Highlights
-
•
Musculoskeletal sarcoma surgery involving the lower limb is associated with significant intraoperative blood loss, with more than 500 ml in 96% of patients.
-
•
Salvaged blood was processed and re-infused in 94% of patients.
-
•
An estimated one-fourth of intraoperative blood loss was returned to the patient through (Intraoperative cell salvage) ICS.
-
•
Cytological analysis of imprints from leucodepletion filters(LDF) and reinfused blood did not reveal evidence of tumour cells.
Keywords: Cell salvage, Transfusion, Musculoskeletal oncology, Reinfusion, Sarcoma
Abbreviations: ICS, Intra-operative cell salvage; LDF, Leucocyte depletion filter; LLE, Lower Limb endoprosthesis; WE, Wide Excision
Abstract
Background
The efficacy and safety of cell salvage for musculoskeletal sarcoma surgery have not been reported, and concerns over re-infusion of tumour cells remain. This study aims to i) describe the intra-operative blood loss and cell salvage reinfusion volumes for lower limb sarcoma and pelvic sarcoma procedures ii) and explore whether there is evidence of tumour cells in reinfused blood.
Methods
Retrospective analysis of 109 consecutive surgical procedures for biopsy-proven sarcoma or bone metastasis performed between 1 July 2015 and 30 October 2019. Salvaged blood was processed and reinfused when intraoperative blood loss exceeded 500 ml. Primary bone tumour (n = 86(79%)) and metastasis (n = 23(21%) constituted the study group and surgeries were classified under hemipelvectomy (n = 43(39%)), lower limb endoprosthesis replacement (LLE) (n = 50(46%)) and wide excision surgery (WE) (n = 16(15%)). Microscopic examination of imprint cytology of leuco-depletion(LD) filters, and peripheral smear examination was performed for reinfused blood.
Results
Median (IQR) intra-operative blood loss was 1750 (600–3000) ml for hemipelvectomy, 850 (600–1200) ml for LLE, and 1000 (550–2000) ml for WE. Salvaged blood was re-infused in 102 of 109 (94%) patients. The mean (SD) volume of re-infusion was 445(4 2 5) ml for hemipelvectomy, 206(1 3 1) ml for LLE, and 184(1 0 6) ml for WE. In total, 64 of 109 (59%) patients received an allogeneic red blood transfusion within 72 h of surgery. Cytology analysis of imprints taken from the filtered blood available in 95(87%) patients and peripheral smear examination of reinfused blood available in 32(29%) patients did not reveal evidence of tumour cells on microscopic examination of any samples.
Conclusion
Our study demonstrates that musculoskeletal sarcoma surgery is associated with significant blood loss, and cell salvage permits reinfusion of autologous blood in most patients. The cytological analysis did not reveal evidence of tumour cells in reinfused blood, consistent with other studies where cell salvage is used for cancer surgery.
1. Introduction
Musculoskeletal sarcoma surgery is associated with significant intraoperative blood loss and allogeneic blood transfusion rates [1], [2]. In complex surgeries involving pelvic resections and wide excision surgery, the average blood loss is around 4500 ml, and nearly 20% of these surgeries require allogenic blood transfusion post-surgery [2], [3]. Increased peri-operative blood loss and blood transfusion are associated with increased mortality rates and increased morbidity in cancer surgery [4], [5]. Strategies to reduce intra-operative blood loss include optimisation of erythropoiesis, use of antifibrinolytic agents [6], and intraoperative cell salvage (ICS) [7]. Although widely used for cancer surgery in some specialties, the role of ICS for musculoskeletal sarcoma surgery has not been reported, and earlier reports raised concerns over the re-infusion of malignant cells into the circulation [8], [9]. Recently, studies have shown the use of ICS to safe in urologic oncology surgery [10] and also in gynaecologic oncology [11].
The Association of Anaesthetists guidelines in 2018 stated that there is no conclusive evidence the ICS induces metastasis or affects cancer prognosis, but recommend considering the use of leucodepletion filters during re-infusion of salvaged blood in cancer surgery [12].
