Abstract
The objective of this experiment was to investigate the influence of dietary tributyrin on reproduction performance and ovary function of broiler breeders with different egg laying rate. Two hundred fifty-six AA broiler breeders (48-wk-old) were allocated to 4 treatment in a 2 × 2 factorial arrangement with the main effects of tributyrin supplementation (0 and 1,000 mg/kg tributyrin [TRI]) and 2 egg laying rate levels (average [AR, 81.01 ± 0.79%] and low [LR, 70.98 ± 0.95%]). The results shown that the LR breeders presented higher egg weight, but lower egg laying rate, qualified egg rate and feed efficiency than the AR breeders (P(laying) < 0.05). Also, the superoxidase dismutase (SOD) activity in magnum was lower while malondialdehyde (MDA) was higher in ovary and magnum of LR breeders than that in the AR breeders (P(laying) < 0.05). Dietary supplementation with tributyrin significantly enhanced egg weight (P(TRI) < 0.05), increased albumen height as well as Haugh unit (HU) in AR breeders (P(interaction) < 0.05), and also had higher total antioxidant capacity (T-AOC) and lower MDA in ovary (P(TRI) < 0.05). The cell apoptosis rate and proapoptosis related gene expression (caspase 8, 9 and Bax) in the ovary of LR breeders was higher, while anti-apoptosis related gene (Bcl-2) expression were lower in LR breeders when compared with the AR breeders (P(laying) < 0.05). Dietary supplementation with tributyrin decreased the cell apoptosis rate and downregulated caspase 9 expression in LR breeders (P(Interaction) < 0.05), up-regulated the Bcl-2 expression in both 2 breeders (P(TRI) < 0.05). These findings suggest that the breeders with lower egg laying rate also characterized by deteriorate ovary function indicated by lower antioxidant capacity and higher cell apoptosis rate. Dietary supplementation with tributyrin increased egg albumen quality, decreased ovarian proapoptosis related gene expression to improve reproductive tract function; and the positive effect on egg albumen quality is more pronounced in average reproductive breeders.
Key words: tributyrin, broiler breeders, egg quality, antioxidant capacity, ovary function
INTRODUCTION
The productive performance of broiler breeders is fundamental to the development of poultry industry. Nutrition has great impact on performance and efficiency of all animals, and a healthy digestive and reproductive system function is critical to maintain production performance and health of the breeders (Yang et al., 2020). Ovary function is the main factor affecting the reproductive performance of layers and breeders (Johnson, 2012), and it is also the organ that is the most sensitive to aging and stress (Rozenboim et al., 2007; Devine et al., 2012; Wang et al., 2018). The follicle utilization rate is extremely low because most follicles are removed from the ovaries before ovulation via a degenerative process known as atresia (Kaipia and Hsueh, 1997; Zhang et al., 2019). With the aging of ovary, the oxidative stress may cause follicular atresia and lead to the declines of fertility (Tatone, 2008; Peters et al., 2020). Follicle atresia in late laying phase (35–50 wk of age) may be the main contributing factor for the inferior total laying performance and the early culling in practice.
Butyrate is produced by microbial fermentation, primarily in the large intestine of animals and birds (Vinolo et al., 2011; Tan et al., 2014). Generally, butyrate known to be served a primary nutrient that provides energy to colonocytes, but also to be involved in regulating multiple functions of gut cells, including gene expression, cell differentiation, immune modulation, and anti-inflammatory (Sengupta et al., 2006). It is also indicated that butyrate and its derivatives (butyric acid, sodium or calcium butyrate, and butyrate glycerides) were found to exert antioxidant effect in animals’ models in vivo or in vitro (Lin et al., 2014; Wu et al., 2016; Memon et al., 2019; Guo et al., 2020). On the same time, lots of studies have demonstrated that sodium butyrate and butyrate glycerides (such as tributyrin and monobutyrin) could improve intestinal health and have beneficial effects on performance of broilers, laying hens and breeders (Czerwinski, et al., 2012; Jahanianand Golshadi, 2015; Kaczmarek et al., 2016; Bedford et al., 2017; Bedford and Gong, 2018; Yang et al., 2018; Zhao et al., 2019; Lan et al., 2020; Feng et al., 2021). However, the response of broiler breeders with different reproduction performance to tributyrin on ovarian antioxidant capacity and function in is not clear.
Therefore, the purpose of the current study was to verify the hypothesis that dietary tributyrin supplementation could promote the reproductive performance of broiler breeders, and whether this effect will be different between broilers with different egg laying rate.
MATERIALS AND METHODS
Birds, Experimental Design
The investigation plan and methods has been authorized with the guidelines of the Animal Care and Use Committee of Sichuan Agricultural University. A total of 256 Arbor Acres broiler breeders at 48 wk of age were randomly assigned to a 2 by 2 factorial design. Treatment composed of 2 egg laying rate levels (average [AR, 81.01 ± 0.79%] and low [LR, 70.98 ± 0.95%]) and 2 levels of tributyrin (control [no additive], 1,000 mg/kg tributyrin [TRI]; based on the previous studies [Zhao et al., 2019]). Each treatment has 8 replicates with 8 birds per replicate. TRI is provided by Shanghai Aladdin Chemical Co., Ltd. (Shanghai, China), with a purity of 98%. The experimental diet was formulated to meet nutrient specifications according to the NRC (1994) in a mash form (Table 1). Broiler breeders were allowed ad libitum access to water and restricted feeding (154 g/d/breeder). The total experimental period was 8 wk. All birds were individually housed in a temperature-controlled room (maintained at 20–22°C) on a 16L:8D photo-period throughout the duration of study.
Table 1.
