Abstract
Objective
Synovial fibrosis is a characteristic symptom of osteoarthritis (OA), which is closely associated with joint pain and stiffness. Previous studies have reported that low-intensity pulsed ultrasound (LIPUS) can alleviate cartilage degradation in OA. However, the functions and mechanisms of LIPUS in OA synovial fibrosis are still unknown.
Methods
The destabilization of the medial meniscus (DMM) mouse model of OA was established in C57 male mice and fibroblast-like synoviocytes (FLS) were isolated from synovial tissue of OA patients. The knee joint diameter, Masson's trichrome (MT) and Hematoxylin-eosin (HE) staining were used to evaluate synovial fibrosis and hyperplasia. The Immunohistochemistry (IHC) staining was performed to detected the expression of synovial fibrosis makers and the activation of Wnt/β-catenin signaling in vivo. FLS were treated with TGF-β1 to serve as an in vitro model of synovial fibrosis, Wnt3a was used to activate the Wnt/β-catenin signaling in cells. Cell proliferation was detected by using EdU assay, cell viability was performed by CCK8 assay. The protein levels of α-SMA, CTGF, Col Ⅰ, β-catenin, active β-catenin, c-Myc and cyclin D1 were examined by western blot and immunofluorescence staining.
Results
Two weeks after the LIPUS treatment, the synovial fibrosis, synovial hyperplasia and synoviocyte proliferation in the DMM model were significantly decreased. In vitro, LIPUS directly inhibited the TGF-β1-induced fibrotic response and proliferation of FLS. Meanwhile, LIPUS suppressed Wnt/β-catenin signaling in the synovium of DMM mice and cultured FLS. More importantly, we found that the synovial fibrosis makers, Wnt/β-catenin pathway downstream proteins and FLS proliferation were significantly decreased in Wnt3a-stimulated FLS following LIPUS treatment.
Conclusions
Our results present a novel role of LIPUS in OA-related synovial fibrosis, which is associated with its ability to repress Wnt/β-catenin signaling in FLS.
The translational potential of this article
This study provides new insight into the clinical application of LIPUS as a therapeutic option to manage synovial fibrosis in OA.
Keywords: Cell proliferation, Fibroblast-like synoviocyte, Low-intensity pulsed ultrasound, Osteoarthritis, Synovial fibrosis, Wnt/β-catenin signaling
1. Introduction
Osteoarthritis (OA) is one of the most common joint diseases in the middle-aged and elderly populations [1]. Previous studies have shown that the pathological changes to the synovium play an essential role in OA progression, which is characterized by hyperplasia, inflammatory cell infiltration, neovascularization, and fibrosis [[1], [2], [3], [4]]. Synovial fibrosis is one of the most important causes of joint pain and stiffness, which seriously affects the exercise ability and quality of life of patients with OA [5,6]. Clinically, it is estimated that more than half of patients with OA have synovial fibrosis [5]. Further studies have shown that fibroblast-like synoviocytes (FLS) are the effector cells for synovial fibrosis [7]. In the synovial tissue of OA patients, inflammation stimulates FLS to proliferate and secrete collagen, which leads to synovial fibrosis [8,9]. Thus, targeting FLS could lead to the development of novel therapies for synovial fibrosis that could improve the quality of life of patients with OA.
Low-intensity pulsed ultrasound (LIPUS) is a non-invasive and safe physical therapy that has been widely used in the clinical treatment of fractures, soft tissue injuries, and other diseases [10,11]. Additionally, previous studies have found that LIPUS can alleviate joint pain and improve the quality of life in OA patients [12]. Our previous study demonstrated that LIPUS improved OA cartilage degeneration by inhibiting the secretion of vascular endothelial growth factor (VEGF) [13]. Zhang et al. reported that LIPUS could inhibit OA synovial inflammation by reducing the production of mature interleukin-1β (IL-1β) in synovial macrophages [14]. Moreover, it has been reported that LIPUS could attenuate joint stiffness in a joint immobilization model, and myocardial interstitial fibrosis in obese diabetic mice [15,16]. However, the role and mechanism of LIPUS in OA synovial fibrosis and FLS are still not explicated.
Wnt/β-catenin signaling is closely associated with fibrosis related diseases [17]. Previous studies have shown that the canonical Wnt signaling pathways is activated in both articular cartilage and synovium during the pathological process of OA [18,19]. In addition, inhibition of Wnt/β-catenin signaling remarkedly alleviate synovial fibrosis in mouse model of OA [19]. On the other hand, it was reported that LIPUS increases the expression of β-catenin in Schwann cells, but have no effect on β-catenin expression in osteosarcoma cells [20,21]. However, the regulation of Wnt/β-catenin signaling in OA FLS by LIPUS remains unclear.
In the present study, we investigated the effect of LIPUS on OA synovial fibrosis in a murine destabilization of the medial meniscus (DMM) model as well as in cultured FLS isolated from the synovial tissue of OA patients, and further explored the mechanism of Wnt/β-catenin signaling in this process.
2. Materials and methods
2.1. Mouse model of OA
The animal protocols in this experiment were approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (no.2019-285). 6–8 weeks old C57BL/6 male mice were purchased (HuaFukang Biotechnology, Beijing, China). The DMM surgery was performed on the right knee joints, as described in our previous study [13]. 20 mice were then randomly divided into two groups, including the DMM group and the DMM + LIPUS group (n = 10, each group). Additionally, we used the left knee joint (healthy knee joint) as the control group. To avoid wound infection, we began treatment with LIPUS (30 mW/cm2, 20 min/d) (Smith & Nephew Exogen 4000, USA) 3 days after DMM surgery (Fig. 1A-C). On days 7 and 14 post-DMM surgery, the diameters of the knee joint of mice in each group were measured, and the horizontal distance between the highest point on both sides of the knee joint was measured with a vernier caliper when bent at 90°. Measurements were recorded 3 times and the average value was calculated [7].
Fig. 1.
The presentation of LIPUS treatment (A) Low-intensity pulsed ultrasound (LIPUS) (Smith & Nephew Exogen 4000, USA) (B and C) After anesthetized by intraperitoneal injection of pentobarbital, right knee joint of each mice was put on the probe of LIPUS with suitable gels (D) FLS was seeded in 3.5-cm-diameter dishes and the LIPUS probe was fixed on the bottom of the 3.5-cm-diameter dishes evenly covered with a coupling gel.
2.2. Histological analysis
The whole knee joint was harvested and fixed in 4 % paraformaldehyde (PFA) for 24 h. Then, samples were decalcified in 15 % EDTA for 14 days and embedded in paraffin. Whole knee joint sagittal sections were obtained at 5-μm sections. Mouse joint sections were stained with Masson's trichrome (MT) to assess the grade of synovial fibrosis. Scoring was performed by two blinded observers (Mengtong Guan and Gailan Wang). Three sections per mouse were scored. The score for each mouse was calculated to evaluate the degree of synovial fibrosis. There was a high level of agreement between the two blinded observers. The scoring was as follows: 0, absent. If present: 1, mild; 2, diffuse [22,23]. Hematoxylin-eosin (HE) staining was used to evaluate synovial thickness, the thickest part of the synovium from ten serial sections of each specimen were selected, and the thickness was measured using Image J software.
