Abstract
Objective
The objective of this study was to evaluate the safety and efficacy of sirolimus (SRL) in the prevention of graft-versus-host disease (GVHD) in recipients following allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Methods
Randomized controlled trials (RCTs) evaluating the safety and efficacy of SRL-based prophylaxis regimens in patients receiving allo-HSCT were obtained from PubMed, Embase, and the Cochrane database. Following specific inclusion and exclusion criteria, studies were selected and screened by two independent reviewers who subsequently extracted the study data. The Cochrane risk bias evaluation tool was used for quality evaluation, and RevMan 5.3 software was used for statistical analysis comparing the effects of SRL-based and non–SRL-based regimens on acute GVHD, chronic GVHD, overall survival (OS), relapse rate, non-relapse mortality (NRM), thrombotic microangiopathy (TMA), and veno-occlusive disease (VOD).
Results
Seven studies were included in this meta-analysis, with a total sample size of 1,673 cases, including 778 cases of patients receiving SRL-based regimens and 895 cases in which patients received non-SRL-based regimens. Our data revealed that SRL containing prophylaxis can effectively reduce the incidence of grade II–IV acute GVHD (RR = 0.75, 95% CI: 0.68∼0.82, p < 0.0001). SRL-based prophylaxis was not associated with an improvement of grade III–IV acute GVHD (RR = 0.78, 95% CI: 0.59∼1.03, p = 0.08), chronic GVHD (p = 0.89), OS (p = 0.98), and relapse rate (p = 0.16). Despite its immunosuppressant effects, SRL-based regimens did not increase bacterial (p = 0.68), fungal (p = 0.70), or CMV (p = 0.10) infections. However, patients receiving SRL-based regimens had increased TMA (p < 0.00001) and VOD (p < 0.00001).
Conclusions
This meta-analysis indicates that addition of sirolimus is an effective alternative prophylaxis strategy for II–IV aGVHD but may cause endothelial cell injury and result in secondary TMA or VOD events.
Keywords: sirolimus, GVHD, HSCT, prophylaxis, TMA
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is currently one of the most effective means to cure hematological malignancies. However, high incidence of graft versus host disease (GVHD) after transplantation results in high non-recurrence of transplantation-related death. The incidence of acute GVHD (aGVHD) is about 40%–75% and is an important factor affecting the overall efficacy of HSCT (1). In addition, chronic GVHD (cGVHD) has become the main cause of late non-relapse mortality (NRM) after HSCT, which also seriously affects the efficacy of transplantation and the quality of patient life. Currently, GVHD prophylaxis regimens among transplant centers are not uniform and mainly include calcineurin inhibitor (CNI), sirolimus (SRL), and posttransplantation cyclophosphamide (PT-Cy)-based regimens.
Our study focuses on the role of SRL in GVHD. SRL is an mTOR inhibitor, possessing antifungal, immunosuppressive, and antitumor properties (2). SRL can inhibit the proliferation and activation of T cells, reduce the release of pro-inflammatory cytokines, and modulate CD4+CD25+ regulatory T (Treg) cells, making it a widely used therapeutic candidate in benign and malignant hematological diseases (3). Accumulating evidence suggests that SRL may play a role in the prevention and treatment of GVHD after HSCT (4). However, some studies have reported that SRL-based regimens did not decrease the incidence of aGVHD (5, 6). Moreover, some have reported that SRL-based prophylaxis was associated with high incidence of thrombotic microangiopathy (TMA) (7). On the other hand, another group reported that the combination of tacrolimus (TAC)/SRL did not pose a higher risk of TMA (8). Therefore, to better understand the efficacy and safety of SRL-based regimens and their impact on GVHD, we performed a meta-analysis of SRL-based GVHD prophylaxis in patients after allo-HSCT.
Materials and Methods
Search Strategy
A literature search was conducted to identify randomized controlled trials (RCTs) evaluating the efficacy of SRL-based prophylaxis in patients after allo-HSCT. The search was conducted through August 2020 in PubMed, Cochrane Library, Embase, and Web of Science. The search terms included “sirolimus,” “rapamycin,” “graft versus host disease” and “GVHD.” The search language was restricted to English.
Inclusion and Exclusion Criteria
Inclusion criteria: the RCT study must include an SRL-based group and a non-SRL-based prophylaxis group. RCTs included patients with hematological malignancies that have received allo-HSCT. The meta-analysis did not exclude studies or patients based on age, gender, source of donor, and level of radiotherapy and chemotherapy before transplantation. Primary outcomes included the incidence of aGVHD and cGVHD. The secondary outcomes included TMA, VOD, and overall survival (OS).
Exclusion criteria are non-RCTs, such as retrospective studies, conference articles, animal experiments, and review articles.
Data Extraction
All data, including the first author of the studies, published year, country of origin, period of enrollment, sample size, median follow-up duration, SRL-based regimens, non-SRL-based regimens, and trial outcomes, were extracted by two independent researchers. Any discrepancies were resolved by discussion and/or consultations with a third independent researcher.
