Abstract
Purpose
Wavelength and temporal frequency have been found to influence refractive development. This study investigated whether retinal dopamine (DA) plays a role in these processes.
Methods
Guinea pigs were randomly divided into nine groups that received different lighting conditions for 4 weeks, as follows: white, green, or blue light at 0, 0.5, or 20.0 Hz. Refractions and axial lengths were measured using streak retinoscopy and A-scan ultrasound imaging. DA and its metabolites were measured by high-pressure liquid chromatography-electrochemical detection.
Results
At 0 Hz, green and blue light produced myopic and hyperopic shifts compared with that of white light. At 0.5 Hz, no significant changes were observed compared with those of green or blue light at 0 Hz, whereas white light at 0.5 Hz induced a myopic shift compared with white light at 0 or 20 Hz. At 20 Hz, green and blue light acted like white light. Among all levels of DA and its metabolites, only vitreous 3, 4-dihydroxyphenylacetic acid (DOPAC) levels and retinal DOPAC/DA ratios were dependent on wavelength, frequency, and their interaction. Specifically, retinal DOPAC/DA ratios were positively correlated with refractions in white and green light conditions. However, blue light (0, 0.5, and 20.0 Hz) produced hyperopic shifts but decreased vitreous DOPAC levels and retinal DOPAC/DA ratios.
Conclusions
The retinal DOPAC/DA ratio, indicating the metabolic efficiency of DA, is correlated with ocular growth. It may underlie myopic shifts from light exposure with a long wavelength and low temporal frequency. However, different biochemical pathways may contribute to the hyperopic shifts from short wavelength light.
Keywords: myopia, wavelength, temporal, dopamine, guinea pigs
Retinal dopamine (DA) plays a pivotal role in modulating refractive development.1–6 As a chemical signal of retinal photoadaptation, retinal DA release and turnover have been shown to be regulated by light in several vertebrates.7–11 Bright light and flickering light can stimulate the ON pathway and intrinsically photosensitive retinal ganglion cells, as well as alter DA synthesis and release.12–14 Inspired by recent epidemiologic studies showing that the outdoor environment plays an important role in the process of refractive development,15–20 a number of experiments with animal models have shown a protective effect of outdoor light on myopic development, possibly via DA signaling.21–26
The spectral composition of light is an important cue that influences refractive development. Studies in fish,27,28 chicks,29,30 guinea pigs,31,32 tree shrews,33,34 and monkeys35,36 have demonstrated that the eye can use wavelength defocus to regulate refractive development. In our previous studies, we have found that guinea pigs use wavelength defocus to determine the sign of defocus, producing increased eye growth under green light and decreased eye growth under blue light.31 Recently, we found that a 20-Hz flickering light flattens the wavelength–defocus effect, making blue and green light act like white light. This flattening effect induces myopic shifts in guinea pigs exposed to blue light and hyperopic shifts in those exposed to green light, as compared with those of corresponding steady conditions.37 In particular, we were interested in whether retinal DA plays a role in these light responses.
The literature on the effects of wavelength on retinal DA in refractive development is fairly limited. Rucker et al.38–40 found that the eyes of chicks exposed to color flicker grew faster, whereas those exposed to luminance flicker grew slower, which were later reported to be linked to retinal DA receptor activity.41 Wang et al.42 investigated how retinal DA depends on the spectrum of light in chicks. However, they found that a comparison of absolute levels of DA and its metabolites had limited value because of high interindividual and interbatch variability. Recently, Strickland et al.43 found that violet light inhibited lens-induced myopia compared with that of white light in mice, but did not change levels of DA and its metabolites. Collectively, these findings indicate that the role of retinal DA in refractive changes caused by different wavelengths of light remains unclear.