2.3. Immunohistochemistry (IHC)
The knee joint sections were dewaxed with xylene and subjected to heat-mediated antigen retrieval before the sections were sealed with goat serum. After incubation with the primary antibody overnight, the secondary antibody and horseradish peroxidase-labeled streptavidin biotin were added. Then, sections were dyed with methyl green. Primary antibodies: alpha-smooth muscle actin (α-SMA) (1:200 dilution; ab32575, Abcam, USA), connective tissue growth factor (CTGF) (1:200 dilution; 23936, Proteintech, China), collagen I (Col I) (1:200 dilution; ab34710, Abcam, USA), β-catenin (1:100 dilution; 9562S, CST, USA).
2.4. Clinical samples and cell culture
Synovial tissue was randomly obtained from OA patients during joint replacement surgery in the Department of Orthopedics, The First Affiliated Hospital of Chongqing Medical University. This study was approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University (no.2019-285), and informed consent was obtained from all study participants.
Human FLS were isolated with 1 mg/mL collagenase type I (2144866, Gibco Life Technologies, USA). Following enzymatic digestion, cells were grown in high glucose DMEM (SH30022.01, HyClone, USA). The FLS were used throughout passages 3–6. The cells were cultured at 37 °C under 5 % CO2, supplemented with 10 % FBS (04-001-1, Biological Industries, Israel) and 1 % penicillin-streptomycin solution (SV30010, HyClone, USA).
2.5. TGF-β1, Wnt3a, and LIPUS treatment
The clinical LIPUS therapeutic system (Smith & Nephew Exogen 4000, USA) was performed. The LIPUS parameters were 30 mW/cm2, 1.5 MHz and 20 % duty cycle, which were consistent with the previous studies [13,14]. FLS were treated with TGF-β1 (10804, Sino Biological, China) at 10 ng/mL for 24 h to induce a fibrotic response [[24], [25], [26]]. In addition, Wnt3a (5036-WN-010, R.D, USA) at 200 ng/mL for 24 h was used to activate the Wnt/β-catenin signaling pathway in FLS. The cells were treated with LIPUS for 20 min (Fig. 1D) and then the lysates were harvested.
2.6. Cell proliferation assay
We used the Cell-Light EdU Apollo567 kit (C10310, Ribobio, China) to evaluate cell proliferation. According to the manufacturer's protocol, the cells were seeded in 3.5-cm-diameter dishes. When Cells were grown to 50 % confluency and starved with 1 % FBS medium overnight. Then, replaced the complete culture medium (10 % FBS) and treated with TGF-β1 and Wnt3a. After LIPUS treatment, a 50 μM EdU culture medium was prepared by diluting EdU solution 1000:1; each 3.5-cm-diameter dish was supplemented with 1 mL for 2 h, and then fixed with 4 % PFA for 20 min. After washing with PBS three times, the cells were stained with APOLLO. Next, the samples were incubated with DAPI for 15 min at 37 °C. Finally, three randomly selected fields were captured using a fluorescence microscope (Olympus, Japan). In vivo, mice were intraperitoneally injected with 5 mg/kg EdU solution 4 h before harvest. Then, the joint tissue was decalcified in 15 % EDTA for 2 weeks. After that, all samples were embedded in frozen section medium and sectioned with a freezing microtome. The staining and imaging methods were the same as those used for the in vitro study.
2.7. Cell viability assay
We performed the cell counting kit-8 (CCK-8) (C6005, UE, China) to evaluate cell viability. FLS were seeded in 96-well plates. Then, treated with TGF-β1 and Wnt3a. After LIPUS treatment, 10 μl CCK-8 solution was added into each well of 96-well plates, followed by further incubation of 2 h at 37 °C according to the manufacturer's instruction. The cell viability was calculated after the absorbance was measured at 450 nm.
2.8. Western blotting
FLS were collected with RIPA lysis buffer (P0013B, Beyotime, China) containing protease inhibitors for protein extraction. Protein concentration was measured using the bicinchoninic acid (BCA) assay kit (P0012, Beyotime, China). Proteins were separated by 12 % SDS-PAGE and transferred to a PVDF membrane. After blocking with 5 % nonfat milk in TBST buffer for 1 h, the membrane was incubated with specific primary antibodies at 4 °C overnight. The membranes were washed three times with TBST, and incubated with secondary antibodies at 37 °C for 1.5 h. The membranes were washed three times with TBST, before immunoreactivity was detected by chemiluminescence. The primary antibodies included α-SMA (1:1000 dilution; ab32575, Abcam, USA), CTGF (1:1000 dilution; 23936, Proteintech, China), Col I (1:1000 dilution; ab34710, Abcam, USA), β-catenin (1:1000 dilution; 9562S, CST, USA), active β-catenin (1:1000 dilution; 8814S, CST, USA), cyclin D1 (1:1000 dilution; 55506T, CST, USA), and c-Myc (1:1000 dilution; H2911, Santa Cruz Biotechnology, USA).
2.9. α-SMA immunofluorescence staining in OA-FLS
The FLS were stimulated by TGF-β1 and treated with LIPUS, then fixed with 4 % PFA for 20 min. After washing three times with PBS, cells were permeabilized with 0.01 % Triton X-100 in PBS for 20 min, and then blocked for 1 h with blocking buffer (P0260, Beyotime). Cells were incubated with α-SMA primary antibody (1:200 dilution; ab32575, Abcam, USA) overnight at 4 °C. Cells were washed three times with PBS before incubation with Alexa Fluor 488 secondary antibody for 1 h at 37 °C. Finally, cells were incubated with DAPI for 15 min at 37 °C.