Methodologic Quality Evaluation
Statistical Analysis
The risk ratio (RR) and 95% confidence interval (CI) were used to analyze extracted dichotomous outcomes. Heterogeneity was assessed using the I2 statistic. An I2 value of greater than 50% and a p value less than 0.10 indicated significant heterogeneity (9). Sensitivity and subgroup analyses were performed to identify and reduce heterogeneity. Meta-analyses were conducted using random effects, regardless of the existence or non-existence of heterogeneity.
Results
Study Selection and Characteristics
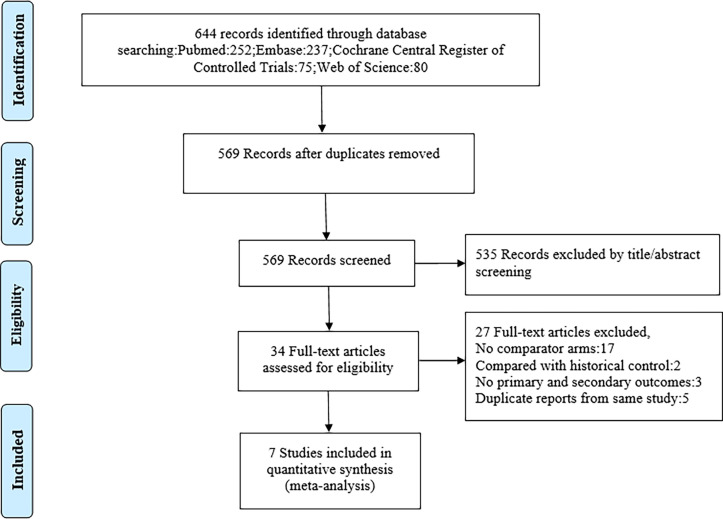
In total, 644 potentially relevant records were identified in database records using the selected search terms ( Figure 1 ). After a thorough screening of the remaining 569 titles and abstracts, 535 non-relevant studies were excluded. The full texts of the remaining 34 studies were assessed, leading to the elimination of 27 studies that did not meet the eligibility criteria. Subsequently, the remaining seven studies were included in our meta-analysis. Characteristics of the studies included in this analysis are listed in Tables 1, 2. Totally, seven studies were included ranging from 74 to 707 patients. In five of these studies, the prophylactic regimen was CNI + methotrexate (MTX) vs. CNI + MTX + SRL (10–14). In two studies, CNI + mycophenolate mofetil (MMF) was compared with CNI + MMF + SRL (15, 16) for GVHD prophylaxis.
Figure 1.
Flow diagram of this study.
Table 1.
Characteristics of the included RCTs, comparing the SRL-based and non–SRL-based groups.
| First author and year | Disease | Age (SRL group) | Sample size SRL/non-SRL | Donors | Conditioning regimen |
|---|---|---|---|---|---|
| Armand 2016 (10) | Lymphoma, except Burkitt lymphoma. | 57 (23–70) | 66/73 | HLA-matched related donors or MUD. | RIC regimen |
| Pulsipher 2014 (11) | High-risk ALL in CR | NA (1–21) | 73/70 | HLA-matched siblings, HLA-matched related or unrelated donors, or single cord blood unit with 4–6/6 matched. | Myeloablative regimen (TBI followed by thiotepa or etoposide and CY) |
| Cutler 2014 (12) | Acute leukemia in remission, MDS, or CML. | 45 (19–59) | 151/153 | HLA-matched sibling. | Myeloablative regimen (TBI in combination with either CY or etoposide) |
| Khimani 2017 (13) | AML, MDS, CML, ALL, CLL, sAA, MM, and lymphoma | 52 (19–74) | 293/414 | HLA-matched sibling HLA-matched or mismatched unrelated donors |
Standard myeloablative Escalated dose busulfan Non-myeloablative Reduced toxicity |
| Pidala 2015 (14) | AML, MDS, CML, ALL, CLL, sAA, MM, and lymphoma | 49 (25–68) | 37/37 | Only 8/8 or more HLA-matched sibling or unrelated donors | Bu/pent, Flu/Mel, Flu/Mel |
| Sandmaier 2019 (15) | Advanced hematological malignancies. | 63 (58–68) | 90/77 | At least 9/10 HLA-matched unrelated donors | Fludarabine+TBI |
| Kornblit 2014 (16) | AML, ALL, MDS, CML, CLL, MM, and lymphoma | 61 (15–76) | 68/71 | HLA-matched or mismatched unrelated donors | Nonmyeloablative regimen (fludarabine and 2 Gy TBI) |
SRL, sirolimus; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; CR, complete remission; MDS, myelodysplastic syndrome; SAA, severe aplastic anemia; MM, multiple myeloma; HLA, human leukocyte antigen; MUD, matched unrelated donor; URD, unrelated donor; TBI, total body irradiation; CY, cyclophosphamide; RIC, reduced-intensity conditioning; Bu, busulfan; ATG, anti-thymocyte globulin; Flu, fludarabine; Mel, melphalan; NMA, non-myeloablative; HCT, allogeneic hematopoietic cell transplantation.