DA release from retinal neurons is known to be stimulated by intermittent illumination.44–46 Flickering light stimulates increased DA synthesis and release compared with that from constant darkness.47–49 High temporal frequency light has been linked to restoring retinal DA release and inhibiting the progression of form-deprivation myopia.50,51 Schwahn and Schaeffel52 found that 12- or 6-Hz light inhibited not only form-deprivation myopia and lens-induced myopia, but also inhibited hyperopia induced by positive lenses. Except for experimental ametropia, studies in cats,53 mice,54 guinea pigs,37,55,56 and chicks51,52,57 have reported that low temporal frequency stimuli produce myopic shifts, whereas high temporal frequency stimuli produce hyperopic shifts. However, further investigation is required to clarify the role of retinal DA in these temporal growth responses.
In the present study, to determine the role of retinal DA in refractive shifts after light exposure at different wavelengths and temporal frequencies, we measured levels of DA and its metabolites based on our previously published study.37 We hypothesized that myopic shifts induced from light with a low temporal frequency and long wavelength would be associated with a lower retinal DA release and turnover, and that the inhibition of myopic shifts by light with a high temporal frequency would be associated with greater retinal DA release and turnover. We also hypothesized that hyperopic shifts by light with a short wavelength would be associated with higher retinal DA release and turnover. The DA-related levels that we measured were as follows: retinal DA, retinal 3,4-dihydroxyphenylacetic acid (DOPAC), retinal DOPAC/DA ratio (an indicator of retinal DA turnover58), and vitreous DOPAC (an index of retinal DA release59).
Methods
Animals and Lighting
Guinea pigs (pigmented, 14 days old, obtained from our laboratory at Fudan University) were divided into nine groups that received different lighting conditions for 4 weeks as follows: white, green, or blue light at 0, 0.5, or 20.0 Hz. The treatment and care of all animals were conducted according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Three types of light-emitting diodes (LEDs: 24 V, 390 Ω, SMD-2835; Rui Gao Xiang Light & Electronic Co. Shanghai, China) were installed: white (color temperature: 5000 K), green (peak value: 530 nm, one-half bandwidth: 30 nm), and blue (peak value: 440 nm, one-half bandwidth: 20 nm). The intensity of the illumination was tested using a luminometer (SMART-SENSOR AR823, China). The irradiance was calibrated by an IL1700 Research Radiometer (International Light Inc, Peabody, MA). Each group was controlled at 500 lux (10.47*10−4 W/cm2, 8.51*10−4 W/cm2, and 13.32*10−4 W/cm2 for white, green, and blue light, respectively). Temporal frequency was controlled by a temporal luminance modulation (Yinuo Automation Co. LTD, Changsha, China; 0.5 Hz: 1-second bright and 1-second dark; 20 Hz: 0.025-second bright and 0.025-second dark). The waveform was a square wave, since square waves are more likely than sinusoidal waves to induce myopia in guinea pigs.55 Illumination followed a 12/12-hour light/dark cycle (on at 6:00 am and off at 6:00 pm).
Optical Measurements
Before experiments and after light exposure for 4 weeks, refraction and axial lengths were measured by streak retinoscopy and A-scan ultrasound imaging (11 MHz; Hiscan A/B, Optikon, Renton, WA), respectively, as previously described.37 All guinea pigs were measured at the same time of day (8:00 am) without lid retractors. Refraction results from three times were averaged for each eye. The axial length measurements were made from the anterior cornea to the retina. Axial length results from 10 readings were averaged for each eye. The light condition was dark during each measurement. Because small eye artifacts60 in this experiment were not removed, the refractions that we measured may have not been entirely accurate with the absolute refractions. However, these small eye artifacts likely did not affect our comparisons between groups.
High-Pressure Liquid Chromatography-Electrochemical Detection
After light exposure for 4 weeks, DA-related levels were analyzed by high-pressure liquid chromatography-electrochemical detection.61–64 All guinea pigs were sacrificed at the same time of day (8:00 am). The whole vitreous and retina from each eye were carefully dissected on ice under a microscope. We carefully dissected away the RPE from the retina and obtained the transparent retina without pigment. Each sample was homogenized in phosphate-buffered saline containing 0.1 mM of ethylenediaminetetra-acetic acid (EDTA). The homogenates were centrifuged at 6000 rpm for 10 minutes at 4 °C. The supernatants were combined with an equal volume of perchloric acid (0.3 M). The samples were centrifuged at 20,000 rpm for 10 minutes at 4 °C, after which the supernatants were collected and assayed.