2.10. Statistical analysis
We used GraphPad Prism 7 software for statistical analysis. The results are represented as mean ± SD. One-way analysis of variance (ANOVA) followed by Tukey's post hoc test was used for comparison among three or four groups. p < 0.05 was regarded as statistically significant; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
3. Results
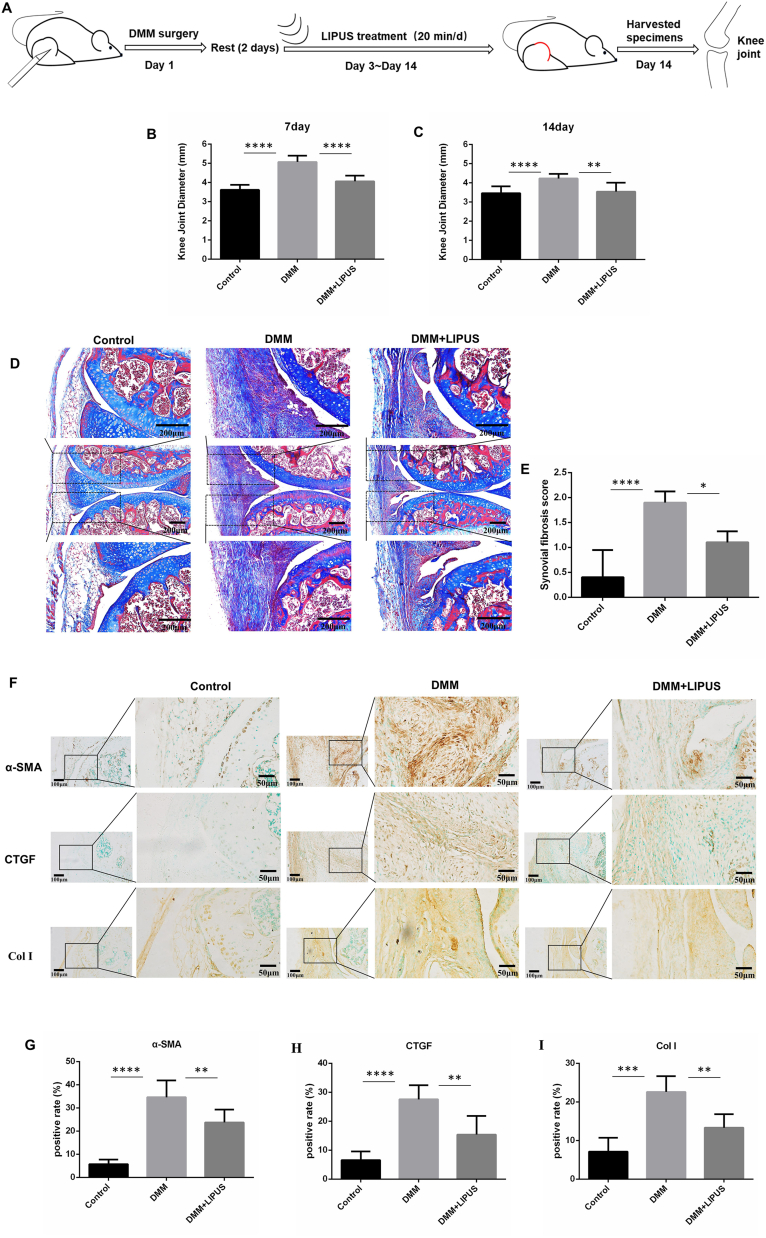
3.1. LIPUS attenuated synovial fibrosis in DMM mice
To explore whether LIPUS alleviated synovial fibrosis in DMM mice, a DMM model was established and mice were then treated with LIPUS for 2 weeks (Fig. 2A). In addition, the knee joint diameter, Masson's trichrome staining, and IHC staining were performed to evaluate synovial fibrosis [7,27]. On days 7 and 14, the diameter of the knee joint in mice in the DMM and DMM + LIPUS groups was significantly larger than that in mice in the control group, and the diameter of the knee joint in mice in the DMM + LIPUS group was significantly less than that in mice in the DMM group (p < 0.05) (Fig. 2B and C). Greater amounts of collagen fibers were observed in mice in the DMM group compared to mice in the DMM + LIPUS group (Fig. 2D). The degree of synovial fibrosis was scored as described previously [23]. Mice in the LIPUS-treated group had a significantly lower synovial fibrosis score than mice in the DMM group on day 14 post-surgery (Fig. 2E). Moreover, α-SMA, CTGF, and Col I, which are considered markers of synovial fibrosis, were evaluated by IHC [5,25,28]. The IHC results revealed a small amount of α-SMA, CTGF, and Col Ⅰ positive regions in mice in the control group. In contrast, α-SMA, CTGF, and Col Ⅰ positive regions, mainly located in the synovial membrane, were increased in mice in the DMM group. Compared to mice in the DMM group, LIPUS attenuated the expression of α-SMA, CTGF, and Col I (Fig. 2F–I). Collectively, these results suggested that LIPUS alleviated synovial fibrosis in DMM mice.
Fig. 2.
LIPUS attenuated synovial fibrosis in DMM mice (A) The DMM-induced OA mice were treated with LIPUS (30 mW/cm2, 20 min/d) as the indicated pattern in schematic diagram (B and C) The joint diameter of mice in each group (n = 7 per group) was calculated by vernier caliper on day 7 and 14 post-DMM surgery (D) The knee joints were harvested on day 14 after DMM surgery and Masson's trichrome staining was performed. The representative images from indicated groups of mice were listed. Scale bar: 200 μm (E) Synovial fibrosis score was performed by two blinded observers in each three sections per mouse (n = 5 per group) (0, absent. If present: 1, mild; 2, diffuse) (F) Immunohistochemical detection of α-SMA, CTGF and Col Ⅰ was performed in synovium of indicated groups of mice. Scale bar: 100 μm or 50 μm. (G–I) Percentage of α-SMA-positive, CTGF-positive and Col Ⅰ-positive rates in the synovium of each group were quantified (n = 5 per group). Statistical analysis was performed by one-way ANOVA, and multiple comparisons were performed using Tukey's test. Data are expressed as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
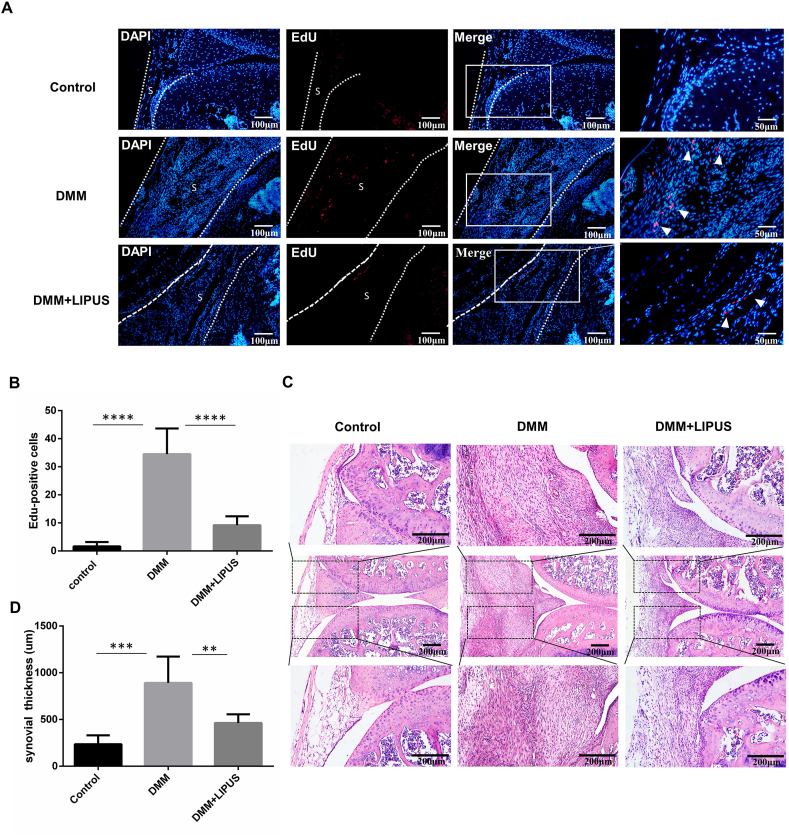
3.2. LIPUS inhibits synoviocyte proliferation and synovial hyperplasia in DMM mice
In the pathological process of OA, excessive synoviocyte proliferation is one of the most important causes of synovial fibrosis [9,29]. To investigate whether synoviocyte proliferation was hindered by LIPUS, we performed an EdU incorporation assay and found that the number of EdU-positive cells in the synovium of mice in the DMM + LIPUS group was significantly decreased in contrast to that of mice in the DMM group at 14 days post-surgery (Fig. 3A and B). In addition, we performed a synovial hyperplasia evaluation by HE staining [30]. The synovial thickness of mice in the DMM and DMM + LIPUS groups was significantly higher than that of mice in the control group. Additionally, compared to mice in the DMM group, the synovial thickness was significantly reduced in mice in the DMM + LIPUS group (Fig. 3C and D). The above results indicated that LIPUS attenuated the synoviocyte proliferation and synovial hyperplasia in DMM mice.
