Abstract
CRM1 is an export receptor mediating rapid nuclear exit of proteins and RNAs to the cytoplasm. CRM1 export cargoes include proteins with a leucine-rich nuclear export signal (NES) that bind directly to CRM1 in a trimeric complex with RanGTP. Using a quantitative CRM1-NES cargo binding assay, significant differences in affinity for CRM1 among natural NESs are demonstrated, suggesting that the steady-state nucleocytoplasmic distribution of shuttling proteins could be determined by the relative strengths of their NESs. We also show that a trimeric CRM1-NES-RanGTP complex is disassembled by RanBP1 in the presence of RanGAP, even though RanBP1 itself contains a leucine-rich NES. Selection of CRM1-binding proteins from Xenopus egg extract leads to the identification of an NES-containing DEAD-box helicase, An3, that continuously shuttles between the nucleus and the cytoplasm. In addition, we identify the Xenopus homologue of the nucleoporin CAN/Nup214 as a RanGTP- and NES cargo-specific binding site for CRM1, suggesting that this nucleoporin plays a role in export complex disassembly and/or CRM1 recycling.
Nuclear export of proteins and RNAs is mediated by soluble, saturable factors. The existence of distinct soluble factors for different classes of export substrates was originally deduced from competition studies (32), and significant progress in their identification has recently been made (for reviews see references 28, 49, and 68).
One class of export substrate carries a short, leucine-rich signal that mediates rapid transport to the cytoplasm, exemplified by the human immunodeficiency virus type 1 (HIV-1) Rev protein that uses its nuclear export signal (NES) to mediate export of genomic and subgenomic HIV-1 mRNAs out of the nucleus (37, 60). We and others identified CRM1 as an export receptor for such leucine-rich NESs, based on several lines of evidence (18, 20, 66). In Saccharomyces cerevisiae and Xenopus laevis oocytes, CRM1 can be inactivated by very different means—through a temperature-sensitive crm1 allele (66) and through binding of the cytotoxin leptomycin B (18, 74), respectively. In both cases, CRM1 inactivation leads to the accumulation of NES-containing substrates in the nucleus, an effect that in Xenopus oocytes can be reversed by overexpression of CRM1.
Further evidence for the export function of CRM1 is its ability to directly interact with leucine-rich NESs (18, 20). This binding is stabilized by cooperative binding of RanGTP (3, 8, 18). Like other small GTPases, Ran switches between the GDP- and GTP-bound states depending on the presence of its GTPase-activating enzyme, RanGAP, which promotes GTP hydrolysis, and its nucleotide exchange factor, RanGEF, which, because of the high GTP/GDP ratio in the cell, promotes RanGDP to RanGTP exchange (reviewed in references 12 and 49). In both vertebrate cells and yeast, RanGAP (named RanGAP1 in vertebrates and Rna1p in yeast) is found in the cytoplasm, whereas RanGEF (RCC1 in vertebrates) is chromatin bound and present in the nucleus. Therefore, a steep RanGTP-RanGDP gradient across the nuclear envelope is predicted, with free RanGTP predicted to be abundant only in the nucleus (22). Based on the RanGTP dependence of high-affinity NES-CRM1 interaction, we concluded that NES binding to CRM1 would be stable in the nucleus and unstable in the cytoplasm, suggesting a mechanism for the unidirectional transport of NES-containing (ribonucleo)proteins, and of exportin-mediated nuclear export in general (reference 18; see also reference 41). Mechanistically similar binding reactions mediate nuclear export of importin α (41), tRNA (2, 42), and the yeast protein Pho4p (34), involving RanGTP-dependent binding of these cargoes to the exportins CAS, exportin t, and Msn5p, respectively.
In addition to RanGTP and NES cargoes, CRM1 interacts with nucleoporins. CRM1 coprecipitates with the nucleoporin CAN/Nup214 from total HeLa extracts, and this interaction is mediated by CAN’s FG-repeat region (17, 19). Similarly, CRM1 binds to the nucleoporin RIP and several other nucleoporin FG-repeats in the yeast two-hybrid assay (55, 67). These nucleoporin-CRM1 interactions most likely reflect those that occur during nuclear export, i.e., passage through the nuclear pore complex (NPC), but their precise role remains to be clarified (56).
Here, we further address the mechanism of CRM1-mediated export. Using a quantitative in vitro CRM1-NES interaction assay, we compared relative strengths of natural NESs and showed that RanBP1 acts as a release factor for CRM1-NES-RanGTP complexes. Using CRM1 affinity chromatography, we identified different classes of proteins from Xenopus egg extract that bind to CRM1 in a RanGTP-dependent way.
MATERIALS AND METHODS
Peptides and recombinant proteins.
Peptides with a minimal purity of 70% were obtained from GenoSys Biotechnologies. Sequences were CLPPLERLTL (HIV-1 Rev), CELALKLAGLDIN (protein kinase inhibitor [PKI]), CVLNLDQQFAGLDLNSADA (An3), CVDEMTKKFGTLTIHDTEK (minute virus of mice [MVM] NS2 wild type) and CVDEMTKKFGTATAHDTEK (MVM NS2 mutant).
z-tagged CRM1 was expressed in Escherichia coli TG1 from plasmid pQE70zz-hCRM1, which encodes two copies of the protein A immunoglobulin G (IgG) binding site (21) in front of the hCRM1 open reading frame (19). z-tagged exportin t was made as previously described (2), and GST-An3N was produced according to the method of Gururajan and Weeks (24). CRM1 and HIV-1 Rev were made as His6 fusions according to the methods described in references 13 and 3, respectively, and RanBP1, Rna1p, RanQ69L, and wild-type Ran were made as described in reference 29. Ran protein was loaded with GTP according to the method described in reference 6. Glutathione S-transferase (GST) protein supplemented with a heart muscle kinase (HMK) sequence was expressed from the pGEX-GTH vector (33) in accordance with standard protocols (Pharmacia Amersham).
CRM1 GAP assay.
Two and a half micrograms of RanGTP was loaded with [γ-32P]GTP (10 mCi/ml, >5,000 Ci/mmol) in the presence of 10 mM EDTA. Loading was stopped by adding MgCl2 to a concentration of 20 mM followed by gel filtration on a Bio-Spin 6 column (BioRad) equilibrated with Ran buffer (40 mM Tris-HCl [pH 8.0], 8 mM MgCl2, 1 mM dithiothreitol, 2 mM GTP, 1 mg of bovine serum albumin [BSA]/ml) containing 500 mM NaCl. Reaction mixtures containing 200 pM Ran[γ-32P]GTP, 0 to 2,000 nM CRM1, and 0 to 1,000 nM NES protein or peptide in 40 μl of reaction buffer (36 mM Tris-HCl [pH 8.0], 75 mM NaCl, 6 mM MgCl2, 0.8 mM dithiothreitol, 0.5 mM GTP, 0.1 mg of BSA/ml, 1 mM phosphate, 1% glycerol) were assembled on ice. After incubation for 20 min, Rna1p was added in 10 μl of Ran buffer to a final concentration of 20 nM and immediately placed at 25°C for 2 or 4 min. Reactions were stopped by adding 1 ml of charcoal suspension (7% [wt/vol] charcoal, 10% [vol/vol] ethanol, 0.1 M HCl, 10 mM KH2PO4) (6), and the mixture was centrifuged for 5 min in an Eppendorf centrifuge. Release of [32P]phosphate was determined by scintillation counting in 0.7 ml of the supernatant.
Oocyte injections.
Microinjection of RNAs and proteins into oocytes, incubations, and extractions were performed as described previously (32, 35). Microinjected 35S-labelled An3Δ21 was produced in rabbit reticulocyte lysate from plasmid pT7-An3Δ21. This plasmid was constructed by ligating a T7 promoter-containing PfuI PCR product encoding amino acids 22 to 697 of An3 (primers 5′-TAA TAC GAC TCA CTA TAG GGA GAC CAC CAT GAA TTC AGC CGA TGC TGA AAG and 5′-TTA GTT GCC CCA CCA GTC) into the SmaI site pUC19. Labelled full-length An3 protein was produced from plasmid pET21a-An3 (55a). GST protein containing an HMK site was labelled by incubation of approximately 5 nmol (150 μg) of protein bound to 15 μl of glutathione-Sepharose 4B (Pharmacia) with 25 U of HMK (Sigma) and 50 μCi of [γ-35S]-ATP (>1,000 Ci/mmol) in 100 μl of HMK buffer (20 mM Tris-HCl [pH 7.5], 100 mM NaCl, 12 mM MgCl2) for 30 min at room temperature in a low-speed shaker. Beads were washed five times with phosphate-buffered saline (PBS)–8.7% (vol/vol) glycerol, eluted three times with 150 μl of 100 mM Tris-HCl [pH 8.0]–120 mM NaCl–20 mM glutathione. The buffer was exchanged with PBS–8.7% (vol/vol) glycerol and concentrated to 80 μl by using a nanosep 10K concentrator (Palfiltron). All microinjected 32P-labelled RNAs were synthesized from plasmids described by Jarmolowski et al. (32), except for fushitarazu (ftz) pre-mRNA, which was synthesized from pGEM2VG1S/B (64).