Composition and nutrient level of basal diet (as-fed basis).
| Item, % | Amount |
|---|---|
| Corn | 69.50 |
| Soybean meal, 43% | 19.00 |
| Soybean oil | 1.00 |
| Calcium carbonate | 8.25 |
| Calcium hydrophosphate | 1.14 |
| L-lsine hydrochloride | 0.08 |
| DL-methionine | 0.11 |
| Threonine | 0.02 |
| NaCl | 0.30 |
| Choline chloride, 50% | 0.10 |
| Vitamin and mineral premix1 | 0.50 |
| Total | 100.00 |
| Analyzed nutrient levels, % | |
| ME2, kcal/kg | 2,780.00 |
| Crude protein | 13.70 |
| Calcium | 3.35 |
| Available phosphorus | 0.35 |
| Lysine | 0.75 |
| Methionine | 0.34 |
| Methionine + cysteine | 0.61 |
Provided per kilogram of diet: vitamin A, 12,000 IU; vitamin D3, 4,000 IU; vitamin E, 100 mg; vitamin K3, 4.0 mg; thiamin, 3.0 mg; riboflavin, 11.5 mg; pyridoxine, 7.2 mg; vitamin B12, 0,02 mg; folic acid, 10.8 mg; niacin, 47.1 mg; pantothenic acid, 21.6 mg; biotin, 0. 6 mg; iron, 80 mg; copper, 20 mg; manganese, 82.5 mg; zinc, 100 mg; selenium, 0.30 mg; iodine, 1.20 mg.
Calculated according to NRC (1994).
Productive Performance and Sample Collection
Egg production, egg weights, and unqualified eggs (egg weight <50 g or >75 g, misshaped egg, dirty egg, and sand-shelled egg) of were measured daily. Feed conversion ratio was calculated as the ratio of grams of total feed intake to grams of total egg weight. The qualified egg rate was defined as the ratio of total qualified eggs to total laid eggs per treatment.
At d 57 of experimental period, blood samples were collected from the wing vein of 2 hens per replicate. Blood samples were then centrifuged at 3,000 × g for 15 min, and then serum was stored at −20°C till analysis. Thereafter, broiler breeders were sacrificed by CO2 suffocation, the ovarian tissues (ovary cortex) and magnum of oviduct were taken and then stored at −80°C till gene expression analysis.
Egg Quality and Egg Hatchability Performance
To determine the egg quality indices, egg samples (4 eggs/replicate, 32 eggs/treatment) were collected at the end of the supplementation period. After individual weighing, each egg was using an eggshell force gauge model II (Robotmation Co., Ltd., Tokyo, Japan) and eggshell thickness gauge to measure strength and thickness respectively. Egg internal quality (including Haugh unit [HU], albumen height, and yolk color) were analyzed via an Egg Multi-tester (EMT-7300, Robotmation Co., Ltd., Tokyo, Japan). Albumen or eggshell ratio was computed as 100 × (albumen weight or eggshell weight [g]/egg weight [g]).
At the end of the experiment, all eggs produced over 5 consecutive days were collected, labeled, and weighted individually, and then stored at 15°C until incubation. Eggs were incubated in a commercial hatchery (Jinling JLZ-2., Yaan, China). Fertility was expressed as the ratio of fertile eggs to total eggs set. The number of eggs that hatched was recorded after 21 d of incubation. Embryonic mortality of eggs was expressed as the ratio of mortalities to set eggs. Hatchability of set eggs was calculated as the ratio of hatching chicks to set eggs.
Serum Reproductive Hormones Assay
Serum concentration of estradiol (E2), follicle stimulating hormone (FSH), testosterone, anti-Müllerian hormone (AMH), and progesterone were assessed by enzyme-linked immunosorbent assay (ELISA) test kits (Nanjing Jiancheng Bioengineering Institute, China) following the manufacturer's protocol.
Magnum and Ovary Antioxidant Capacity
Commercial kits were used to analyze the antioxidant capacity of ovary and magnum, including activities of superoxide dismutase (SOD), total antioxidant capacity (T-AOC) and malondialdehyde (MDA) content according to the manufacture (SOD, A001-1; T-AOC, A015; MDA, A003-1; Nanjing Jiancheng Biotechnology, Nanjing, China).
Ovary Apoptosis Related mRNA Expression by Real-Time PCR
Total RNA for ovary tissue was extracted with TRIzol reagent (TaKaRa, Dalian, China) and cDNA was synthesized via reverse transcription. ABI Prism 7000 detection system in a 2-step protocol with SYBR Green (TaKaRa, Dalian, China) were used to conduct the quantitative real-time PCR. The primer information for all the genes (Caspase 3, Caspase 8, Caspase 9, Bcl2 and Bax) is listed in Table 2. Each sample was assayed in triplicate and β–actin was used as the housekeeping gene. Gene expression was calculated by using the 2−ΔΔCT method.
Table 2.
Related gene and primer information.1
| Genes1 | Orientation | Primer sequences (5′-3′) | Product size | Accession number |
|---|---|---|---|---|
| β-actin | Forward | GCTACAGCTTCACCACCACA | 90 | NM_205518.1 |
| Reverse | TCTCCTGCTCGAAATCCAGT | |||
| Caspase 9 | Forward | TATGGTGGAGGACATGCAGA | 99 | XM_424580.5 |
| Reverse | AATATTGGGAAGGCCTGCTT | |||
| Caspase 3 | Forward | AAAGATGGACCACGCTCAGG | 204 | NM_204725 |
| Reverse | TGAACGAGATGACAGTCCGG | |||
| Caspase 8 | Forward | GCTGTATCCTATCCCACG | 125 | KM_016991 |
| Reverse | TCATCAGGCACTCCTTT | |||
| Bax | Forward | GTACGTCAATGTGGTCACCC | 210 | XM_015274882 |
| Reverse | TGGGATAATGCTGGGGTTGA | |||
| Bcl2 | Forward | ACCATGAATGAAACCGTGCC | 181 | NM_205339.2 |
| Reverse | TTGTCGTAGCCTCTTCTCCC |
Abbreviation: Bax, B lymphoma 2 associated X protein, Bcl2, B lymphoma cell 2.