Table 2.
Characteristics of the included RCTs, comparing the SRL-based and non–SRL-based groups.
| First author and year | SRL usage and dosage | Grouping scheme | SRL administration time | Follow-up time (months) | SRL-based benefit outcomes (p < 0.05) |
|---|---|---|---|---|---|
| Armand 2016 (10) | 12 mg orally on day -3, then 4 mg daily to 360 days, with 5~12 ng/ml | TAC+MTX vs. TAC+MTX+SRL | -3 days~+360 days | 22 (NA) | II–IV aGVHD, yes; III–IV aGVHD, no; cGVHD, no; relapse, no; TMA, no; PFS, no; OS, no |
| Pulsipher 2014 (11) | 4 mg/m2 on day 0, maintaining for 6 months, with 3~12 ng/ml level, followed by a 1-month taper | TAC+MTX vs. TAC+MTX+SRL | 0 days~+180 days | 26 (23~38) | II–IV aGVHD, yes; III–IV aGVHD, no; cGVHD, no; relapse, no; TMA, no; VOD, no; OS, no; NRM, no |
| Cutler 2014 (12) | Started on day -3 with 12 mg, followed by a daily dose of 4 mg, maintaining a 3~12-ng/ml level | TAC+MTX vs. TAC+SRL | -3 days~+100 days | 24 (NA) | II–IV aGVHD, no; III–IV aGVHD, yes; cGVHD, no; relapse, no; TMA, no; VOD, no; OS, no; NRM, no |
| Khimani 2017 (13) | 9 mg oral loading dose on day -1, kept at 5–14 ng/ml concentration, and continued for at least 1 year | TAC+MTX vs. TAC+SRL | -1 day~+365 days | 23.7(11.1-73.1) | II–IV aGVHD, yes; III–IV aGVHD, no; cGVHD, no; relapse, no; VOD, no; OS, yes; NRM, no |
| Pidala 2015 (14) | Started on day -1 with 9 mg, maintaining 5~14 ng/ml level, continued for at least 1 year | TAC+MTX vs. TAC+SRL | -1 day~+365 days | 41(27~60) | II–IV aGVHD, yes; cGVHD, yes; relapse, yes; TMA, no; VOD, no; OS, no; NRM, no. |
| Sandmaier 2019 (15) | Started on day -3 at 2 mg/day, maintaining 3~12 ng/ml to day 150, and tapered off by day 180 | CsA+MMF vs. CsA+MMF+SRL | -3 days~+180 days | 48 (31–60) | II–IV aGVHD, yes; III–IV aGVHD, yes; cGVHD, no; relapse, no; OS, yes; PFS, no; NRM, yes |
| Kornblit 2014 (16) | Started on day -3 at 2 mg, maintaining 3–12 ng/ml. Stopped on day 80 without a taper | TAC+MMF vs. TAC+MMF+SRL | -3 days~+80 days | 59 (6–101) | II–IV aGVHD, yes; III–IV aGVHD, no; cGVHD, no; relapse, no; EFS, no; OS, no; PFS, no |
TAC, tacrolimus; MTX, methotrexate; CSA, cyclosporine; MMF, mycophenolate mofetil; aGVHD, acute graft-versus-host disease; OS, overall survival; PFS, progression-free survival; NRM, non-relapse mortality; TMA, thrombotic microangiopathy; VOD, veno-occlusive disease.