Pull-down assays.
For pull-downs from Xenopus extract, 100 μl of interphase egg extract (1) was supplemented with 440 mM NaCl, 0.9% Triton X-100, protease inhibitors (0.5 mg of Perfabloc SC/ml, 10 μg of E64/ml, 50 μg of antipain/ml, 0.7 μg of pepstatin/ml, and 500 U of aprotinin/ml), and phosphatase inhibitors (0.1 mM Na3VO4 and 0.1 mM NaF) and incubated on ice for 15 min. The extract was subsequently diluted 1:5 in 20 mM HEPES-KOH (pH 7.5)–5 mM MgCl2–0.2 mM GTP containing protease and phosphatase inhibitors and filtered through a 0.45-μm-pore-size low-protein binding membrane. The filtrate was added to 12.5 μl of IgG Sepharose FastFlow (Pharmacia) to which 100 pmol (15 μg) of z-tagged CRM1 or exportin t had been bound. RanQ69LGTP and/or MVM NS2 NES peptide were added at 3 and 40 μM, respectively, and the mixture was rotated for 2 h at 4°C. Beads were subsequently washed four times with 500 μl of 20 mM HEPES-KOH (pH 7.5)–250 mM NaCl–0.25% Triton X-100–5 mM MgCl2, and proteins were eluted on ice with 25 μl of 0.2% sodium dodecyl sulfate (SDS).
For RanBP1- and Rna1p-dependent dissociation, 3 μg of GST-An3N was bound to 5 μl of glutathione-Sepharose 4B in 50 μl of PBS–8.7% (vol/vol) glycerol for 2 h at 4°C in a low-speed shaker. Beads were washed once and resuspended in 50 μl of PBS-glycerol containing 0.5 μM CRM1 and 2 μM RanGTP and incubated as described above for 1 h. Beads were washed twice and incubated in 20 μl of PBS-glycerol for 5 min with 50 nM RanBP1 and/or 250 nM Rna1p at room temperature. Flowthrough fractions were collected, and the beads were washed three times with PBS-glycerol and finally eluted with SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer.
Pull-downs of in vitro-translated An3 proteins (see above) were performed by incubation of 0.5 μl of labelled An3-containing reticulocyte lysate with 5 μl of IgG-Sepharose beads to which 5 μg (35 pmol) of z-tagged CRM1 had been bound in 50 μl of a buffer containing 500 mM NaCl, 20 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 5 mg of BSA/ml, 0.01% Triton X-100 (LBG buffer), and 2 μM RanGTP in the absence or presence of 0.4 mM competitor peptide for 1 h at 4°C in a low-speed shaker. Unbound proteins were collected, and beads were washed three times with 500 μl of LBG buffer. Bound proteins were eluted with SDS-PAGE sample buffer.
Nanoelectrospray mass spectrometry.
The bands of interest were excised and the proteins were digested in gel with trypsin in a buffer containing 33% of H218O in order to label the C-terminal part of the tryptic peptides. The resulting peptide mixtures were desalted on a Poros R2 column and eluted directly into a nanoelectrospray needle (73). All tandem mass spectrometry experiments were carried on a triple quadrupole mass spectrometer (API III; PE-Sciex, Ontario, Canada). X92 was identified in a nonredundant database as ATP-dependent RNA helicase An3 (SwissProt P24346), while X94 and X280 were de novo sequenced by using a differential scanning technique (72). It was possible to read out the complete sequence of 5 peptides for X94 (LFTSSTTVVLK, SEHALFSR, ANPLLLNTCK, DPVLSESER, and QEDLLNR) and 10 peptides for X280 (YLQLLYK, DILVTVQPK, SNLLVLSNK, LFDYPADLPK, NPAPFYPVK, ELHSFFLELK, DVELQDFQK, PQQDMGELATK, EELAHFQK, and EAAPACGPR). Note that L denotes leucine or isoleucine, since these amino acids have identical masses.
cDNA cloning.
cDNA was synthesized by using reverse degenerate primer MA80 (5′-TCICCCATRTCYTGYTGIGG; I denotes inosine) derived from X280 peptide sequence PQQDMGE with Superscript II reverse transcriptase (Gibco BRL) from 0.5 μg of total RNA from Xenopus laevis stage V to VI oocytes. Two overlapping CAN-encoding cDNAs were subsequently amplified with degenerate primers MA83 (5′-TAYTTYTTYGGIGARGG), MA79 (5′-CARCARGAYATGGGIGAR) and 3XCAN2 (5′-YTGYTTICCYTTIGGISWCCARCA) derived, respectively, from X280 peptide sequences YFFGEG and QQDMGE and from a sequence identical between human and Drosophila melanogaster CAN (CWSPKGKQ). The amplified cDNAs were used to isolate cDNAs in Xenopus oocyte and four cell stage embryonic phage libraries representing the full-length mRNA. The full-length cDNA was cloned into pBluescriptSK(−) (T3-XCAN) and used as a template for in vitro transcription and translation. The Xenopus CAN sequence is available under EMBL accession no. AJ243889.
RESULTS
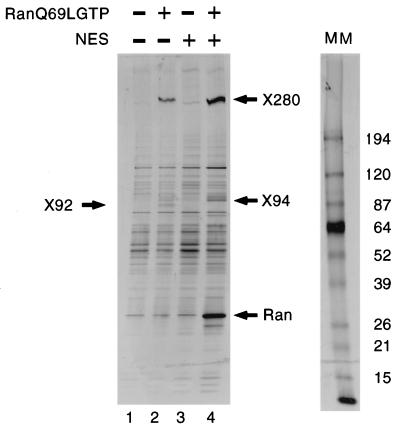
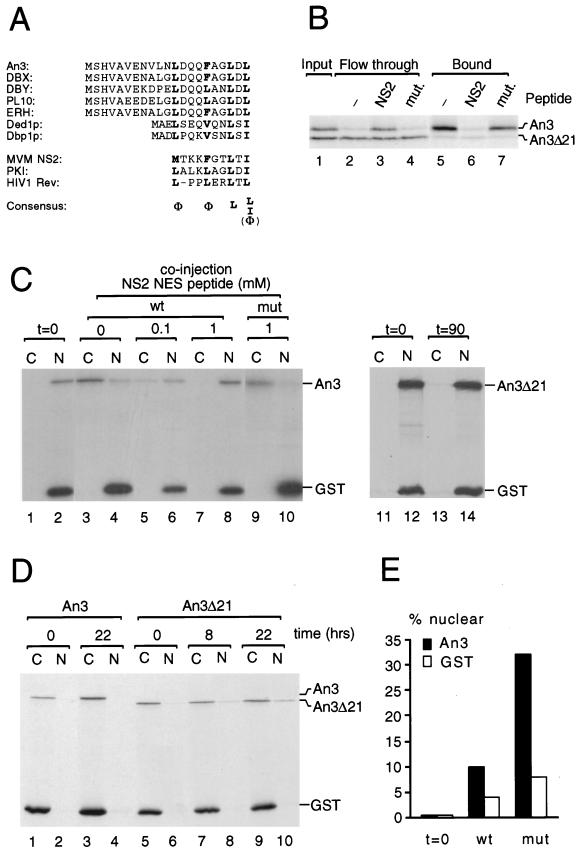
To identify new CRM1-interacting factors, we analyzed binding of proteins from X. laevis egg extract to a z-tagged hCRM1 column in the presence or absence of a nonhydrolyzable form of RanGTP, RanQ69LGTP (38). As shown in Fig. 1, several proteins that specifically bound to CRM1 in the presence of RanGTP were recovered (compare lanes 1 and 2). Some of these proteins, e.g., X92, could be competed with an excess of NES peptide (lane 4) and thus behaved like NES cargo proteins, whereas others, e.g., X280 and X94, exhibited increased binding in the presence of saturating amounts of NES peptide (lanes 2 and 4). The free NES peptide used for competition is a sequence from the MVM NS2 protein (11, 52), which binds particularly strongly to CRM1 (reference 7 and this study). By scaling up the binding reactions presented in lanes 2 and 4, enough material was obtained for sequence analysis by mass spectrometry. Sequences of tryptic peptides of X92 that were identical to those from the X. laevis An3 protein, a DEAD-box helicase with putative homologues in human, mouse, and budding yeast, were obtained. Its primary sequence revealed a putative NES sequence within the first 21 amino acids (Fig. 2A) that is conserved in mammals and yeast. To test whether this sequence mediated RanGTP-dependent CRM1 interaction, full-length An3 and a mutant lacking the first 21 amino acids (An3Δ21) were produced in rabbit reticulocyte lysate and selected on z-tagged CRM1-Sepharose in the presence of RanGTP. Full-length An3, but not the Δ21 mutant, was significantly retained on the CRM1 column (Fig. 2B, lanes 2 and 5), and this binding could be competed with free NS2 NES peptide (lanes 3 and 6) but not with a mutant NES peptide (lanes 4 and 7).
