Abstract
In this retrospective study of 105 severe acute respiratory coronavirus virus 2 (SARS-CoV-2)–infected cancer patients with longitudinal nasopharyngeal sampling, the duration of viral shedding and time to attain cycle threshold >30 was longer in patients with hematologic malignancy than in those with solid tumors. These findings have important public health implications.
Keywords: SARS CoV-2, COVID-19, Cancer, Viral shedding
The duration of shedding for respiratory viruses can be longer in severely immunocompromised patients.1 The consequences of persistent infection include the risk of in-host evolution of viral variants, treatment ineffectiveness, and longer transmissibility. For severe acute respiratory coronavirus virus 2 (SARS-CoV-2)–infected patients with immunocompromising conditions, replication competent virus has been detected for >20 days and up to 2 months after infection.2–4
To assess viral viability, culture techniques are not readily available in clinical diagnostic laboratories. As an alternative, information on viability can be gained from assessing the viral load measured by the PCR cycle threshold (Ct). In immunocompetent persons, live virus recovery is rare after day 11 of illness, and the probability diminishes with the interval days from disease onset and higher Ct.5,6 In this study, we analyzed infection resolution predictors using Ct value from serially collected nasopharyngeal swabs of cancer patients with coronavirus disease 2019 (COVID-19).
Methods
Memorial Sloan Kettering Cancer Center is a 514-bed tertiary-care cancer center. All consecutive cases of symptomatic and laboratory-confirmed SARS-CoV-2 infection diagnosed from March 10 through April 24, 2020, were included if they had >1 serial nasopharyngeal sample collected after diagnosis and through June 30, 2020. Clinical data were abstracted from medical records. The institutional review board granted a Health Insurance Portability and Accountability Act waiver of authorization to conduct this study.
Laboratory methods
The SARS-CoV-2 PCR was either a laboratory-developed test or the cobas SARS-CoV-2 test (Roche Molecular Diagnostics, Indianapolis, Indiana) targeting the ORF1a/b and E genes.7 Because the differences between the Ct values for the 2 targets in each assay and between all 3 tests were small (Supplementary Methods online), analyses were focused on 1 target (target 1: N2, N, or ORF).
Statistical analysis
The Wilcoxon signed rank-sum test was used for continuous variables and the Fisher exact test for categorical variables. To visualize differences in Ct values by cancer type [ie, solid tumor (ST) vs hematologic malignancy (HT)], we plotted the values for all positive tests and the first sustained negative test by days since first positive test. Kaplan-Meier product limit estimates were generated to compare time to PCR-confirmed resolution by cancer type. Patients who did not resolve by the end of the observation period were censored following the last test. Plots were constructed using 4 different definitions of COVID-19 onset and resolution as follows: model 1, date of first positive test to date of first sustained negative test; model 2, date of first positive test to date of first sustained test result with Ct >30; model 3, date of symptom onset to date of first sustained negative test; and model 4, date of symptom onset to date of first sustained test result with Ct >30. In models 3 and 4, asymptomatic patients were removed from the analysis and date of first positive test was imputed for patients with unknown date of symptom onset.
Factors associated with time to PCR-confirmed resolution were identified using Cox proportional hazards regression. Models were constructed using stepwise selection for each of the 4 definitions of onset and resolution described above. Variables that differed significantly for patients with solid tumors (STs) versus hematologic tumors (HTs) at α = 0.2 in the bivariate analyses were assessed for inclusion in the final models. Statistical analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC).
Results
Patient characteristics
Overall, 105 patients with cancer and PCR-confirmed infection were included in the analysis. The median follow-up was 23 days (range, 0–86 days) until sustained negative PCR test or end of the study period; 40 patients (38%) required hospitalization and 21 died (20%). Supplementary Table 1 (online) summarizes the COVID-19 disease course. Hydroxychloroquine was commonly used in both groups of patients: 37 ST patients and 23 HT patients). Only 2 patients received treatment with remdesivir, both with underlying HT.