Risk-of-Bias Assessment
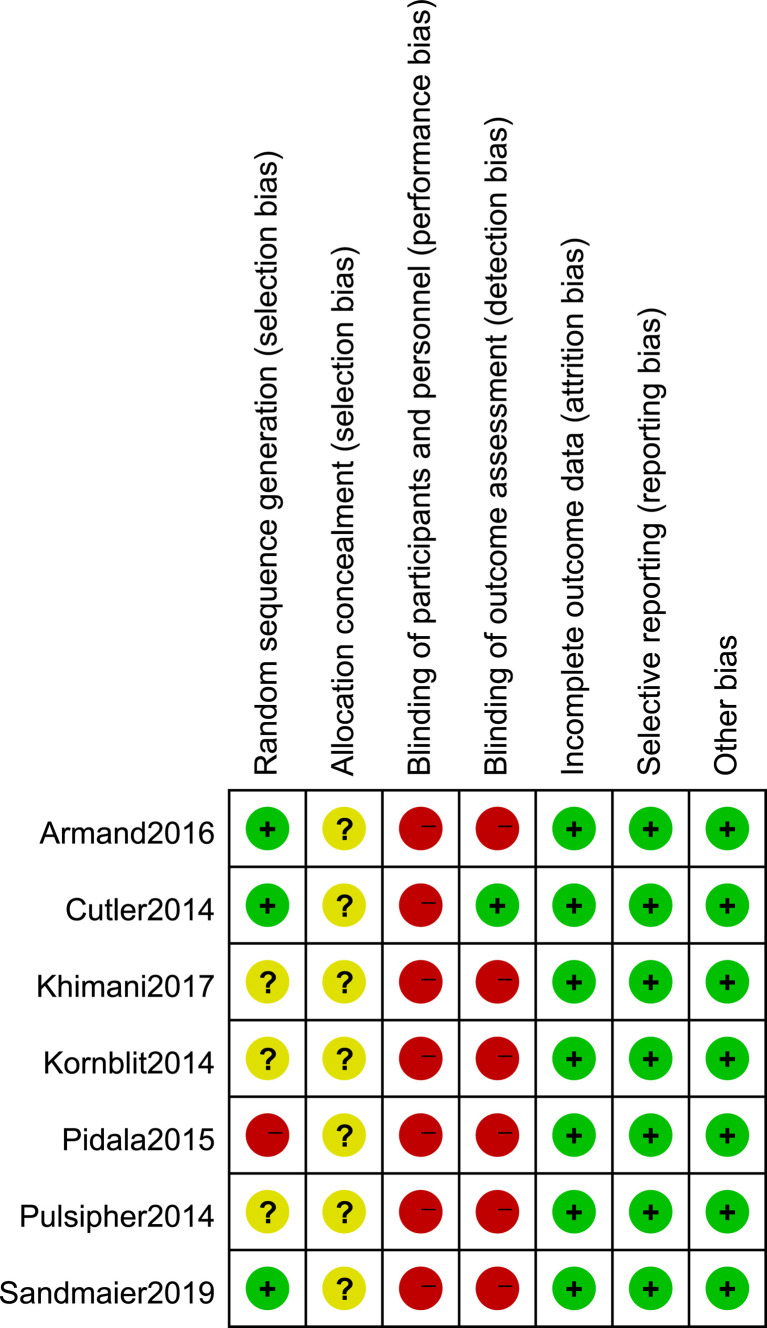
The quality evaluation of the included studies was performed according to the Cochrane handbook. The risk-of-bias assessment was performed to address six aspects: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. In the selection bias assessment, three papers described the use of computer-generated random sequences, one paper randomized according to age and donor type, and the other three papers did not mention their grouping method. Seven studies did not mention their method for allocation concealment, while one mentioned the method of blinding data extraction. Seven studies were all low risk on other bias assessment ( Figure 2 ).
Figure 2.
Risk of bias of included randomized controlled trials.
Study Outcome Analysis
Primary Outcomes: GVHD, Including aGVHD and cGVHD
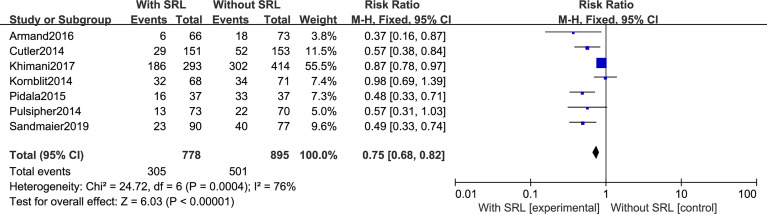
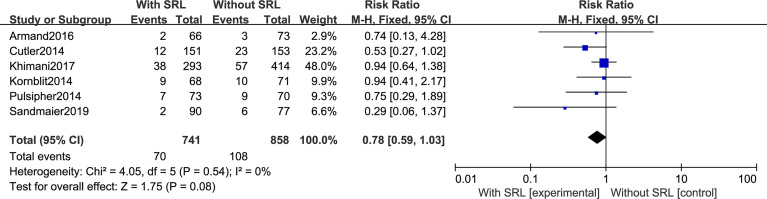
All seven studies reported the incidence of Grade II–IV aGVHD. Interstudy heterogeneity was observed to be significant, and the random-effect model was used (I2 = 76%, p < 0.0001). By Stata analysis, Begg’s test showed that p = 0.548 > 0.5, which suggests that there is no publication bias. Based on the results of modeling, SRL-based regimens significantly decreased the incidence of Grade II–IV aGVHD (RR, 0.75; 95% CI, 0.68–0.82; Figure 3). Interestingly, six studies reported the incidence of Grade III–IV aGVHD and the results did not support that addition of SRL can also reduce Grade III–IV aGVHD (RR, 0.78; 95% CI, 0.59–1.03, p = 0.08; Figure 4).
Figure 3.
The effect of addition of SRL to prophylaxis on Grade II to IV aGVHD.
Figure 4.
The effect of addition of SRL to prophylaxis on Grade III to IV aGVHD.