FIG. 1.
Different classes of proteins from Xenopus egg extract are retained by a CRM1 column. Eluates of a z-tagged CRM1 IgG-Sepharose column that had been incubated with Xenopus egg extract in the absence (lane 1) or presence (lanes 2 and 4) of 2 μM RanQ69LGTP and/or 50 μM MVM NS2 NES peptide (lanes 3 and 4) were separated on a gradient SDS–5 to 20% polyacrylamide gel and visualized by silver staining. Positions of bound X92, X94, X280, and RanQ69LGTP are indicated. A molecular mass (MM) marker (in kilodaltons) is shown at the right.
FIG. 2.
An3 is a shuttling DEAD-box helicase. (A) A conserved N-terminal NES sequence in the An3-Ded1p family of RNA helicases is revealed by alignment of the amino termini of Xenopus An3 (23), human DBX and DBY (43), mouse ERH (65) and PL10 (44), and S. cerevisiae Ded1p (31) and Dbp1p (30). The proposed leucine-rich NES consensus sequence is shown at the bottom (9, 37). The Φ sign denotes amino acids M, V, I, L, F, or W. (B) An3 binds to CRM1 via its extreme N terminus. A mixture of in vitro-translated 35S-labelled An3 and An3Δ21 (lane 1) were incubated with a z-tagged CRM1 column in the presence of 2 μM RanGTP and in the absence (−) or presence of 0.4 mM NS2 NES peptide (NS2) or a mutated (mut.) peptide as indicated. Bound (lanes 5 to 7) or flowthrough (lanes 2 to 4) fractions were analyzed by SDS-PAGE and fluorography. Positions of An3 and An3Δ21 are indicated at the right. (C) An3 is exported by the CRM1 pathway. A mixture of in vitro-translated 35S-labelled An3 (lanes 1 to 10) or An3Δ21 (lanes 11 to 14) and [γ-35S]ATP-labelled GST was microinjected into Xenopus oocyte nuclei in the presence of 0, 0.1, or 1 mM NS2 NES peptide or a mutated NES peptide (mut) as indicated. GST forms a multimer that remains in the compartment of injection to serve as injection and dissection control. Oocytes were dissected into nuclear (N) and cytoplasmic (C) fractions immediately (lanes 1 to 2 and 11 to 12) or after an incubation of 90 min (lanes 3 to 10 and 13 to 14), and labelled proteins were visualized by SDS-PAGE and fluorography. Positions of An3, An3Δ21, and GST are indicated. (D) An3 is imported into nuclei of Xenopus stage V and VI oocytes. The same mixture of proteins as described for panel C, as indicated, was microinjected into the cytoplasm of Xenopus oocytes that were dissected into nuclear (N) and cytoplasmic (C) fractions after 0 (lanes 1 to 2 and 5 to 6), 8 (lanes 7 to 8), or 22 h (lanes 3 to 4 and 9 to 10). (E) Nuclear accumulation of An3 in stage IV oocytes. Radiolabelled wild-type (wt) or Leu-19/21-Ala (mut) An3 were injected together with [35S]GST into the cytoplasm of stage IV oocytes and dissected as described above at t = 0 or after 8 h. Nuclear accumulation was quantified by with phosphorimager analysis after SDS-PAGE.
When full-length An3 was injected into Xenopus oocyte nuclei (Fig. 2C, lanes 1 to 10), the protein was completely exported in 90 min (lanes 3 to 4), whereas An3Δ21 remained in the nucleus (lanes 11 to 14). An3 export could be blocked by coinjection of a large excess of wild-type NES peptide (lanes 5 to 8) but not by a mutant peptide (lanes 9 to 10). In addition, An3 carrying a more subtle disruption of the NES, that is, a substitution of Leu-19 and Leu-21 with alanine residues, is also defective in export (data not shown). Thus, the predicted NES sequence of An3 functions as an NES both in vitro and in vivo. When full-length An3 was injected into the cytoplasm, no nuclear accumulation was observed, even after overnight incubations. However, the Δ21 mutant slowly accumulated in the nucleus (Fig. 2D), demonstrating that An3 is a shuttling protein in these stage V and VI oocytes, but with a much higher export rate than import rate. Even under conditions where NES export was drastically reduced by leptomycin B or microinjection of NES peptide, we could not detect significant nuclear accumulation of wild-type An3 (data not shown). However, in stage IV oocytes, a small percentage (5 to 10%) of wild-type An3 that had been injected into the cytoplasm was recovered from the nuclear fraction after 8 h of incubation, while around 30% of the Leu-19 and Leu-21 NES mutant protein was nuclear at this time point (Fig. 2E). Together, these data indicate that nuclear import of An3 is more efficient in stage IV oocytes than in stage V and VI oocytes and that the slow accumulation of the Δ21 mutant in later stages is an intrinsic property of the wild-type protein.
NES sequences bind to CRM1 with different affinities.
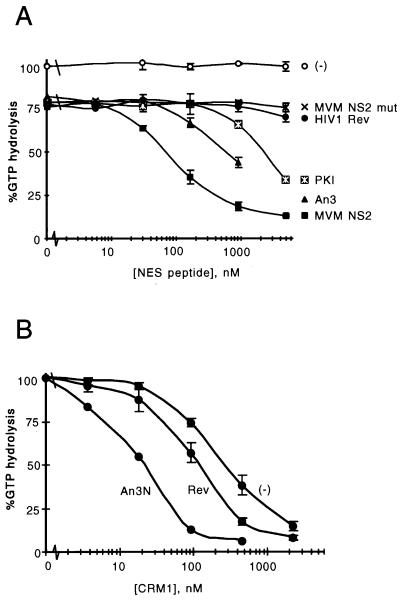
To obtain quantitative data on the affinity of NES sequences for CRM1, we used a system developed to study interactions between RanGTP and interaction partners (6), such as members of the importin β family (19, 21). For the purpose of this article, the assay will be termed the CRM1 GAP assay. This assay uses the inability of RanGAP to stimulate Ran’s GTPase activity when Ran is in a complex with an importin β family member, thereby allowing quantification of unbound RanGTP (6). One hundred nanomolar recombinant CRM1 was incubated with 200 pM [γ-32P]GTP-loaded Ran in the presence of different concentrations of NES peptides from HIV-1 Rev, PKI (70), Xenopus An3, and wild-type or mutant MVM NS2. Subsequently, 20 nM Rna1p (the Schizosaccharomyces pombe RanGAP) was added, and after a further incubation, released [32P]phosphate was measured (see Materials and Methods). As shown in Fig. 3A, the affinities of the NES peptides for CRM1 differed significantly. The MVM NS2 NES showed the highest affinity, whereas An3 and PKI displayed intermediate affinities and HIV-1 Rev NES displayed low affinity for CRM1, at a level almost indistinguishable from that of mutant NS2. The 25% protection of GTP hydrolysis observed in the absence of peptide most likely represents low-level NES-independent binding of RanGTP to CRM1, observable because of the high CRM1 concentration in the assay. Alternatively, it could represent a low level of exchange activity of CRM1.
