| 1 | 20 (18.9) | 12 | 9 | 4 | |
| 2 | 3 (2.8) | 0 | 3 | 3 | |
| HPV | |||||
| Positive | 44 (41.5) | 31 | 9 | 4 | .013 |
| Negative | 62 (58.5) | 26 | 28 | 8 | |
| Smoking status | |||||
| Nonsmoker | 22 (20.8) | 12 | 9 | 1 | |
| Smoker (current or ex) | 84 (79.2) | 45 | 28 | 11 | |
| Drinking history | |||||
| Yes | 29 | 16 | 10 | 3 | .964 |
| None | 76 | 40 | 27 | 9 | |
| Tumor stage | |||||
| T1 | 11 (10.4) | 5 | 5 | 1 | .332 |
| T2 | 45 (42.5) | 29 | 11 | 5 | |
| T3 | 21 (19.9) | 12 | 8 | 1 | |
| T4 | 29 (27.2) | 11 | 13 | 5 | |
| Nodal stage | |||||
| N0 | 22 (20.8) | 12 | 9 | 1 | .227 |
| N1 | 34 (32.1) | 23 | 7 | 4 | |
| N2 | 43 (40.6) | 20 | 18 | 5 | |
| N3 | 7 (6.5) | 2 | 3 | 2 | |
| AJCC stage | |||||
| I | 29 (27.3) | 20 | 6 | 3 | .123 |
| II | 16 (15.1) | 10 | 4 | 2 | |
| III | 14 (13.2) | 9 | 5 | 0 | |
| IV | 47 (44.4) | 18 | 22 | 7 | |
| Tumor location | |||||
| Palatine tonsils | 72 (68.0) | 43 | 21 | 8 | .163 |
| Base of the tongue | 26 (24.4) | 10 | 14 | 2 | |
| Posterior pharyngeal wall | 4 (3.8) | 1 | 2 | 1 | |
| Soft palate | 4 (3.8) | 3 | 0 | 1 | |
| Treatment | |||||
| Radiotherapy | 17 (16.1) | 11 | 5 | 1 | .743 |
| Chemoradiotherapy | 84 (79.2) | 43 | 30 | 11 | |
| TOVS + ND | 5 (4.7) | 3 | 2 | 0 | |
Abbreviations: AJCC, American Joint Committee on Cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; HPV, human papillomavirus; HS-mGPS, high-sensitivity modified Glasgow prognostic score; ND, neck dissection; TOVS, transoral videolaryngoscopic surgery.
Appropriate statistical test (Student t test, Mann-Whitney U, χ2 test, or Fisher exact test) conducted between HS-mGPS.
Relationships Between OS and the HS-mGPS
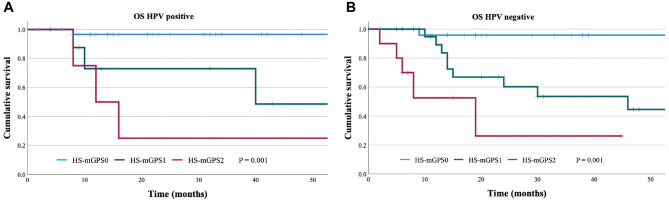
The Kaplan-Meier curves for OS with HPV-positive patients were compared according to the HS-mGPS (Figure 1A), which revealed 3-year OS rates of 96.6% for the HS-mGPS0 group, 48.6% for the HS-mGPS1 group, and 25.0% for the HS-mGPS2 group. Significant differences in the OS outcomes were observed between the HS-mGPS0, HS-mGPS1, and HS-mGPS2 groups (HR, 4.36; 95% CI, 1.76-10.80; P = .001). The Kaplan-Meier curves for OS with HPV-negative patients were also compared according to the HS-mGPS (Figure 1B), which revealed 3-year OS rates of 95.8% for the HS-mGPS0 group, 53.5% for the HS-mGPS1 group, and 26.2% for the HS-mGPS2 group. Significant differences in the OS outcomes were observed between the HS-mGPS0, HS-mGPS1, and HS-mGPS2 groups (HR, 4.73; 95% CI, 2.17-10.27; P < .0001).
Figure 1.
Kaplan-Meier overall survival (OS) curves for the HS-mGPS. HS-mGPS was significantly associated with (A) HPV-positive (log-rank P = .001) and (B) HPV-negative (log-rank P = .001) patients. HPV, human papillomavirus; HS-mGPS, high-sensitivity modified Glasgow prognostic score.