The aims of this study are to describe the intra-operative blood loss and cell salvage reinfusion volumes for musculoskeletal sarcoma procedures and explore whether there is evidence of tumour cells in reinfused blood.
2. Methods
We performed a retrospective cohort study of consecutive patients who underwent lower limb surgery for musculoskeletal sarcoma and received ICS for their surgery at the Nuffield Orthopaedic Centre, Oxford University Hospitals NHS Foundation Trust, Oxford, UK, between 1 July 2015 and 30 October 2019. Indication for surgery was classified as primary bone tumour or metastasis. Procedures performed were classified as hemipelvectomy, lower limb endoprosthesis (LLE) replacement, and wide excision surgery (WE). Hemipelvectomy included internal hemipelvectomy (type I, type II, and type III) and external hemipelvectomy cases; LLE included patients who needed prosthesis replacement after tumour excision or in metastatic cases/fractures; WE included patients where wide excision was performed that did not require reconstruction. Finding are reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. The study received approval from the local Research Ethics Committee, and the reference number granted was 6649.
All patients had their diagnosis and treatment plan finalized at the multidisciplinary team (MDT) meeting. The procedures were performed by two surgeons (DW and MG). All patients received general anaesthesia with or without neuraxial anaesthesia. Intravenous 1 g tranexamic acid was administered to all patients before skin incision. Routine thromboprophylaxis of low-molecular-weight heparin was commenced 6 h post-surgery. During the study period, there was no change in routine anaesthesia or surgical technique.
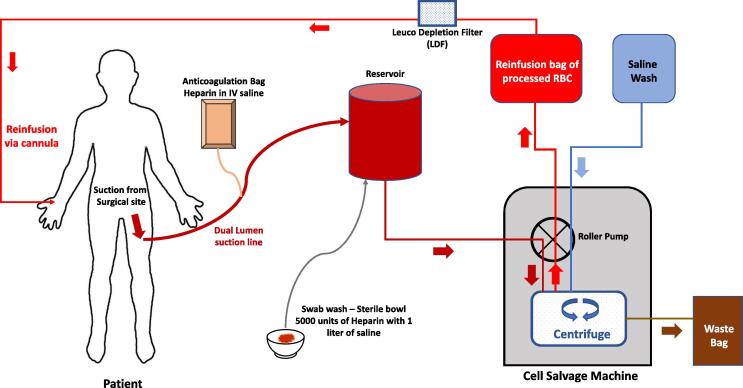
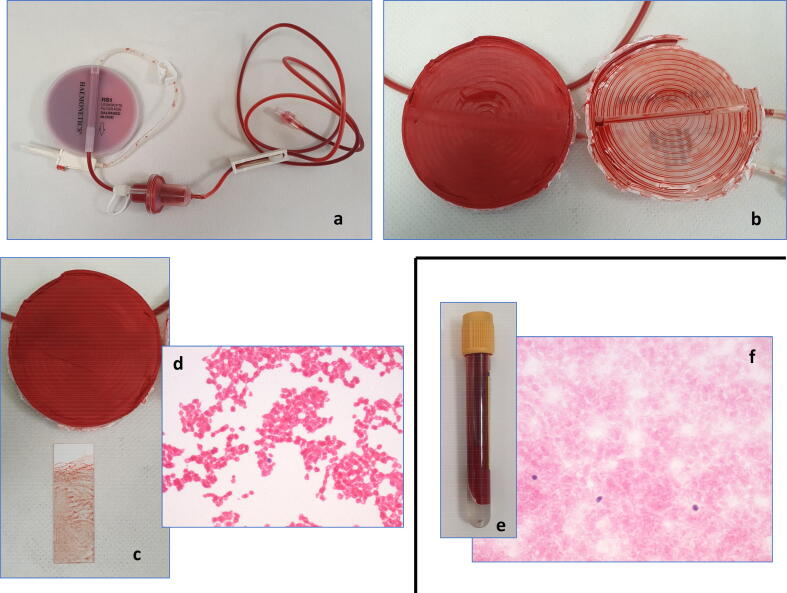
ICS was used in all musculoskeletal sarcoma surgeries from 1 July 2015, where intraoperative blood loss was expected to exceed 500 ml. The anaesthetist explained to all patients about the potential risks and benefits and a specific informed consent was obtained prior to surgery. Cell salvage was performed using Sorin XtraTM machine (LivaNova, London, UK), and the size of the bowl was selected based on the volume of salvaged blood (55–225 ml) (Fig. 1). Leucocyte depletion filter (LDF) (40 µm)was used in all cases. Wound irrigation was performed using saline. All procedures were commenced with the ICS system in ‘collect only’ mode. The salvaged blood was also then processed to re-suspend the red blood cells with a haematocrit of 60% once the estimated blood loss exceeded 500 ml.