FIG. 3.
Quantitative analysis of NES-CRM1 affinity in vitro using the CRM1 GAP assay. (A) Comparison between CRM1-NES affinities as measured from CRM1-dependent protection of Rna1p-stimulated GTP hydrolysis on Ran, as a function of increasing concentrations of peptides representing wild-type or mutated MVM NS2 NES, HIV-1 Rev NES, protein kinase inhibitor (PKI) NES, or An3 NES. In all series, CRM1 is present at 100 nM, except series marked “(−),” where the effect of MVM NS2 wild type is measured in the absence of CRM1; the RanGTP concentration in all reaction mixtures is 200 pM. (B) Comparison between CRM1-dependent protection of RanGTP hydrolysis in the presence or absence of 1 μM HIV-1 Rev or GST-An3N as a function of increasing concentrations of CRM1.
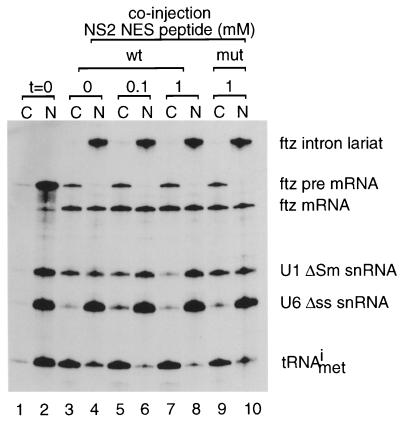
To investigate whether these in vitro affinities have significance in vivo, we injected the NS2 (wild type and mutant) and HIV-1 Rev NESs into Xenopus oocyte nuclei together with a mixture of 32P-labelled in vitro-transcribed RNAs (Fig. 4). The RNA mixture included an initiator methionyl tRNA, a ftz pre-mRNA that is spliced into a ftz mRNA, and U1 and U6 snRNAs. U1 snRNA is exported via the CRM1 pathway (15, 18), whereas tRNA and ftz mRNA follow different export pathways. U6 snRNA does not leave the nucleus and is included as an internal control. As shown in Fig. 4, U1 snRNA export is inhibited by coinjection of the high-affinity NES peptide, whereas tRNA export, pre-mRNA splicing, and mRNA export are unaffected (lanes 1 to 8). U1 snRNA export was not inhibited by coinjection of a mutant NS2 NES peptide (lanes 9 to 10). In contrast to the NS2 NES, microinjection of free HIV-1 Rev peptide has no effect of U1 snRNA export (data not shown), consistent with its weak affinity in the CRM1 GAP assay. To test whether the relative affinities of the free NES peptides were representative of the NES sequences in a more natural structural context, we compared the binding affinities of recombinant His-tagged HIV-1 Rev and a GST fusion of the first 238 amino acids of An3 (GST-An3N) in the CRM1 GAP assay. In this experiment, a fixed concentration (1 μM) of the NES protein was used and recombinant CRM1 was added at increasing concentrations. As shown in Fig. 3B, the differences in affinity found between the free peptides of HIV-1 Rev and An3 are duplicated using the proteins as substrates, indicating that the affinity of the NES peptide for CRM1 reflects the affinity of the NES-containing protein in the CRM1 GAP assay.
FIG. 4.
An NES peptide that functions as a specific CRM1 inhibitor in vivo. A mixture of 32P-labelled in vitro-transcribed ftz pre-mRNA, U1ΔSm snRNA, U6Δss snRNA, and initiator methionyl-tRNA (tRNAmeti) were injected into Xenopus oocyte nuclei in the presence of 0, 0.1, or 1 mM MVM NS2 NES peptide (wt) or a mutated version thereof (mut) as indicated above the lanes. After 0 (lanes 1 and 2) or 90 min (lanes 3 to 10), nuclear (N) and cytoplasmic (C) fractions were obtained and RNAs were purified and analyzed by denaturing PAGE and autoradiography. Positions of the injected RNAs as well as the intron lariat and spliced product of the ftz pre-mRNA are indicated.
RanBP1 dissociates CRM1 export complexes.
The protection against RanGAP-activated RanGTP hydrolysis by binding to importin β can be reversed by RanBP1 (5, 16), and it was proposed that RanBP1 dissociates RanGTP from the complex and presents it to RanGAP1. However, RanBP1 also contains a leucine-rich NES (61) and accumulates in the nucleus upon saturation of the CRM1 pathway (59) and therefore might also be expected to stimulate CRM1-dependent RanGTPase protection, i.e., having the opposite effect.
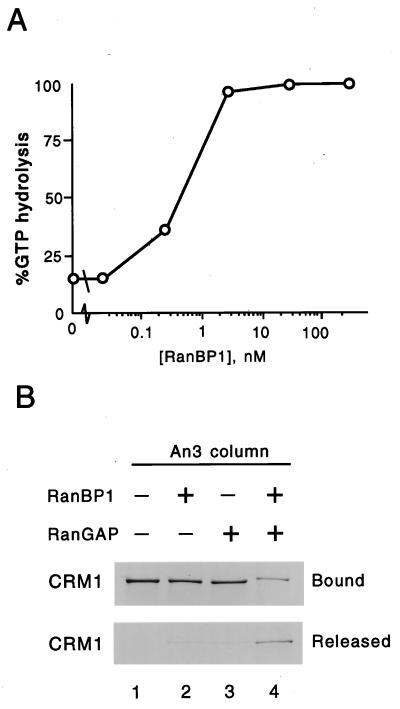
Recombinant CRM1-NES-RanGTP trimeric complexes were therefore assembled, and RanGAP-stimulated RanGTP hydrolysis was tested in the presence of increasing concentrations of RanBP1 (Fig. 5A). While in this experiment RanGTP was present at 100 nM, low nanomolar concentrations of RanBP1 were sufficient to completely disassemble the CRM1 complexes, indicating that RanBP1 functions as a catalytic release factor rather than a cargo under these assay conditions. To obtain more direct evidence for the combined effect of RanBP1 and RanGAP on complex disassembly, we bound CRM1 and RanGTP to a GST-An3N column and added RanBP1, RanGAP1, or both (Fig. 5B). Significant amounts of CRM1 were released from the column in the presence of both RanGAP and RanBP1 (compare lanes 1 and 4) but not when either protein was added singly (lanes 2 to 3) or not at all (lane 1).
FIG. 5.
The CRM1-NES-RanGTP complex is disassembled under the influence of RanBP1 and RanGAP. (A) Dissociation of a complex of CRM1, MVM NS2 NES, and RanGTP as a function of increasing concentrations of RanBP1 using the CRM1 GAP assay. The CRM1, MVM NS2 NES, RanGTP, and the RanGAP Rna1p were present at 1 μM, 4 μM, 100 nM, and 20 nM, respectively. (B) A trimeric complex that had been formed by binding of 0.5 μM CRM1 and 2 μM RanGTP to a GST-An3N column (equivalent to 1.5 μM) was incubated without (lane 1) or with (lanes 2 and 4) 0.05 μM RanBP1 and/or 0.25 μM Rna1p (lanes 3 and 4) for 5 min at room temperature, and bound (upper panel) and released (lower panel) CRM1 was analyzed by SDS-PAGE and silver staining.
Proteins that bind to CRM1 export complexes.
Whereas An3 acted in all respects as a CRM1 export cargo, the other category of CRM1-binding proteins purified from Xenopus egg extract was not competed by a large excess of NES peptide (Fig. 1), indicating a different mode of interaction. X280 and X94 were purified on a preparative scale and sequenced. The two proteins appeared to be present in near-stoichiometric amounts. The 280-kDa protein yielded 10 sequences, of which only 1, EE(L/I)AHFQK, matched that of human CAN. Since the Xenopus homologue of CAN was reported to migrate at 200 kDa (46, 51, 57) and considering the borderline significance of the single database match, a fragment of X280 cDNA was cloned by using degenerate reverse transcriptase PCR. Primers were designed on the basis of the peptide sequences obtained and sequence information from a small region of CAN that is identical in human and Drosophila (reference 71; see Materials and Methods). Combining multiple pairs of degenerate primers, a cDNA was amplified whose predicted amino acid sequence contained three of the X280 peptides (excluding primer-encoded regions) in the same reading frame and that therefore very likely encoded part of the 280-kDa protein. Moreover, the encoded protein sequence shared a significant homology (54% identity; 70% similarity) to the N-terminal 600 amino acids of human CAN. The full-length cDNA, which was isolated from Xenopus cDNA phage libraries, encodes a protein with homology to human CAN over its entire length and directed synthesis of a 280-kDa protein in rabbit reticulocyte lysate in vitro (data not shown). For X94, five tryptic peptide sequences were obtained, three of which showed significant homology to human Nup88 (Table 1). Because human Nup88 is found in a complex with CAN (4, 19), X94 most likely represents Xenopus Nup88.