SARS-CoV-2 testing
After excluding nonsustained negative tests for 7 patients, 404 total tests were performed: 242 in ST patients and 162 in HT patients. Of these, 27 nonnasopharyngeal samples were excluded: 12 (5%) in ST patients and 15 (9%) in HT patients (P = .09).
In total, 377 tests were analyzed: 230 (61%) from 70 ST patients and 147 (39%) from 35 HT patients. The median number of tests performed for patients with was 3 (range, 2–7) for ST patients and 3 (range, 2–15) for HT patients (P = .18). Moreover, for HT patients, 67% of samples were analyzed by the laboratory, 25% were analyzed using cobas, and 8% were analyzed using rapid testing platforms. For ST patients, 71% of samples were analyzed by the laboratory, 18% were analyzed using cobas, and 10% were analyzed using rapid testing platforms (P = .21).
Resolution of SARS-CoV-2
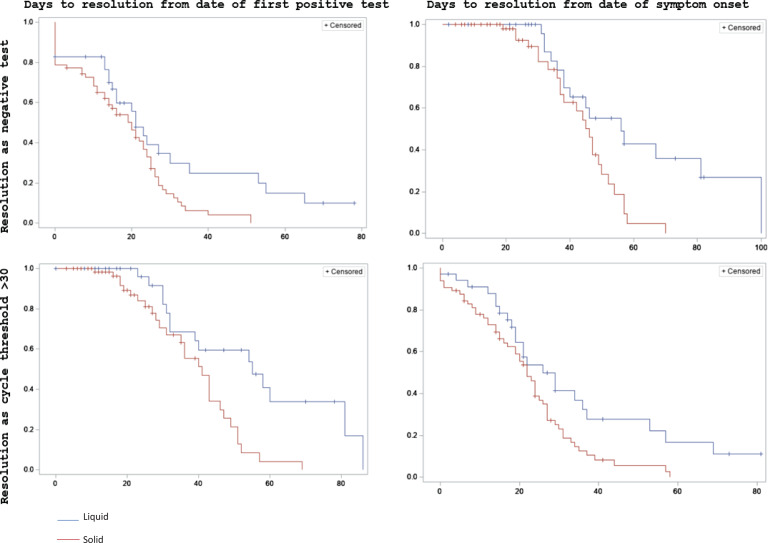
Supplemental Figure 1 (online) shows plots of Ct values by days since the first positive test. HT and ST patients had similar Ct values for 40 days following the first positive test, but only patients with HT remained positive after 45 days. Figure 1 shows the Kaplan-Meier product-limit survival estimates for 4 methods of defining infection onset and resolution. HT patients had longer time to resolution versus than ST patients in all 4 models. Probability of resolution began similarly in both groups and then diverged considerably after 25 days in model 1, after 40 days in model 2, after 50 days in model 3, and after 40 days in model 4. Table 1 shows the results of multivariable Cox proportional hazards models. Tumor type was the only factor significantly associated with time to resolution in all 4 models. Presence of symptoms, Hispanic ethnicity, and asthma were also significant predictors of time to resolution in some models.
Fig. 1.
Kaplan-Meier product-limit survival estimates for time to SARS-CoV-2 resolution in patients with cancer. Plots show survival estimates using different definitions of infection onset (date of first positive test vs date of symptom onset) and resolution (negative result versus cycle threshold >30). The x-axes show days from onset to resolution and y-axes show probabilities of resolution. Patients with solid tumors are represented in red and patients with hematologic tumors are represented in blue.
Table 1.
