Abstract
Growing evidence implicates an association between psychosocial stress and oxidative stress (OxSt) although there are not yet reliable biomarkers to study this association. We used a Trier Social Stress Test (TSST) and compared the response of a healthy control group (HC; N=10) against the response of a schizophrenia group (SCZ; N=10) that is expected to have higher levels of OxSt. Because our previous study showed inconsistent changes in conventional molecular markers for stress responses in the neuroendocrine and immune systems, we analyzed the same serum samples using a separate reducing capacity assay that provides a more global measurement of OxSt. This assay uses the moderately strong oxidizing agent iridium (Ir) to probe a sample's reducing capacity. Specifically, we characterized OxSt by this Ir-reducing capacity assay (Ir-RCA) using two measurement modalities (optical and electrochemical) and we tuned this assay by imposing an input voltage sequence that generates multiple output metrics for data-driven analysis. We defined five OxSt metrics (one optical and four electrochemical metrics) and showed: (i) internal consistency among each metric in the measurements of all 40 samples (baseline and post TSST for N=20); (ii) all five metrics were consistent with expectations of higher levels of OxSt for the SCZ group (three individual metrics showed statistically significant differences); and (iii) all five metrics showed higher levels of OxSt Post-TSST (one metric showed statistically significant difference). Using multivariant analysis, we showed that combinations of OxSt metrics could discern statistically significant increases in OxSt for both the SCZ and HC groups 90 min after the imposed acute psychosocial stress.
Keywords: Oxidative stress, Psychosocial stress, Trier social stress test (TSST), Schizophrenia, Electrochemistry, Receiver operating characteristic (ROC)
Graphical abstract
Highlights
-
•
Ir-reducing capacity assay (Ir-RCA) provides a robust global measure of oxidative stress in serum.
-
•
The multiple oxidative stress (OxSt) output metrics of this Ir-RCA are useful for data-driven analysis.
-
•
The combination of OxSt metrics can discern significant increases in OxStwithin 90 mins of an imposed psychosocial stress.
The primary conclusions from this study are: the Ir-RCA provides robust global measurements of OxSt that can detect changes over comparatively short time scales (i.e., hours); and the results from this study support growing evidence for an association between psychosocial and oxidative stress.
1. Introduction
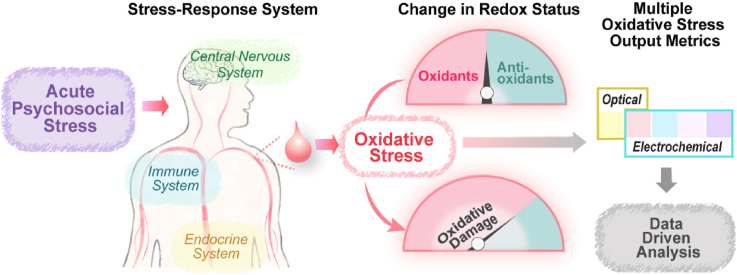
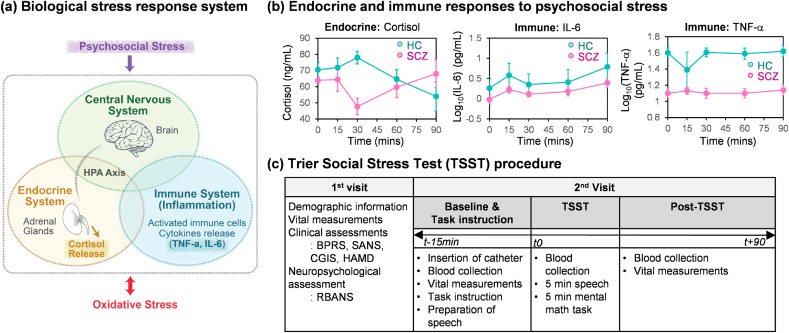
Fig. 1a illustrates that the biological response to external stresses (e.g., psychosocial stresses) involves a complex interconnection among the nervous, endocrine, and immune systems [1,2]. In response to stress, the activated hypothalamic-pituitary-adrenal (HPA) axis in the neuroendocrine system leads to secretion of the stress hormone cortisol that serves to interconnect the CNS with the immune system [[3], [4], [5], [6], [7]]. Also, activation of the immune system can result in the generation of cytokines that affect the CNS (e.g., neuroinflammation) and other systems [5,[8], [9], [10], [11], [12]]. Thus, it appears that cortisol and cytokines are key components for bidirectional communication between the CNS and immune system.
Fig. 1.
Psychosocial stress and oxidative stress. (a) The integrated biological stress response system includes the central nervous, endocrine, and immune systems. (b) Cortisol and pro-inflammatory cytokines were used as molecular biomarkers in a prior study involving acute psychosocial stress (results are adapted with permission under a Creative Commons Attribution License from Ref. [18]). The same serum samples were used for the work reported here. (c) Summary of the Trier Social Stress Test procedure.
Previously, we designed a pilot study to investigate the response to acute psychosocial stresses by the endocrine system (as measured by cortisol) and the immune system (as measured by the pro-inflammatory biomarkers, TNF-α, and IL-6). Because the stress-response system of Fig. 1a is implicated in the pathology of schizophrenia [[13], [14], [15], [16], [17]], we also focused on a comparison between persons diagnosed with schizophrenia (SCZ; N=10) and healthy controls (HC; N=10) [18]. In that study, we employed the Trier Social Stress Test (TSST) protocol that is one of the most widely used laboratory stress tests to examine the effects of acute stress on psychological and physiological functioning in humans [[19], [20], [21], [22], [23], [24], [25]]. The results from that previously-published study are replotted in Fig. 1b and show significant changes in cortisol levels during that short term study. Specifically, the HC group showed a transient increase in cortisol levels following the imposed stress, while the SCZ group showed a markedly different response pattern. The results with the two pro-inflammatory biomarkers were more difficult to interpret. At all measurement times, these two pro-inflammatory biomarkers were lower for the SCZ vs HC group which is inconsistent with evidence linking inflammation and schizophrenia [[26], [27], [28]]. In addition, both groups showed trends of gradual increases in IL-6 but no changes in TNF-α: recent studies suggest psychological stress activates the immune system [1,15,29,30] although inconsistent responses of inflammatory biomarkers across studies have been reported [9,26,31,32]. These discrepancies in inflammation biomarkers have been explained by: i) the immune response susceptibility to various confounding factors [9,33]; ii) inter-individual differences [26,34]; and iii) inconsistencies in sample collection, storage and measurement protocols [26,35].
Fig. 1a also suggests that psychosocial stress is linked to oxidative stress (OxSt) although the mechanistic details remain unclear. While OxSt is believed to result from the excessive generation of reactive oxygen species (ROS), there is growing evidence that ROS also perform important redox signaling functions [[36], [37], [38], [39]] and are embedded within a broader network of redox reactions (i.e., the redox interactome [36,40]) important for maintaining homeostasis. Further, this redox network appears to serve as an interface: at the molecular level with other biological networks (e.g., transcription and protein interaction networks) [41,42]; and at a systems level between exposure to various internal and external stressors (the exposome) [43] and the biological stress-responses [7,[44], [45], [46]]. Thus, there is increasing interest in understanding the relationships among ROS, redox signaling, OxSt and the integrated stress response systems [7,36,40,47,48].
There is no generally-accepted measurement for OxSt but various molecular biomarkers have been considered [[49], [50], [51], [52], [53], [54]]. Such molecular biomarkers include the reactive oxidants (e.g., ROS and RNS) [50,54,55], the endogenously generated protective antioxidants (e.g., GSH and ascorbic acid) [56,57], proteins for antioxidant protection (e.g., defense enzymes) or the ROS-induced damage (e.g., lipid peroxidation and protein carbonylation) [[58], [59], [60], [61], [62], [63], [64]]. An alternative approach to assess OxSt is to obtain a global measurement of antioxidant status that integrates the contributions from various molecular species [[65], [66], [67], [68], [69]].
We are developing a new global measurement for the OxSt using an Ir-reducing capacity assay (Ir-RCA) that probes a serum sample using the moderately strong oxidant K2IrCl6(IV) (designated IrOX) [70,71] to determine the electron-donating activities of the various serum components (e.g., glutathione, ascorbate and proteins). In our first report [70], we showed that the IrOX could be reduced upon incubation with diluted serum sample, which could be detected by electrochemical and optical measurements. In addition, the Ir-RCA results were compared with a commercial Cu-reducing capacity assay (Cu-RCA). Ir-RCA could discern higher levels of OxSt in the serum from a SCZ group (N=10) vs a HC group (N=5) while a commercial Cu-RCA could not discern differences in OxSt between the SCZ and HC groups. In our second report [71] using only an optical measurement, we validated that the Ir-RCA can discern higher OxSt in the SCZ group (N=73) vs HC group (N=45).
Here, we modified the Ir-RCA and investigated its ability to discern changes in OxSt in response to acute psychosocial stress. Specifically, we modified the assay to detect reducing capacity in serum using independent optical and electrochemical measurements, and we imposed a sequence of electrical voltage inputs to enrich the output signal to generate multiple metrics suitable for data-driven analysis. We applied this assay method to the same serum samples collected from our previous study (i.e., in Fig. 1b [18]). Consistent with our previous studies [70,71], we observed that this Ir-RCA can discern higher levels of OxSt in the SCZ group vs the HC group. More importantly, our measurements showed that increases in OxSt for both groups were observable within 2 h of the imposed psychosocial stress. By incorporating an enhanced data analytics approach, our results demonstrate the value of an Ir-RCA in providing a global indicator of OxSt. Moreover, our data reinforces the link between psychosocial stress and oxidative stress.