In HPV-positive patients, univariate analyses revealed that OS was associated with PS (P = .003) and the HS-mGPS (P = .001) (Table 2). Multivariable analysis revealed that the HS-mGPS independently predicted OS (HR, 2.970; 95% CI, 0.979-9.016; P = .045). In HPV-negative patients, univariate analyses revealed that OS was associated with PS (P < .001) and the HS-mGPS (P < .001) (Table 2). Multivariable analysis revealed that the HS-mGPS independently predicted OS (HR, 5.778; 95% CI, 2.247-14.856; P < .001).
Table 2.
Univariate and Multivariable of Overall Survival and Disease-Free Survival in HPV-Positive and HPV-Negative Oropharyngeal Squamous Cell Carcinoma.
| Variable | Overall survival |
Disease-free survival |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate, HR (95% CI) | P valuea | Multivariable, HR (95% CI) | P valueb | Univariate, HR (95% CI) | P valuea | Multivariable, HR (95% CI) | P valueb | |
| HPV positive (n = 44) | ||||||||
| Age (continuous) | 0.94 (0.18-4.85) | .94 | 0.70 (0.19-2.54) | .58 | ||||
| Sex (males vs females) | 0.44 (0.00-3.16) | .63 | 0.90 (0.12-6.90) | .92 | ||||
| ECOG PS (0 vs 1-2) | 5.18 (1.74-15.40) | <.01 | 2.21 (0.63-7.77) | .22 | 1.94 (0.89-4.23) | .10 | ||
| Tumor stage (1, 2, 3, 4) | 1.23 (0.50-3.05) | .65 | 0.85 (0.43-1.68) | .63 | ||||
| Nodal stage (0, 1, 2, 3) | 1.04 (0.32-3.38) | .94 | 1.90 (0.86-4.20) | .11 | ||||
| AJCC stage (I, II, III, IV) | 0.77 (0.26-2.30) | .64 | 0.89 (0.43-1.81) | .74 | ||||
| Tumor location (palatine tonsils vs others) | 0.13 (0.00-77.14) | .53 | 0.71 (0.23-2.25) | .57 | ||||
| Smoking status (nonsmoker vs ex or current) | 0.65 (0.13-3.38) | .61 | 0.36 (0.12-1.12) | .08 | ||||
| Drinking history (yes or none) | 0.62 (0.14-2.77) | .53 | 0.74 (0.24-2.28) | .60 | ||||
| HS-mGPS (0, 1, 2) | 4.364 (1.76-10.80) | <.01 | 2.97 (0.98-9.02) | .04 | 1.90 (1.00-3.63) | .04 | ||
| HPV negative (n = 62) | ||||||||
| Age (continuous) | 0.95 (0.34-2.64) | .94 | 0.85 (0.43-1.70) | .65 | ||||
| Sex (males vs females) | 0.39 (0.05-2.98) | .63 | 0.68 (0.21-2.23) | .52 | ||||
| ECOG PS (0 vs 1-2) | 4.38 (2.17-8.85) | <.01 | 4.22 (2.00-8.88) | <.01 | 1.60 (0.96-2.67) | .07 | ||
| Tumor stage (1, 2, 3, 4) | 1.23 (0.50-3.05) | .65 | 1.50 (1.05-2.16) | .03 | 1.36 (0.94-1.96) | .11 | ||
| Nodal stage (0, 1, 2, 3) | 1.45 (0.85-2.46) | .94 | 1.65 (1.08-2.50) | .02 | 1.31 (0.82-2.10) | .26 | ||
| AJCC stage (I, II, III, IV) | 1.51 (0.70-3.27) | .64 | 2.11 (1.11-4.01) | .02 | 1.28 (0.54-2.73) | .63 | ||
| Tumor location (palatine tonsils vs others) | 0.98 (0.49-1.94) | .53 | 1.07 (0.71-1.63) | .74 | ||||
| Smoking status (nonsmoker vs ex or current) | 1.05 (0.50-2.21) | .61 | 0.97 (0.60-1.57) | .90 | ||||
| Drink | 0.872 (0.28-2.75) | .53 | 0.86 (0.40-1.86) | .71 | ||||
| HS-mGPS (0, 1, 2) | 4.73 (2.18-10.27) | <.01 | 5.78 (2.25-14.86) | <.01 | 2.54 (1.59-4.07) | <.01 | 2.37 (1.46-3.86) | <.01 |
Abbreviations: AJCC, American Joint Committee on Cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; HPV, human papillomavirus; HR, hazard ratio; HS-mGPS, high-sensitivity modified Glasgow prognostic score.