Statistical Analysis
Data were analyzed as a 2 × 2 factorial using the general linear model procedures of SAS 9.2 (SAS Institute, Cary, NC), with a model that included the main effects of egg laying rate and TRI, as well as their interaction. Means were compared by using Tukey's range test to determine significant differences among means with a significant level of P < 0.05.
RESULTS
Reproduction Performance, Egg Quality and Incubation Performance
At presented in Table 3, the LR breeders presented higher egg weight but lower egg laying rate, qualified egg rate and feed efficiency than the AR breeders (P(laying) < 0.05). No difference was found in egg quality between LR and AR breeders (P(laying) > 0.05; Table 4). Dietary supplementation with tributyrin significantly improved egg weight compared with no tributyrin addition (P(TRI) < 0.05). Dietary supplementation with tributyrin significantly enhanced egg weight (P(TRI) < 0.05) and increased albumen height together with HU in AR breeders (P(interaction) < 0.05).
Table 3.
Effect of glycerol tributyrate on reproduction performance of broiler breeders with different egg-laying rate.1
| Item | Laying rate, % | Egg weight, g | FCR | Qualified egg rate, % | |
|---|---|---|---|---|---|
| Laying | TRI | ||||
| AR | − | 78.58a | 64.10b | 3.12b | 93.23a |
| AR | + | 78.67a | 66.99a | 2.92b | 93.80a |
| LR | − | 68.52b | 66.86b | 3.39a | 89.22b |
| LR | + | 69.69b | 68.34a | 3.31a | 91.57b |
| SEM | 1.17 | 0.43 | 0.07 | 1.34 | |
| P-value | <0.01 | <0.01 | 0.05 | 0.03 | |
| P-value | |||||
| Laying | <0.01 | <0.01 | 0.02 | 0.03 | |
| TRI | 0.31 | 0.01 | 0.51 | 0.43 | |
| Laying × TRI | 0.47 | 0.89 | 0.16 | 0.54 | |
Abbreviations: AR, average egg laying rate; LR, low egg laying rate; TRI, 1,000 mg/kg tributyrin.
Means with different superscripts within a column differ significantly (P ≤ 0.05).
Each mean represents 8 layers/replicate, 8 replicates/treatment.
Table 4.
Effect of glycerol tributyrate on egg quality of broiler breeders with different egg-laying rate.1
| Item | Eggshell strength, kg/cm3 | Albumen height, mm | York color | Haugh unit | Yolk weight ratio, % | Egg shell weight ratio, % | Eggshell thickness, mm−2 | Egg white ratio, % | |
|---|---|---|---|---|---|---|---|---|---|
| Laying | TRI | ||||||||
| AR | − | 3.59 | 6.12b | 8.11 | 74.60b | 33.67 | 9.89 | 30.57 | 59.54 |
| AR | + | 3.64 | 6.61a | 8.12 | 78.51a | 32.75 | 9.96 | 30.18 | 59.86 |
| LR | − | 3.55 | 6.18b | 8.20 | 76.69b | 33.81 | 9.98 | 30.16 | 59.86 |
| LR | + | 3.55 | 6.25b | 8.17 | 77.38b | 32.65 | 9.90 | 30.08 | 60.02 |
| SEM | 0.19 | 0.28 | 0.16 | 2.03 | 0.37 | 0.16 | 0.49 | 0.39 | |
| P-value | 0.29 | 0.02 | 0.51 | 0.05 | 0.57 | 0.34 | 0.12 | 0.11 | |
| P-value | |||||||||
| Laying | 0.50 | 0.99 | 0.31 | 0.78 | 0.97 | 0.52 | 0.28 | 0.76 | |
| TRI | 0.11 | 0.22 | 0.69 | 0.23 | 0.32 | 0.37 | 0.39 | 0.44 | |
| Laying × TRI | 0.61 | 0.02 | 0.30 | <0.01 | 0.74 | 0.14 | 0.20 | 0.37 | |
Abbreviations: AR, average egg laying rate; LR, low egg laying rate; TRI, 1000 mg/kg tributyrin.
Means with different superscripts within a column differ significantly (P ≤ 0.05).
Each mean represents 8 layers/replicate, 8 replicates/treatment.
There were no significant differences in incubation performance parameters measured in this study (embryo mortality, fertility, hatchability of set eggs, and health born chicken rate) caused by different laying rate breeders or dietary TRI supplementation (Table 5, P > 0.05).
Table 5.
Effect of glycerol tributyrate on hatchability of broiler breeders with different egg-laying rate.1
| Item | Embryonic mortality, % | Fertility, % | Hatchability of set eggs, % | Health chicken rate, % | |
|---|---|---|---|---|---|
| Laying | TRI | ||||
| AR | − | 8.12 | 95.47 | 88.95 | 88.23 |
| AR | + | 7.98 | 96.34 | 89.55 | 88.60 |
| LR | − | 8.49 | 95.22 | 89.45 | 89.44 |
| LR | + | 8.34 | 97.40 | 89.11 | 89.48 |
| SEM | 2.14 | 1.51 | 2.65 | 2.78 | |
| P-value | 0.87 | 0.94 | 0.96 | 0.94 | |
| P-value | |||||
| Laying | 0.97 | 0.81 | 0.83 | 0.77 | |
| TRI | 0.98 | 0.64 | 0.87 | 0.69 | |
| Laying × TRI | 0.63 | 0.74 | 0.64 | 0.73 | |
Abbreviations: AR, average egg laying rate; LR, low egg laying rate; TRI, 1,000 mg/kg tributyrin.