2. Materials and methods
2.1. Chemicals
The following were purchased from Sigma-Aldrich: K2IrCl6 (IV), and phosphate buffered saline (PBS, pH 7.4). The water (>18 MΩ) used in this study was obtained from a Super Q water system (Millipore). A stock solution of 10 mM K2IrCl6 (IrOX) was prepared in PBS (pH 7.4) and its aliquot was stored in −80 °C freezer.
2.2. Study participants
The recruitment of participants was described in the previous report [18] and more details are provided in the Supplementary Material.
2.3. Design of the study
This study consists of two visits as described in Fig. 1c.
2.3.1. Consent and screening in the first visit
In the first visit, all participants signed informed consent and completed a screening to evaluate eligibility. Details of screening process were previously described [18] and are provided in the Supplementary Material. During the first visit, the participants completed various clinical assessments to evaluate psychiatric symptoms including the Structured Clinical Interview for the DSM-IV Diagnoses (SCID) [72], Brief Psychiatric Rating Scale (BPRS) (18-item) [73], Hamilton Depression Scale (HAMD) [74], Scale for the Assessment of Negative Symptoms (SANS) [75], and the Clinical Global Impression Scale (CGIS) [76]. Participants also completed the Repeatable Battery for the Assessments of Neuropsychiatric Status (RBANS) [77].
2.3.2. Trier social stress test (TSST) in the second visit
Upon arrival of the second visit, participants had an intravenous catheter inserted and then the baseline blood was drawn (i.e., 15 min before TSST). After the participants watched the introducing video about the role-playing and prepared for their speech for 15 min, they returned to the room with the “panel of judges” to deliver their speech and complete the mental math (e.g., TSST). Blood was drawn just prior to the TSST (t=0), and 15, 30, 60, 90 min post stressor as described in Fig. 1c. In this study, we assayed the serum samples collected at 15 min before TSST (t-15 min: Baseline) and 90 min after TSST (t+90 min: post-TSST). More details for TSST are provided in Supplementary Material.
2.4. Biochemical analysis (cortisol, cytokines, C-reactive protein)
The analysis of serum cortisol and cytokines (IL-6 and TNF-α) was described in the previous study [18] and Supplementary Material. Here, we used the previously published results and we measured serum C-reactive protein (CRP) using a commercial Human C-Reactive Protein ELISA Kit (MilliporeSigma, MO, USA), following manufacturer's recommended protocol.
2.5. Ir-reducing capacity assay (Ir-RCA)
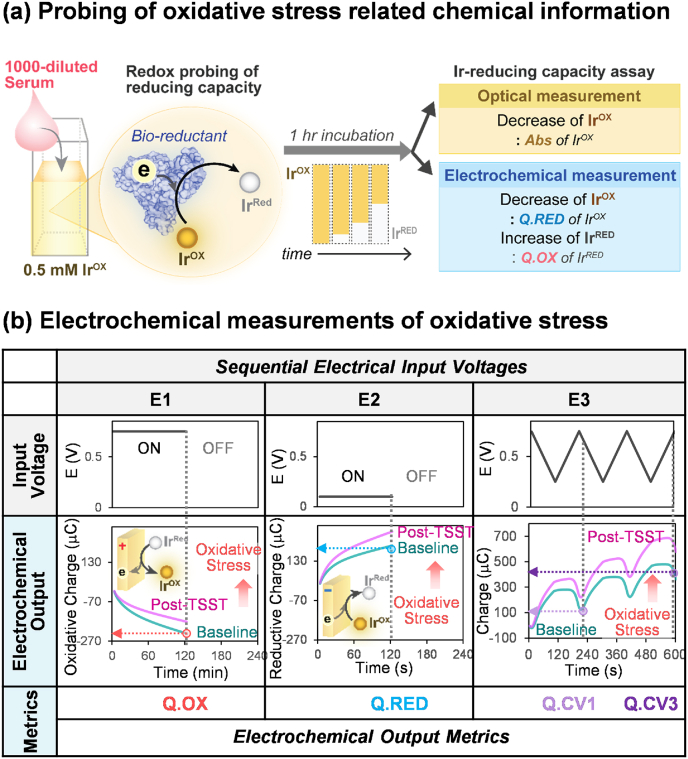
Before the measurement, we thawed each aliquot of serum and a 10 mM IrOX stock solution that had all been frozen at −80 °C. Sample solution (3000 μL) was prepared by diluting serum (1000-fold) and IrOX stock solution (20-fold) with 0.1 M PBS. After 1-h incubation at room temperature, two 200 μL aliquots of each sample were transferred to each of 2 wells of a 96 well microplate for duplicate optical absorbance measurements at 490 nm using a standard absorbance microplate reader (iMark, Bio-Rad Laboratories, Inc.). At the same time, 2600 μL of the samples were analyzed electrochemically (Fig. S1 provides more information of an electrochemical system); and an imposed voltage sequence that included a 2 min wait (i.e., OFF) period followed by a sequence of 3 separate voltage inputs (between each input the samples were stirred and a 2 min OFF period was used). Fig. 2b explains each imposed voltage input and its electrochemical output in detail.
Fig. 2.
Optical and electrochemical metrics of the Ir-reducing capacity assay. (a) A redox-mediator (K2IrCl6, IrOX) is used to detect oxidative stress by probing the reducing capacities of a diluted serum sample. During a 1 h incubation, IrOX is converted to IrRed by accepting electrons from the sample's reductants. Independent measures of the residual IrOX are obtained optically (Abs at 490 nm) and electrochemically (reductive charge; Q.RED), while measures of the generated IrRed are obtained electrochemically (oxidative charge; Q.OX). (b) For the electrochemical measurement, three sequential electrical input voltages are imposed: (i) E1 is a 2-min imposed oxidation voltage (+0.7 V vs Ag/AgCl) that yields one metric (the cumulative oxidative charge during these 2 min; Q.OX); (ii) E2 is a 2-min imposed reduction voltage (+0.1 V vs Ag/AgCl) that yields a second metric (the cumulative reductive charge during these 2 min; Q.RED); and (iii) E3 is a 10-min imposed cyclic voltage (CV) that generates 2 additional metrics (Q.CV1 and Q.CV3) that reflect the dynamic balance between the reduction of the residual IrOX and oxidation of the generated IrRed. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Two independent analyses of the 40 serum samples were performed over a 60-day period. At each analysis, each sample was measured in triplicate using both optical and electrochemical methods, and the triplicate measurements were averaged for the further data analysis.
2.6. Statistical analysis
Statistical analyses were performed with R version 3.4.3. Group differences in demographic information, clinical assessments, and Ir-reducing capacity measurements were assessed using Kruskal-Wallis test for continuous variables and chi-squared test for categorical variables. The intraclass correlation coefficient (ICC) is calculated using the ‘irr’ package [78] based on an absolute-agreement, one-way random effect analysis of variance (ANOVA) model [79,80]. Spearman's correlation coefficients were calculated to examine the relationship between the 5 OxSt metrics and the 5 clinical assessments. A logistic regression model [[81], [82], [83]] (‘nnet’ package) [84], was used to assess the combination effect of multiple OxSt metrics on i) the discerning ability of SCZ group from HC group and ii) discriminating ability of post-stressed status relative to baseline. We used Akaike Information Criterion (AIC) to estimate the quality of each model. The discriminating ability was evaluated using ROC (Receiver Operating Characteristic) metric with AUC (Area Under Curve) (i.e., c-statistic)) and p-values [[85], [86], [87], [88], [89], [90], [91]].
3. Results
Table 1 summarizes the characteristics of the HC group (N=10) and the persons with SCZ (N=10) at baseline study entry [18]. Table 1 shows that there is no significant difference between the two groups for age, gender, race, current smoking status or perceived stress. However, the psychiatric symptoms assessments (BPRS, SANS, HAMD, CGIS) shows the significantly higher scores and the RBANS shows significantly lower scores in the SCZ group compared with HC group as expected. With respect to physiological measurements, there is no significant difference in pulse or systolic blood pressure, while the SCZ group had a significantly higher diastolic blood pressure.
Table 1.