P value from Kaplan-Meier log-rank test.
P value from Cox regression log-likelihood ratio test.
Relationships Between DFS and the HS-mGPS
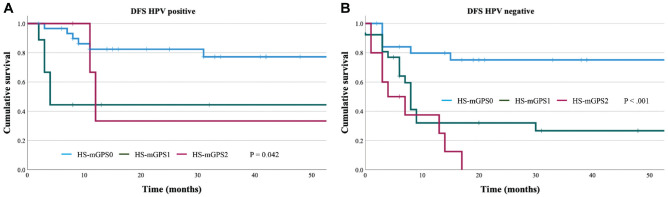
The Kaplan-Meier curves for DFS with HPV-positive patients were compared according to the HS-mGPS (Figure 2A), which revealed 3-year DFS rates of 77.3% in the HS-mGPS0 group, 44.4% in the HS-mGPS1 group, and 33.3% in the HS-mGPS2 group. Significant differences in the DFS outcomes were observed between the HS-mGPS0, HS-mGPS1, and HS-mGPS2 groups (HR, 1.900; 95% CI, 0.995-3.628; P = .042). The Kaplan-Meier curves for DFS with HPV-negative patients were also compared according to the HS-mGPS (Figure 2B), which revealed 3-year OS rates of 75.1% for the HS-mGPS0 group, 26.7% for the HS-mGPS1 group, and 0.0% for the HS-mGPS2 group. Significant differences in the DFS outcomes were observed between the HS-mGPS0, HS-mGPS1, and HS-mGPS2 groups (HR, 2.540; 95% CI, 1.585-4.072; P < .0001).
Figure 2.
The Kaplan-Meier disease-free survival curves for the HS-mGPS. HS-mGPS was significantly associated with (A) HPV-positive (log-rank P = .042) and (B) HPV-negative (log-rank P < .001) patients. HPV, human papillomavirus; HS-mGPS, high-sensitivity modified Glasgow prognostic score.
In HPV-positive patients, univariate analyses revealed that DFS was associated with the HS-mGPS (P < .042) (Table 2). In HPV-negative patients, univariate analyses revealed that DFS was associated with tumor stage (P = .027), nodal stage (P = .02), AJCC stage (P = .023), and the HS-mGPS (P < .001) (Table 2). Multivariable analysis revealed that DFS was only independently predicted by the HS-mGPS (HR, 2.374; 95% CI, 1.459-3.861; P < .001).
Discussion
The present study revealed that the HS-mGPS was significantly associated with outcomes among patients with OPC in the univariate analyses. However, only the HS-mGPS was independently associated with OS and DFS in the multivariable analysis regardless of HPV infection. Therefore, the results from this study indicate that the HS-mGPS may be used for predicting outcomes in all patients with OPC.
The inflammation-based GPS system is based on elevated serum CRP concentrations and hypoalbuminemia.9 Elevated serum CRP concentrations reflect a state of systemic inflammation and are generally associated with a higher cancer risk and poorer prognosis.10 Hypoalbuminemia reflects the hypercatabolic state of cancer cachexia, which is caused by cytokine activation, and it is commonly observed in patients with cancer.11 Previous studies have indicated that the GPS was superior to white blood cell count, neutrophil count, platelet count, the neutrophil-lymphocyte ratio, and the Edinburgh Clinical Risk Score for predicting survival among patients with cancer.12 Several studies have also investigated the association between the mGPS and outcomes among patients with head and neck cancer. Nakayama et al13 were the first to report the prognostic value of the mGPS in this setting, and Iuchi et al5 subsequently reported that combining the mGPS and conventional TNM staging provided more accurate prognostication. In addition, Chen et al14 have reported that the GPS may have prognostic value and guide personalized treatment among patients with metastatic nasopharyngeal carcinoma who received cisplatin-based palliative chemotherapy.