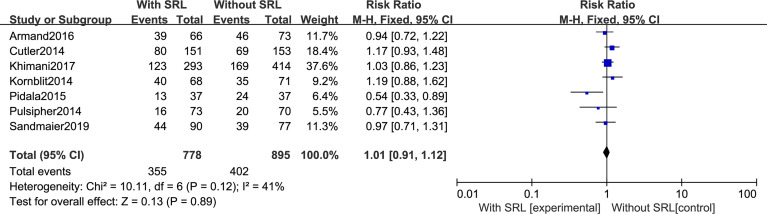
Additionally, SRL-based prophylaxis also cannot reduce the incidence of cGVHD. All seven studies reported that the incidence of cGVHD was not impacted by SRL-based prophylaxis regimens. There was no statistical difference in cGVHD prevention between SRL-based and non-SRL groups (RR, 1.01; 95% CI, 0.91-1.12, P=0.89; Figure 5).
Figure 5.
The effect of addition of SRL to prophylaxis on chronic GVHD.
Secondary Outcomes: TMA, VOD, and OS
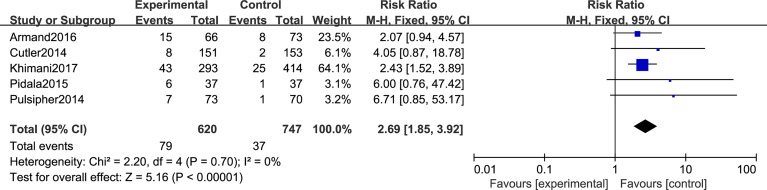
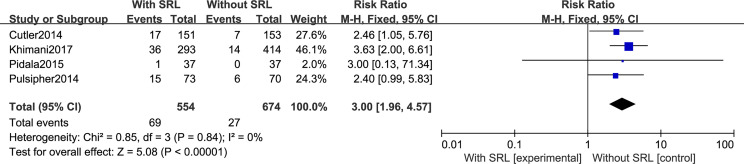
Regarding TMA, five studies included in this meta-analysis analyzed the risk of developing TMA. Pooled analysis suggested that all the SRL-based regimens led to an increased TMA incidence (RR, 2.69; 95% CI, 1.85–3.92, p < 0.00001; Figure 6). Regarding VOD risk, four studies mentioned that SRL-based prophylaxis increased the incidence of VOD, and only one reported that there was no VOD on either arm. Pooled analysis also hinted that SRL-based regimens increase VOD risk (RR, 3.00; 95% CI, 1.96–4.57, p < 0.00001; Figure 7). It is hypothesized that rapamycin can cause endothelial cell damage through macrophage activation (17) and calcium overload (18).
Figure 6.
The effect of SRL-based and non-SRL-based prophylaxis on TMA.
Figure 7.
Impact of SRL-based prophylaxis versus non-SRL-based prophylaxis on VOD.
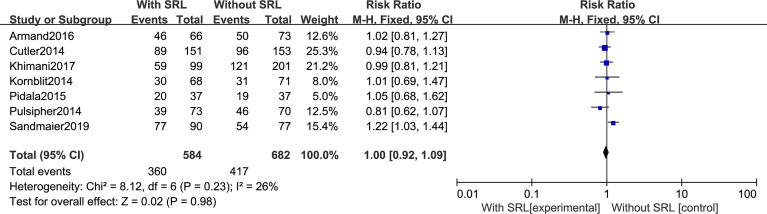
Our analysis includes seven RCTs to analyze OS. The pooled meta-analysis showed that there are no differences in OS (RR, 1.00; 95% CI, 0.92–1.09, p = 0.98; Figure 8). Moreover, SRL-based interventions had no statistical effect on PFS, relapse, NRM, bacterial and fungal infection, and CMV reactivation (Supplementary File 1 ).
Figure 8.
Impact of SRL-based prophylaxis versus non-SRL-based prophylaxis on OS.
Publication Bias Assessment and Sensitivity Analysis
Stata 15.0 software was used to test the publication bias. The results showed that there was no significant publication bias (p > 0.05). The heterogeneity of two indexes (II–IV aGVHD and bacterial infection) displayed that the I2 value was greater than 50%. We used sensitivity analysis with Stata software to determine the stability of the results. The sensitivity analysis showed that the research results were stable and reliable ( Supplementary File 2 ).
Discussion
Effective prophylaxis of GVHD is the key to successful allo-HSCT. Sirolimus, in combination with a calcineurin inhibitor or post-transplantation cyclophosphamide, is a common regimen for GVHD prophylaxis. This meta-analysis specifically focuses on sirolimus-based GVHD prophylaxis, but there are indeed many other prophylaxis regimens, each with their own advantages and disadvantages depending on the transplant setting and GVHD risk factors.
In this study, we systematically evaluated the effect of SRL on GVHD as a prophylactic drug and included seven RCTs. Statistical results show that SRL prophylaxis regimens can significantly reduce the incidence of Grade II–IV aGVHD. There was no significant difference between the SRL group and the non-SRL group in reducing the incidence of III–IV aGVHD and cGVHD, which indicated that the sirolimus-containing regimen may not prevent all forms of GVHD. This suggests that additional steps may be necessary to prevent cGVHD in the clinic in patients treated with SRL-based regimens.