Each mean represents 8 layers/replicate, 8 replicates/treatment.
Serum Reproductive Hormone
As shown in Table 6, the serum AMH level were lower in LR group compared to the AR breeders (P(laying) < 0.05). The concentration of E2, FSH, testosterone, and progesterone were not affected by dietary tributyrin or in layers with different egg laying rate (P > 0.05).
Table 6.
Effect of glycerol tributyrate on blood hormone levels of broiler breeders with different egg-laying rate.1
| Item | E2, pmol/L | FSH, U/L | Testosterone, nmol/L | AMH, pg/mL | Progesterone, pmol/L | |
|---|---|---|---|---|---|---|
| Laying | TRI | |||||
| AR | − | 63.12 | 7.34 | 197.12 | 229.11a | 1811.07 |
| AR | + | 69.34 | 7.17 | 191.54 | 244.45a | 1827.62 |
| LR | − | 64.11 | 6.69 | 174.1 | 164.98b | 1817.08 |
| LR | + | 66.54 | 7.51 | 188.64 | 147.41b | 1724.34 |
| SEM | 3.47 | 0.39 | 13.49 | 16.6 | 114.27 | |
| P-value | 0.85 | 0.13 | 0.69 | 0.01 | 0.27 | |
| P-value | ||||||
| Laying | 0.65 | 0.61 | 0.85 | 0.02 | 0.23 | |
| TRI | 0.84 | 0.08 | 0.92 | 0.17 | 0.26 | |
| Laying × TRI | 0.47 | 0.12 | 0.24 | 0.57 | 0.26 | |
Abbreviations: AMH, anti-Müllerian hormone; AR, average egg laying rate; E2, estradiol; FSH, follicle stimulating hormone; LR, low egg laying rate; TRI, 1,000 mg/kg tributyrin.
Means with different superscripts within a column differ significantly (P ≤ 0.05).
Each mean represents 2 layers/replicate, 8 replicates/treatment.
Antioxidant Capacity of Magnum and Ovary
When compared with the AR breeders, the SOD activity in magnum was lower and MDA was higher in ovary and magnum in LR breeders (P(laying) < 0.05; Table 7). Dietary supplementation with tributyrin significantly increased T-AOC and lower MDA in ovary (P(TRI) < 0.05).
Table 7.
Effect of glycerol tributyrate on magnum and ovary antioxidant capacity of broiler breeders with different egg laying rate.1
| Ovary |
Magnum |
||||||
|---|---|---|---|---|---|---|---|
| Item | SOD | T-AOC | MDA | SOD | T-AOC | MDA | |
| Laying | TRI | ||||||
| AR | − | 181.41 | 0.60b | 1.65b | 34.19a | 0.19 | 0.59a |
| AR | + | 191.03 | 0.78a | 1.23c | 35.98a | 0.18 | 0.51a |
| LR | − | 183.22 | 0.56b | 2.15a | 29.10b | 0.15 | 0.46b |
| LR | + | 164.08 | 0.89a | 1.67b | 30.01b | 0.13 | 0.32b |
| SEM | 14.21 | 0.11 | 0.10 | 2.24 | 0.03 | 0.07 | |
| P-value | 0.55 | 0.01 | 0.05 | 0.01 | 0.94 | 0.03 | |
| P-value | |||||||
| Laying | 0.60 | 0.91 | <0.01 | <0.01 | 0.73 | 0.05 | |
| TRI | 0.99 | <0.01 | 0.03 | 0.42 | 0.95 | 0.02 | |
| Laying 0 ×TRI | 0.19 | 0.35 | 0.37 | 0.69 | 0.62 | 0.33 | |
Abbreviations: AMH, anti-Müllerian hormone; AR, average egg laying rate; E2, estrogen 2, FSH, follicle stimulation hormone; LR, low egg laying rate; TRI, 1,000 mg/kg tributyrin.
Means with different superscripts within a column differ significantly (P ≤ 0.05).
Each mean represents 2 layers/replicate, 8 replicates/treatment.