TABLE 1.
Xenopus X94 tryptic peptides are homologous to human Nup88
| Peptide or nucleoporin | Sequencea |
|---|---|
| X94 peptide 1 | LFTSSTTVVLK |
| :*****:: ** | |
| Nup88 | 163 RFFTSSTSLTLK 174 |
| X94 peptide 2 | QEDILNR |
| ****:** | |
| Nup88 | 632 KQEDIMNR 639 |
| X94 peptide 3 | DPVLSESER |
| ****:*** | |
| Nup88 | 650 ELPVLSDSER 659 |
In the alignment, asterisks denote amino acid identity and colons denote similarity; numbers refer to the amino acid positions in human Nup88.
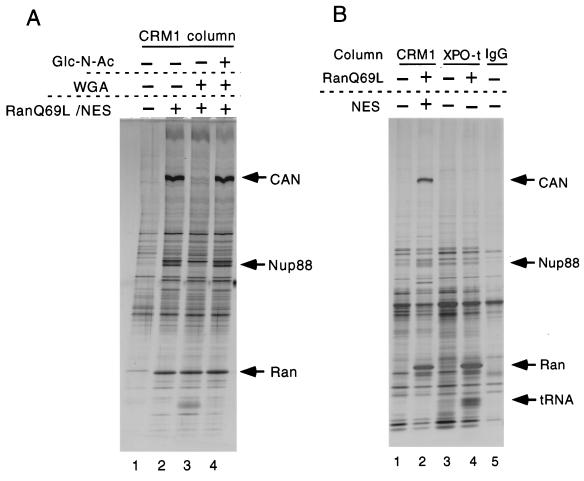
To confirm that X280 and X94 are nucleoporins, we tested whether the X280 and X94 interaction with CRM1 was sensitive to wheat germ agglutinin (WGA). This compound binds to O-linked N-acetylglucosamine-modified nucleoporins (25, 27) and thereby inhibits several import and export pathways (14, 54, 58). As shown in Fig. 6A, 270 μg of WGA/ml dramatically reduced the association of X280 and X94 to the CRM1 column (lanes 2 to 3), an effect that was completely reversed by saturating the WGA with 250 mM N-acetylglucosamine (lane 4). Note that CRM1-NES-RanGTP trimer formation is not affected by association of CAN and Nup88, as seen by the equal amounts of RanQ69LGTP precipitated in lanes 2 to 4. To test whether the CAN-Nup88 subcomplex associates with cargo-loaded exportins in general, we compared proteins bound to the CRM1 column with those binding to an exportin t column in the presence or absence of RanQ69LGTP. Exportin t is a nuclear export receptor for tRNA (2, 42). As shown in Fig. 6B, the exportin t column can bind tRNA (which was present in the egg extract at a concentration of approximately 0.5 μM [26]) only in the presence of RanGTP (compare lanes 3 and 4). However, no association with CAN or Nup88 was observed (compare lanes 2 and 4).
FIG. 6.
Xenopus homologues of nucleoporins CAN and Nup88 specifically bind to CRM1 in a RanGTP-dependent, WGA-sensitive manner. (A) Proteins bound to a z-tagged CRM1 column from total Xenopus egg extract in the absence (lane 1) or presence (lanes 2 to 4) of 2 μM RanQ69LGTP and 10 μM MVM NS2 NES and in the presence or absence of 270 μg of WGA/ml and/or 250 mM N-acetylglucosamine (Glc-N-Ac) as indicated were analyzed by SDS–5 to 20% PAGE and silver staining. Positions of Xenopus CAN, Nup88, and RanQ69LGTP are indicated. (B) Proteins from Xenopus extract bound to a CRM1 column in the presence or absence of RanQ69LGTP and NES peptide (lanes 1 and 2) were compared with those bound to a z-tagged exportin t (XPO-t) column in the presence or absence of RanQ69LGTP (lanes 3 and 4). IgG-Sepharose (lane 5) served as an additional control. Analysis was as described for panel A, and positions of CAN, Nup88, RanQ69LGTP, and tRNA are indicated.
DISCUSSION
In this study, knowledge of the CRM1-dependent, NES-mediated nuclear export pathway was broadened by biochemical screening of CRM1-interacting factors from Xenopus egg extract and was deepened by quantitative evaluation of the role of components whose function was predicted from earlier work. A comparison of leucine-rich NESs of several proteins revealed that the archetypal NES from the HIV-1 Rev protein has relatively low affinity for CRM1. The highest affinity NES in this study, that of the MVM NS2 protein, is, similar to the proposed NES “consensus” (9), quite leucine poor. The relatively low affinity of Rev for CRM1 might be a way to ensure that Rev is preferentially exported to the cytoplasm when multimerized on its target RNA (37, 48, 60). Perhaps only the multimer generates a sufficiently high local concentration to remain in a stable complex with CRM1 and RanGTP. The observation that a free Rev NES peptide cannot specifically compete with the CRM1 export pathway in Xenopus oocytes at any concentration tested (unpublished data) is consistent with this idea. In contrast, either the higher-affinity MVM NS2 NES as a peptide or the Rev peptide when conjugated in multiple copies to BSA can function efficiently as competitive inhibitor of CRM1 export (Fig. 2C and 4) (15). The structural basis of leucine-rich NES-CRM1 recognition remains to be elucidated but is likely to depend on hydrophobic amino acids correctly separated by charged or polar residues (9, 36), where leucine may not always confer the optimal hydrophobic contact.
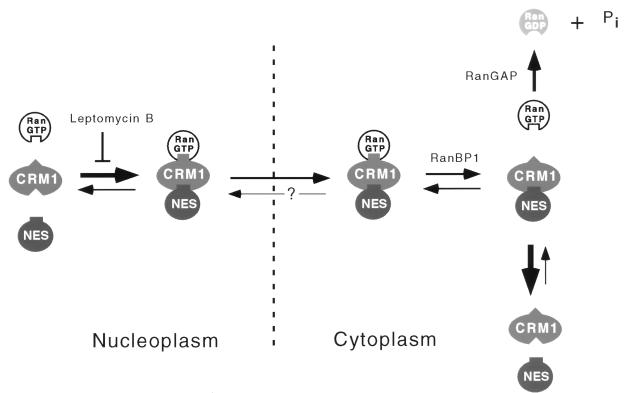
When in complex with the importins and exportins tested so far, RanGTP is resistant to RanGAP-stimulated GTP hydrolysis (references 5, 16, 41, and 42, and this study). However, RanBP1, and presumably RanBP1-like domains in RanBP2-Nup358, destabilize these complexes, thereby relieving the RanGAP resistance (5, 16). For CRM1, a potentially complicating factor is that RanBP1 itself contains a functional leucine-rich NES (61). However, we found that RanBP1’s activity in destabilizing RanGTP-NES-CRM1 complexes is indistinguishable from its activity on other complexes. In Fig. 7, RanBP1’s role in NES-mediated, CRM1-dependent nuclear export is schematically represented, in the context of other solution equilibria detected in our studies. Note that in this model, RanBP1-initiated dissociation of the export complex is made irreversible by hydrolysis of GTP, which is in agreement with the observation that in the presence of RanBP1 alone very little complex dissociation is observed (Fig. 5B). The fact that saturation of CRM1-mediated export in Xenopus oocytes (59) or inactivation of CRM1 in budding yeast (69) leads to nuclear RanBP1 accumulation suggests strongly that RanBP1 is a genuine CRM1 export substrate. Export of vertebrate RanBP1 requires a leucine-rich NES located at the C terminus of the protein (61). The biochemical data presented here suggest strongly that an additional factor will be required to allow formation of stable RanGTP-RanBP1-CRM1 export complexes.
FIG. 7.