High-pressure liquid chromatography-electrochemical detection was carried out at the Neuron Science Institute at the Chinese Academy of Sciences, Shanghai. The investigators carrying out HPLC were blinded to the experimental groupings. The HPLC system that we used was equipped with an electrochemical detector (Coulochem III, ESA Biosciences Inc, Chelmsford, MA), model 5041 analytical cell, and an ESA 584 HPLC pump (ESA Biosciences Inc). The column (Eclipse Plus C18, 3.5 µm, 2.1*150 mm; Agilent Technologies, Santa Clara, CA) was kept at 35 °C with a column heater. The mobile phase consisted of 100 mM of NaH2PO4, 50 mM of citric acid buffer (pH 3.0, adjusted with sodium hydroxide), 200 mg/L of sodium 1-octanesulfonate, 7% to 10% methanol, and 50 µM of EDTA. The flow rate of the mobile phase was 0.2 mL⁄min. Twenty microliters of the sample were injected onto the column. The data were collected and analyzed by a Chromeleon chromatography workstation (Thermo Fisher Scientific, Waltham, MA).
Statistical Analysis
In this study, data were collected from both eyes of each guinea pig. We presented the data as the average of the right and left eyes of each guinea pig. A two-way ANOVA (factors: wavelength and frequency) was performed, followed by post hoc tests using Tukey's honestly significant difference (HSD). The correlations between DA-related levels and refractions, and the correlations between irradiance and DA-related levels were analyzed by linear correlation analysis. A two-tailed P value of less than 0.05 indicated statistical significance.
Results
Table shows results from the nine lighting conditions: white, green, or blue light at 0, 0.5, or 20 Hz. The number of guinea pigs in each condition is shown as (N). The ocular components are reported as refractions and axial lengths, while DA-related levels are reported as retinal DA levels, retinal DOPAC levels, retinal DOPAC/DA ratios, and vitreous DOPAC levels.
Table.
Refractive Development and DA Metabolism (Mean ± SEM)
| Retina | ||||||||
|---|---|---|---|---|---|---|---|---|
| Wavelength | Frequency | N | Refraction D | Axial Length mm | DOPAC (pg/mg) | DA (pg/mg) | DOPAC/DA | Vitreous DOPAC (pg/µL) |
| White | 0 Hz | 15 | 4.48 ± 0.36 | 7.82 ± 0.06 | 87.36 ± 29.28 | 88.56 ± 32.42 | 1 ± 0.07 | 35.28 ± 5.72 |
| 0.5 Hz | 12 | 3.2 ± 0.47 | 8.02 ± 0.07 | 96.13 ± 30.59 | 111.20 ± 31.48 | 0.86 ± 0.06 | 29.88 ± 4.61 | |
| 20 Hz | 12 | 4.58 ± 0.40 | 7.84 ± 0.07 | 85.16 ± 20.52 | 78.55 ± 17.02 | 1.10 ± 0.09 | 26.29 ± 1.48 | |
| Green | 0 Hz | 11 | 3.58 ± 0.33 | 7.98 ± 0.05 | 87.12 ± 16.77 | 98.57 ± 21.44 | 0.89 ± 0.08 | 22.74 ± 3.01 |
| 0.5 Hz | 13 | 3.28 ± 0.54 | 8.00 ± 0.06 | 99.20 ± 14.37 | 115.05 ± 16.19 | 0.87 ± 0.05 | 32.00 ± 6.77 | |
| 20 Hz | 12 | 4.53 ± 0.49 | 7.85 ± 0.07 | 108.15 ± 21.28 | 109.52 ± 24.34 | 1 ± 0.10 | 26.47 ± 4.37 | |
| Blue | 0 Hz | 13 | 5.42 ± 0.68 | 7.73 ± 0.08 | 84.58 ± 9.15 | 102.96 ± 16.38 | 0.83 ± 0.08 | 27.44 ± 6.70 |
| 0.5 Hz | 11 | 5.5 ± 0.61 | 7.74 ± 0.08 | 93.22 ± 19.19 | 121.83 ± 22.96 | 0.77 ± 0.05 | 27.98 ± 6.73 | |
| 20 Hz | 12 | 5.06 ± 0.66 | 7.79 ± 0.06 | 89.39 ± 24.62 | 130.60 ± 28.71 | 0.69 ± 0.04 | 23.37 ± 5.17 | |
Refractions and Axial Lengths
Before the 4-week light exposure, there were no significant differences among the nine groups in terms of refraction or axial length (P > 0.05, all). After 4 weeks of light exposure, refractions and axial lengths were both dependent on wavelength (ANOVA, P < 0.001, both), frequency (ANOVA, P < 0.001, both), and their interaction (ANOVA, P < 0.001, both).