Fig. 3.
LIPUS inhibits synoviocyte proliferation and synovial hyperplasia (A) The mice were subjected to EdU assay and knee joints were harvested on day 14 after DMM surgery. S represents synovium. The dotted lines indicate the boundary of the synovium. Nuclei were stained by DAPI (blue) and EdU-positive cells appear red. Scale bar: 100 μm and 50 μm (B) Quantification of EdU-positive cells (n = 3 per group) (C) Representative hematoxylin and eosin-stained images of knee joints from indicated groups of mice. Scale bar = 200 μm (D) Synovial hyperplasia was measured by synovial thickness (n = 5 per group). Statistical analysis was performed by one-way ANOVA, and multiple comparisons were performed using Tukey's test. Data are expressed as mean ± SD. ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
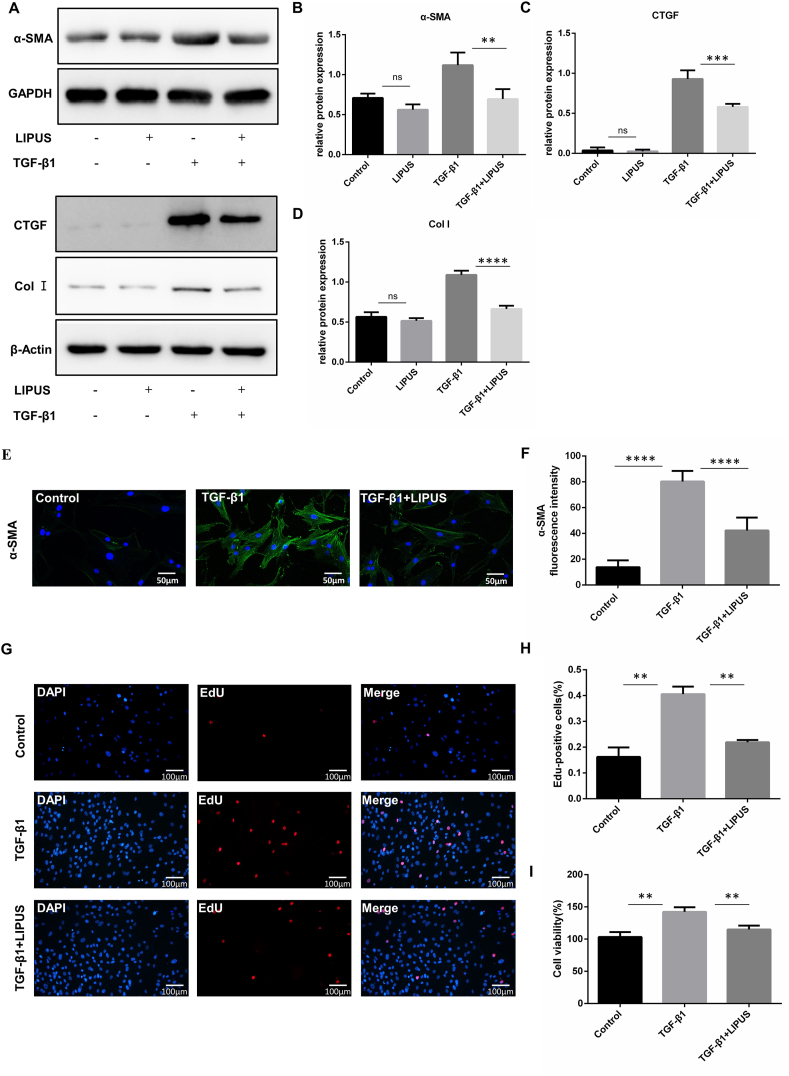
3.3. LIPUS directly inhibits the TGF-β1-induced fibrotic response and proliferation of FLS
TGF-β plays an important role in the occurrence and development of fibrotic diseases. Previous studies have demonstrated that TGF-β induces synovial fibrotic changes characterized by cell proliferation, Col I accumulation, and promoting the differentiation of FLS into a myofibroblast-like phenotype [9,25]. Therefore, we stimulated FLS with TGF-β1 to serve as an in vitro model of synovial fibrosis. In addition, we applied LIPUS after TGF-β1 exposure in FLS and detected the expression levels of fibrosis-related proteins by western blotting. In FLS treated with TGF-β1, we found that TGF-β1 significantly increased the expression of α-SMA, CTGF, and Col I compared to the control group. LIPUS attenuated TGF-β1-induced α-SMA, CTGF, and Col I expression in FLS (Fig. 4A–D). In addition, we performed immunofluorescence staining to evaluate the expression of α-SMA in FLS. The results showed that LIPUS significantly reduced the expression of α-SMA induced by TGF-β1, thereby inhibiting FLS differentiation into myofibroblast-like phenotype (Fig. 4E and F). Furthermore, LIPUS treatment remarkedly inhibited TGF-β1-induced FLS proliferation and viability (Fig. 4G–I). The above results indicated that LIPUS directly alleviated the fibrotic response and proliferation induced by TGF-β1.
Fig. 4.