Demographic information for healthy controls and schizophrenia group (*; p<0.05).
| Variable | Healthy Controls (HC, N =10) | Schizophrenia Group (SCZ, N=10) | Test Statistics |
|---|---|---|---|
| Age/years | 35.4 ± 12.3 | 40.9 ± 14.7 | χ2= 0.46, p = 0.49 |
| Sex/Male | 10 (50%) | 10 (50%) | χ2= 0.20, p = 1 |
| Race | χ2= 0.28, p = 1 | ||
| African American | 7 (70%) | 7 (70%) | |
| Caucasian | 2 (20%) | 3 (30%) | |
| Mixed | 1 (10%) | ||
| Smoker | 3 (30%) | 4 (40%) | χ2= 0.95, p = 0.62 |
| Clinical Assessment (Symptoms) | |||
| *BPRS Total Score | 19.6 ± 1.8 | 33.2 ± 5.3 | χ2= 12.59, p = 3.9 × 10−4 |
| *SANS Total Score | 1.0 ± 2.8 | 25.7 ± 9.2 | χ2= 15.26, p = 9.36 × 10−5 |
| *HAMD | 0.67 ± 1.4 | 8.8 ± 5.8 | χ2= 11.61, p = 6.57 × 10−4 |
| *CGIS | 1.0 ± 0.0 | 4.5 ± 0.8 | χ2= 16.59, p = 4.63 × 10−5 |
| *RBANS Total Score | 86.4 ± 15.1 | 71.0 ± 16.0 | χ2= 4.66, p = 0.031 |
| Perceived Stress Scale | 11.2 ± 9.7 | 14.5 ± 5.2 | χ2= 0.57, p = 0.45 |
| Physiological Measurement | |||
| Systolic Blood Pressure (mmHg) | 122.7 ± 21.7 | 117.7 ± 13.1 | χ2= 0.036, p = 0.85 |
| *Diastolic Blood Pressure (mmHg) | 67.2 ± 10.8 | 74.9 ± 5.2 | χ2= 4.18, p = 0.041 |
| Pulse (bpm) | 73 ± 14.4 | 83.9 ± 12.9 | χ2= 1.56, p = 0.21 |
3.1. Description of Ir-RCA and OxSt metrics
Fig. 2a illustrates the redox probing method (i.e., Ir-RCA) used in this study. IrOX is incubated with diluted serum samples and accepts electrons from serum components converting the yellow-colored IrOX into its colorless reduced form (IrRed). This decrease in IrOX and increase of IrRed are measured both optically and electrochemically after 1-h incubation. The optical absorbance (Abs) at 490 nm measures the yellow-color associated with remaining IrOX in the sample after the 1-h incubation: samples from persons with higher levels of OxSt (i.e., less serum reducing capacity) are expected to have more IrOX and thus higher Abs values.
Fig. 2b shows that we used three independent electrochemical measurements of IrOX and IrRred. Specifically, an electrode was inserted into the sample and three different input voltage sequences were imposed while measuring the output current responses. Importantly, electrochemistry measures a small region of the sample (we estimate ∼0.15% of the sample volume) [92] and thus allows multiple measurements to be performed while minimally altering the sample (note between each of the three voltage input sequences we stirred the sample to refresh the measurement region and then used a 2 min OFF time).
The first voltage input (designated E1) was a 2-min step change to an oxidative value (+0.7 V vs Ag/AgCl reference electrode). The number of electrons transferred during this oxidative pulse (Q.OX; μC) provides a measure of how much IrRed was formed during the 1-h incubation: Fig. 2b illustrates that samples from persons with higher levels of OxSt are expected to form less IrRed and show a smaller Q.Ox response (by convention, oxidative Q values have a negative value and thus a smaller response corresponds to a less negative Q.Ox).
The second voltage input (E2) was a 2-min step change to a moderately reductive value sufficient to reduce IrOx (+0.1 V vs Ag/AgCl). The number of electrons transferred during this reductive pulse (Q.RED; μC) provides a measure of how much IrOX remained in the sample after the 1-h incubation: Fig. 2b illustrates that samples from persons with higher levels of OxSt are expected to have higher IrOX and thus larger Q.RED values.
The final voltage input (E3) was a 3-cycle (10 min) oscillating cyclic voltage (CV) between the oxidative value (+0.75 V) and a reductive value (+0.25 V). Fig. 2b shows an oscillating output Q is observed with a trend toward larger (i.e., reductive) values and we characterized this output response by two metrics: the first metric is the minimum charge after the first cycle (Q.CV1) and the second is the final charge after the third cycle (Q.CV3). This sequence provides an independent measure of the IrOX remaining in the sample after the 1-h incubation: Fig. 2b illustrates that serum from persons with higher levels of OxSt are expected to have higher IrOX levels and thus higher Q.CV1 and Q.CV3 values. Here, we defined five output metrics of Ir-RCA including Abs, Q.OX, Q.RED, Q.CV1 and Q.CV3 as OxSt metrics.
3.2. Reliability of Ir-RCA
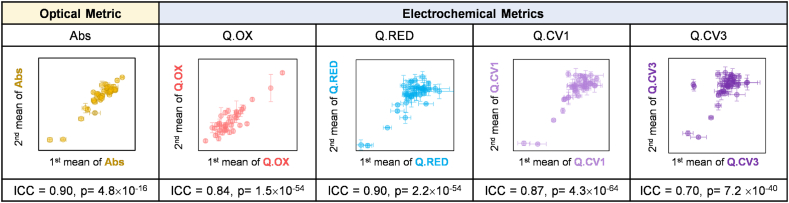
To investigate the reliability of Ir-RCA, we analyzed the 40 samples (20 samples at baseline and 20 samples post TSST) at two different times that spanned 60 days. At each analysis, a single aliquot for each of the 40 frozen serum samples (-80°C) was thawed, and measured in triplicate to generate our 5 OxSt metrics (Abs, Q.Ox, Q.RED, Q.CV1 and Q.CV3). The triplicate measurements of each analysis were averaged to 1st mean and 2nd mean. Fig. 3 represents the correlation between two mean values of each sample for each metric.
Fig. 3.
Reliability of Ir-RCA. The cross plots of the individual metrics show good agreement between two independent analyses of 40 samples (20: Baseline, 20: Post TSST) that were performed at two different times spaced 60-days apart. Each point represents the mean value and the error bars are for triplicate measurements for each sample in the 1st and 2nd analysis. The intraclass correlation coefficient (ICC) is calculated to assess the agreement of 6 replicate measurements for each of the 5 metrics.
To quantify the reliability of our assay, we calculated the intraclass correlation coefficient (ICC) [79,93,94] which assesses the absolute agreement between different analyses. Each calculated ICC for 5 metric measurements was relatively high indicating a good agreement for the 6 replicate measurements. Importantly, the high reliability of this assay indicates that the assay is measuring a stable feature in the serum (i.e., it is not measuring unstable reactive species) and it is not sensitive to the presence of air (i.e., no precautions were made to exclude oxygen when drawing the blood, processing the serum or assaying the samples).
3.3. Correlation between OxSt metrics and clinical assessments
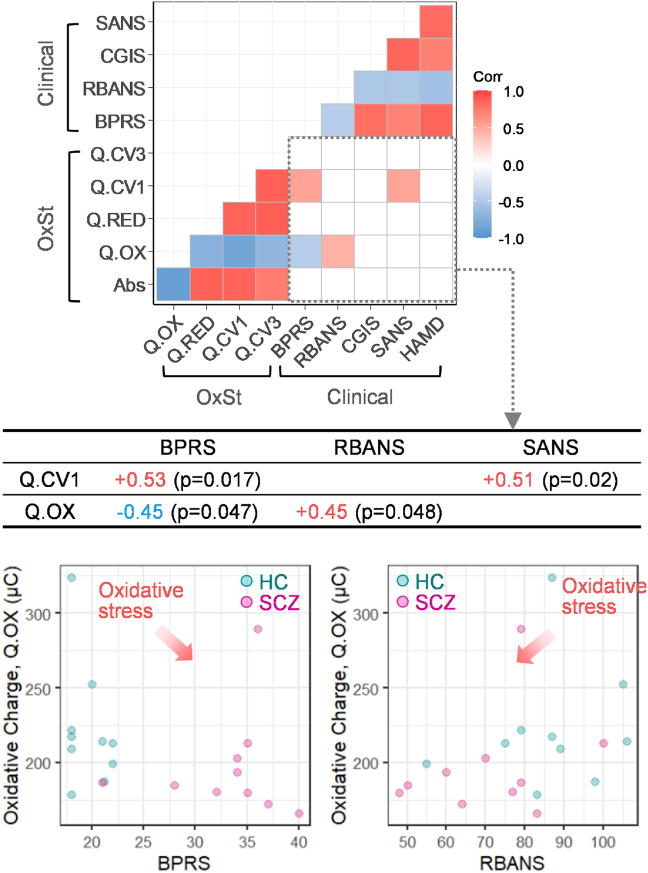
Fig. 4 presents the correlation heat map between 5 OxSt metrics and 5 clinical assessments at the baseline. Each colored cell of the heat map indicates a statistically significant correlation between variables. First, the lower left region of this heat map shows the strong correlations between the 5 OxSt metrics, which supports the internal consistency of the optical and electrochemical metrics of OxSt. Second, the upper right region of the heat map of Fig. 4 shows strong correlations between the 5 different clinical assessments (Fig. S2 provides correlation plots). The symptom severity-related assessments (BPRS, CGIS, SANS) show positive correlations with each other, but a negative correlation with RBANS scales that assess cognition [32,[95], [96], [97]].
Fig. 4.
Correlations of the metrics from the Ir-RCA with clinical assessments. Heat map of Spearman correlation coefficients of the 5 OxSt metrics and 5 clinical assessments (BPRS, RBANS, CGIS, SANS, HAMD) at baseline show: excellent internal consistency among the 5 OxSt metrics (lower left); strong internal consistency among the clinical assessments (upper right); and some correlation between OxSt metrics and clinical assessments (lower right). Q.OX metric shows correlation with BPRS and RBANS and Q.CV1 shows a correlation with BPRS and SANS: these correlations indicate that symptom severity is associated with OxSt.