Fig. 1.
Schematic diagram showing the routine intra-operative set-up for intraoperative cell salvage.
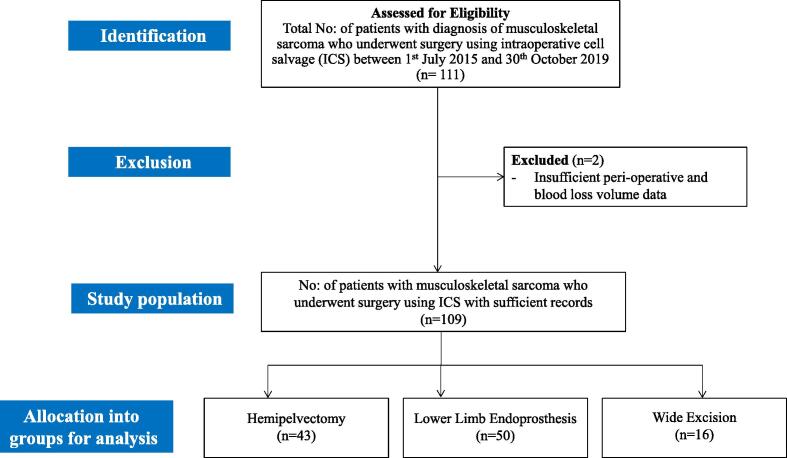
Data were collected from local electronic health records and cell salvage databases. The collected data included: patient demographics (age, sex, BMI, ASA grade); diagnosis made at MDT; procedure performed; cell salvage records (calculated intra-operative blood loss, re-infused blood volume and haematocrit (Hct) measurements); and perioperative Hb measurements. Records were also reviewed for reinfusion hypotension in the postoperative period. Patients with incomplete record details were excluded from the study (Fig. 2). In line with national guidelines [13], the haemoglobin red blood cell transfusion threshold was70 g/l in our hospital, except in patients with acute coronary syndrome where the threshold was 80 g/l. Transfusions of red blood cells on the day of surgery were defined as those taking place before midnight.
Fig. 2.
Flowchart depicting the study methodology and categorization of cases.
Estimated intra-operative blood loss was calculated according to the formula [14]:
Blood loss (ml) = Estimated blood volume (ml) * [(Hct procedure start (%) – Hct pretransfusion %) / (Hct procedure start (%) + Hct pretransfusion (%))/2]
Re-infusion volume was collected from the cell salvage records. The proportion of estimated blood loss re-infused was calculated as [15]:
Cell salvage efficiency (%) = (Re-infusion volume (ml) * Re-infusion Hct (%)/ Hct procedure start (%) * Estimated blood loss (ml)).
The volume of salvaged blood was standardised to haematocrit 60%.