LIPUS directly inhibits the TGF-β1-induced fibrotic response and proliferation of FLS. FLS were stimulated with TGF-β1 for 24 h and treated with or without LIPUS for 20 min (A–D) The protein levels of α-SMA, CTGF and Col Ⅰ were measured by western blot (n = 3 per group) (E) Immunofluorescence assay was taken to measure the expression of α-SMA in FLS. Scale bar:50 μm (F) Quantification of α-SMA fluorescence intensity (n = 5 per group) (G) The EdU assay was taken to detect the proliferation of FLS. Nuclei were stained with DAPI (blue) and EdU-positive cells appear red. Scale bar: 100 μm (H) Quantification of EdU-positive cells in three randomly selected microscope fields. EdU Positive rate was calculated as total number cells divided by positive cells (n = 3 per group) (I) CCK8 assay was performed to examine the FLS viability. Statistical analysis was performed by one-way ANOVA, and multiple comparisons were performed using Tukey's test. Data are expressed as mean ± SD. ns, not significant, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
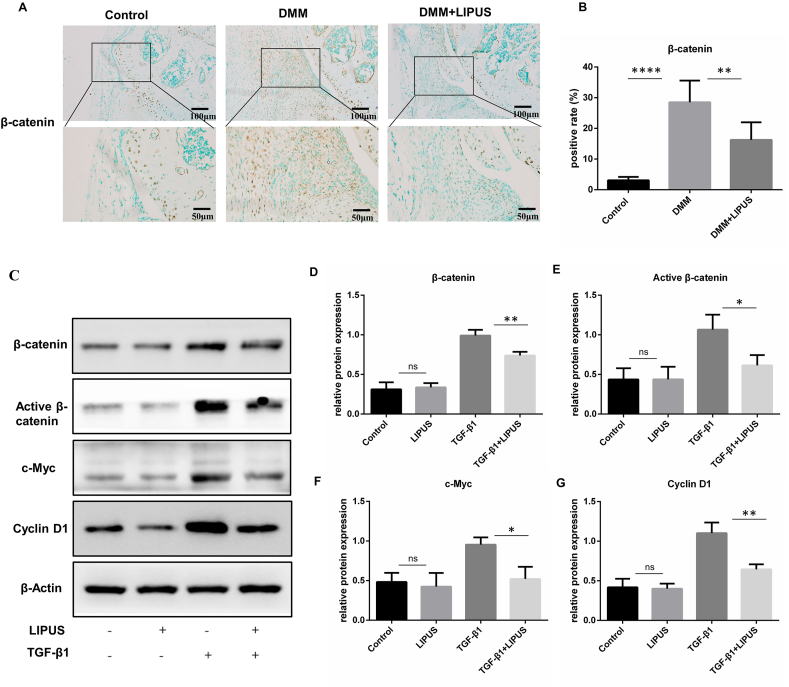
3.4. LIPUS suppresses Wnt/β-catenin signaling in the synovium of DMM mice and cultured FLS
The Wnt/β-catenin pathway is tightly associated with cell proliferation, fibrosis, embryonic skeletal formation, tissue repair, and joint homeostasis [17,31]. A previous study reported that Wnt/β-catenin signaling was activated in the FLS of DMM mice and OA patients, and pharmacological inhibition of Wnt/β-catenin signaling attenuated the proliferation of FLS [19]. Thus, we further determined the activation of Wnt/β-catenin signaling in the synovium of mice by IHC. The results revealed that β-catenin- positive cells were increased in synovium of the DMM group compared with control group. In addition, the number of β-catenin-positive cells in DMM mice treated with LIPUS was decreased, indicating that LIPUS inhibited Wnt/β-catenin signaling in the synovium of DMM mice (Fig. 5A and B). To Further investigate the effect of LIPUS on Wnt/β-catenin signaling in vitro, we detected the expression of β-catenin, activate β-catenin and the downstream proliferation-related proteins (c-Myc and cyclin D1) in FLS treated with TGF-β1 by western blotting. The results showed that TGF-β1 increased the expression of β-catenin, activate β-catenin, c-Myc and cyclin D1 compared with the control. In addition, the protein level of β-catenin, activate β-catenin, c-Myc and cyclin D1 was significantly downregulated by LIPUS in TGF-β1-treated FLS (Fig. 5C–G). These data proved that LIPUS suppresses Wnt/β-catenin signaling in TGF-β1-treated FLS.
Fig. 5.
LIPUS suppresses Wnt/β-catenin signaling in vivo and in vitro (A and B) Immunohistochemical detection of β-catenin was performed in synovium of indicated groups of mice (n = 5 per group). Scale bar:100 μm and 50 μm (C–G) FLS were stimulated with TGF-β1 for 24 h and treated with or without LIPUS for 20 min, and then the protein levels of β-catenin, active β-catenin, c-Myc and cyclin D1 were measured by western blot (n = 3 per group). Statistical analysis was performed by one-way ANOVA, and multiple comparisons were performed using Tukey's test. Data are expressed as mean ± SD. ns, not significant, ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗∗p < 0.0001.
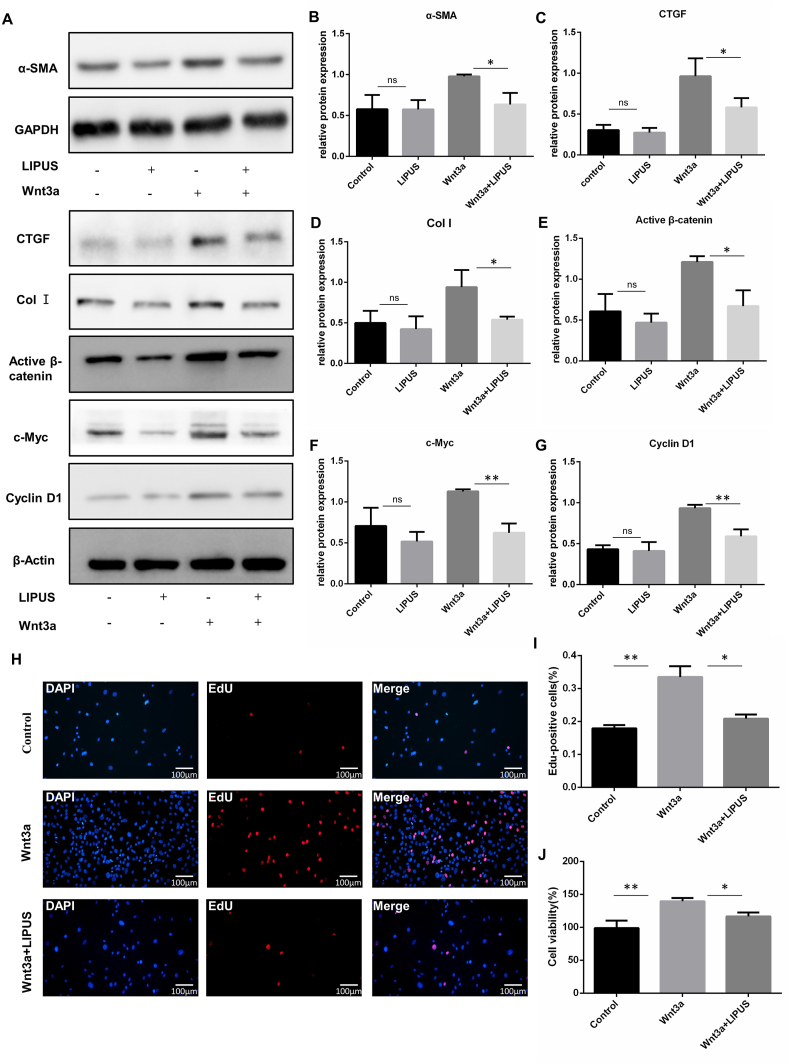
3.5. LIPUS inhibits FLS proliferation and fibrotic response by regulating Wnt/β-catenin signaling
To identify whether LIPUS modulates FLS proliferation and fibrotic response by regulating the Wnt/β-catenin pathway, FLS were stimulated with Wnt3a recombinant protein to stimulate the Wnt/β-catenin pathway in the absence or presence of LIPUS. The results showed that LIPUS inhibited the activation of Wnt/β-catenin signaling and the expression of fibrosis markers (α-SMA, CTGF and Col I) in FLS (Fig. 6A–G). In addition, we found that the FLS proliferation level and cell viability were significantly increased in Wnt3a-stimulated FLS, after LIPUS treatment, the FLS proliferation level and cell viability were significantly decreased (Fig. 6H–J). These data suggested that LIPUS inhibited the proliferation and fibrotic response of FLS by suppressing the Wnt/β-catenin signaling pathway.