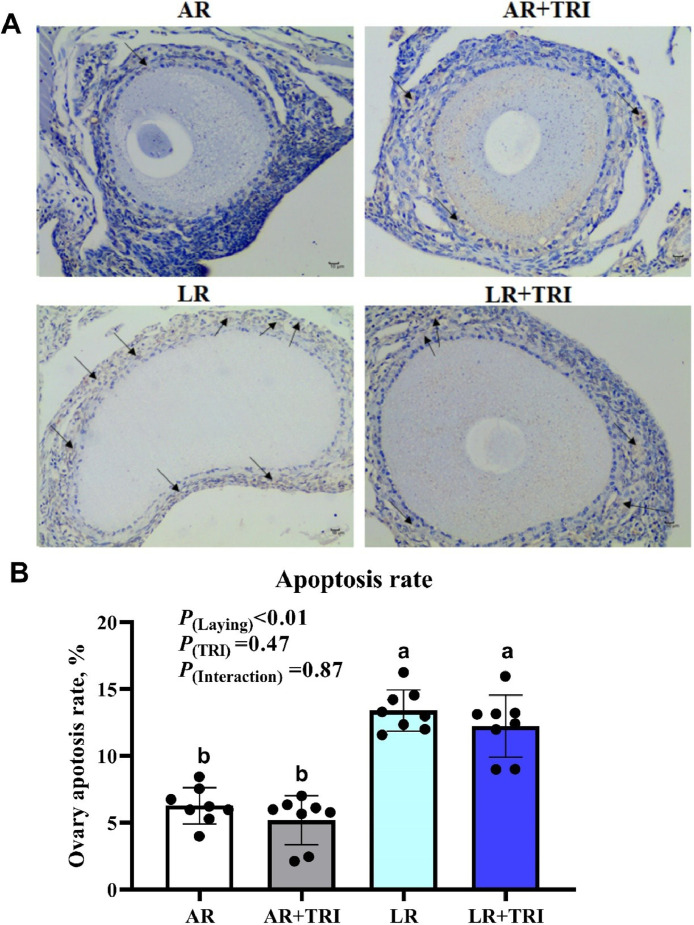
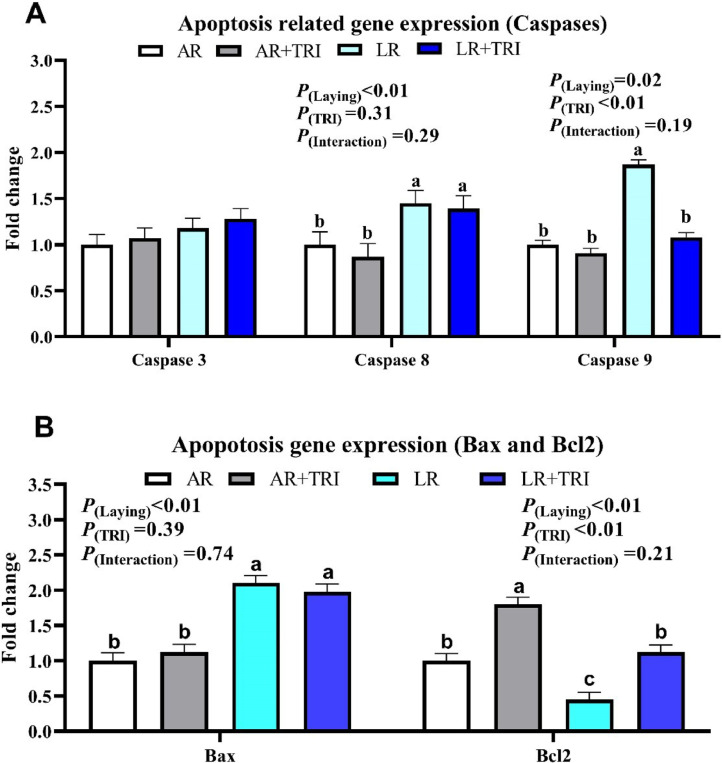
Ovary Apoptosis Rate and Apoptosis Related Gene Expression
As presented in Figure 1, Figure 2, LR breeders had higher cell apoptosis rate and u-regulated the proapoptosis related gene expression (caspase 8, 9 and Bax) in the ovary as compared with the AR breeders, while antiapoptosis related gene expression (Bcl-2) were also lower in LR breeders (P(laying) < 0.05). Dietary supplementation with tributyrin decreased the cell apoptosis rate and downregulated caspase 9 expression in LR breeders (P(Interaction) < 0.05), upregulated the Bcl-2 expression in both 2 breeders (P(TRI) < 0.05). The caspase 3 gene expression was not altered by experimental treatment (P > 0.05).
Figure 1.
The effect of a tributyrin on ovary apoptosis of broiler breeders with different egg-laying rate. Each means represents 1 layers/replicate, 8 replicates/treatment. (A) Ovary apoptosis (TUNEL). Apoptotic color is light yellow or brown yellow (as shown by black arrow), negative expression is blue with white background. (B) Ovary apoptosis rate. Abbreviations: AR, average egg laying rate; LR, low egg laying rate; TRI, 1,000 mg/kg tributyrin.
Figure 2.
The effect of tributyrin on ovary apoptosis related gene expression of broiler breeders with different egg-laying rate. Each means represents 1 layer/replicate, 8 replicates/treatment. (A) Caspase 3, caspase 8, caspase 9 expression; (B) Bax and Bcl2 expression. Abbreviations: AR, average egg laying rate; LR, low egg laying rate; TRI, 1,000 mg/kg tributyrin.
DISCUSSION
Broiler breeders often experience a reduction in their reproductive performance, especially as they become older or under stress. In our study, we found that AR breeders has higher egg laying rate and feed efficiency than that of LR breeders, which is in consistent with our previous study (Zhao et al., 2019; Yang et al., 2020; Wang et al., 2021) and other studies (Tallentire et al., 2016); however, the specific reason is not elucidated. In the current study, we found that dietary supplementation with tributyrin led to an increase in reproductive performance (egg weight and feed efficiency) and albumen quality (albumen height and HU), and the improving effect on albumen quality was found only in AR breeders, which is in accordance with the result of our previous study (Zhao et al., 2019). The intestine health and microbiota balance are important for nutrient digestion, absorption and utilization in poultry. Breeders with higher productive performance were found to have lower crypt depth and higher villus/crypt ratio (Zhao et al., 2019). Butyric acid has been shown to play an important role in maintaining the integrity of the intestinal mucosa and to exert potent anti-inflammatory, antioxidative effects, moderate immune response and improve growth performance in broilers (Zhang et al., 2011; Jahanian and Golshadi, 2015; Kaczmarek et al., 2016; Zou et al., 2019; Elnesr et al.,2020). The positive effect of tributyrin on egg weight and feed efficiency could be attributed to enhanced breeders’ intestinal health and improved gastrointestinal functionality. Similarly, Feng et al. (2021) also shown that supplementation of monobutyrin at 250 mg/kg level increased egg weight, but had no effect on egg production rate and feed conversion rate in broiler breeders. Furthermore, interestingly, the improving effect of tributyrin on albumen quality is only observed in AR breeders in current study. The reason why the result in albumen quality in not consistent between LR and AR breeders is not clear. It has been found that the feed efficiency is higher in higher rate breeders, which may indicate that the nutrient utilization was more efficient in higher rate breeders (Yang et al., 2020; Wang et al., 2021). This may result in different egg quality and also led to differentiation when feed tributyrin on albumen quality. Since, we also found that the positive effect on egg weight and albumen quality of other dietary treatments (pectic oligosaccharide and Enterococcus feacium) was more pronounced in breeders with an average egg-laying rate (Zhao et al., 2019), this may indicate that it is worth to take the layer psychology status into account when use the dietary strategy.