Third, the lower right region of the heat map of Fig. 4 shows the correlations between the 5 OxSt metrics and the 5 different clinical assessments. Importantly, Fig. S2a shows the response of each metric is consistent with expectations that higher OxSt is associated with more severe symptoms [[98], [99], [100]] and lower cognition [95,97]. As indicated in the Table of Fig. 4, four of these correlations are statistically significant. The BPRS assessment of psychiatric symptoms shows a negative correlation with the Q.OX metric (r = −0.45, p = 0.047) and a positive correlation with the Q.CV1 metric (r = +0.53, p = 0.017). Also, the SANS assessment of negative symptoms shows a positive correlation with the Q.CV1 metric (r = +0.51, p = 0.02). The RBANS assessment of cognition shows a positive correlation with the Q.OX metric (r = +0.45, p = 0.048). Thus, the OxSt metrics are consistent with clinical expectations and, in some cases, statistically significant correlations were observed between individual metrics and clinical assessments despite the small sample size.
When we checked the correlation between the 5 OxSt metrics and other biochemical markers including cortisol and inflammation markers (CRP, IL-6, TNF-α) in Fig. S2a, only one metric, Q.OX, shows a positive correlation (r = +0.46, p = 0.005) with TNF-α. The relationship is not consistent with the other studies that show an elevation of TNF-α levels with higher level of OxSt biomarkers [101,102].
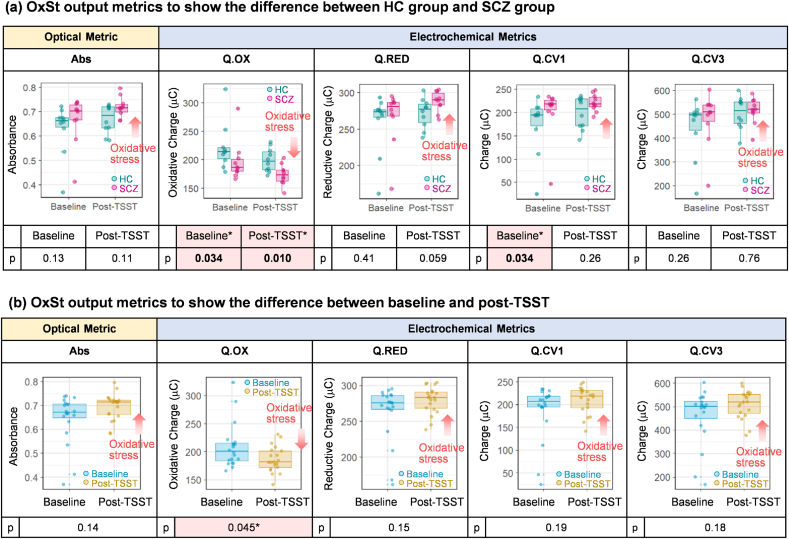
3.4. Discriminating abilities of individual OxSt metrics
In addition to identifying correlations to baseline clinical assessments, we also examined the abilities of the individual assay metrics to discern differences between the two groups (SCZ and HC) and the two states (pre- and post-TSST). Fig. 5a shows the ability of Ir-mediated OxSt assay to discriminate the SCZ group from the HCs at baseline and post-TSST using the 5 OxSt metrics. The first important observation in Fig. 5a is that all 5 metrics consistently show higher OxSt for the SCZ (vs HC) group both at baseline and post-TSST. The differences between SCZ and HC groups for three of these metrics was statistically significant (Q.OX at baseline (p=0.034) and post TSST (p=0.010), and Q.CV1 at baseline (p=0.034)). This observation agrees with our previous studies that show higher OxSt for persons diagnosed with SCZ vs healthy controls [70,71,103]. The second important observation in Fig. 5a is that all 5 metrics changed in the direction of increasing OxSt in post-TSST compared with baseline for both the SCZ and HC groups. Fig. 5b shows results when the groups were combined to consider only the effect of the TSST. Again, all five metrics showed the expected responses that OxSt was higher post-TSST compared to baseline and one metric showed a statistically significant difference (Q.OX (p=0.045)). These two observations - increasing OxSt for the SCZ group (vs. HC) and post-TSST (vs. pre-TSST) - are consistent with the expectations (Note: Fig. S3 provides additional plots to show the changes of OxSt metrics for individual samples pre/post TSST and Fig. S4 provides additional analysis of the difference of OxSt metrics between pre and post TSST).
Fig. 5.
Discriminating abilities of individual OxSt metrics. (a) Five OxSt metrics including one optical metric (Abs) and four electrochemical metrics (Q.OX, Q.RED, Q.CV1 and Q.CV3) consistently show the higher oxidative stress for the SCZ group vs HC group at baseline and post-TSST. Three metrics shows the statistically significant difference between the two groups (*; p<0.05, Q.OX at baseline, post-TSST, Q.CV1 at baseline). (b) Five OxSt metrics for the combined group show consistently increasing OxSt for post-TSST vs baseline. One metric (Q.OX) shows the statistically significant difference between baseline and post-TSST (*; p<0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
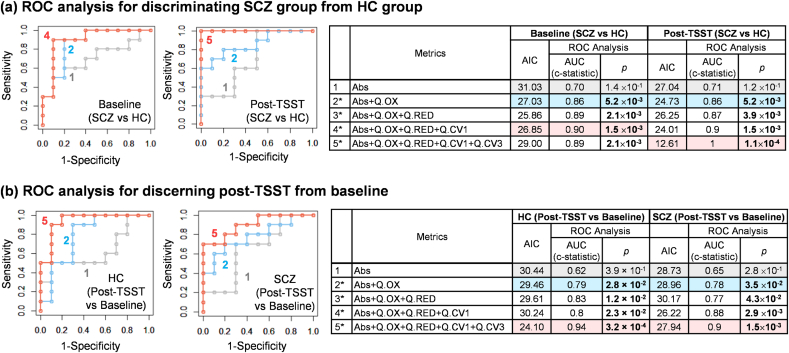
3.5. Combining effect of multiple OxSt metrics
In Fig. 6, we investigated the additive effect of multiple metrics on discerning higher levels of oxidative stress using a logistic regression model [[81], [82], [83]]. First, Fig. 6a estimates the probabilities for discriminating the SCZ group from HC group using one or combinations of multiple OxSt metrics. The goodness-of-fit for each model is evaluated using the Akaike Information Criterion (AIC) [104] and the discriminating power is evaluated using ROC curve analysis [[86], [87], [88], [89], [90], [91]]. The table in Fig. 6a presents the calculated AIC and the AUC values (i.e., c-statistic) and the p-values from ROC curve for each combination of multiple metrics at the baseline and post-TSST (Fig. S5 provides the ROC analyzed results for each single metric). While the ROC analysis with a single metric (Abs) shows lower AUC with higher p-value (>0.05), the combination of OxSt metrics increased the AUC and the discriminating ability became statistically more significant (p-value < 0.05). This result indicates that the combination of multiple metrics increased the power for discriminating the SCZ and HC groups based on their levels of OxSt.
Fig. 6.
Receiver operating characteristic (ROC) analyses for assessing the combination effect of multiple metrics. (a) ROC analysis for discerning SCZ group from HC group at baseline and post-TSST. (b) ROC analysis for discerning post-TSST from baseline for HC group and SCZ group. ROC curves and Tables show that the combination of multiple OxSt metrics increased its discerning ability (SCZ vs. HC, Post-TSST vs. Baseline) with higher AUC (c-statistic) and lower p-value (*; p<0.05).
In Fig. 6b, a logistic model is used to estimate the probabilities to discern the post-TSST from baseline (pre-TSST) using one or combinations of multiple OxSt metrics. The table in Fig. 6b shows that the combination of OxSt metrics increases the AUC (c-statistic) and lowers the p-value. This result indicates that the combination of multiple OxSt metrics by logistic regression model can improve the ability to discern increases in OxSt post-TSST.
4. Discussion
There is growing evidence for an integrative stress response system [7,105,106] that includes the immune [11], nervous [2] and endocrine systems, and also that persons with schizophrenia may have dysfunctions in this stress response. In a previous pilot study using a short-time psychosocial stress [18], we observed that the response in cortisol levels (a marker of HPA axis response) for people with SCZ was markedly different from healthy controls (HC) as expected from other studies [6,7,105,107]. However, changes in the immune response biomarkers (TNF-α and IL-6) were less consistent with expectations. First, these measurements showed lower levels of TNF-α and IL-6 for the SCZ group compared to HC group (Fig. 1b), and these cytokine levels did not correlate to clinical measures of symptom severity (Fig. 4). These results are inconsistent with other studies and the neuroimmune hypothesis of schizophrenia [1,12,19,108,109]. Second, after psychosocial stress, IL-6 increased but TNF-α remained unchanged in both the SCZ and HC groups (Fig. 1b) which is also inconsistent across studies and expectations (based on a neuroimmune hypotheses). These inconsistencies suggest that pro-inflammatory cytokines may not be robust biomarkers [110].
There is also evidence that HPA activation and inflammation are linked to oxidative stress (OxSt) [19,26,111,112]. To examine this link, we used an Ir reducing capacity assay (Ir-RCA) to measure the same samples collected from this previous study [18]. In contrast to traditional molecular biomarkers or measures of oxidation [49,51,54,113,114], the Ir-RCA provides a more global measure that is detected through relatively simple optical and electrochemical methods. Here, we extended the Ir-RCA from a single optical [71] or electrochemical output metric [70,103] to multiple independently-measured output metrics useful for a data-driven analysis [41,[115], [116], [117]]. Each of the five output metrics showed that the SCZ group had higher OxSt compared with the HC group which is consistent with expectations [70,71,103] (3 of these metrics showed statistically-significant differences: Fig. 5). Further, each of the five output metrics showed that increased OxSt is associated with greater symptom severity and lower cognition which is also consistent with expectations [[118], [119], [120], [121], [122], [123]] (2 of these metrics showed statistically-significant correlation: Fig. 4). As expected, combining output metrics increased the discriminating power of the Ir-RCA (e.g., Fig. 6a).