Fig. 6.
LIPUS inhibits FLS proliferation and fibrosis by regulating Wnt/β-catenin signaling. FLS were stimulated with Wnt3a for 24 h and treated with or without LIPUS for 20 min (A–G) The protein levels of α-SMA, CTGF, Col Ⅰ, active β-catenin, c-Myc and cyclin D1 were measured by western blot (n = 3 per group) (H) The EdU assay was taken to detect the proliferation of FLS. Nuclei were stained with DAPI (blue) and EdU-positive cells appear red. Scale bar: 100 μm (I) Quantification of EdU-positive cells in three randomly selected microscope fields. EdU-positive rate was calculated as total number cells divided by positive cells (n = 3 per group) (J) CCK8 assay was performed to examine the FLS viability. Statistical analysis was performed by one-way ANOVA, and multiple comparisons were performed using Tukey's test. Data are expressed as mean ± SD. ns, not significant, ∗p < 0.05 and ∗∗p < 0.01.
4. Discussion
The synovial membrane is an essential part of the synovial joint, and synovial fibrosis results in joint pain and loss of normal joint function in patients with OA [5]. Various factors influence synovial fibrosis, and studies have shown that synovial macrophages mediate osteophytes, synovial fibrosis, and other pathological processes of OA [29]. Zhang et al. reported that inhibited synovial macrophage pyroptosis could alleviate synovial fibrosis in OA [27]. Furthermore, another study found that cartilage-specific ablation of mTOR could significantly reduce synovial fibrosis [32]. It has further been reported that LIPUS could regulate the function of synovial macrophages and chondrocytes in OA [13,14,33], demonstrating that the inhibition of synovial fibrosis by LIPUS may be a comprehensive effect. In OA synovial membranes, synovial macrophages produce tumor necrosis factor and IL-1β to promote the inflammatory and destructive response of FLS, as well as stimulate the proliferation of the synovium [34]. These results suggest that LIPUS may indirectly inhibit the proliferation of FLS through synovial macrophages. In the present study, our data showed that LIPUS can directly inhibit the activation of FLS.
To date, LIPUS has been widely used in the treatment of fractures, soft tissue injuries, and inflammation diseases [11,14,35]. However, previous studies have shown that the effects of LIPUS on cell proliferation are not constant. Xu et al. reported that LIPUS suppressed rat visceral preadipocyte proliferation and promoted apoptosis [36]. In contrast, some studies found that LIPUS could promote the proliferation of human amnion-derived mesenchymal stem cells [37] and chondrocytes [33]. However, it has also been reported that LIPUS has little effect on epithelial cell proliferation [38]. In our study, we found that LIPUS reduced the proliferation of FLS in vivo and in vitro. In addition, the role of LIPUS varies among fibroblasts in different tissues. In skin tissue, LIPUS promotes wound healing by increasing the migration of skin fibroblasts to the wound site [39]. In periodontal ligament fibroblasts, LIPUS suppresses LPS-PG, IL-1β, and TNF-α induced an inflammatory response by phosphorylation of Rho-associated kinase 1 [40]. Therefore, LIPUS may play a different role in different tissues and cells, and it is necessary to investigate the cell-specific effect of LIPUS in different diseases.
Numerous studies have shown that TGF-β enhances OA progression. In the synovial membrane, TGF-β signaling induces the proliferation of FLS and the accumulation of type I and III collagen, leading to synovial fibrosis [8,24]. Our study demonstrated that LIPUS directly inhibits the TGF-β1-induced fibrotic response in FLS.
Furthermore, previous studies have shown that the canonical Wnt pathway is highly expressed in osteoarthritis, which leads to the increase of FLS proliferation level and exacerbates synovial fibrosis [19]. Inhibition of Wnt/β-catenin signaling can effectively alleviate the process of synovial fibrosis and reduce the proliferation of FLS [19]. In this study, we found that LIPUS significantly inhibited the Wnt/β-catenin signaling activation, FLS proliferation and fibrotic response in the DMM model as well as in cultured FLS isolated from the synovial tissue of OA patients. Previous studies have found that LIPUS has different effects on Wnt/β-catenin signaling in different cells [20,21]; our results provide evidence that LIPUS attenuated FLS proliferation and fibrotic response by inhibiting of Wnt/β-catenin signaling, although additional studies are needed to clarify the precise molecular mechanisms.
5. Conclusions
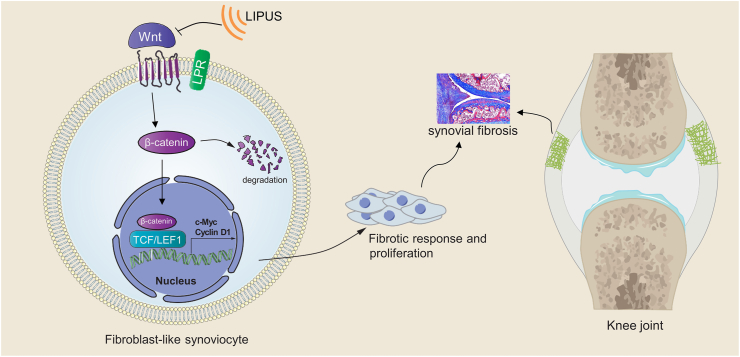
In brief, our study is the first to demonstrate that LIPUS could inhibit the proliferation and fibrotic response of FLS through the Wnt/β-catenin pathway, thereby alleviating synovial fibrosis in OA (Fig. 7). This study provides new insight into the clinical application of LIPUS as a therapeutic option to manage synovial fibrosis in OA. In future, we will further study the optimal parameters of LIPUS in the treatment of synovial fibrosis and the deeper molecular mechanism.
Fig. 7.
Schematic diagram. LIPUS alleviates synovial fibrosis in OA by inhibiting the fibrotic response and proliferation of FLS, and this effect is mainly exerted through the inhibition of Wnt/β-catenin signaling.
Credit author statement
Conception and design of study: B.Liao, M.T.Guan, H.Chen, K.T.Li; acquisition of data: B.Liao, R.B.Zhang, J.L.Huang, M.Liu; analysis and/or interpretation of data: B.Liao, Q.Y.Tan, M.T.Guan, G.L,Wang. Drafting the manuscript: B.Liao; revising the manuscript critically for important intellectual content: Y.Zhu, D.Q.Bai, Q.Y.Tan.
Funding
This study was supported by grants from the Natural Science Foundation of Chongqing [grant numbers cstc2020jcy-j-msxmX0935] and the National Natural Science Foundation of China [grant numbers 82002402 and 81871853].
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
All persons who have made substantial contributions to the work reported in the manuscript (e.g., technical help, writing and editing assistance, general support), but who do not meet the criteria for authorship, are named in the Acknowledgements and have given us their written permission to be named. If we have not included an Acknowledgements, then that indicates that we have not received substantial contributions from non-authors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2021.08.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
figs1.