A model for directionally of CRM1-mediated nuclear export. In the nucleus, trimeric complex formation between CRM1, NES cargo, and RanGTP is promoted by high RanGTP concentration. This interaction can, however, be prevented by the cytotoxin leptomycin B (18, 74). The trimeric complex traverses the NPC, a process that is reversible under certain conditions (69). In the cytoplasm RanBP1 or RanBP1-like domains in RanBP2-Nup358 destabilize the trimeric complex, a process that is made irreversible by GTP hydrolysis on Ran stimulated by RanGAP. Note that RanBP1 (or RanBP1-like domains) and RanGAP are present in the cytoplasm or on the cytoplasmic face of NPCs.
The biochemical screening of CRM1-binding factors from total Xenopus egg extract revealed two classes of RanGTP-dependent interacting proteins, NES containing and non-NES containing. A major NES-containing protein in Xenopus egg extract was the DEAD-box helicase An3, which is shown to shuttle between the nucleus and the cytoplasm in a CRM1-dependent manner. An3 was first identified in Xenopus oocytes as a protein encoded by an abundant maternal mRNA localized to the animal hemisphere (23). An3 has homologues in budding yeast, human, and mouse (30, 43, 44, 65). The Xenopus protein was shown to have RNA helicase activity in vitro (24). In oocytes as well as during embryogenesis, the majority of An3 protein is cytoplasmic. However, a fraction of the protein exhibits a changing subnuclear distribution in oocyte differentiation (45), with nucleolar localization in stages II to V and absence of nuclear localization by stage VI. Indeed, we find that nuclear import of An3 in stage IV oocytes is more efficient than in stage V and VI oocytes, suggesting that the nuclear accumulation of An3 is regulated at the level of import. Attempts to define a nuclear function of this shuttling RNA helicase are in progress. In S. cerevisiae, the putative An3 homologue, Ded1p, has been shown to function in translational initiation both in vivo and in vitro (10). Interestingly, the strong NES consensus sequence is conserved in Ded1p, suggesting that Ded1p is also a shuttling protein in yeast and may have an additional nuclear function.
Although we have presented only one NES substrate in this study, we observed several other proteins that bind CRM1 in a RanGTP-dependent, leptomycin B- or NES-peptide-sensitive manner, using less stringent binding conditions than the ones used in the experiment shown in Fig. 1. Nevertheless, we expect that many more NES-containing substrates fall below our present detection levels. It is possible that some RanGTP-CRM1-NES complexes are more sensitive to endogenous RanBP1 than others, but this would not distort the relative amounts retained on the CRM1 column, due to a 30- to 40-fold molar excess of added RanQ69LGTP that would overwhelm any effect of RanBP1.
The non-NES-containing class of CRM1-interacting proteins included the Xenopus homologues of the nucleoporins CAN/Nup214 and Nup88. Earlier, indirect evidence has suggested that the Xenopus homologue of human CAN has a relative mobility of about 200 kDa (46, 51, 57). At least for the p200 from Xenopus egg extract (46, 51), the difference with the apparent molecular mass of Xenopus CAN in this report (280 kDa) could be due to an underestimation of the size of the large protein in gels with a relatively high percentage of polyacrylamide. Mammalian and yeast homologues of CAN and Nup88 were earlier found in a complex with each other (4, 4a, 27a) and with CRM1 (19), and the interaction between the CAN-Nup88 subcomplex and CRM1 shown to be mediated by CAN’s FG repeat (17, 19). Here we show in addition that their interaction with CRM1 is RanGTP and NES dependent, indicating that this complex is specific for cargo-loaded CRM1. Since CAN is localized predominantly at the cytoplasmic face of the NPC (40, 57), the CRM1-CAN interaction may represent the termination site of NES export. Topologically, CAN is an attractive site for this, with the presence of RanBP1-like domains in RanBP2-Nup358 (75, 76) and RanBP2-Nup358-bound SUMO-modified RanGAP1 (47, 50, 62) that mediate CRM1 export complex disassembly nearby (see above). Stable binding of CRM1 export complexes to CAN would also be a way to inhibit their possible reimport. Finally, complex disassembly at the cytoplasmic face of the NPC would make subsequent recycling of CRM1 to the nucleus faster than if disassembly took place in the cytosol. In this context, there is a striking parallel with the situation at the nucleoplasmic face of the NPC. Under experimental conditions similar to those described here, the interaction of the import receptor importin β with Nup153 is dissociated by RanGTP (63). Recent observations suggest that RanGTP is not required for NPC translocation per se (13, 39, 53). In this light, it would be interesting to test whether more generally RanGTP’s role instead is to promote exportin-nucleoporin association at the cytoplasmic face and importin-nucleoporin dissociation at the nucleoplasmic face of the NPC.
ACKNOWLEDGMENTS
We thank Ursula Bodendorf and Nathalie Salome (TMV, Heidelberg, Germany), Anne Uv and Anna Wickberg (University of Umeå, Umeå, Sweden), and Karsten Weis (University of California, Berkeley) for communicating unpublished results; Gert-Jan Arts and Scott Kuersten for sharing materials; and members of the Mattaj lab and Judith Boer for valuable discussions and comments on the manuscript. The Resource Center of the German Human Genome Project is acknowledged for the screening of Xenopus cDNA libraries.
P.A., M.F., and M.O. were supported by EMBL short-term, EMBL long-term, and Deutsche Forschungsgemeinschaft fellowships, respectively.
REFERENCES
- 1.Arts G-J, Englmeier L, Mattaj I W. Energy- and temperature-dependent in vitro export of RNA from synthetic nuclei. Biol Chem. 1997;378:641–649. doi: 10.1515/bchm.1997.378.7.641. [DOI] [PubMed] [Google Scholar]
- 2.Arts G-J, Fornerod M, Mattaj I W. Identification of a nuclear export receptor for tRNA. Curr Biol. 1998;8:305–314. doi: 10.1016/s0960-9822(98)70130-7. [DOI] [PubMed] [Google Scholar]
- 3.Askjaer P, Jensen T H, Nilsson J, Englmeier L, Kjems J. The specificity of the CRM1-Rev nuclear export signal interaction is mediated by RanGTP. J Biol Chem. 1998;273:33414–33422. doi: 10.1074/jbc.273.50.33414. [DOI] [PubMed] [Google Scholar]
- 4.Bastos R, Ribas de Pouplana L, Enarson M, Bodoor K, Burke B. Nup84, a novel nucleoporin that is associated with CAN/Nup214 on the cytoplasmic face of the nuclear pore complex. J Cell Biol. 1997;137:989–1000. doi: 10.1083/jcb.137.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Belgareh N, Snay-Hodge C, Pasteau F, Dagher S, Cole C N, Doye V. Functional characterization of a Nup159p-containing nuclear pore subcomplex. Mol Biol Cell. 1998;9:3475–3492. doi: 10.1091/mbc.9.12.3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bischoff F R, Görlich D. RanBP1 is crucial for the release of RanGTP from importin β-related nuclear transport factors. FEBS Lett. 1997;419:249–254. doi: 10.1016/s0014-5793(97)01467-1. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff F R, Ponstingl H. Catalysis of guanine nucleotide exchange of Ran by RCC1 and stimulation of hydrolysis of Ran-bound GTP by Ran-GAP1. Methods Enzymol. 1995;257:135–144. doi: 10.1016/s0076-6879(95)57019-5. [DOI] [PubMed] [Google Scholar]
- 7.Bodendorf, U., and N. Salome. Unpublished results.