Although many studies suggest that the chronic psychosocial stress is linked to OxSt and the pathologies of diverse diseases [[6], [7], [8],45,46,124,125], few studies focus on the association between acute psychosocial stress and OxSt [[126], [127], [128]]. Jansakova et al. [127] examined the acute effect of psychosocial stress on OxSt using saliva samples from children. They found increases in the total antioxidant capacity (as measured by ferric ion reducing antioxidant power) after exposure to acute psychosocial stress (e.g., TSST) during the stress day, and higher lipid peroxidation on stress day than control day but no significant difference prior to and post TSST. Wiegand et al. [126] found a higher expression of the microRNA, which is implicated in regulating the production of reactive species, in the saliva of healthy subjects after the psychosocial stress test (TSST). While both studies were performed in healthy groups, we investigated for the first time the acute effect of psychosocial stress on the OxSt in SCZ and HC groups. All five output metrics in our study showed an increase in OxSt after the TSST compared with the baseline (Fig. 5) and one metric (Q.OX) showed a statistically significant difference between baseline and post-TSST. Further, the results in Fig. 5 implicate an association between psychosocial stress and OxSt. The combination of OxSt metrics was well-fitted with the model to distinguish post-TSST from baseline (Fig. 6b). While our results indicate that the TSST test was associated with increased OxSt for both the SCZ and HC groups, our Ir-RCA could not discern differences in response between these two groups (using linear mixed effects model in Fig. S4).
There are several strengths and limitations of this study. One strength is that the Ir-RCA appears to access stable features of the serum samples. Specifically, we measured samples that had been stored at −80 °C for 3 years, and our replicate measurements that were performed 60 days apart showed strong interclass correlations (Fig. 3). Presumably, this assay is detecting stable differences in the oxidation state of proteins and other antioxidants that provide a stable historical record of the oxidative context that had been experienced by the serum at the time of sampling (e.g., trace levels of reactive species are expected to rapidly decay during sample processing while oxidized amino acid residues are expected to be stable) [71]. A second strength is the cost and simplicity of the Ir-RCA, as well as the ability to make independent measurements to facilitate a data-driven analysis. A third strength is that all 5 metrics measured in this study show responses consistent with expectations (greater oxidative stress for the SCZ group and for both groups post-TSST). One limitation of this study is that, since the optical and electrochemical metrics generated by the Ir-RCA reflect a summation of all oxidizable species (i.e., antioxidants) in the sample, it is generally not possible to relate the Ir-RCA measurements to individual chemical species or molecular mechanisms of OxSt. A second limitation of the Ir-RCA is that there are several assay variables that could be adjusted (e.g., mediator types/levels, electrochemical input sequences, and output response quantification) and optimizing this assay for clinical analysis is limited by the small volumes of sample available for methods development. A final limitation is the small sample size of this pilot study (N=10 schizophrenia and N=10 control). Thus, while this study supports the growing evidence for a link between psychosocial stress, inflammation, and oxidative stress, larger studies are needed to confirm these findings.
In summary, the results from this study indicate that acute psychosocial stresses can lead to detectable increases in oxidative stress over comparatively short times (<2 h).
Declaration of competing interest
All authors declare no conflicts of interest related to this work.
Acknowledgements
This work was supported by National Science Foundation (CBET-1932963), United States and Defense Threat Reduction Agency (HDTRA-11910021), United States.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.102138.
Contributors
EK, DLK, and GFP designed the study and wrote the first draft of the manuscript. JR built an electrochemical analyzer. EK performed the assay. MG collected serum samples. EK, ZZ and SC undertook the statistical analysis. All authors contributed to edit the manuscript and have approved the final manuscript.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Buckley P.F. Neuroinflammation and schizophrenia. Curr. Psychiatr. Rep. 2019;21:1–3. doi: 10.1007/s11920-019-1050-z. [DOI] [PubMed] [Google Scholar]
- 2.Manley K., Han W., Zelin G., Lawrence D.A. Crosstalk between the immune, endocrine, and nervous systems in immunotoxicology. Curr. Opin. Toxicol. 2018;10:37–45. doi: 10.1016/j.cotox.2017.12.003. [DOI] [Google Scholar]
- 3.Stephens M.A.C., McCaul M.E., Wand G.S. Neurobiol. Alcohol Depend. 2014. The potential role of glucocorticoids and the HPA Axis in alcohol dependence; pp. 429–450. [DOI] [Google Scholar]
- 4.Bellavance M.-A., Rivest S., Gualillo O., Servizo S.(, Fantuzzi G. 2014. The HPA – Immune axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasahara E., Inoue M. Cross-talk between HPA-axis-increased glucocorticoids and mitochondrial stress determines immune responses and clinical manifestations of patients with sepsis. Redox Rep. 2015;20:1–10. doi: 10.1179/1351000214Y.0000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen A.P., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G. Biological and psychological markers of stress in humans: focus on the trier social stress test. Neurosci. Biobehav. Rev. 2014;38:94–124. doi: 10.1016/j.neubiorev.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Godoy L.D., Rossignoli M.T., Delfino-Pereira P., Garcia-Cairasco N., Umeoka E. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front. Behav. Neurosci. 2018;12:127. doi: 10.3389/fnbeh.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiavone S., Trabace L. Inflammation, stress response, and redox dysregulation biomarkers: clinical outcomes and pharmacological implications for psychosis. Front. Psychiatr. 2017;8:1–10. doi: 10.3389/fpsyt.2017.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Müller N. Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr. Bull. 2018;44:973–982. doi: 10.1093/schbul/sby024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller N., Weidinger E., Leitner B., Schwarz M.J. The role of inflammation in schizophrenia. Front. Neurosci. 2015;9:372. doi: 10.3389/fnins.2015.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steptoe A., Hamer M., Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav. Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Watkins C.C., Andrews S.R. Clinical studies of neuroinflammatory mechanisms in schizophrenia. Schizophr. Res. 2016;176:14–22. doi: 10.1016/j.schres.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi T. Conversion of psychological stress into cellular stress response: roles of the sigma-1 receptor in the process. Psychiatr. Clin. Neurosci. 2015;69:179–191. doi: 10.1111/pcn.12262. [DOI] [PubMed] [Google Scholar]
- 14.Jorgensen A., Broedbaek K., Fink-Jensen A., Knorr U., Greisen Soendergaard M., Henriksen T., Weimann A., Jepsen P., Lykkesfeldt J., Enghusen Poulsen H., Balslev Jorgensen M. Increased systemic oxidatively generated DNA and RNA damage in schizophrenia. Psychiatr. Res. 2013;209:417–423. doi: 10.1016/j.psychres.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 15.Leza J.C., Bueno B., Bioque M., Arango C., Parellada M., Do K., O'Donnell P., Bernardo M. Inflammation in schizophrenia: a question of balance. Neurosci. Biobehav. Rev. 2015;55:612–626. doi: 10.1016/j.neubiorev.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Perkins D.O., Jeffries C.D., Do K.Q. Potential roles of redox dysregulation in the development of schizophrenia. Biol. Psychiatr. 2020;88:326–336. doi: 10.1016/J.BIOPSYCH.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coughlin J.M., Yang K., Marsman A., Pradhan S., Wang M., Ward R.E., Bonekamp S., Ambinder E.B., Higgs C.P., Kim P.K., Edwards J.A., Varvaris M., Wang H., Posporelis S., Ma S., Tsujimura T., Edden R.A.E., Pomper M.G., Sedlak T.W., Fournier M., Schretlen D.J., Cascella N.G., Barker P.B., Sawa A. A multimodal approach to studying the relationship between peripheral glutathione, brain glutamate, and cognition in health and in schizophrenia. Mol. Psychiatr. 2020 doi: 10.1038/s41380-020-00901-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glassman M., Wehring H.J., Pocivavsek A., Sullivan K.M., Rowland L.M. Peripheral cortisol and inflammatory response to a psychosocial stressor in people with schizophrenia. J. Neuropsychiatry. 2018;2:4. doi: 10.21767/2471-8548.10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dantzer R. Neuroimmune interactions: from the brain to the immune system And vice versa. Physiol. Rev. 2018;98:477–504. doi: 10.1152/physrev.00039.2016.-Because. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen A.P., Kennedy P.J., Dockray S., Cryan J.F., Dinan T.G., Clarke G. The trier social stress test: principles and practice. Neurobiol. Stress. 2017;6:113–126. doi: 10.1016/j.ynstr.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narvaez Linares N.F., Charron V., Ouimet A.J., Labelle P.R., Plamondon H. A systematic review of the Trier Social Stress Test methodology: issues in promoting study comparison and replicable research. Neurobiol. Stress. 2020;13:100235. doi: 10.1016/j.ynstr.2020.100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodman W.K., Janson J., Wolf J.M. Meta-analytical assessment of the effects of protocol variations on cortisol responses to the Trier Social Stress Test. Psychoneuroendocrinology. 2017;80:26–35. doi: 10.1016/j.psyneuen.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 23.Labuschagne I., Grace C., Rendell P., Terrett G., Heinrichs M. An introductory guide to conducting the trier social stress test. Neurosci. Biobehav. Rev. 2019;107:686–695. doi: 10.1016/j.neubiorev.2019.09.032. [DOI] [PubMed] [Google Scholar]