Reproductive hormones, including estrogen, FSH and progesterone, play a critical role in follicle mutation and ovulation to regulate fertility of females and have long-term effects on metabolic homeostasis of body (Kabir et al., 2015; Gea et al., 2020). These serum reproductive hormones are closely related to the egg production rate of broiler breeders, in this experiment we found that the serum AMH levels were lower in LR breeders, and the addition of tributyrin in the diet has no significant effect on serum reproductive hormones concentration. The AMH was mainly secreted by growing follicles, and it is a marker of ovarian reserve and aging (Visser et al., 2007). Therefore, this may serve as an explanation for the lower egg laying rate of LR breeders.
Ovary is the main regulator for female reproductive function, as it regulates follicle development and reproductive hormones secretion and produces mature oocytes (Regan et al., 2018). Growing evidence demonstrates that oxidative stress damage has been considered to be the mechanism of ovarian aging, and several antioxidants have been used to delay ovarian aging (Liu et al., 2018; Yang et al., 2019). MDA is well known as a marker of lipid peroxidation and antioxidant status, and it was also estimated in patients with ovarian cancer (White et al., 2014). Members of the enzymatic antioxidant system, such as SOD, CAT, GSH-ST, and GPx, play important roles in protecting the organism from oxidative damage (Mishra and Jha, 2019). Supportably, our results suggest that LR breeders had lower antioxidant capacity in ovary, as indicated by high levels of MDA and lower SOD activity, which may also explain the lower reproduction performance (egg laying rate and feed efficiency) in this study. As shown in our current study, supplementation with tributyrin improved antioxidant capacity in LR breeders’ ovary and magnum this may also be the possible reason for improving effect of tributyrin on albumen quality. It has been previously demonstrated that sodium butyrate had antioxidative effect in the colon of healthy humans (Hamer et al., 2009) and also alleviated oxidative stress induced by lipopolysaccharide in the intestinal cells (Russo et al., 2012). Lin et al. (2014) found that sodium butyrate improved reproductive performance and changing the gene expression involved in radical scavenging in rats. Liu et al. (2021) also shown that sodium butyrate supplementation improved growth performance and antioxidant function (increase glutathione peroxidase activity) in pre-weaned dairy calves. Similarly, previous studies have shown that sodium butyrate supplementation increased SOD activity and reduced the MDA level of broilers (Zhang et al., 2011; Wu et al., 2016; Lan et al., 2020) and breeder rooster (Alhaj et al., 2018).
Apoptosis in the granulosa cells was closely associated with the dominant follicle selection and follicular atresia (Johnson, 2012; Regan et al., 2018). In current study, we observed that the cell apoptosis rate was higher in ovary of low reproductive performance breeders. This result is in consistent with our previous studies, where we found that breeders with higher egg laying rate had lower ovarian cell apoptosis rate (Yang et al., 2020; Wang et al., 2021). In fact, the balance and imbalance of apoptosis and Bax/Bcl-2 affect the structure and function of the ovary (Hussein, 2005). In our study, the relative expression of proapoptosis factors (caspases 8, caspase 9, Bax) in the ovary of AP breeders was lower than that of LR breeders, suggesting that the number of atretic follicles in the AP breeders might have been lower than that of LP breeders; while this hypothesis needs to be confirmed in future studies, it could provide an explanation for the different egg laying rate between the 2 groups of breeders. Furthermore, we also found dietary supplementation with tributyrin downregulated the mRNA expression of caspase 9 and upregulated Bcl-2 expression in both AR and LR breeders. In accordance with our result, Lin et al. (2014) found that butyrate supplementation increases Bcl2 expression in rats, resulting in higher Bcl-2/Bax ratio. However, the literature about butyric acid or its derivatives in ovary function of poultry is not well elucidated, which needed to be explored in future study.
CONCLUSIONS
These findings suggest that the breeders with lower egg laying rate also characterized by deteriorate ovary function indicated by lower antioxidant capacity and higher cell apoptosis. Dietary supplementation with tributyrin increased egg albumen quality, decreased the ovary apoptosis related gene expression to improve reproductive tract function; and the positive effect on egg albumen quality is more pronounced in average reproductive breeders.
ACKNOWLEDGMENTS
We are grateful for the following projects, National Natural Science Foundation of China (31872792, 31402031), and Sichuan Provincial Science and Technology Projects (Grant No. 2019YFH0062, 2018NZ20009) for financial support. This study was partially supported by the 111 Project too.