Meta-analysis showed that the prophylactic regimen containing SRL could reduce acute GVHD but had no effect on OS and PFS. It is known that factors affecting OS and PFS in patients with HSCT are complex, and SRL prophylaxis is not the only factor. Khimani et al. (13) divided the patients into four subgroups according to the hematopoietic cell transplantation–comorbidity index (HCT-CI: creatinine, ejection fraction, FEV1, aspartate aminotransferase, ALT, and bilirubin) (19). Patients with HCT-CI ≥ 4 had significantly worse OS with MTX/TAC than the SRL/TAC group. Thus, it is possible that SRL may improve outcomes in high-risk populations. In addition, SRL combined with post-transplant cyclophosphamide can also provide better survival outcomes in patients after allo-HSCT (20–23). Additional RCTs are required to carefully assess the role of SRL in transplant patient outcomes.
TMA and VOD are both thrombotic diseases that commonly develop post-transplant, and, at present, the pathogenesis of these diseases is unknown. It has been reported that the occurrence of TMA and VOD is related to different regimens, aGVHD, bacterial or fungal infection, HLA mismatch, and combination of drugs (tacrolimus and sirolimus), which caused endothelial injury, thrombosis, and microcirculatory fibrin deposition. Meta-analysis showed that the SRL-based prophylaxes increased the risk of TMA and VOD, suggesting that administration of SRL should be stopped in time when a toxic reaction arises caused by thrombogenesis. However, it should be noted that the occurrence of TMA is not necessarily caused by SRL. Pidala et al. (14) reported that the occurrence of TMA in their study was related to use of tacrolimus, which indicates that TMA may also be a result of the combined effect of SRL and TAC. SRL-induced TMA may be attributable to enhanced platelet activation and aggregation, leading to endothelial damage. Another theory involves the pharmacokinetic interaction between sirolimus and calcineurin inhibitors, which may potentially lead to increased serum and kidney levels of these agents (24). TMA is a multifactorial complication, which may be caused by pathogenic microorganism infection (CMV, BK virus, etc.), calcineurin inhibitor and mTOR inhibitor, GVHD, cytokines (IL-6, INF-a, etc), and neutrophil extracellular traps (NET). TMA might primarily be an effect of high-dose Bu treatment, especially in combination with tacrolimus-based GVHD prophylaxis (25).
The addition of SRL on the basis of the prevention of the two drugs may aggravate the immunosuppression and increase the chance of infection in the later stage. However, there were contrary opinions that sirolimus-based GVHD prophylaxis significantly reduced CMV reactivation (26). Additionally, SRL also exerts anti-EB viral (27) and antifungal (28) actions. Our meta-analysis showed that the SRL-containing regimen neither increased nor decreased bacterial, fungal, and CMV infection. Therefore, more RCTs are needed to confirm this conclusion.
In the seven articles, almost all recipients received identical transplants, including HLA-matched sibling or unrelated donors, with no more than a single allele disparity. Only one paper mentioned mismatched unrelated donor (MMUD); in this report, in the SRL/TAC group, 15% of the patients received MMUD, and in the TAC/MTX group, 22% patients received MMUD (13). Although donor source mismatch unrelated and matched sibling have no difference in OS rate according to the GVHD prophylaxis group, it is worth noting that SRL/TAC has been associated with improved OS among patients at high risk for GVHD based on subgroup analysis (13). There are many factors affecting the clinical outcomes of allo-HSCT, including disease status, conditioning regimen, and GVHD prophylaxis. Whether SRL-based prophylaxis is suitable for haploid transplantation requires additional clinical studies.