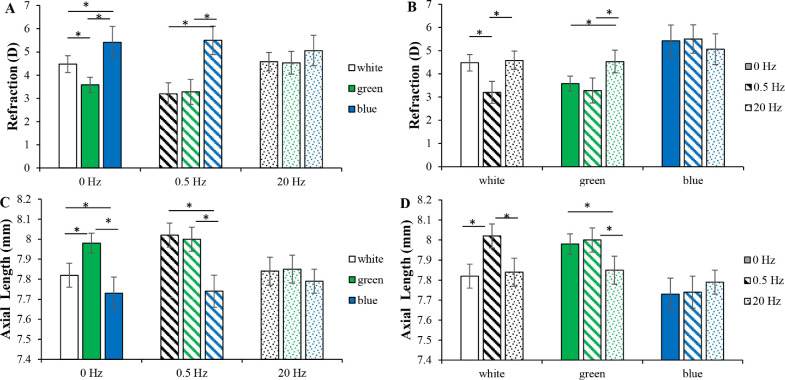
Figures 1A and B show the absolute values of refractions at week 4. At 0 Hz, green and blue light produced myopic and hyperopic shifts compared with that of white light (Tukey's HSD, P < 0.001, both). At 0.5 Hz, no significant differences were observed compared with those of green or blue light at 0 Hz (Tukey's HSD, P = 0.219 and P = 0.752, respectively), whereas white light at 0.5 Hz induced a myopic shift compared with white light at 0 or 20 Hz (Tukey's HSD, P < 0.001, both). At 20 Hz, green and blue light acted like white light, with a hyperopic shift in green light at 20 Hz compared with green light at 0 or 0.5 Hz (Tukey's HSD, P < 0.001, both). Blue light protected against temporal frequency-induced changes in refraction. However, there was a trend that refraction in blue light at 20 Hz was less hyperopic than blue light at 0 or 0.5 Hz (Tukey's HSD, P = 0.093 and P = 0.053, respectively).
Figure 1.
Refractions and axial lengths. (A) Refractions in different wavelengths at the same frequency conditions. At 0 Hz, green and blue light produced myopic and hyperopic shifts compared with that of white light. At 0.5 Hz, no significant changes were observed compared with those of green or blue light at 0 Hz. (B) Refractions at different frequencies in the same wavelength conditions. White light at 0.5 Hz induced a myopic shift compared with that of white light at 0 or 20 Hz. Green light at 20 Hz produced a more hyperopic shift than that of green light at 0 or 0.5 Hz. (C) Axial lengths in different wavelengths at the same frequency conditions. (D) Axial lengths at different frequencies in the same wavelength conditions. The trends in axial lengths were consistent with those of refractions across the lighting conditions. *P < 0.05. Error bars denote the standard errors of the mean (SEMs).