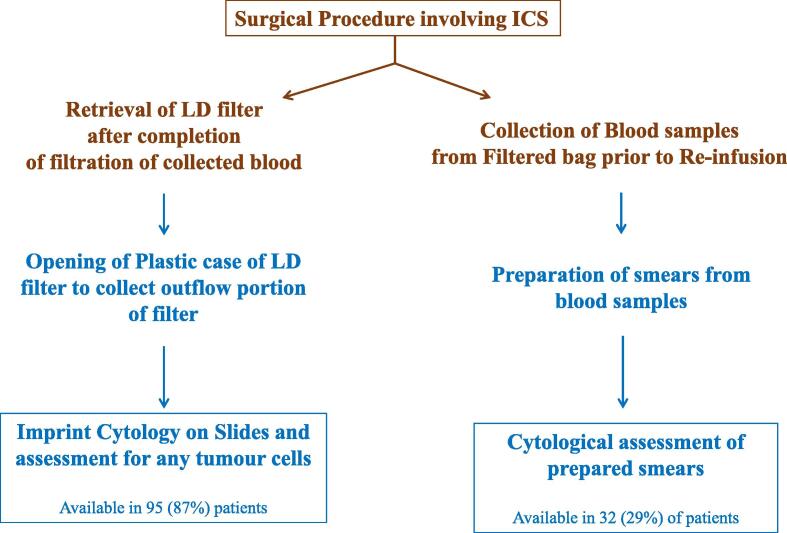
To analyse whether there were malignant tumour cells in the re-infused blood, touch imprint samples were taken from the plastic case on the outflow portion of the filter (Fig. 3). Imprint cytology is a well-recognised simple technique for preparation of specimen for assessment [16]. The sample which needs analysis is pressed onto a glass slide, which is then fixed and stained for assessment. Samples of blood for re-infusion were taken and two peripheral smears were prepared. Two touch imprints were taken on 2–2 slides from the central and peripheral portion of the filter. The slides were air-dried, fixed in ether-alcohol solution(1:1), and stained with haematoxylin and eosin. Microscopic examination of the slides was performed by a histopathologist (OZ).
Fig. 3.
Flowchart depicting the method of assessment of LD filters and re-infused blood for malignant cells. Steps and arrows in ‘brown’ were done in operation theatre; steps and arrows in ‘dark blue’ were performed in laboratory by pathologist. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3. Results:
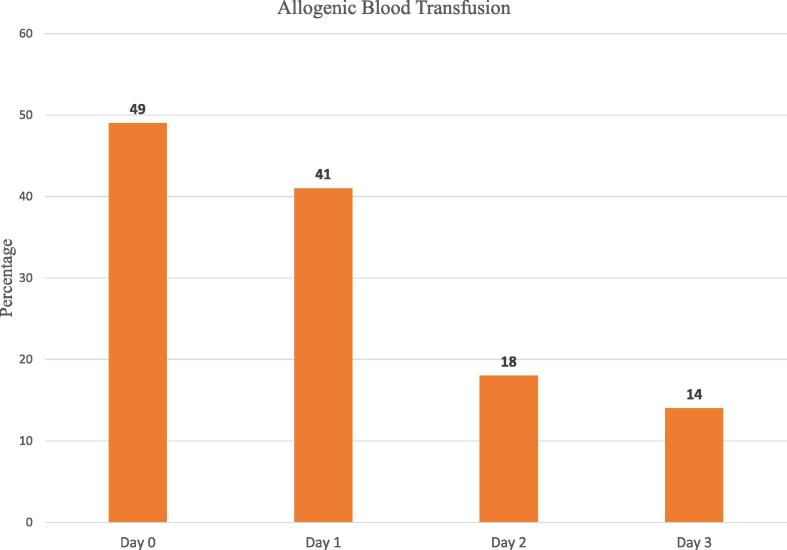
A total of 111 patients of biopsy proven sarcoma of the pelvis or lower limb underwent surgery using ICS during the study period. Two patients had incomplete cell salvage data and were excluded from the study (Fig. 2). The indication for surgery was primary bone tumour in 86 cases (79%) and metastasis in 23 cases (21%). Surgery consisted of hemipelvectomy in 43 patients(39%), lower limb endoprosthesis replacement (LLE) in 50 patients (46%) and wide excision surgery (WE) in 16 patients (15%). Intraoperative blood loss was more than 500 ml in 96%(105/109) of patients. A sufficient volume of blood was salvaged for processing and re-infusion in 94%(102/109) of procedures. An estimated one-fourth of intra-operative blood volume loss was returned to the patient (Table 1) through ICS. In total, 64 of 109 (59%) patients received an allogenic red blood transfusion within 72 h of surgery (Fig. 4).