Due to different classifications of HSCT, such as HLA-matched or mismatched, sibling, or unrelated donors, the prophylaxis schemes of GVHD are different. To minimize heterogeneity, we strictly chose seven papers for this meta-analysis; from the results, addition of sirolimus could be an effective and safe prophylaxis option for GVHD, although the association of SRL with increased thrombotic complications must be carefully monitored and effectively managed. While SRL has some advantages, it is not a first choice for GVHD prophylaxis; however, subgroup analysis may identify additional advantages in high-risk (≥HCT-CI) groups. More adequately powered RCTs are required to better understand the impact of SRL-based GVHD prophylactic regimens.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
YF wrote the original draft and revised the manuscript. XC and YF completed the literature search, data extraction, data analysis, and chart making. KC, HS, LW, TC, HY, SY, and XZ edited this review. XC, XZ, and YF funded the work. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Chongqing Science and Technology Commission joint project (2021MSXM299) and the Natural Science Foundation of Chongqing (cstc2019jcyj-msxmX0273, cstc2020jcyj-msxmX1086 and cstc2020jcyj-msxmX0448), Science and Technology Innovation Capacity Promotion Project of Army Medical University (2019XLC3014), Special Projects in the Frontier of Military Medicine Natural Science of Xinqiao Hospital (2018 YQYLY002), and National Key Research Program (2017YFA0105502).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.683263/full#supplementary-material
References
- 1.Fuchs EJ, Huang XJ, Miller JS. HLA-Haploidentical Stem Cell Transplantation for Hematologic Malignancies. Biol Blood Marrow Transplant (2010) 16:S57–63. 10.1016/j.bbmt.2009.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu T, Sun D, Chen Y, Ouyang L. Targeting Mtor for Fighting Diseases: A Revisited Review of mTOR Inhibitors. Eur J Med Chem (2020) 199:112391. 10.1016/j.ejmech.2020.112391 [DOI] [PubMed] [Google Scholar]
- 3.Feng YM, Xiao YS, Yan HJ, Wang P, Zhu W, Cassady K, et al. Sirolimus as Rescue Therapy for Refractory/Relapsed Immune Thrombocytopenia: Results of a Single-Center, Prospective, Single-Arm Study. Front Med (2020) 7:110. 10.3389/fmed.2020.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng Y, Chen X, Cassady K, Zou Z, Yang S, Wang Z, et al. The Role of Mtor Inhibitors in Hematologic Disease: From Bench to Bedside. Front Oncol (2021) 10:611690. 10.3389/fonc.2020.611690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston L, Florek M, Armstrong R, McCune JS, Arai S, Brown J, et al. Sirolimus and Mycophenolate Mofetil as GVHD Prophylaxis in Myeloablative, Matched-Related Donor Hematopoietic Cell Transplantation. Bone Marrow Transplant (2012) 47:581–8. 10.1038/bmt.2011.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furlong T, Kiem HP, Appelbaum FR, Carpenter PA, Deeg HJ, Doney K, et al. Sirolimus in Combination With Cyclosporine or Tacrolimus Plus Methotrexate for Prevention of Graft-Versus-Host Disease Following Hematopoietic Cell Transplantation From Unrelated Donors. Biol Blood Marrow Transplant (2008) 14:531–7. 10.1016/j.bbmt.2008.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutler C, Henry NL, Magee C, Li S, Kim HT, Alyea E, et al. Sirolimus and Thrombotic Microangiopathy After Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2005) 11:551–7. 10.1016/j.bbmt.2005.04.007 [DOI] [PubMed] [Google Scholar]
- 8.Labrador J, Lopez-Corral L, Lopez-Godino O, Vazquez L, Cabrero-Calvo M, Perez-Lopez R, et al. Risk Factors for Thrombotic Microangiopathy in Allogeneic Hematopoietic Stem Cell Recipients Receiving GVHD Prophylaxis With Tacrolimus Plus MTX or Sirolimus. Bone Marrow Transplant (2014) 49:684–90. 10.1038/bmt.2014.17 [DOI] [PubMed] [Google Scholar]
- 9.Higgins JP, Thompson SG. Quantifying Heterogeneity in a Meta-Analysis. Stat Med (2002) 21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 10.Armand P, Kim HT, Sainvil MM, Lange PB, Giardino AA, Bachanova V, et al. The Addition of Sirolimus to the Graft-Versus-Host Disease Prophylaxis Regimen in Reduced Intensity Allogeneic Stem Cell Transplantation for Lymphoma: A Multicentre Randomized Trial. Br J Haematol (2016) 173:96–104. 10.1111/bjh.13931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pulsipher MA, Langholz B, Wall DA, Schultz KR, Bunin N, Carroll WL, et al. The Addition of Sirolimus to Tacrolimus/Methotrexate GVHD Prophylaxis in Children With ALL: A Phase 3 Children’s Oncology Group/Pediatric Blood and Marrow Transplant Consortium Trial. Blood (2014) 123:2017–25. 10.1182/blood-2013-10-534297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cutler C, Logan B, Nakamura R, Johnston L, Choi S, Porter D, et al. Tacrolimus/Sirolimus vs Tacrolimus/Methotrexate as GVHD Prophylaxis After Matched, Related Donor Allogeneic HCT. Blood (2014) 124:1372–7. 