Figures 1C and D show the absolute values of axial lengths at week 4. At 0 Hz, axial length grew faster in green light and slower in blue light than that of white light (Tukey's HSD, P < 0.05, both). At 0.5 Hz, no significant differences were observed compared with those of green or blue light at 0 Hz (Tukey's HSD, P = 0.620 and P = 0.727, respectively), whereas axial length in white light at 0.5 Hz grew faster than that at 0 or 20 Hz (Tukey's HSD, P < 0.001, both). At 20 Hz, green and blue light acted like white light, with a shorter axial length in green light at 20 Hz than green light at 0 or 0.5 Hz (Tukey's HSD, P < 0.001, both). Blue light protected against temporal frequency-induced changes in axial length as well.
Retinal DA and DOPAC Levels
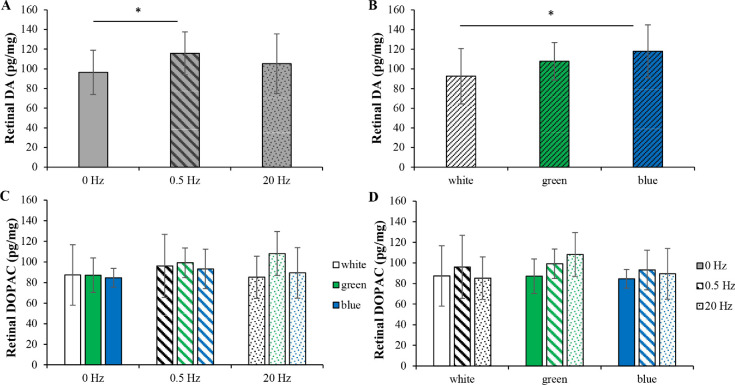
Figures 2A and B show the retinal DA levels across the nine conditions after 4 weeks of light exposure. Retinal DA levels were dependent on wavelength (ANOVA, P = 0.004) and frequency (ANOVA, P = 0.041), but not their interaction (ANOVA, P = 0.196). Figure 2A shows the data pooled across wavelengths. The retinal DA level was significantly higher at 0.5 Hz than at 0 Hz (Tukey's HSD, P = 0.030), whereas the retinal DA level was not significantly different between 20 and 0.5 Hz (Tukey's HSD, P = 0.348). Figure 2B shows the data pooled across frequencies. The retinal DA level in blue light was significantly higher than that in white light but not green light (Tukey's HSD, P = 0.004 and P = 0.388, respectively).
Figure 2.
Retinal DA and DOPAC levels. (A) Retinal DA levels as a function of frequency when the data were pooled across wavelengths. The retinal DA level was significantly higher at 0.5 Hz than at 0 Hz. However, the retinal DA level at 20 Hz was not significantly different from that at 0.5 Hz. (B) Retinal DA levels as a function of wavelength when the data were pooled across frequencies. The retinal DA level in blue light was significantly higher than that in white light but not green light. (C) Retinal DOPAC levels in different wavelengths at the same frequency conditions. (D) Retinal DOPAC levels at different frequencies in the same wavelength conditions. There were no significant differences in retinal DOPAC levels across the conditions. *P < 0.05. Error bars denote SEMs.
Figures 2C and D show the retinal DOPAC levels across the nine conditions after exposure to different light conditions for 4 weeks. Wavelength, frequency, as well as their interaction, had no effects on retinal DOPAC levels (ANOVA, P > 0.05, all).
Retinal DOPAC/DA Ratios
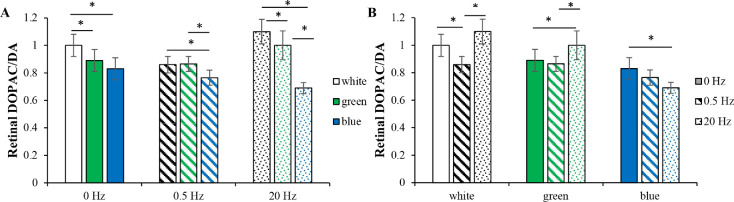
Figures 3A and B show the retinal DOPAC/DA ratios across the nine conditions after 4 weeks of light exposure. The ratios were dependent on wavelength (ANOVA, P < 0.001), frequency (ANOVA, P = 0.001), and their interaction (ANOVA, P < 0.001).