Table 1.
Patient characteristics according to type of surgery performed. Intra-operative blood loss values are in median (IQR). Rest all values in mean (SD) or number (proportion).
| Hemipelvectomy | Lower Limb Endoprosthesis replacement | Wide Excision | |
|---|---|---|---|
| No: of Patients | 43 (39.4%) | 50 (45.9%) | 16 (14.7%) |
| Age (in years) | 53.8 (18.4) | 64.4 (14.6) | 60.5 (26.4) |
| Sex (%Female); % | 32.5% | 56% | 37.5% |
| Body Mass Index (BMI) | 26.9 (5.1) | 26.3 (5.7) | 28.4 (5.5) |
| Intra-operative blood loss (in ml) | 1750 (600–3000) | 850 (600–1200) | 1000 (550–2000) |
| Volume of re-infused blood (in ml)a | 445 (4 2 5) | 206 (1 3 1) | 184 (1 0 6) |
| Proportion of blood loss salvaged; % | 30.5 (11.5) | 29.7 (12.3) | 23.2 (10.8) |
Excludes all cases where inadequate blood was available for re-infusion. Standardized to hematocrit 60%.
Fig. 4.
Proportion of patient cohort (n = 109) receiving allogeneic blood transfusions. Day 0 graph includes transfusions that occurred intra-operatively and post-operatively until midnight on the day of surgery.
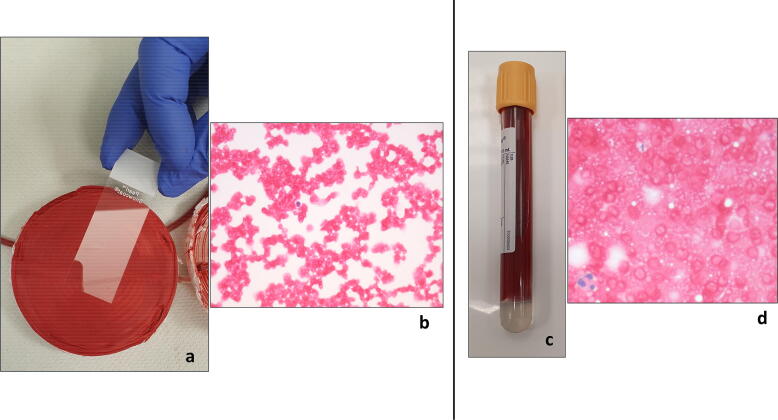
Cytology analysis of imprints taken from the LD filter, which were available in 95(87%) patients, and peripheral smears of re-infused bloods which were available in 32 (29%) of patients, did not reveal evidence of tumour cells on microscopic examination of any samples (Fig. 5 & Fig. 6). As per records of the 102 patients who received re-infusion, no patient was reported to have had reinfusion hypotension.
Fig. 5.
Retrieval of LD filters and samples of blood for re-infusion from surgery (a). Breaking open the LD filter in the laboratory (b) and imprints taken on slides from the outflow portion (c) revealed no evidence of any tumour cells (d) on microscopic examination. Peripheral smears of samples of blood for re-infusion (e) showed red blood cells with occasional monocytes (f). There was no evidence of any malignant cell. The patient underwent wide excision surgery for biopsy-proven undifferentiated pleomorphic sarcoma. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
Touch imprint of outflow portion of LD filter in a lower limb endoprosthesis surgery for biopsy-proven osteosarcoma of the distal femur shows red blood cells and very few occasional lymphocytes. No atypical cells are present. Haematoxylin-eosin (H&E) staining’ magnification × 400 (b). Touch imprint of the blood for re-infusion after filtered through the LDF shows red blood cells and occasional neutrophil granulocytes. No atypical cells or tumour cells are seen. Haematoxylin-eosin (H&E) staining’ magnification × 400. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Our study demonstrates that musculoskeletal sarcoma surgery involving the pelvis and lower limb is associated with significant blood loss, and ICS permits reinfusion of autologous blood in the majority of the patients. In cases where sufficient blood was processed for re-infusion, it equated to approximately two units of red blood cells per patient who underwent a hemipelvectomy and nearly one unit in LLE and WE surgeries. Almost a quarter of intraoperative blood loss was salvaged and re-infused. Cytological analysis of imprints of filtered blood did not show any evidence of malignant cells.