DISCLOSURES
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
REFERENCES
- Alhaj H.W., Li Z., Shan T., Dai P., Zhu P., Li Y., Alsiddig M.A., Abdelghani E., Li C. Effects of dietary sodium butyrate on reproduction in adult breeder roosters. Anim. Reprod. Sci. 2018;196:111–119. doi: 10.1016/j.anireprosci.2018.07.002. [DOI] [PubMed] [Google Scholar]
- Bedford A., Gong J. Implication of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018;5:151–159. doi: 10.1016/j.aninu.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford A., Yu H., Squires E.J., Leeson S., Gong J. Effects of supplementation level and feeding schedule of butyrate glycerides on the growth performance and carcass composition of broiler chickens. Poult. Sci. 2017;96:3221–3228. doi: 10.3382/ps/pex098. [DOI] [PubMed] [Google Scholar]
- Czerwiński J., HØjberg O., Smulikowska S., Engberg R.M., Mieczkowska A. Effects of sodium butyrate and salinomycin upon intestinal microbiota, mucosal morphology and performance of broiler chickens. Arch. Anim. Nutr. 2012;66:102–116. doi: 10.1080/1745039x.2012.663668. [DOI] [PubMed] [Google Scholar]
- Devine P.J., Perreault S.D., Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol. Reprod. 2012;86:27. doi: 10.1095/biolreprod.111.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnesr S.S., Alagawany M., Elwan H.A.M., Fathi M.A., Farag M.R. Effect of sodium butyrate on intestinal health of poultry-a review. J. Anim. Sci. 2020;20:29–41. [Google Scholar]
- Feng X., Kong X., Zheng L., Qi Q., Long L., Gong L., Huang W., Zhang H. Effects of monobutyrin supplementation on egg production, biochemical indexes, and gut microbiota of broiler breeders. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gea M., Toso A., Schilirȯ T. Estrogenic activity of biological samples as a biomarker. Sci. Total Environ. 2020;740 doi: 10.1016/j.scitotenv.2020.140050. [DOI] [PubMed] [Google Scholar]
- Guo W., Liu J., Sun J., Gong Q., Ma H., Kan X., Cao Y., Wang J., Fu S. Butyrate alleviates oxidative stress by regulating NRF2 nuclear accumulation and H3K9/14 acetylation via GPR109A in bovine mammary epithelial cells and mammary glands. Free Radical Bio. Med. 2020;152:728–742. doi: 10.1016/j.freeradbiomed.2020.01.016. [DOI] [PubMed] [Google Scholar]
- Hamer H.M., Jonkers D.M.A.E., Bast A., Vanhoutvin S.A.L.W., Fischer M.A.J.G., Kodde A., Troost F.J., Venema K., Brummer R.J.M. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin. Nutr. 2009;28:88–93. doi: 10.1016/j.clnu.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Hussein M.R. Apoptosis in the ovary: molecular mechanisms. Hum. Reprod. Update. 2005;11:162–177. doi: 10.1093/humupd/dmi001. [DOI] [PubMed] [Google Scholar]
- Jahanian R., Golshadi M. Effect of dietary supplementation of butyric acid glycerides on performance, immunological responses, ileal microflora, and nutrient digestibility in laying hens fed different basal diets. Livest. Sci. 2015;178:228–236. [Google Scholar]
- Johnson P.A. Follicle selection in avian ovary. Reprod. Domest. Anim. 2012;47:283–287. doi: 10.1111/j.1439-0531.2012.02087.x. [DOI] [PubMed] [Google Scholar]
- Kabir E.R., Rahman M.S., Rahman I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Phar. 2015;40:241–258. doi: 10.1016/j.etap.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Kaczmarek S.A., Barri A., Hejdysz M., Rutkowski A. Effect of different doses of coated butyric acid on growth performance and energy utilization in broilers. Poult. Sci. 2016;95:851–859. doi: 10.3382/ps/pev382. [DOI] [PubMed] [Google Scholar]
- Kaipia A., Hsueh A.J.W. Regulation of ovarian follicle atresia. Annu. Rev. Physiol. 1997;59:349–363. doi: 10.1146/annurev.physiol.59.1.349. [DOI] [PubMed] [Google Scholar]
- Lan R., Zhao Z., Li S., An L. Sodium butyrate as an effective feed additive to improve performance, liver function, and meat quality in broiler under hot climatic conditions. Poult. Sci. 2020;99:5491–5500. doi: 10.1016/j.psj.2020.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Fang Z.F., Che L.Q., Xu S.Y., Wu D., Wu C.M., Wu X.Q. Use of sodium butyrate as an alternative to dietary fiber: effects on the embryonic development and anti-oxidative capacity of rats. PLoS One. 2014;9:e97838. doi: 10.1371/journal.pone.0097838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Liu X., Mi Y., Li J., Zhang C. Grape seed proanthocyanidin extract prevents ovarian aging by inhibiting oxidative stress in hens. Oxid. Med. Cell Longev. 2018;2018 doi: 10.1155/2018/9390810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., La A.L.T.Z., Evans A., Gao S., Yu Z., Bu D., Ma L. Supplementation with sodium butyrate improves growth and antioxidant function in dairy calves before weaning. J. Anim. Sci. Biotechnol. 2021;12:2. doi: 10.1186/s40104-020-00521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon M.A., Dai H., Wang Y., Xu T., Aabdin Z.U., Bilal M.S., Chandra R.A., Shen X. Efficacy of sodium butyrate in alleviating mammary oxidative stress induced by sub-acute ruminal acidosis in lactating goats. Microb. Pathogen. 2019;137 doi: 10.1016/j.micpath.2019.103781. [DOI] [PubMed] [Google Scholar]
- Mishra B., Jha R. Oxidative stress in the poultry gut: potential challenges and interventions. Front. Vet. Sci. 2019;6:66. doi: 10.3389/fvets.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . Nat’l Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. 9th rev. ed. [Google Scholar]