Figure 3.
Retinal DOPAC/DA ratios. (A) Retinal DOPAC/DA ratios in different wavelengths at the same frequency conditions. At 0- and 20-Hz conditions, the retinal DOPAC/DA ratios in green and blue light were both lower than those in white light. At 0.5 Hz, the retinal DOPAC/DA ratios in white and green light were similar. Across different temporal frequencies, there was a trend that retinal DOPAC/DA ratios in blue light were lower than those in white and green light. (B) Retinal DOPAC/DA ratios at different frequencies in the same wavelength conditions. In green and blue light, 0.5 Hz produced no significant differences compared with those at 0 Hz. However, white light at 0.5 Hz reduced the retinal DOPAC/DA ratio compared with white light at 0 or 20 Hz. In green light, 20 Hz increased the retinal DOPAC/DA ratio compared with that at 0 or 0.5 Hz. However, in blue light, 20 Hz decreased the retinal DOPAC/DA ratio compared with that at 0 Hz. *P < 0.05. Error bars denote SEMs.
Across different temporal frequencies, retinal DOPAC/DA ratios in green and blue light were both lower than those in white light, except at 0.5 Hz, at which the ratios in white and green light were similar (Tukey's HSD), steady (both P < 0.05), at 0.5 Hz, green vs white (P = 0.900) and blue vs white (P = 0.049), and at 20 Hz (both P < 0.05). The retinal DOPAC/DA ratios in blue light were lower than those of white and green light, except for blue vs green light at 0 Hz (Tukey's HSD, at 0 Hz blue vs white [P < 0.001], blue vs green [P = 0.185]; at 0.5 Hz [both P < 0.05], and at 20 Hz [both P < 0.05]). This phenomenon may have been due to increased retinal DA levels with unaffected retinal DOPAC levels in blue light groups (Fig. 3A).
In white light, 20 and 0 Hz each showed a higher retinal DOPAC/DA ratio than that at 0.5 Hz (Tukey's HSD; P = 0.003 and P < 0.001, respectively). In green light, 20 Hz showed a higher retinal DOPAC/DA ratio than that at 0 or 0.5 Hz (Tukey's HSD, P = 0.022 and P = 0.005, respectively). In blue light, 20 Hz exhibited a lower retinal DOPAC/DA ratio than that at 0 Hz (Tukey's HSD, P = 0.005). This phenomenon may have been due to increased retinal DA levels with unaffected retinal DOPAC levels in blue light at 20 Hz (Fig. 3B).
Vitreous DOPAC Levels
Figures 4A and B show the vitreous DOPAC levels across the nine conditions after 4 weeks of light exposure. Vitreous DOPAC levels were dependent on wavelength (ANOVA, P = 0.015), frequency (ANOVA, P = 0.012), and their interaction (ANOVA, P = 0.002).
Figure 4.
Vitreous DOPAC levels. (A) Vitreous DOPAC levels in different wavelengths at the same frequency conditions. At 0 Hz, vitreous DOPAC levels in green and blue light were both lower than that in white light. However, at 0.5 and 20 Hz, no significant changes were observed among white, green, and blue lights. (B) Vitreous DOPAC levels at different frequencies in the same wavelength conditions. Across different wavelengths, there was a trend that vitreous DOPAC levels at 20 Hz were lower than those at 0.5 Hz. In white light, 0.5 and 20 Hz both decreased the vitreous DOPAC levels compared with that at 0 Hz. In green light, 0.5 Hz increased the vitreous DOPAC level compared with that at 0 Hz. *P < 0.05. Error bars denote SEMs.