Our reported estimated total blood loss was comparable to previously published studies. Our median estimated total blood loss was 1750 ml for hemipelvectomy. A review of 137 pelvic resections for sarcoma demonstrated nearly 45% of patients have blood loss exceeding 3000 ml [2]. Another study of 28 pelvic resections for tumours, reported the average blood loss to be 4793 ml [1]. As per the recent recommendation by the association of anaesthetists [17], ICS should be considered for all procedure with anticipated intraoperative blood loss exceeding 500 ml of blood loss, which accounts for 96% patient in our cohort. The proportion of patients with sufficient blood salvaged for re-infusion in our cohort was higher for musculoskeletal sarcoma surgery that is reported for other orthopaedic surgeries where ICS is employed [14], [17]. Studies analysing re-infusion rates following ICS in cohorts involving revision arthroplasty patients demonstrated sufficient volume for salvage in 76% [15] and 54% [18] of patients, respectively.
Nearly 28% of intra-operative blood loss was re-infused after salvage in our cohort. This is lower than a similar study performed at our institution of ICS in revision hip arthroplasty patients, where an estimated 35% was salvaged for re-infusion [15]. One probable reason is difficulty containing blood loss during sarcoma surgery since the surgical fields can be larger compared with revision hip arthroplasty.
Our reported blood loss for different musculoskeletal sarcoma procedures can guide clinicians as to the estimated blood that can be salvaged using ICS and the blood products that may be required.
There has been uncertainty around the safety of ICS in cancer surgery, and debate regarding their safety still exists. Studies have demonstrated that circulating tumour cells are often present in cancer patients who undergo surgery, irrespective of ICS use. Moreover, very few of these are thought to be capable of causing metastasis [19]. The use of LDF reduces the number of malignant cells with no adverse effect on the final quality of the blood available for re-infusion [19] and recent guidelines advocate using LDF in ICS for cancer surgery [12], [18]. Our histological analysis of slides of reinfused blood from 95 patients with a range of sarcoma pathologies who had their blood filtered through an LDF, did not show any evidence of any malignant cells. This is reassuring to surgeons and clinicians regarding the use of ICS in sarcoma surgery. An observational study of flow-cytometric evaluation of filtered blood through LDF in spine tumour surgery showed the tumour cell count to be zero in 8 of the 11 samples. It demonstrated LDF to be an effective tool in removing tumour cells [20]. The safety of ICS in cancer surgery has been supported by studies of hepatocellular carcinoma surgery [21] where ICS use did not show an increased rate of metastasis or recurrence. National Institute for Health and Care Excellence (NICE) guidelines recommend using ICS in patients undergoing radical prostatectomy and cystectomy for cancer [21]. Recently, encouraging results of ICS use in surgery involving urology oncology [10] and metastasis [21], [22] strongly suggest that ICS can be strongly considered in cancer surgery, including musculoskeletal tumours. Another major advantage of the use of ICS is may reduce complication associated with allogenic transfusion [12].
Cost-effectiveness of ICS has not been considered in this study, but is improved by using the cell salvage machine in ‘collect only’ mode until adequate blood has been salvaged for reinfusion [10], [15]. ICS has demonstrated cost-effectiveness in high bleeding risk surgical settings, with clear economic benefits of cell salvage evident over the use of peri-operative allogeneic blood transfusion [23].