- 8.Bogerd H P, Echarri A, Ross T M, Cullen B R. Inhibition of human immunodeficiency virus Rev and human T-cell leukemia virus Rex function, but not Mason-Pfizer monkey virus constitutive transport element activity, by a mutant human nucleoporin targeted to Crm1. J Virol. 1998;72:8627–8635. doi: 10.1128/jvi.72.11.8627-8635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogerd H P, Fridell R A, Benson R E, Hua J, Cullen B R. Protein sequence requirements for function of the human T-cell leukemia virus type 1 Rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Mol Cell Biol. 1996;16:4207–4214. doi: 10.1128/mcb.16.8.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuang R Y, Weaver P L, Liu Z, Chang T H. Requirement of the DEAD-box protein ded1p for messenger RNA translation. Science. 1997;275:1468–1471. doi: 10.1126/science.275.5305.1468. [DOI] [PubMed] [Google Scholar]
- 11.Cotmore S F, Tattersall P. Alternate splicing in a parvoviral nonstructural gene links a common amino-terminal sequence to downstream domains which confer radically different localization and turnover characteristics. Virology. 1990;177:477–487. doi: 10.1016/0042-6822(90)90512-p. [DOI] [PubMed] [Google Scholar]
- 12.Dahlberg J E, Lund E. Functions of the GTPase Ran in RNA export from the nucleus. Curr Opin Cell Biol. 1998;10:400–408. doi: 10.1016/s0955-0674(98)80017-3. [DOI] [PubMed] [Google Scholar]
- 13.Englmeier L, Olivo J C, Mattaj I W. Receptor-mediated substrate translocation through the nuclear pore complex without nucleotide triphosphate hydrolysis. Curr Biol. 1999;9:30–41. doi: 10.1016/s0960-9822(99)80044-x. [DOI] [PubMed] [Google Scholar]
- 14.Finlay D R, Newmeyer D D, Price T M, Forbes D J. Inhibition of in vitro nuclear transport by a lectin that binds to nuclear pores. J Cell Biol. 1987;104:189–200. doi: 10.1083/jcb.104.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer U, Huber J, Boelens W C, Mattaj I W, Lührmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 16.Floer M, Blobel G, Rexach M. Disassembly of RanGTP-karyopherin β complex, an intermediate in nuclear protein import. J Biol Chem. 1997;272:19538–19546. doi: 10.1074/jbc.272.31.19538. [DOI] [PubMed] [Google Scholar]
- 17.Fornerod M, Boer J, van Baal S, Morreau H, Grosveld G. Interaction of cellular proteins with the leukemia specific fusion proteins DEK-CAN and SET-CAN and their normal counterpart, the nucleoporin CAN. Oncogene. 1996;13:1801–1808. [PubMed] [Google Scholar]
- 18.Fornerod M, Ohno M, Yoshida M, Mattaj I W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 19.Fornerod M, van Deursen J, van Baal S, Reynolds A, Davis D, Murti K G, Fransen J, Grosveld G. The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88. EMBO J. 1997;16:807–816. doi: 10.1093/emboj/16.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 21.Görlich D, Dabrowski M, Bischoff F R, Kutay U, Bork P, Hartmann E, Prehn S, Izaurralde E. A novel class of RanGTP binding proteins. J Cell Biol. 1997;138:65–80. doi: 10.1083/jcb.138.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Görlich D, Panté N, Kutay U, Aebi U, Bischoff F R. Identification of different roles for RanGDP and RanGTP in nuclear protein import. EMBO J. 1996;15:5584–5594. [PMC free article] [PubMed] [Google Scholar]
- 23.Gururajan R, Perry O K H, Melton D A, Weeks D L. The Xenopus localized messenger RNA An3 may encode an ATP-dependent RNA helicase. Nature. 1991;349:717–719. doi: 10.1038/349717a0. [DOI] [PubMed] [Google Scholar]
- 24.Gururajan R, Weeks D L. An3 protein encoded by a localized maternal mRNA in Xenopus laevis is an ATPase with substrate-specific RNA helicase activity. Biochim Biophys Acta. 1997;1350:169–182. doi: 10.1016/s0167-4781(96)00155-8. [DOI] [PubMed] [Google Scholar]
- 25.Hanover J A, Cohen C K, Willingham M C, Park M K. O-linked N-acetylglucosamine is attached to proteins of the nuclear pore. Evidence for cytoplasmic and nucleoplasmic glycoproteins. J Biol Chem. 1987;262:9887–9894. [PubMed] [Google Scholar]
- 26.Hausen P, Riebesell M. The early development of Xenopus laevis. Berlin, Germany: Springer-Verlag; 1991. [Google Scholar]
- 27.Holt G D, Snow C M, Senior A, Haltiwanger R S, Gerace L, Hart G W. Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J Cell Biol. 1987;104:1157–1164. doi: 10.1083/jcb.104.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Hurwitz M E, Strambio-de-Castillia C, Blobel G. Two yeast nuclear pore complex proteins involved in mRNA export form a cytoplasmically oriented subcomplex. Proc Natl Acad Sci USA. 1998;95:11241–11245. doi: 10.1073/pnas.95.19.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izaurralde E, Adam S. Transport of macromolecules between the nucleus and the cytoplasm. RNA. 1998;4:351–364. [PMC free article] [PubMed] [Google Scholar]
- 29.Izaurralde E, Kutay U, von Kobbe C, Mattaj I W, Görlich D. The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus. EMBO J. 1997;16:6535–6547. doi: 10.1093/emboj/16.21.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jamieson D J, Beggs J D. A suppressor of yeast spp81/ded1 mutations encodes a very similar putative ATP-dependent RNA helicase. Mol Microbiol. 1991;5:805–812. doi: 10.1111/j.1365-2958.1991.tb00753.x. [DOI] [PubMed] [Google Scholar]
- 31.Jamieson D J, Rahe B, Pringle J, Beggs J D. A suppressor of a yeast splicing mutation (prp8-1) encodes a putative ATP-dependent RNA helicase. Nature. 1991;349:715–717. doi: 10.1038/349715a0. [DOI] [PubMed] [Google Scholar]
- 32.Jarmolowski A, Boelens W C, Izaurralde E, Mattaj I W. Nuclear export of different classes of RNA is mediated by specific factors. J Cell Biol. 1994;124:627–635. doi: 10.1083/jcb.124.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen T H, Jensen A, Kjems J. Tools for the production and purification of full-length, N- or C-terminal 32P-labeled protein, applied to HIV-1 Gag and Rev. Gene. 1995;162:235–237. doi: 10.1016/0378-1119(95)00328-4. [DOI] [PubMed] [Google Scholar]
- 34.Kaffman A, Rank N M, O’Neill E M, Huang L S, O’Shea E K. The receptor Msn5 exports the phosphorylated transcription factor Pho4 out of the nucleus. Nature. 1998;396:482–486. doi: 10.1038/24898. [DOI] [PubMed] [Google Scholar]
- 35.Kambach C, Mattaj I W. Intracellular distribution of the U1A protein depends on active transport and nuclear binding to U1 snRNA. J Cell Biol. 1992;118:11–21. doi: 10.1083/jcb.118.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim F J, Beeche A A, Hunter J J, Chin D J, Hope T J. Characterization of the nuclear export signal of human T-cell lymphotropic virus type 1 Rex reveals that nuclear export is mediated by position-variable hydrophobic interactions. Mol Cell Biol. 1996;16:5147–5155. doi: 10.1128/mcb.16.9.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kjems, J., and P. Askjaer. The Rev protein and its cellular partners. In K. T. Jeang (ed.), HIV: molecular mechanisms and clinical applications, in press. Academic Press, San Diego, Calif. [DOI] [PubMed]
- 38.Klebe C, Bischoff F R, Ponstingl H, Wittinghofer A. Interaction of the nuclear GTP-binding protein Ran with its regulatory proteins RCC1 and RanGAP1. Biochemistry. 1995;34:639–647. doi: 10.1021/bi00002a031. [DOI] [PubMed] [Google Scholar]
- 39.Kose S, Imamoto N, Tachibana T, Shimamoto T, Yoneda Y. Ran-unassisted nuclear migration of a 97-kDa component of nuclear pore-targeting complex. J Cell Biol. 1997;139:841–849. doi: 10.1083/jcb.139.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraemer D, Wozniak R W, Blobel G, Radu A. The human CAN protein, a putative oncogene product associated with myeloid leukemogenesis, is a nuclear pore complex protein that faces the cytoplasm. Proc Natl Acad Sci USA. 1994;91:1519–1523. doi: 10.1073/pnas.91.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kutay U, Bischoff F R, Kostka S, Kraft R, Görlich D. Export of importin α from the nucleus is mediated by a specific nuclear transport factor. Cell. 1997;90:1061–1071. doi: 10.1016/s0092-8674(00)80372-4. [DOI] [PubMed] [Google Scholar]