- 24.Vors O., Marqueste T., Mascret N. The trier social stress test and the trier social stress test for groups: qualitative investigations. PloS One. 2018;13 doi: 10.1371/journal.pone.0195722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frisch J.U., Häusser J.A., Mojzisch A. The Trier Social Stress Test as a paradigm to study how people respond to threat in social interactions. Front. Psychol. 2015;6 doi: 10.3389/fpsyg.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Köhler-Forsberg O., Rey R., Hospitalier Le Vinatier C., Jasmina Mallet F., Publique Hopitaux De Paris A., Fond G., Lançon C., Korchia T., Auquier P., Boyer L. The role of inflammation in the treatment of schizophrenia. Front. Psychiatry | Www.Frontiersin.Org. 2020;1:160. doi: 10.3389/fpsyt.2020.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng T., McEvoy J.P., Miller B.J. Longitudinal study of inflammatory markers and psychopathology in schizophrenia. Schizophr. Res. 2020;224:58–66. doi: 10.1016/j.schres.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Mongan D., Ramesar M., Föcking M., Cannon M., Cotter D. Role of inflammation in the pathogenesis of schizophrenia: a review of the evidence, proposed mechanisms and implications for treatment. Early Interv. Psychiatry. 2020;14:385–397. doi: 10.1111/eip.12859. [DOI] [PubMed] [Google Scholar]
- 29.Kirkpatrick B., Miller B.J. Inflammation and schizophrenia. Schizophr. Bull. 2013;39:1174–1179. doi: 10.1093/schbul/sbt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickerson F., Stallings C., Origoni A., Schroeder J., Katsafanas E., Schweinfurth L., Savage C., Khushalani S., Yolken R. Inflammatory markers in recent onset psychosis and chronic schizophrenia. Schizophr. Bull. 2016;42:134–141. doi: 10.1093/schbul/sbv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopresti A.L., Maker G.L., Hood S.D., Drummond P.D. A review of peripheral biomarkers in major depression: the potential of inflammatory and oxidative stress biomarkers. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;48:102–111. doi: 10.1016/j.pnpbp.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Sayana P., Colpo G.D., Simões L.R., Giridharan V.V., Teixeira A.L., Quevedo J., Barichello T. A systemic review of evidence for the role of inflammatory biomarkers in bipolar patients. J. Psychiatr. Res. 2017;92:160–182. doi: 10.1016/j.jpsychires.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Llibre A., Duffy D. Immune response biomarkers in human and veterinary research, Comp. Immunol. Microbiol. Infect. Dis. 2018;59:57–62. doi: 10.1016/j.cimid.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lange C., Deutschenbaur L., Borgwardt S., Lang U.E., Walter M., Huber C.G. Experimentally induced psychosocial stress in schizophrenia spectrum disorders: a systematic review. Schizophr. Res. 2017;182:4–12. doi: 10.1016/j.schres.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Kim Y.K., Amidfar M., Won E. A review on inflammatory cytokine-induced alterations of the brain as potential neural biomarkers in post-traumatic stress disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2019;91:103–112. doi: 10.1016/j.pnpbp.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Santolini J., Wootton S.A., Jackson A.A., Feelisch M. The Redox architecture of physiological function. Curr. Opin. Physiol. 2019;9:34–47. doi: 10.1016/j.cophys.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sies H., editor. Oxidative Stress:Eustress and Distress. Academic Press; 2020. [DOI] [Google Scholar]
- 38.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020:1–21. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 39.Sun Y., Lu Y., Saredy J., Wang X., Drummer C., IV, Shao Y., Saaoud F., Xu K., Liu M., Yang W.Y., Jiang X., Wang H., Yang X. ROS systems are a new integrated network for sensing homeostasis and alarming stresses in organelle metabolic processes. Redox Biol. 2020;37:101696. doi: 10.1016/J.REDOX.2020.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cortese-Krott M.M., Koning A., Kuhnle G.G.C., Nagy P., Bianco C.L., Pasch A., Wink D.A., Fukuto J.M., Jackson A.A., Van Goor H., Olson K.R., Feelisch M. The reactive species interactome: evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxidants Redox Signal. 2017;27:684–712. doi: 10.1089/ars.2017.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dennis K.K., Go Y.M., Jones D.P. Redox systems biology of nutrition and oxidative stress. J. Nutr. 2019;149:553–565. doi: 10.1093/jn/nxy306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansen J.M., Jones D.P., Harris C. The redox theory of development. Antioxidants Redox Signal. 2020;32:715–740. doi: 10.1089/ars.2019.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vermeulen R., Schymanski E.L., Barabási A.L., Miller G.W. The exposome and health: where chemistry meets biology. Science. 2020;367:392–396. doi: 10.1126/science.aay3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhama K., Latheef S.K., Dadar M., Samad H.A., Munjal A., Khandia R., Karthik K., Tiwari R., Yatoo M.I., Bhatt P., Chakraborty S., Singh K.P., Iqbal H.M.N., Chaicumpa W., Joshi S.K. Biomarkers in stress related diseases/disorders: diagnostic, prognostic, and therapeutic values. Front. Mol. Biosci. 2019;6:91. doi: 10.3389/fmolb.2019.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghezzi P., Floridi L., Boraschi D., Cuadrado A., Manda G., Levic S., D'Acquisto F., Hamilton A., Athersuch T.J., Selley L. Oxidative stress and inflammation induced by environmental and psychological stressors: a biomarker perspective. Antioxidants Redox Signal. 2018;28:852–872. doi: 10.1089/ars.2017.7147. [DOI] [PubMed] [Google Scholar]
- 46.Atrooz F., Liu H., Salim S. Prog. Mol. Biol. Transl. Sci. 2019. Stress, psychiatric disorders, molecular targets, and more; pp. 77–105. [DOI] [PubMed] [Google Scholar]
- 47.Go Y.M., Jones D.P. Redox biology: interface of the exposome with the proteome, epigenome and genome. Redox Biol. 2014;2:358–360. doi: 10.1016/j.redox.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang R.S., Oldham W.M., Maron B.A., Loscalzo J. Systems biology approaches to redox metabolism in stress and disease states. Antioxidants Redox Signal. 2018;29:953–972. doi: 10.1089/ars.2017.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalle-Donne I., Rossi R., Colombo R., Giustarini D., Milzani A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 50.Frustaci A., Neri M., Cesario A., Adams J.B., Domenici E., Dalla Bernardina B., Bonassi S. Oxidative stress-related biomarkers in autism: systematic review and meta-analyses. Free Radic. Biol. Med. 2012;52:2128–2141. doi: 10.1016/j.freeradbiomed.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 51.Ho E., Karimi Galougahi K., Liu C.-C.C., Bhindi R., Figtree G.A. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol. 2013;1:483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bloomer R.J., Fisher-Wellman K.H. Blood oxidative stress biomarkers: influence of sex, exercise training status, and dietary intake. Gend. Med. 2008;5:218–228. doi: 10.1016/J.GENM.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Gagné F. Biochem. Ecotoxicol. Princ. Methods. Academic Press; 2014. Oxidative stress; pp. 103–115. [DOI] [Google Scholar]
- 54.Flatow J., Buckley P., Miller B.J. Meta-analysis of oxidative stress in schizophrenia. Biol. Psychiatr. 2013;74:400–409. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bitanihirwe B.K.Y., Woo T.U.W. Oxidative stress in schizophrenia: an integrated approach. Neurosci. Biobehav. Rev. 2011;35:878–893. doi: 10.1016/j.neubiorev.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao J.K., Leonard S., Reddy R. Altered glutathione redox state in schizophrenia. Dis. Markers. 2013 doi: 10.1155/2006/248387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conus P., Seidman L.J., Fournier M., Xin L., Cleusix M., Baumann P.S., Ferrari C., Cousins A., Alameda L., Gholam-Rezaee M., Golay P., Jenni R., Woo T.U.W., Keshavan M.S., Eap C.B., Wojcik J., Cuenod M., Buclin T., Gruetter R., Do K.Q. N-acetylcysteine in a double-blind randomized placebo-controlled trial: toward biomarker-guided treatment in early psychosis. Schizophr. Bull. 2018 doi: 10.1093/schbul/sbx093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stadtman E.R., Levine R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 59.Berlett B.S. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 60.Javitt D.C., Kantrowitz J.T. third ed. Springer US; Boston, MA: 2009. Handbook of Neurochemistry and Molecular Neurobiology Schizophrenia. [DOI] [Google Scholar]