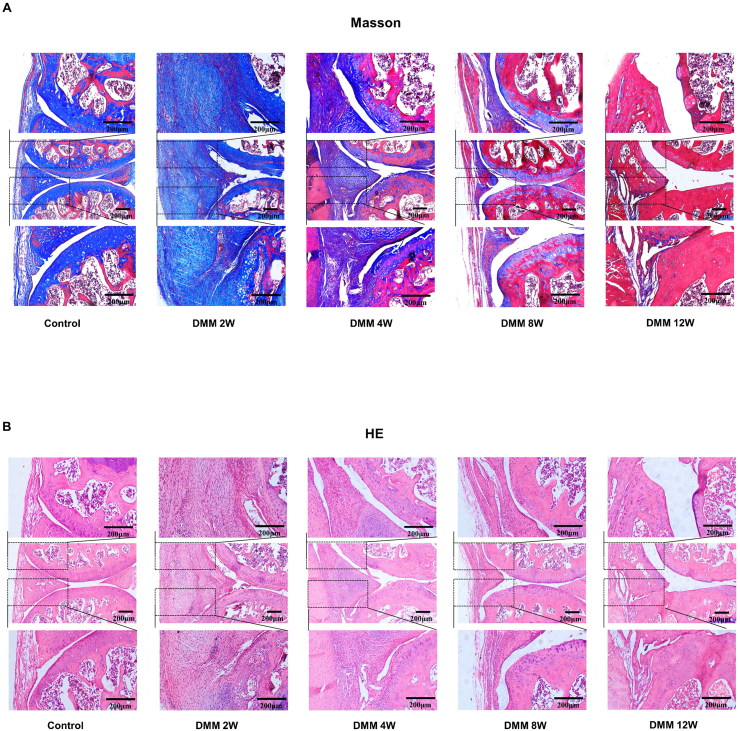
Histological evaluation of the synovium at different time points after DMM. Histologic sections of the knee joints at different time points (2w, 4w, 8w, 12w) after DMM surgery were analyzed by Masson’s trichrome and H&E staining. Representative sections stained with Masson's trichrome (A) and H&E (B). Scale bar: 200μm.
References
- 1.Scanzello C.R., Goldring S.R. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2):249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathiessen A., Conaghan P.G. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19(1):18. doi: 10.1186/s13075-017-1229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J., Wang Y., Zhang H., Gao W., Lu M., Liu W. Forkhead box C1 promotes the pathology of osteoarthritis by upregulating β-catenin in synovial fibroblasts. FEBS J. 2020;287(14):3065–3087. doi: 10.1111/febs.15178. [DOI] [PubMed] [Google Scholar]
- 4.Sun A.R., Udduttula A., Li J., Liu Y., Ren P.G., Zhang P. Cartilage tissue engineering for obesity-induced osteoarthritis: physiology, challenges, and future prospects. J Orthopaedic Trans. 2021;26:3–15. doi: 10.1016/j.jot.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remst D.F., Blaney Davidson E.N., van der Kraan P.M. Unravelling osteoarthritis-related synovial fibrosis: a step closer to solving joint stiffness. Rheumatology. 2015;54(11):1954–1963. doi: 10.1093/rheumatology/kev228. [DOI] [PubMed] [Google Scholar]
- 6.O'Neill T.W., Felson D.T. Mechanisms of osteoarthritis (OA) pain. Curr Osteoporos Rep. 2018;16(5):611–616. doi: 10.1007/s11914-018-0477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L., Zhang L., Huang Z., Xing R., Li X., Yin S. Increased HIF-1alpha in knee osteoarthritis aggravate synovial fibrosis via fibroblast-like synoviocyte pyroptosis. Oxid Med Cell Longev. 2019;2019:6326517. doi: 10.1155/2019/6326517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song C., Xu X., Wu Y., Ji B., Zhou X., Qin L. Study of the mechanism underlying hsa-miR338-3p downregulation to promote fibrosis of the synovial tissue in osteoarthritis patients. Mol Biol Rep. 2019;46(1):627–637. doi: 10.1007/s11033-018-4518-8. [DOI] [PubMed] [Google Scholar]
- 9.Shen J., Li S., Chen D.J.B.R. TGF-β signaling and the development of osteoarthritis. Bone Res. 2014;2:73–79. doi: 10.1038/boneres.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin G., Amanda R.M., Lin M., Xin Z., Tom L. Effects and mechanisms of low-intensity pulsed ultrasound for chronic prostatitis and chronic pelvic pain syndrome. Int J Mol Sci. 2016;17(7):1057. doi: 10.3390/ijms17071057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busse J.W., Bhandari M., Kulkarni A.V., Tunks E. The effect of low-intensity pulsed ultrasound therapy on time to fracture healing: a meta-analysis. CMAJ (Can Med Assoc J) : Canad Med Assoc J. 2002;166(4):437–441. [PMC free article] [PubMed] [Google Scholar]
- 12.Jia L., Wang Y., Chen J., Chen W.J.S.R. Efficacy of focused low-intensity pulsed ultrasound therapy for the management of knee osteoarthritis: a randomized, double blind, placebo-controlled trial. Sci Rep. 2016;6:35453. doi: 10.1038/srep35453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan M., Zhu Y., Liao B., Tan Q., Qi H., Zhang B. Low-intensity pulsed ultrasound inhibits VEGFA expression in chondrocytes and protects against cartilage degeneration in experimental osteoarthritis. FEBS open bio. 2020;10(3):434–443. doi: 10.1002/2211-5463.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B., Chen H., Ouyang J., Xie Y., Chen L.J.A. SQSTM1-dependent autophagic degradation of PKM2 inhibits the production of mature IL1B/IL-1β and contributes to LIPUS-mediated anti-inflammatory effect. Autophagy. 2020;16(7):1262–1278. doi: 10.1080/15548627.2019.1664705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itaya N., Yabe Y., Hagiwara Y., Kanazawa K., Koide M., Sekiguchi T. Effects of low-intensity pulsed ultrasound for preventing joint stiffness in immobilized knee model in rats. Ultrasound Med Biol. 2018;44(6):1244–1256. doi: 10.1016/j.ultrasmedbio.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Yuto M., Tomohiko S., Kumiko E., Ryo K., Yuta K., Yosuke I. Low-intensity pulsed ultrasound ameliorates cardiac diastolic dysfunction in mice-A possible novel therapy for HFpEF. Cardiovasc Res. 2020;19 doi: 10.1093/cvr/cvaa221. cvaa221. [DOI] [PubMed] [Google Scholar]
- 17.Lam A.P., Gottardi C.J. beta-catenin signaling: a novel mediator of fibrosis and potential therapeutic target. Curr Opin Rheumatol. 2011;23(6):562–567. doi: 10.1097/BOR.0b013e32834b3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dell'Accio F., De Bari C., El Tawil N.M., Barone F., Mitsiadis T.A., O'Dowd J. Activation of WNT and BMP signaling in adult human articular cartilage following mechanical injury. Arthritis Res Ther. 2006;8(5):R139. doi: 10.1186/ar2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caressa L., Brian W., Sarah L., Andrew S., Madhur S., Evgeny R. Inhibition of Wnt/β-catenin signaling ameliorates osteoarthritis in a murine model of experimental osteoarthritis. JCI Insight. 2018;3(3) doi: 10.1172/jci.insight.96308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren C., Chen X., Du N., Geng S., Hu Y., Liu X. Low-intensity pulsed ultrasound promotes Schwann cell viability and proliferation via the GSK-3β/β-catenin signaling pathway. Int J Biol Sci. 2018;14(5):497–507. doi: 10.7150/ijbs.22409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawai Y., Murata H., Koto K., Matsui T., Horie N., Ashihara E. Effects of low-intensity pulsed ultrasound on osteosarcoma and cancer cells. Oncol Rep. 2012;28(2):481–486. doi: 10.3892/or.2012.1816. [DOI] [PubMed] [Google Scholar]