However, several studies have recently suggested that the HS-mGPS is superior to the mGPS as a prognostic marker for many cancer types.15,16 A large retrospective study of patients with resectable gastric cancer also revealed that the mGPS and HS-mGPS provided good preoperative prediction of OS outcomes, although the HS-mGPS was found to be superior based on multivariable Cox regression analysis.16 Another study compared the prognostic values of the GPS, mGPS, HS-mGPS, and other inflammation-based markers among patients with resectable non–small cell lung cancer, which also revealed that the HS-mGPS provided better ability to predict OS (vs the GPS and mGPS).17 Proctor et al15 reported that the HS-mGPS provided better prognostic value than the GPS and mGPS in a large cohort of patients with cancer. Furthermore, Hanai et al6 demonstrated that the HS-mGPS is superior to the mGPS as a prognostic predictor for head and neck cancer, and that study included patients with HSCC. However, there is limited evidence regarding the prognostic value of the HS-mGPS among patients with OPC, and the present study revealed that the HS-mGPS was an independent prognostic marker in cases of OPC. Therefore, when considered together, our findings and previous findings suggest that the HS-mGPS is a significant and independent predictor of survival outcomes and may be superior to the mGPS.
Huang et al18 identified that patients with p16-positive oropharyngeal cancer with high circulating neutrophil levels have a reduced OS and RFS (relapse-free survival). Interestingly, this association was not seen in the p16-negative oropharyngeal patients. Furthermore, higher levels of circulating lymphocytes were predictive of improved RFS and marginally improved OS in the p16-positive population but not in the p16-negative patients. In addition, in a study by Ward et al,19 patients with HPV-positive OPC with high or moderate tumor-infiltrating lymphocyte expression had significantly improved survival compared to patients with HPV-positive low tumor-infiltrating lymphocytes and HPV-negative patients regardless of lymphocyte expression. This would suggest within the HPV oropharyngeal cancer population the systemic and local inflammatory environment may be important for determination of clinical outcomes. In both studies, there is a significant minority of HPV-positive patients (20%) who have poor OS. In this study, even HPV-positive patients with HS-mGPS2 had a very poor 3-year OS rate of 25% and DFS of 12.2%. Therefore, one of the causes of poor prognosis in HPV-positive patients may be poor HS-mGPS.
Although the underlying mechanism is unclear, Hanai et al6 have reported that the precise CRP concentration can be used to accurately identify patients with poor expected prognoses. Technological advances have also permitted very accurate measurements of inflammatory markers, including CRP, even at relatively small values.20 In addition, there is increasing evidence that CRP concentrations of >0.3 mg/dL can predict a poor prognosis among patients with and without cancer,21 and Zacho et al22 have reported that elevated CRP concentrations are associated with increased risks of cardiovascular death, cancer death, and all-cause death. As elevated CRP concentrations do not per se cause early death, they are more likely a marker of hidden and potentially fatal inflammatory disease.22 Thus, even slight changes in inflammation might be related to potential cachexia and a subsequent poor prognosis, which could explain the ability of the HS-mGPS to independently predict survival outcomes and possibly identify cases of cancer cachexia.
This study has 2 important limitations. First, a small single-center retrospective study is prone to various sources of bias, including selection bias. Second, the HS-mGPS scores were calculated retrospectively, not by the clinicians who were making the treatment decisions, which is another potential source of information bias. The number of cases was limited, and patients who underwent various treatments were included in the population. However, if any bias were present, it would lead to nondifferential misclassification, as the treatment would be performed regardless of the HS-mGPS. Therefore, this may negate any potential bias in this respect. Therefore, to validate our findings, a larger-scale study incorporating the treatment modality should be performed.
Conclusion
The present study revealed that the HS-mGPS have prognostic value among patients with OPC, although only the HS-mGPS was independently associated with OS and DFS in the multivariable analysis. The greater prognostic value of the HS-mGPS may related to it being a more sensitive index of inflammation. Therefore, we suggest using the HS-mGPS for prognostication among patients with OPC.
Authors Contributions
Hiroyuki Iuchi, acquired and organized retrospective data, drafted initial rough draft of work, approved final version; Junichiro Ohori, acquired and organized retrospective data, interpreted data; Yumi Ando, analyzed retrospective data, revised work; Takeshi Tokushige, interpreted data, revised multiple drafts; Megumi Haraguchi, analyzed retrospective data, revised multiple drafts; Masaru Yamashita, approved final version.
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: None.