Our study has limitations. The use of ICS for the patients in our cohort was at the discretion of the surgeon and the anaesthetist, when they estimated blood loss to exceed 500 ml. The volume of blood loss and cell salvage efficiency are estimations that do not account for intra-operative fluid therapy and blood transfusions. Retrospective studies have an inherent bias of few missing data, which was also evident in our study. The surgical technique and anaesthesia technique may have influenced blood loss, which was not assessed in this study. Musculoskeletal sarcoma patients are a broad heterogeneous group, unlike many other cancers, making it challenging to draw generalised conclusions. We adopted imprint cytology of filtered blood to look for malignant cells, whereas flow cytometry has been shown to have higher sensitivity. Long-term data in larger cohorts is required to examine the rate of disease recurrence. This will be invaluable in determining whether there is a longer-term association between the use of ICS and disease recurrence.
5. Conclusion
Our study demonstrates that musculoskeletal sarcoma surgery is associated with significant blood loss and use of ICS permits reinfusion of autologous blood in most patients. Cytological analysis did not reveal evidence of tumour cells in reinfused blood, consistent with other studies where cell salvage is used for cancer surgery. Future studies should aim to analyse if any increased risk of metastasis associated with use of ICS in sarcoma surgery and also analyse the cost effectiveness of ICS.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Guo W., Li D., Tang X., Yang Y., Ji T. Reconstruction with modular hemipelvic prostheses for periacetabular tumor. Clin. Orthop. Relat. Res. 2007;461:180–188. doi: 10.1097/BLO.0b013e31806165d5. [DOI] [PubMed] [Google Scholar]
- 2.Tang X., Guo W., Yang R., Tang S., Ji T. Evaluation of blood loss during limb salvage surgery for pelvic tumours. Int. Orthop. 2009;33(3):751–756. doi: 10.1007/s00264-008-0695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai A., Kadota H., Yamaguchi U., Morimoto Y., Ozaki T., Beppu Y. Blood loss and transfusion associated with musculoskeletal tumor surgery. J. Surg. Oncol. 2005;92(1):52–58. doi: 10.1002/(ISSN)1096-909810.1002/jso.v92:110.1002/jso.20375. [DOI] [PubMed] [Google Scholar]
- 4.Tai Y.H., Wu H.L., Mandell M.S., Tsou M.Y., Chang K.Y. The association of allogeneic blood transfusion and the recurrence of hepatic cancer after surgical resection. Anaesthesia. 2020;75(4):464–471. doi: 10.1111/anae.v75.410.1111/anae.14862. [DOI] [PubMed] [Google Scholar]
- 5.Schiergens T.S., Rentsch M., Kasparek M.S., Frenes K., Jauch K.W., Thasler W.E. Impact of perioperative allogeneic red blood cell transfusion on recurrence and overall survival after resection of colorectal liver metastases. Dis. Colon Rectum. 2015;58(1):74–82. doi: 10.1097/DCR.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 6.Shah A., Palmer A., Klein A.A. Strategies to minimize intraoperative blood loss during major surgery. Br. J. Surg. 2020;107(2):e26–e38. doi: 10.1002/bjs.11393. [DOI] [PubMed] [Google Scholar]
- 7.Esper S.A., Waters J.H. Intra-operative cell salvage: a fresh look at the indications and contraindications. Blood transfusion = Trasfusione del sangue. 2011;9(2):139–147. doi: 10.2450/2011.0081-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaw P.B., Sentany M., Link W.J., Wahle W.M., GGlover, J. L. Tumor cells carried through autotransfusion. Contraindication to intraoperative blood recovery? JAMA. 1975;231(5):490–491. [PubMed] [Google Scholar]
- 9.Autologous blood transfusions. Council on Scientific Affairs. (1986). JAMA, 256(17), 2378–2380. [PubMed]
- 10.Ferroni M.C., Correa A.F., Lyon T.D., Davies B.J., Ost M.C. The use of intraoperative cell salvage in urologic oncology. Rev. Urol. 2017;19(2):89–96. doi: 10.3909/riu0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagarsheth N.P., Sharma T., Shander A., Awan A. Blood salvage use in gynecologic oncology. Transfusion. 2009;49(10):2048–2053. doi: 10.1111/j.1537-2995.2009.02256.x. [DOI] [PubMed] [Google Scholar]
- 12.Klein A.A., Bailey C.R., Charlton A.J., Evans E., Guckian-Fisher M., McCrossan R., Nimmo A.F., Payne S., Shreeve K., Smith J., Torella F. Association of Anaesthetists guidelines: cell salvage for peri-operative blood conservation 2018. Anaesthesia. 2018;73(9):1141–1150. doi: 10.1111/anae.14331. [DOI] [PubMed] [Google Scholar]
- 13.NICE guideline [NG24]. Recommendations, Blood transfusion. Guidance. NICE ; 2015. Available from : https://www.nice.org.uk/guidance/ng24/chapter/Recommendations. [Accessed 13 May 2021].