- 42.Kutay U, Lipowsky G, Izaurralde E, Bischoff F R, Schwarzmaier P, Hartmann E, Görlich D. Identification of a tRNA-specific nuclear export receptor. Mol Cell. 1998;1:359–369. doi: 10.1016/s1097-2765(00)80036-2. [DOI] [PubMed] [Google Scholar]
- 43.Lahn B T, Page D C. Functional coherence of the human Y chromosome. Science. 1997;278:675–680. doi: 10.1126/science.278.5338.675. [DOI] [PubMed] [Google Scholar]
- 44.Leroy P, Alzari P, Sassoon D, Wolgemuth D, Fellous M. The protein encoded by a murine male germ cell-specific transcript is a putative ATP-dependent RNA helicase. Cell. 1989;57:549–559. doi: 10.1016/0092-8674(89)90125-6. [DOI] [PubMed] [Google Scholar]
- 45.Longo F J, Mathews L, Gururajan R, Chen J, Weeks D L. Changes in nuclear localization of An3, a RNA helicase, during oogenesis and embryogenesis in Xenopus laevis. Mol Reprod Dev. 1996;45:491–502. doi: 10.1002/(SICI)1098-2795(199612)45:4<491::AID-MRD12>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 46.Macaulay C, Meier E, Forbes D J. Differential mitotic phosphorylation of proteins of the nuclear pore complex. J Biol Chem. 1995;270:254–262. doi: 10.1074/jbc.270.1.254. [DOI] [PubMed] [Google Scholar]
- 47.Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell. 1997;88:97–107. doi: 10.1016/s0092-8674(00)81862-0. [DOI] [PubMed] [Google Scholar]
- 48.Malim M H, Cullen B R. HIV-1 structural gene expression requires the binding of multiple Rev monomers to the viral RRE: implications for HIV-1 latency. Cell. 1991;65:241–248. doi: 10.1016/0092-8674(91)90158-u. [DOI] [PubMed] [Google Scholar]
- 49.Mattaj I W, Englmeier L. Nucleocytoplasmic transport: the soluble phase. Annu Rev Biochem. 1998;67:265–306. doi: 10.1146/annurev.biochem.67.1.265. [DOI] [PubMed] [Google Scholar]
- 50.Matunis M J, Wu J, Blobel G. SUMO-1 modification and its role in targeting the Ran GTPase-activating protein, RanGAP1, to the nuclear pore complex. J Cell Biol. 1998;140:499–509. doi: 10.1083/jcb.140.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meier E, Miller B R, Forbes D J. Nuclear pore complex assembly studied with a biochemical assay for annulate lamellae formation. J Cell Biol. 1995;129:1459–1472. doi: 10.1083/jcb.129.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naeger L K, Cater J, Pintel D J. The small nonstructural protein (NS2) of the parvovirus minute virus of mice is required for efficient DNA replication and infectious virus production in a cell-type-specific manner. J Virol. 1990;64:6166–6175. doi: 10.1128/jvi.64.12.6166-6175.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakielny S, Dreyfuss G. Import and export of the nuclear protein import receptor transportin by a mechanism independent of GTP hydrolysis. Curr Biol. 1998;8:89–95. doi: 10.1016/s0960-9822(98)70039-9. [DOI] [PubMed] [Google Scholar]
- 54.Neuman de Vegvar H E, Dahlberg J E. Nucleocytoplasmic transport and processing of small nuclear RNA precursors. Mol Cell Biol. 1990;10:3365–3375. doi: 10.1128/mcb.10.7.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neville M, Stutz F, Lee L, Davis L I, Rosbash M. The importin-β family member Crm1p bridges the interaction between Rev and the nuclear pore complex during nuclear export. Curr Biol. 1997;7:767–775. doi: 10.1016/s0960-9822(06)00335-6. [DOI] [PubMed] [Google Scholar]
- 55a.Ogniewski, V., and D. L. Weeks. Unpublished results.
- 56.Ohno M, Fornerod M, Mattaj I W. Nucleocytoplasmic transport: the last 200 nanometers. Cell. 1998;92:327–336. doi: 10.1016/s0092-8674(00)80926-5. [DOI] [PubMed] [Google Scholar]
- 57.Panté N, Bastos R, McMorrow I, Burke B, Aebi U. Interactions and three-dimensional localization of a group of nuclear pore complex proteins. J Cell Biol. 1994;126:603–617. doi: 10.1083/jcb.126.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Panté N, Jarmolowski A, Izaurralde E, Sauder U, Baschong W, Mattaj I W. Visualizing nuclear export of different classes of RNA by electron microscopy. RNA. 1997;3:498–513. [PMC free article] [PubMed] [Google Scholar]
- 59.Pasquinelli A E, Powers M A, Lund E, Forbes D, Dahlberg J E. Inhibition of mRNA export in vertebrate cells by nuclear export signal conjugates. Proc Natl Acad Sci USA. 1997;94:14394–14399. doi: 10.1073/pnas.94.26.14394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pollard V W, Malim M H. The HIV-1 Rev protein. Annu Rev Microbiol. 1998;52:491–532. doi: 10.1146/annurev.micro.52.1.491. [DOI] [PubMed] [Google Scholar]
- 61.Richards S A, Lounsbury K M, Carey K L, Macara I G. A nuclear export signal is essential for the cytosolic localization of the Ran binding protein, RanBP1. J Cell Biol. 1996;134:1157–1168. doi: 10.1083/jcb.134.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saitoh H, Sparrow D B, Shiomi T, Pu R T, Nishimoto T, Mohun T J, Dasso M. Ubc9p and the conjugation of SUMO-1 to RanGAP1 and RanBP2. Curr Biol. 1998;8:121–124. doi: 10.1016/s0960-9822(98)70044-2. [DOI] [PubMed] [Google Scholar]
- 63.Shah S, Tugendreich S, Forbes D. Major binding sites for the nuclear import receptor are the internal nucleoporin Nup153 and the adjacent nuclear filament protein Tpr. J Cell Biol. 1998;141:31–49. doi: 10.1083/jcb.141.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siebel C W, Rio D C. Regulated splicing of the Drosophila P transposable element third intron in vitro: somatic repression. Science. 1990;248:1200–1208. doi: 10.1126/science.2161558. [DOI] [PubMed] [Google Scholar]
- 65.Sowden J, Putt W, Morrison K, Beddington R, Edwards Y. The embryonic RNA helicase gene (ERH): a new member of the DEAD box family of RNA helicases. Biochem J. 1995;308:839–846. doi: 10.1042/bj3080839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stade K, Ford C S, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- 67.Stutz F, Izaurralde E, Mattaj I W, Rosbash M. A role for nucleoporin FG repeat domains in export of human immunodeficiency virus type 1 Rev protein and RNA from the nucleus. Mol Cell Biol. 1996;16:7144–7150. doi: 10.1128/mcb.16.12.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stutz F, Rosbash M. Nuclear RNA export. Genes Dev. 1998;12:3303–3319. doi: 10.1101/gad.12.21.3303. [DOI] [PubMed] [Google Scholar]
- 69.Weis, K. Personal communication.
- 70.Wen W, Meinkoth J L, Tsien R Y, Taylor S S. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 71.Wickberg, A., and A. Uv. Personal communication.
- 72.Wilm, M., L. Neubauer, A. Taylor, A. Shevchenko, and A. Bachi. De novo sequencing of proteins using the differential scanning technique. Submitted for publication. [DOI] [PubMed]
- 73.Wilm M, Shevchenko A, Houthaeve T, Breit S, Schweigerer L, Fotsis T, Mann M. Femtomole sequencing of proteins from polyacrylamide gels by nanoelectrospray mass spectrometry. Nature. 1996;379:466–469. doi: 10.1038/379466a0. [DOI] [PubMed] [Google Scholar]
- 74.Wolff B, Sanglier J J, Wang Y. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleocytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem Biol. 1997;4:139–147. doi: 10.1016/s1074-5521(97)90257-x. [DOI] [PubMed] [Google Scholar]
- 75.Wu J, Matunis M J, Kraemer D, Blobel G, Coutavas E. Nup358, a cytoplasmically exposed nucleoporin with peptide repeats, Ran-GTP binding sites, zinc fingers, a cyclophilin A homologous domain, and a leucine-rich region. J Biol Chem. 1995;270:14209–14213. doi: 10.1074/jbc.270.23.14209. [DOI] [PubMed] [Google Scholar]
- 76.Yokoyama N, Hayashi N, Seki T, Panté N, Ohba T, Nishii K, Kuma K, Hayashida T, Miyata T, Aebi U, et al. A giant nucleopore protein that binds Ran/TC4. Nature. 1995;376:184–188. doi: 10.1038/376184a0. [DOI] [PubMed] [Google Scholar]