References
- 1.Elrefaey S, Massaro MA, Chiocca S, Chiesa F, Ansarin M.HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014;34:299-309. [PMC free article] [PubMed] [Google Scholar]
- 2.Moore KA, II, Mehta V.The growing epidemic of HPV-positive oropharyngeal carcinoma: a clinical review for primary care providers. J Am Board Fam Med. 2015;28:498-503. [DOI] [PubMed] [Google Scholar]
- 3.Misiukiewicz K, Camille N, Gupta V, et al. The role of HPV status in recurrent/metastatic squamous cell carcinoma of the head and neck. Clin Adv Hematol Oncol. 2014;12:812-819. [PubMed] [Google Scholar]
- 4.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iuchi H, Kyutoku T, Ito K, Matsumoto H, Ohori J, Yamashita M.Impacts of inflammation-based prognostic scores on survival in patients with hypopharyngeal squamous cell carcinoma. OTO Open. 2020;4(4):2473974X20978137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanai N, Sawabe M, Kimura T, et al. The high-sensitivity modified Glasgow prognostic score is superior to the modified Glasgow prognostic score as a prognostic predictor for head and neck cancer. Oncotarget. 2018;9(97):37008-37016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng C, Min F, Qiuyan W, Zhang X, Song T, Wu S.High-sensitivity modified Glasgow prognostic score (HS-mGPS) is superior to the mGPS in esophageal cancer patients treated with chemoradiotherapy. Oncotarget. 2017;8(59):99861-99870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [DOI] [PubMed] [Google Scholar]
- 9.Al Murri AM, Bartlett JM, Canney PA, Doughty JC, Wilson C, McMillan DC.Evaluation of an inflammation-based prognostic score (GPS) in patients with metastatic breast cancer. Br J Cancer. 2006;94(2):227-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allin KH, Bojesen SE, Nordestgaard BG.Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. 2009;27(13):2217-2224. [DOI] [PubMed] [Google Scholar]
- 11.Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A.Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34(1):94-104. [DOI] [PubMed] [Google Scholar]
- 12.Ramsey S, Lamb GW, Aitchison M, Graham J, McMillan DC.Evaluation of an inflammation-based prognostic score in patients with metastatic renal cancer. Cancer. 2007;109(2):205-212. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama M, Tabuchi K, Hara A.Clinical utility of the modified Glasgow prognostic score in patients with advanced head and neck cancer. Head Neck. 2015;37(12):1745-1749. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Sun P, Dai QS, Weng HW, Li HP, Ye S.The Glasgow prognostic score predicts poor survival in cisplatin-based treated patients with metastatic nasopharyngeal carcinoma. PLoS One. 2014;9(11):e112581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Proctor MJ, Horgan PG, Talwar D, Fletcher CD, Morrison DS, McMillan DC.Optimization of the systemic inflammation-based Glasgow prognostic score: a Glasgow Inflammation Outcome Study. Cancer. 2013;119(12):2325-2332. [DOI] [PubMed] [Google Scholar]
- 16.Takeno S, Hashimoto T, Shibata R, et al. The high-sensitivity modified Glasgow prognostic score is superior to the modified Glasgow prognostic score as a prognostic predictor in patients with resectable gastric cancer. Oncology. 2014;87(4):205-214. [DOI] [PubMed] [Google Scholar]
- 17.Osugi J, Muto S, Matsumura Y, Higuchi M, Suzuki H, Gotoh M.Prognostic impact of the high-sensitivity modified Glasgow prognostic score in patients with resectable non–small cell lung cancer. J Cancer Res Ther. 2016;12(2):945-951. [DOI] [PubMed] [Google Scholar]
- 18.Huang SH, Waldron JN, Milosevic M, et al. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes in oropharyngeal cancer stratified by human papillomavirus status. Cancer. 2015;121(4):545-555. [DOI] [PubMed] [Google Scholar]
- 19.Ward MJ, Thirdborough SM, Mellows T, Riley C, Harris S, Suchak K, et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer. 2014;110(2):489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rifai N, Tracy RP, Ridker PM.Clinical efficacy of an automated high-sensitivity C-reactive protein assay. Clin Chem. 1999;45(12):2136-2141. [PubMed] [Google Scholar]
- 21.Tuomisto K, Jousilahti P, Sundvall J, Pajunen P, Salomaa V.C-reactive protein, interleukin-6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality: a population-based, prospective study. Thromb Haemost. 2006;95(03):511-518. [DOI] [PubMed] [Google Scholar]
- 22.Zacho J, Tybjaerg-Hansen A, Nordestgaard BG.C-reactive protein and all-cause mortality—the Copenhagen City Heart Study. Eur Heart J. 2010;31(13):1624-1632. [DOI] [PubMed] [Google Scholar]