- 61.Davison J., O'Gorman A., Brennan L., Cotter D.R. A systematic review of metabolite biomarkers of schizophrenia. Schizophr. Res. 2018;195:32–50. doi: 10.1016/j.schres.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 62.Yang J., Chen T., Sun L., Zhao Z., Qi X., Zhou K., Cao Y., Wang X., Qiu Y., Su M., Zhao A., Wang P., Yang P., Wu J., Feng G., He L., Jia W., Wan C. Potential metabolite markers of schizophrenia. Mol. Psychiatr. 2013;18:67–78. doi: 10.1038/mp.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koga M., V Serritella A., Sawa A., Sedlak T.W. Implications for reactive oxygen species in schizophrenia pathogenesis. Schizophr. Res. 2016;176:52–71. doi: 10.1016/j.schres.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 64.Valko M., Leibfritz D., Moncol J., Cronin M.T.D., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Huang D., Ou B., Prior R.L., Boxin O.U., Prior R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 66.Deepa G., Ayesha S., Nishtha K., Thankamani M. Comparative evaluation of various total antioxidant capacity assays applied to phytochemical compounds of indian culinary spices. Int. Food Res. J. 2013;20:1711–1716. doi: 10.3390/12071496. [DOI] [Google Scholar]
- 67.Ghiselli A., Serafini M., Natella F., Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic. Biol. Med. 2000;29:1106–1114. doi: 10.1016/S0891-5849(00)00394-4. [DOI] [PubMed] [Google Scholar]
- 68.Woodford F.P., Whitehead T.P. Is measuring serum antioxidant capacity clinically useful? Ann. Clin. Biochem. An Int. J. Biochem. Med. 1998;35:48–56. doi: 10.1177/000456329803500105. [DOI] [PubMed] [Google Scholar]
- 69.Rice-Evans C., Miller N.J. Methods Enzymol. Academic Press; 1994. [241 Total antioxidant status in plasma and body fluids; pp. 279–293. [DOI] [PubMed] [Google Scholar]
- 70.Kim E., Winkler T.E., Kitchen C., Kang M., Banis G., Bentley W.E., Kelly D.L., Ghodssi R., Payne G.F. Redox probing for chemical information of oxidative stress. Anal. Chem. 2017;89:1583–1592. doi: 10.1021/acs.analchem.6b03620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim E., Keskey Z., Kang M., Kitchen C., Bentley W.E., Chen S., Kelly D.L., Payne G.F. Validation of oxidative stress assay for schizophrenia. Schizophr. Res. 2019;212:126–133. doi: 10.1016/j.schres.2019.07.057. [DOI] [PubMed] [Google Scholar]
- 72.First M.B. American Psychiatric Press; 1997. User's Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders SCID-I: Clinician Version, Abridged.https://books.google.com/books/about/User_s_Guide_for_the_Structured_Clinical.html?id=H91VmCO__O0C accessed. [Google Scholar]
- 73.Overall J.E., Gorham D.R. The Brief psychiatric rating scale. Psychol. Rep. 1962;10:799–812. doi: 10.2466/pr0.1962.10.3.799. [DOI] [Google Scholar]
- 74.Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andreasen N.C., Olsen S. Negative v positive schizophrenia: definition and validation. Arch. Gen. Psychiatr. 1982;39:789–794. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- 76.Guy W. 1976 edition. Open Library, DHEW Publication; Rockville, MD: 1976. ECDEU Assessment Manual for Psychopharmacology.https://openlibrary.org/books/OL24341821M/ECDEU_assessment_manual_for_psychopharmacology#edition-details National Institute of Mental Health, USA. accessed. [Google Scholar]
- 77.Schatz P. Encycl. Clin. Neuropsychol. 2018. Repeatable Battery for the assessment of neuropsychological status; pp. 2990–2991. [DOI] [Google Scholar]
- 78.M G., J L., Singh I.F.P. Irr: various coefficients of interrater reliability and agreement. R Packag. Version 0.84.1. 2019 https://cran.r-project.org/package=irr [Google Scholar]
- 79.Shrout P.E., Fleiss J.L. Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. http://www.ncbi.nlm.nih.gov/pubmed/18839484 accessed. [DOI] [PubMed] [Google Scholar]
- 80.Bartko J.J. The intraclass correlation coefficient as a measure of reliability. Psychol. Rep. 1966;19:3–11. doi: 10.2466/pr0.1966.19.1.3. [DOI] [PubMed] [Google Scholar]
- 81.Sierra-Sánchez Á., Garrido-Martín D., Lourido L., González-González M., Díez P., Ruiz-Romero C., Sjöber R., Droste C., De Las Rivas J., Nilsson P., Blanco F., Fuentes M. Screening and validation of novel biomarkers in osteoarticular pathologies by comprehensive combination of protein array technologies. J. Proteome Res. 2017;16:1890–1899. doi: 10.1021/acs.jproteome.6b00980. [DOI] [PubMed] [Google Scholar]
- 82.Hedeker D. A mixed-effects multinomial logistic regression model. Stat. Med. 2003;22:1433–1446. doi: 10.1002/sim.1522. [DOI] [PubMed] [Google Scholar]
- 83.Jalali A., Kitching M., Martin K., Richardson C., Murphy T.B., FitzGerald S.P., Watson R.W., Perry A.S. Integrating inflammatory serum biomarkers into a risk calculator for prostate cancer detection. Sci. Rep. 2021;11:2525. doi: 10.1038/s41598-021-81965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.W N.V., Ripley B.D. Springer; Fourth: 2002. Modern Applied Statistics with S.https://www.stats.ox.ac.uk/pub/MASS4/ [Google Scholar]
- 85.Harrell F.E. Springer International Publishing; Cham: 2015. Regression Modeling Strategies. [DOI] [Google Scholar]
- 86.Mazzara S., Rossi R.L., Grifantini R., Donizetti S., Abrignani S., Bombaci M. CombiROC: an interactive web tool for selecting accurate marker combinations of omics data. Sci. Rep. 2017;7 doi: 10.1038/srep45477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Royston P., Altman D.G. Visualizing and assessing discrimination in the logistic regression model. Stat. Med. 2010;29:2508–2520. doi: 10.1002/sim.3994. [DOI] [PubMed] [Google Scholar]
- 88.Maria Araujo Martins A., Kumar Bharti S., Carolina de Mattos Zeri A., Mep C., Rodrigo Alborghetti M., Elvira Pizzigatti Correa M., Whangbo J., Shi X., Aparecida Aricetti J., Aparecida da Silva A., Cristina Martins Miranda E., Luis Sforca M., Caldana C., Gerszten R.E., Ritz J. Clinical metabolomics identifies blood serum branched chain amino acids as potential predictive biomarkers for chronic graft vs. Host disease. Front. Oncol. | Www.Frontiersin.Org. 2019;1:141. doi: 10.3389/fonc.2019.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rathnayake D., Chang T., Udagama P. Selected serum cytokines and nitric oxide as potential multi-marker biosignature panels for Parkinson disease of varying durations: a case-control study. BMC Neurol. 2019;19 doi: 10.1186/s12883-019-1286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Austin P.C., Merlo J. Intermediate and advanced topics in multilevel logistic regression analysis. Stat. Med. 2017;36:3257–3277. doi: 10.1002/sim.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kumar N.P., Hissar S., Thiruvengadam K., V Banurekha V., Suresh N., Shankar J., E S., G N.S., K S., G J., A M.A., Baskaran D., Tripathy S., Swaminathan S., Babu S. Discovery and validation of a three-cytokine plasma signature as a biomarker for diagnosis of pediatric tuberculosis. Front. Immunol. 2021;12:1. doi: 10.3389/fimmu.2021.653898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weusten S.J.C., de Groot M.T., van der Schaaf J. A comparative study of the stability of hexachloroiridate and hexacyanoferrate in electrochemical mass transfer measurements. J. Electroanal. Chem. 2020;878:114512. doi: 10.1016/j.jelechem.2020.114512. [DOI] [Google Scholar]
- 93.Hallgren K.A. Computing inter-rater reliability for observational data: an overview and tutorial. Tutor. Quant. Methods Psychol. 2012;8:23–34. doi: 10.20982/tqmp.08.1.p023. http://www.ncbi.nlm.nih.gov/pubmed/22833776 accessed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bartko J.J., Shrout P.E., Fleiss J.L. The intraclass correlation coefficient as a measure of reliability. Psychol. Rep. 1966;86:3–11. doi: 10.2466/pr0.1966.19.1.3. [DOI] [PubMed] [Google Scholar]
- 95.Zhang X.Y., Chen da C., Xiu M.H., Tang W., Zhang F., Liu L., Chen Y., Liu J., Yao J.K., Kosten T.A.R.T.R., Chen D.C., Xiu M.H., Tang W., Zhang F., Liu L., Chen Y., Liu J., Yao J.K., Kosten T.A.R.T.R., Kosten T.A.R.T.R. Plasma total antioxidant status and cognitive impairments in schizophrenia. Schizophr. Res. 2012;139:66–72. doi: 10.1016/j.schres.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 96.Dickerson F., Stallings C., Origoni A., Boronow J., Yolken R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr. Res. 2007;93:261–265. doi: 10.1016/j.schres.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 97.Zhang X.Y., Chen D.C., Xiu M.H., De Yang F., Tan Y.L., He S., Kosten T.A.T.R., Kosten T.A.T.R. Thioredoxin, a novel oxidative stress marker and cognitive performance in chronic and medicated schizophrenia versus healthy controls. Schizophr. Res. 2013;143:301–306. doi: 10.1016/j.schres.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 98.Akyol M., Herken H., Uz E., FadıllıoǧluFadıllıoǧlu E., SögSöǧüt S., Asuman Savas H. The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients the possible role of oxidant/antioxidant imbalance. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2002;26:995–1005. doi: 10.1016/s0278-5846(02)00220-8. [DOI] [PubMed] [Google Scholar]