- 22.Morko J., Kiviranta R., Joronen K., Säämänen A.M., Vuorio E., Salminen-Mankonen H. Spontaneous development of synovitis and cartilage degeneration in transgenic mice overexpressing cathepsin K. Arthritis Rheum. 2005;52(12):3713–3717. doi: 10.1002/art.21423. [DOI] [PubMed] [Google Scholar]
- 23.Joronen K., Ala-aho R., Majuri M.L., Alenius H., Kähäri V.M., Vuorio E. Adenovirus mediated intra-articular expression of collagenase-3 (MMP-13) induces inflammatory arthritis in mice. Ann Rheum Dis. 2004;63(6):656–664. doi: 10.1136/ard.2003.009720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qadri M.M., Jay G.D., Ostrom R.S., Zhang L.X., Elsaid K.A. cAMP attenuates TGF-beta's profibrotic responses in osteoarthritic synoviocytes: involvement of hyaluronan and PRG4. Am J Physiol Cell Physiol. 2018;315(3):C432–C443. doi: 10.1152/ajpcell.00041.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaamonde-Garcia C., Malaise O., Charlier E., Deroyer C., Neuville S., Gillet P. 15-Deoxy-Delta-12, 14-prostaglandin J2 acts cooperatively with prednisolone to reduce TGF-beta-induced pro-fibrotic pathways in human osteoarthritis fibroblasts. Biochem Pharmacol. 2019;165:66–78. doi: 10.1016/j.bcp.2019.03.039. [DOI] [PubMed] [Google Scholar]
- 26.Watson R.S., Gouze E., Levings P.P., Bush M.L., Kay J.D., Jorgensen M.S. Gene delivery of TGF-β1 induces arthrofibrosis and chondrometaplasia of synovium in vivo. Lab Invest. 2010;90(11):1615. doi: 10.1038/labinvest.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L., Xing R., Huang Z., Zhang N., Zhang L., Li X. Inhibition of synovial macrophage pyroptosis alleviates synovitis and fibrosis in knee osteoarthritis. Mediat Inflamm. 2019;2019:2165918. doi: 10.1155/2019/2165918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blaney Davidson E.N., Vitters E.L., Mooren F.M., Oliver N., Berg W.B., van der Kraan P.M. Connective tissue growth factor/CCN2 overexpression in mouse synovial lining results in transient fibrosis and cartilage damage. Arthritis Rheum. 2006;54(5):1653–1661. doi: 10.1002/art.21795. [DOI] [PubMed] [Google Scholar]
- 29.Blom A.B., van Lent P.L., Holthuysen A.E., van der Kraan P.M., Roth J., van Rooijen N. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage. 2004;12(8):627–635. doi: 10.1016/j.joca.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Valverde-Franco G., Hum D., Matsuo K., Lussier B., Pelletier J.P., Fahmi H. The inVivo effect of prophylactic subchondral bone protection of osteoarthritic synovial membrane in bone-specific ephb4-overexpressing mice. Am J Pathol. 2015;185(2):335–346. doi: 10.1016/j.ajpath.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Jiao R., Chen H., Wan Q., Zhang X., Dai J., Li X. Apigenin inhibits fibroblast proliferation and reduces epidural fibrosis by regulating Wnt3a/beta-catenin signaling pathway. J Orthop Surg Res. 2019;14(1):258. doi: 10.1186/s13018-019-1305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y., Vasheghani F., Li Y.H., Blati M., Simeone K., Fahmi H. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann Rheum Dis. 2015;74(7):1432–1440. doi: 10.1136/annrheumdis-2013-204599. [DOI] [PubMed] [Google Scholar]
- 33.Uddin S.M., Richbourgh B., Ding Y., Hettinghouse A., Komatsu D.E., Qin Y.X. Chondro-protective effects of low intensity pulsed ultrasound. Osteoarthritis Cartilage. 2016;24(11):1989–1998. doi: 10.1016/j.joca.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sellam J., Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6(11):625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 35.da Silva Junior E.M., Mesquita-Ferrari R.A., França C.M., Andreo L., Bussadori S.K., Fernandes K.P.S. Modulating effect of low intensity pulsed ultrasound on the phenotype of inflammatory cells. Biomed pharmacother. 2017;96:1147–1153. doi: 10.1016/j.biopha.2017.11.108. [DOI] [PubMed] [Google Scholar]
- 36.Xu T., Gu J., Li C., Guo X., Tu J., Zhang D. Low-intensity pulsed ultrasound suppresses proliferation and promotes apoptosis via p38 MAPK signaling in rat visceral preadipocytes. Am J Tourism Res. 2018;10(3):948–956. [PMC free article] [PubMed] [Google Scholar]
- 37.Ling L., Wei T., He L., Wang Y., Wang Y., Feng X. Low-intensity pulsed ultrasound activates ERK1/2 and PI3K-Akt signalling pathways and promotes the proliferation of human amnion-derived mesenchymal stem cells. Cell Prolif. 2017;50(6) doi: 10.1111/cpr.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill G.E., Fenwick S., Matthews B.J., Chivers R.A., Southgate J. The effect of low-intensity pulsed ultrasound on repair of epithelial cell monolayers in vitro. Ultrasound Med Biol. 2005;31(12):1701–1706. doi: 10.1016/j.ultrasmedbio.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Roper J., Williamson R., Bally B., Cowell C., Brooks R., Stephens P. Ultrasonic stimulation of mouse skin reverses the healing delays in diabetes and aging by activation of Rac1. J Invest Dermatol. 2015;135(11):2842–2851. doi: 10.1038/jid.2015.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kusuyama J., Nakamura T., Ohnishi T., Albertson B., Ebe Y., Eiraku N. Low-intensity pulsed ultrasound promotes bone morphogenic protein 9-induced osteogenesis and suppresses inhibitory effects of inflammatory cytokines on cellular responses via Rho-associated kinase 1 in human periodontal ligament fibroblasts. J Cell Biochem. 2019;120(9):14657–14669. doi: 10.1002/jcb.28727. [DOI] [PubMed] [Google Scholar]