- 14.Gross J.B. Estimating allowable blood loss: corrected for dilution. Anesthesiology. 1983;58(3):277–280. doi: 10.1097/00000542-198303000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Palmer A.J.R., Lloyd T.D., Gibbs V.N., Shah A., Dhiman P., Booth R., Murphy M.F., Taylor A.H., Kendrick B.J.L., McGill A, Alvand A, Carr A.J. The role of intra-operative cell salvage in patient blood management for revision hip arthroplasty: a prospective cohort study. Anaesthesia. 2020;75(4):479–486. doi: 10.1111/anae.v75.410.1111/anae.14989. [DOI] [PubMed] [Google Scholar]
- 16.Bell Z., Cameron I., Dace J.S. Imprint cytology predicts axillary node status in sentinel lymph node biopsy. Ulster Med. J. 2010;79(3):119–122. [PMC free article] [PubMed] [Google Scholar]
- 17.Carroll C., Young F. Intraoperative cell salvage. BJA education. 2021;21(3):95–101. doi: 10.1016/j.bjae.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh T.S., Palmer J., Watson D., Biggin K., Seretny M., Davidson H., Harkness M., Hay A. Multicentre cohort study of red blood cell use for revision hip arthroplasty and factors associated with greater risk of allogeneic blood transfusion. Br. J. Anaesth. 2012;108(1):63–71. doi: 10.1093/bja/aer326. [DOI] [PubMed] [Google Scholar]
- 19.Cata J.P., Wang H., Gottumukkala V., Reuben J., Sessler D.I. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br. J. Anaesth. 2013;110(5):690–701. doi: 10.1093/bja/aet068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar N., Lam R., Zaw A.S., Malhotra R., Tan J., Tan G., Setiobudi T. Flow cytometric evaluation of the safety of intraoperative salvaged blood filtered with leucocyte depletion filter in spine tumour surgery. Ann. Surg. Oncol. 2014;21(13):4330–4335. doi: 10.1245/s10434-014-3950-9. [DOI] [PubMed] [Google Scholar]
- 21.Muscari Fabrice, Suc Bertrand, Vigouroux Dominique, Duffas Jean-Pierre, Migueres Isabelle, Mathieu Anne, Lavayssiere Laurence, Rostaing Lionel, Fourtanier Gilles. Blood salvage autotransfusion during transplantation for hepatocarcinoma: does it increase the risk of neoplastic recurrence? Transplant Int. Off. J. Eur. Soc. Organ Transplantat. 2005;18(11):1236–1239. doi: 10.1111/tri.2005.18.issue-1110.1111/j.1432-2277.2005.00207.x. [DOI] [PubMed] [Google Scholar]
- 22.N. Kumar, N. Ravikumar, J. Tan, K. Akbary, R.S. Patel, R. Kannan. Current Status of the Use of Salvaged Blood in Metastatic Spine Tumour Surgery Neurospine, 15 (3) 2018 206-215 Doi: 10.14245/ns.1836140.070. [DOI] [PMC free article] [PubMed]
- 23.Lim G., Melnyk V., Facco F.L., Waters J.H., Smith K.J. Cost-effectiveness Analysis of Intraoperative Cell Salvage for Obstetric Hemorrhage. Anesthesiology. 2018;128(2):328–337. doi: 10.1097/ALN.0000000000001981. [DOI] [PMC free article] [PubMed] [Google Scholar]