- Peters A.E., Mihalas B., Bromfield E.G., Roman S.D., Nixon B., Sutherland J.M. Autophagy in female fertility: a role in oxidative stress and aging. Antioxid. Redox Sign. 2020;32:550–568. doi: 10.1089/ars.2019.7986. [DOI] [PubMed] [Google Scholar]
- Regan S.L.P., Knight P.G., Yovich J.L., Stanger J.D., Leung Y., Arfuso F., Almahbobi G., Dharmarajan A. The effect of ovarian reserve and receptor signalling on granulosa cell apoptosis during human follicle development. Mol. Cell. Endocrinol. 2018;470:219–227. doi: 10.1016/j.mce.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Russo I., Lucianl A., De Cicco P., Troncone E., Ciacci C. Butyrate attenuates lipopolysaccharide-induced inflammantion in intestinal cells and Crohn's mucosa through modulation of antioxidant defense machinery. PLoS One. 2012;7:e32841. doi: 10.1371/journal.pone.0032841. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rozenboim I., Tako E., Gal-Garber O., Proudman J.A., Uni Z. The effect of heat stress on ovarian function of laying hens. Poult. Sci. 2007;86:1760–1765. doi: 10.1093/ps/86.8.1760. [DOI] [PubMed] [Google Scholar]
- Sengupta S., Muir J.G., Gibson P.R. Does butyrate protect from colorectal cancer. J. Gastrogenterol. Hepatol. 2006;21:209–218. doi: 10.1111/j.1440-1746.2006.04213.x. [DOI] [PubMed] [Google Scholar]
- Tan J., Mckenzie C., Potamitis M., Thorburn A.N., Mackay C.R., Macia L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014;121:91–118. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- Tallentire C.W., Leinonen I., Kyriazakis I. Breeding for efficiency in the broiler chicken: a review. Agron. Sustain. Dev. 2016;36:66. [Google Scholar]
- Tatone C. Oocyte senescence: a firm link to age-related female subfertility. Gynecol. Endocrinol. 2008;24:59–63. doi: 10.1080/09513590701733504. [DOI] [PubMed] [Google Scholar]
- Vinolo M.A.R., Rodrigues H.G., Nachbar R.T., Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser J.A., Durlinger A.L.L., Peters I.J.J., van den Heuvel E.R., Rose U.M., Lramer P., de Jong F.H., Themmen A.P.N. Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Müllerian hormone null mice. Endocrinol. 2007;148:2301–2308. doi: 10.1210/en.2006-1265. [DOI] [PubMed] [Google Scholar]
- Wang J., Wan C., Zhao S., Yang Z., Celi P., Ding X., Bai S., Zeng Q., Mao X., Xu S., Zhang K., Li M. Differential analysis of gut microbiota and effect of dietary Enterococcus feacium supplementation in broiler breeders with high or low laying performance. Poult. Sci. 2021;100:1109–1119. doi: 10.1016/j.psj.2020.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Qian X., Gao Q., Lv C., Xu J., Jin H., Zhu H. Quercetin increases the antioxidant capacity of the ovary in menopausal rats and in ovarian granulosa cell culture in vitro. J. Ovarian Res. 2018;11:51. doi: 10.1186/s13048-018-0421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M., J. Cohen, C. Hummel, R. Burky, and A. Cruz. 2014. Chapter 5 - The role of oxidative stress in ovarian cancer: implications for the treatment of patients. Pages 41–50 in Cancer, Oxidative Stress and Dietary Antioxidants. Elsevier Inc. Amsterdam, Netherland.
- Wu Y., Zhou Y., Lu C.H., Ahmad H., Zhang H., He J., Zhang L., Wang T. Influence of butyrate loaded clinoptilolite dietary supplementation on growth performance, development of intestine and antioxidant capacity in broiler chickens. PLoS One. 2016;11:1–15. doi: 10.1371/journal.pone.0154410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yin F., Yang Y., Lepp D., Yu H., Ruan Z., Yang C., Yin Y., Hou Y., Leeson S., Gong J. Dietary butyrate glycerides modulate intestinal microbiota composition and serum metabolites in broilers. Sci. Rep. 2018;8:4940. doi: 10.1038/s41598-018-22565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Cheung H.H., Zhang C., Wu J., Chan W.Y. Melatonin as potential targets for delaying ovarian aging. Curr. Drug Targets. 2019;20:16–28. doi: 10.2174/1389450119666180828144843. [DOI] [PubMed] [Google Scholar]
- Yang Z., Zhang C., Wang J., Celi P., Ding X., Bai S., Zeng Q., Mao X., Zhuo Y., Xu S., Yan H., Zhang K., Shan Z. Characterization of the intestinal microbiota of broiler breeders with different egg laying rate. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.599337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.B., Xu Y.X., Liu H.L., Pan Z.X. MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis. Reprod. Biol. Endocrin. 2019;17:9–20. doi: 10.1186/s12958-018-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.H., Jiang Y., Zhu Q.F., Gao F., Dai S.F., Chen J., Zhou G.H. Sodium butyrate maintains growth performance by regulating the immune response in broiler chickens. Br. Poult. Sci. 2011;52:292–301. doi: 10.1080/00071668.2011.578121. [DOI] [PubMed] [Google Scholar]
- Zhao S.J., Zhang K.Y., Ding X.M., Celi P., Yan L., Bai S.P., Zeng Q.F., Mao X.B., Xu S.Y., Wang J.P. The impact of dietary supplementation of different feed additives on performances of broiler breeders characterized by different egg-laying rate. Poult. Sci. 2019;98:6091–6099. doi: 10.3382/ps/pez316. [DOI] [PubMed] [Google Scholar]
- Zou X., Ji J., Qu H., Wang J., Shu D.M., Wang Y., Liu T.F., Li Y., Luo C.L. Effects of sodium butyrate on intestinal health and gut microbiota composition during intestinal inflammation progression in broilers. Poult. Sci. 2019;98:4449–4456. doi: 10.3382/ps/pez279. [DOI] [PubMed] [Google Scholar]