- 99.Zhang X.Y., Zhou D.F., Cao L.Y., Zhang P.Y., Wu G.Y. Elevated blood superoxide dismutase in neuroleptic-free schizophrenia: association with positive symptoms. Psychiatr. Res. 2003;117:85–88. doi: 10.1016/S0165-1781(02)00303-7. [DOI] [PubMed] [Google Scholar]
- 100.Matsuzawa D., Obata T., Shirayama Y., Nonaka H., Kanazawa Y., Yoshitome E., Takanashi J., Matsuda T., Shimizu E., Ikehira H., Iyo M., Hashimoto K. Negative correlation between brain glutathione level and negative symptoms in schizophrenia: a 3T 1H-mrs study. PloS One. 2008;3 doi: 10.1371/journal.pone.0001944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu S., Zhao L., Fan Y., Lv Q., Wu K., Lang X., Li Z., Yi Z., Geng D. Interaction between TNF-α and oxidative stress status in first-episode drug-naïve schizophrenia. Psychoneuroendocrinology. 2020;114 doi: 10.1016/j.psyneuen.2020.104595. [DOI] [PubMed] [Google Scholar]
- 102.Maes M., Sirivichayakul S., Matsumoto A.K., Michelin A.P., de Oliveira Semeão L., de Lima Pedrão J.V., Moreira E.G., Barbosa D.S., Carvalho A.F., Solmi M., Kanchanatawan B. Lowered antioxidant defenses and increased oxidative toxicity are hallmarks of deficit schizophrenia: a nomothetic network psychiatry approach. Mol. Neurobiol. 2020;57:4578–4597. doi: 10.1007/s12035-020-02047-5. [DOI] [PubMed] [Google Scholar]
- 103.Kang M., Kim E., Chen S., Bentley W.E., Kelly D.L., Payne G.F. Signal processing approach to probe chemical space for discriminating redox signatures. Biosens. Bioelectron. 2018;112:127–135. doi: 10.1016/J.BIOS.2018.04.032. [DOI] [PubMed] [Google Scholar]
- 104.Maydeu-Olivares A., García-Forero C. Int. Encycl. Educ. Elsevier; 2010. Goodness-of-fit testing; pp. 190–196. [DOI] [Google Scholar]
- 105.Schiavone S., Trabace L. Inflammation, stress response, and redox dysregulation biomarkers: clinical outcomes and pharmacological implications for psychosis. Front. Psychiatr. 2017;8 doi: 10.3389/fpsyt.2017.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Santos-Ribeiro D., Godinas L., Pilette C., Perros F. The integrated stress response system in cardiovascular disease. Drug Discov. Today. 2018;23:920–929. doi: 10.1016/j.drudis.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 107.Mondelli V., Ciufolini S., Murri M.B., Bonaccorso S., Di Forti M., Giordano A., Marques T.R., Zunszain P.A., Morgan C., Murray R.M., Pariante C.M., Dazzan P. Cortisol and inflammatory biomarkers predict poor treatment response in first episode psychosis, Schizophr. Bull. (Arch. Am. Art) 2015;41:1162–1170. doi: 10.1093/schbul/sbv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Czarny P., Wigner P., Galecki P., Sliwinski T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;80:309–321. doi: 10.1016/j.pnpbp.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 109.Goldsmith D.R., Rapaport M.H., Miller B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatr. 2016;21:1696–1709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Momtazmanesh S., Zare-Shahabadi A., Rezaei N., Garcia-Gutierrez M.S., Schiavone S., Arsenijevic N.N. 2019. Cytokine Alterations in Schizophrenia: an Updated Review; p. 1. Article. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Steullet P., Cabungcal J.H., Monin A., Dwir D., O'Donnell P., Cuenod M., Do K.Q. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: a “central hub” in schizophrenia pathophysiology? Schizophr. Res. 2016;176:41–51. doi: 10.1016/j.schres.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tomasik J., Rahmoune H., Guest P.C., Bahn S. Neuroimmune biomarkers in schizophrenia. Schizophr. Res. 2016;176:3–13. doi: 10.1016/j.schres.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 113.Frustaci A., Neri M., Cesario A., Adams J.B., Domenici E., Dalla Bernardina B., Bonassi S. Oxidative stress-related biomarkers in autism: systematic review and meta-analyses. Free Radic. Biol. Med. 2012;52:2128–2141. doi: 10.1016/j.freeradbiomed.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 114.Lai C.-Y., Scarr E., Udawela M., Everall I., Chen W.J., Dean B. Biomarkers in schizophrenia: a focus on blood based diagnostics and theranostics. World J. Psychiatr. 2016;6:102. doi: 10.5498/wjp.v6.i1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pouget J.G. The emerging immunogenetic architecture of schizophrenia. Schizophr. Bull. 2018;44:993–1004. doi: 10.1093/schbul/sby038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nedic Erjavec G., Konjevod M., Nikolac Perkovic M., Svob Strac D., Tudor L., Barbas C., Grune T., Zarkovic N., Pivac N. Short overview on metabolomic approach and redox changes in psychiatric disorders. Redox Biol. 2018;14:178–186. doi: 10.1016/j.redox.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dean K.R., Hammamieh R., Mellon S.H., Abu-Amara D., Flory J.D., Guffanti G., Wang K., Daigle B.J., Jr., Gautam A., Lee I., Yang R., Almli L.M., Saverio Bersani F., Chakraborty N., Donohue D., Kerley K., Kim T.-K., Laska E., Young Lee M., Lindqvist D., Lori A., Lu L., Misganaw B., Muhie S., Newman J., Price N.D., Qin S., Reus V.I., Siegel C., Somvanshi P.R., Thakur G.S., Zhou Y., Hood L., Ressler K.J., Wolkowitz O.M., Yehuda R., Jett M., Doyle F.J., III, Marmar C. Multi-omic biomarker identification and validation for diagnosing warzone-related post-traumatic stress disorder. Mol. Psychiatr. 2020;25:3337–3349. doi: 10.1038/s41380-019-0496-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li X.F., Zheng Y.L., Xiu M.H., Chen D.C., Kosten T.R., Zhang X.Y. Reduced plasma total antioxidant status in first-episode drug-naive patients with schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35:1064–1067. doi: 10.1016/j.pnpbp.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 119.Tsai M.C., Liou C.W., Lin T.K., Lin I.M., Huang T.L. Changes in oxidative stress markers in patients with schizophrenia: the effect of antipsychotic drugs. Psychiatr. Res. 2013;209:284–290. doi: 10.1016/j.psychres.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 120.Coughlin J.M., Hayes L.N., Tanaka T., Xiao M., Yolken R.H., Worley P., Leweke F.M., Sawa A. Reduced superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with early psychosis in association with clinical features. Schizophr. Res. 2017;183:64–69. doi: 10.1016/j.schres.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 121.Chien Y.L., Hwu H.G., Hwang T.J., Hsieh M.H., Liu C.C., Lin-Shiau S.Y., Liu C.M. Clinical implications of oxidative stress in schizophrenia: acute relapse and chronic stable phase. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2020;99 doi: 10.1016/j.pnpbp.2020.109868. [DOI] [PubMed] [Google Scholar]
- 122.González-Blanco L., García-Portilla M.P., García-Álvarez L., de la Fuente-Tomás L., Iglesias García C., Sáiz P.A., Rodríguez-González S., Coto-Montes A., Bobes J. Oxidative stress biomarkers and clinical dimensions in first 10 years of schizophrenia. Rev. Psiquiatía Salud Ment. 2018;11:130–140. doi: 10.1016/j.rpsm.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 123.Loughland C.M., Lewin T.J., Carr V.J., Sheedy J., Harris A.W. RBANS neuropsychological profiles within schizophrenia samples recruited from non-clinical settings. Schizophr. Res. 2007;89:232–242. doi: 10.1016/j.schres.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 124.Case A.J., Roessner C.T., Tian J., Zimmerman M.C. Mitochondrial superoxide signaling contributes to norepinephrine-mediated T-lymphocytecytokine profiles. PloS One. 2016;11 doi: 10.1371/journal.pone.0164609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moshfegh C.M., Elkhatib S.K., Collins C.W., Kohl A.J., Case A.J. Autonomic and redox imbalance correlates with T-Lymphocyte inflammation in a model of chronic social defeat stress. Front. Behav. Neurosci. 2019;13:103. doi: 10.3389/fnbeh.2019.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wiegand C., Heusser P., Klinger C., Cysarz D., Büssing A., Ostermann T., Savelsbergh A. Stress-associated changes in salivary microRNAs can be detected in response to the Trier Social Stress Test: an exploratory study. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-25554-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Janšáková K., Belica I., Rajčániová E., Rajčáni J., Kyselicová K., Celušáková H., Laznibatová J., Ostatníková D. The acute effect of psychosocial stress on the level of oxidative stress in children. Int. J. Psychophysiol. 2021;161:86–90. doi: 10.1016/j.ijpsycho.2021.01.007. [DOI] [PubMed] [Google Scholar]
- 128.Meyer T., Wirtz P.H. Mechanisms of mitochondrial redox signaling in psychosocial stress-responsive systems: new insights into an old story. Antioxidants Redox Signal. 2018;28:760–772. doi: 10.1089/ars.2017.7186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.