10.1182/blood-2014-04-567164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khimani F, Kim J, Chen L, Dean E, Rizk V, Betts B, et al. Predictors of Overall Survival Among Patients Treated With Sirolimus/Tacrolimus vs Methotrexate/Tacrolimus for GvHD Prevention. Bone Marrow Transplant (2017) 52:1003–9. 10.1038/bmt.2017.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pidala J, Kim J, Alsina M, Ayala E, Betts BC, Fernandez HF, et al. Prolonged Sirolimus Administration After Allogeneic Hematopoietic Cell Transplantation Is Associated With Decreased Risk for Moderate-Severe Chronic Graft-Versus-Host Disease. Haematologica (2015) 100:970–7. 10.3324/haematol.2015.123588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandmaier BM, Kornblit B, Storer BE, Olesen G, Maris MB, Langston AA, et al. Addition of Sirolimus to Standard Cyclosporine Plus Mycophenolate Mofetil-Based Graft-Versus-Host Disease Prophylaxis for Patients After Unrelated Non-Myeloablative Haemopoietic Stem Cell Transplantation: A Multicentre, Randomised, Phase 3 Trial. Lancet Haematol (2019) 6:e409–18. 10.1016/S2352-3026(19)30088-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kornblit B, Maloney DG, Storer BE, Maris MB, Vindelov L, Hari P, et al. A Randomized Phase II Trial of Tacrolimus, Mycophenolate Mofetil and Sirolimus After Non-Myeloablative Unrelated Donor Transplantation. Haematologica (2014) 99:1624–31. 10.3324/haematol.2014.108340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SJ, Kang SJ, Virmani R, Nakano M, Ueda Y. In-Stent Neoatherosclerosis: A Final Common Pathway of Late Stent Failure. J Am Coll Cardiol (2012) 59:2051–7. 10.1016/j.jacc.2011.10.909 [DOI] [PubMed] [Google Scholar]
- 18.Long C, Cook LG, Hamilton SL, Wu GY, Mitchell BM. FK506 Binding Protein 12/12.6 Depletion Increases Endothelial Nitric Oxide Synthase Threonine 495 Phosphorylation and Blood Pressure. Hypertension (2007) 49:569–76. 10.1161/01.HYP.0000257914.80918.72 [DOI] [PubMed] [Google Scholar]
- 19.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic Cell Transplantation (HCT)-Specific Comorbidity Index: A New Tool for Risk Assessment Before Allogeneic HCT. Blood (2005) 106:2912–9. 10.1182/blood-2005-05-2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montoro J, Roldan E, Pinana JL, Barba P, Chorao P, Quintero A, et al. Ex Vivo T-Cell Depletion vs Post-Transplant Cyclophosphamide, Sirolimus, and Mycophenolate Mofetil as Graft-vs-Host Disease Prophylaxis for Allogeneic Hematopoietic Stem Cell Transplantation. Eur J Haematol (2021) 106:114–25. 10.1111/ejh.13529 [DOI] [PubMed] [Google Scholar]
- 21.Jaiswal SR, Chatterjee S, Mukherjee S, Ray K, Chakrabarti S. Pre-Transplant Sirolimus Might Improve the Outcome of Haploidentical Peripheral Blood Stem Cell Transplantation With Post-Transplant Cyclophosphamide for Patients With Severe Aplastic Anemia. Bone Marrow Transplant (2015) 50:873–5. 10.1038/bmt.2015.50 [DOI] [PubMed] [Google Scholar]
- 22.Jaiswal SR, Bhakuni P, Zaman S, Bansal S, Bharadwaj P, Bhargava S, et al. T Cell Costimulation Blockade Promotes Transplantation Tolerance in Combination With Sirolimus and Post-Transplantation Cyclophosphamide for Haploidentical Transplantation in Children With Severe Aplastic Anemia. Transpl Immunol (2017) 43-44:54–9. 10.1016/j.trim.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 23.Jaiswal SR, Bhakuni P, Aiyer HM, Soni M, Bansal S, Chakrabarti S. CTLA4Ig in an Extended Schedule Along With Sirolimus Improves Outcome With a Distinct Pattern of Immune Reconstitution Following Post-Transplantation Cyclophosphamide-Based Haploidentical Transplantation for Hemoglobinopathies. Biol Blood Marrow Transplant (2020) 26:1469–76. 10.1016/j.bbmt.2020.05.005 [DOI] [PubMed] [Google Scholar]
- 24.Shayani S, Palmer J, Stiller T, Liu X, Thomas SH, Khuu T, et al. Thrombotic Microangiopathy Associated With Sirolimus Level After Allogeneic Hematopoietic Cell Transplantation With Tacrolimus/Sirolimus-Based Graft-Versus-Host Disease Prophylaxis. Biol Blood Marrow Transplant (2013) 19:298–304. 10.1016/j.bbmt.2012.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassan Z, Hellstrom-Lindberg E, Alsadi S, Edgren M, Hagglund H, Hassan M. The Effect of Modulation of Glutathione Cellular Content on Busulphan-Induced Cytotoxicity on Hematopoietic Cells In Vitro and In Vivo . Bone Marrow Transplant (2002) 30:141–7. 10.1038/sj.bmt.1703615 [DOI] [PubMed] [Google Scholar]
- 26.Marty FM, Bryar J, Browne SK, Schwarzberg T, Ho VT, Bassett IV, et al. Sirolimus-Based Graft-Versus-Host Disease Prophylaxis Protects Against Cytomegalovirus Reactivation After Allogeneic Hematopoietic Stem Cell Transplantation: A Cohort Analysis. Blood (2007) 110:490–500. 10.1182/blood-2007-01-069294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krams SM, Martinez OM. Epstein-Barr Virus, Rapamycin, and Host Immune Responses. Curr Opin Organ Transplant (2008) 13:563–8. 10.1097/MOT.0b013e3283186ba9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a New Antifungal Antibiotic. I. Taxonomy of the Producing Streptomycete and Isolation of the Active Principle. J Antibiot (Tokyo) (1975) 28:721–6. 10.7164/antibiotics.28.721 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.