Multivariable Cox Proportional Hazards Analysis of Factors Associated With Time to SARS-CoV-2 Resolution
| Factors Associated With Time to SARS-CoV-2 Resolution | Hazard Ratio (95% CI) |
|---|---|
| Model 1. First positive test to first negative test | |
| Hematologic tumor (reference, solid tumor) | 2.34 (1.11–5.13) |
| Symptomatic | 9.13 (2.17–38.37) |
| Hispanic/Latinx ethnicity | 2.85 (1.28–6.36) |
| Asthma | 4.09 (1.12–14.89) |
| Model 2. First positive test to first cycle threshold >30 | |
| Hematologic tumor (reference, solid tumor) | 1.71 (1.004–2.902) |
| Model 3. Symptom onset to first negative test | |
| Hematologic tumor (reference, solid tumor) | 2.21 (1.06–4.62) |
| Hispanic/Latinx ethnicity | 5.86 (2.33–14.73) |
| Asthma | 5.68 (1.51–21.39) |
| Model 4. Symptom onset to first cycle threshold >30 | |
| Hematologic tumor (reference, solid tumor) | 1.73 (1.004–2.98) |
| Hispanic/Latinx ethnicity | 2.05 (1.07–3.92) |
Note. Tumor type, race, ethnicity, chronic kidney disease, chronic obstructive pulmonary disease, asthma, receipt of PD1 inhibitor, receipt of rituximab, presence of symptoms, fever, and cough were considered for inclusion in models 1 and 2. Tumor type, race, ethnicity, chronic kidney disease, chronic obstructive pulmonary disease, asthma, receipt of PD1 inhibitor, receipt of rituximab, fever, and cough were considered for inclusion in models 3 and 4. Final models were determined using stepwise selection.
Discussion
Our study on the viral kinetics of SARS-CoV-2 shedding in patients with immunocompromising conditions due to cancer demonstrated a longer time to infection resolution in HT patients than ST patients. This finding was consistent across all 4 models using different criteria for infection resolution. Our findings collectively suggest that the time to viral clearance or reaching sustained Ct >30 after SARS-CoV-2 infection is longer in HT patients. Consequently, the period of infectiousness may be longer in HT patients infected with SARS CoV-2. Another important finding of our study is that the Ct values were comparable during early illness for both cancer types.
The SARS CoV-2 viral burden in the upper airway is highest at symptom onset, and the probability of recovering live virus decreases 3–5 days after symptom onset. Studies examining PCR Ct cutoffs beyond which the likelihood of SARS CoV-2 recovery in culture is low show the a CT range of 24–34.8,9 Our data corroborate findings from previous reports on the predisposition of HT patients to develop persistent infection.4,10 Public health surveillance efforts must incorporate longitudinal viral genomic surveillance from persistently infected HT patients to detect emergent SARS CoV-2 variants.
Our study has several limitations. This was a nonsystematic, single-center study. However, we did not observe significant testing variances. There are potential limitations in both symptom ascertainment and lack of correlation between symptoms and PCR. Our analysis may not have detected all predictors of resolution due to the small sample size. Finally, Ct has limitations as a quantitative measure for viral load, yet it remains the only practical tool for clinicians to guide complex decisions.
In summary, our study findings show a longer time to infection resolution in patients with hematologic malignancy. These findings have important implications for infection control measures and public health surveillance efforts.
Acknowledgments
Financial support
This work was supported by National Cancer Institute Cancer Center (grant no. P30 CA 008748).
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/ice.2021.378.
click here to view supplementary material
References
- 1.Richardson L, Brite J, Del Castillo M, et al. Comparison of respiratory virus shedding by conventional and molecular testing methods in patients with haematological malignancy. Clin Microbiol Infect 2016;22:380:e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aydillo T, Gonzalez-Reiche AS, Aslam S,, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med 2020;383:2586–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Kampen JJA, van de Vijver D, Fraaij PLA, et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat Commun 2021;12:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med 2020;383:2291–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinzerling A, Stuckey MJ, Scheuer T, et al. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient—Solano County, California, February 2020. Morb Mortal Wkly Rep 2020;69:472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020. ;382:2081–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med 2020;26:1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bullard J, Dust K, Funk D, et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis 2020;71:2663–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La Scola B, Le Bideau M, Andreani J, et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis 2020;39:1059–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baang JH, Smith C, Mirabelli C, et al. Prolonged severe acute respiratory syndrome coronavirus 2 replication in an immunocompromised patient. J Infect Dis 2021;223:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/ice.2021.378.
click here to view supplementary material