At 0 Hz, the vitreous DOPAC levels in green and blue light were both lower than that in white light (Tukey's HSD, P < 0.001 and P = 0.003, respectively). However, at 0.5 and 20 Hz, no significant changes were found among white, green, and blue lights (Tukey's HSD, P > 0.05, all). The vitreous DOPAC levels may be predominantly affected by temporal frequency. At the same frequency, the vitreous DOPAC levels in different wavelength were similar (Fig. 4A).
Across different wavelengths, there was a trend that vitreous DOPAC levels at 20 Hz were lower than those at 0.5 Hz, although this trend was not significant for white light (Tukey's HSD: white, P = 0.176; green, P = 0.039; and blue, P = 0.049). In white light, 0.5 and 20.0 Hz both had lower vitreous DOPAC levels than that at 0 Hz (Tukey's HSD, P = 0.038 and P < 0.001, respectively). In green light, the vitreous DOPAC level at 0 Hz was lower than that at 0.5 Hz (Tukey's HSD, P < 0.001) (Fig. 4B).
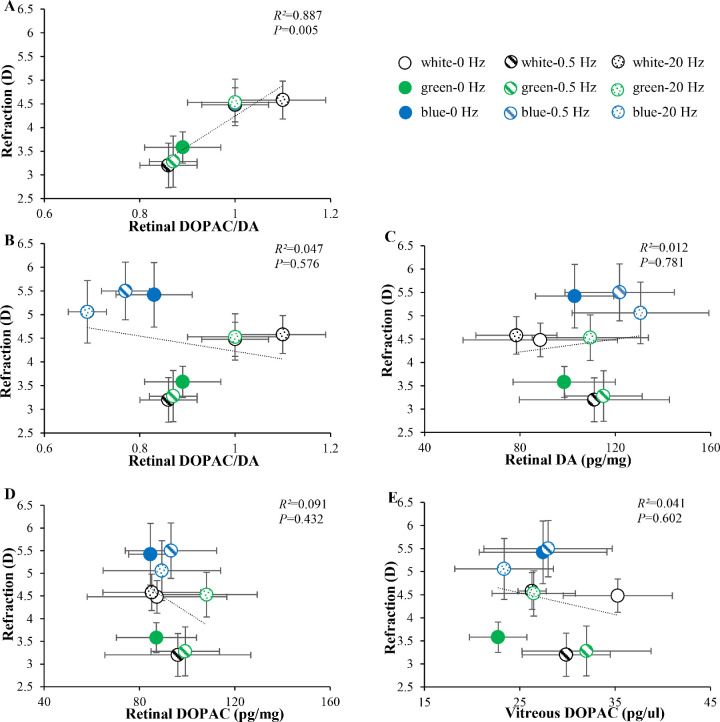
Relationship Between DA-Related Levels and Refractions
Figure 5 shows the relationship between DA-related levels and refractions. There was a significant positive correlation between the retinal DOPAC/DA ratios and refractions in white and green light (Fig. 5A, linear correlation analysis, R² = 0.887, P = 0.005). In white light, 0.5 Hz produced a myopic shift with lower retinal DOPAC/DA ratios than those at 0 and 20 Hz (Tukey's HSD, P < 0.05, both). Green light at 0 and 0.5 Hz produced myopic shifts accompanied by lower retinal DOPAC/DA ratios, while green light at 20 Hz inhibited myopic shifts, accompanied by higher retinal DOPAC/DA ratios (Tukey's HSD, P < 0.05, all). However, the three blue light groups (0, 0.5, and 20.0 Hz) were outliers in the correlation map (Fig. 5B), which produced the highest refractions but lowest retinal DOPAC/DA ratios.
Figure 5.
Relationship between DA-related levels and refractions. (A) Relationship between refractions vs retinal DOPAC/DA ratios in white and green light conditions. (B) Relationship between refractions vs retinal DOPAC/DA ratios across the nine conditions. (C–E) Relationships between refractions vs retinal DA levels, retinal DOPAC levels, and vitreous DOPAC levels across the nine conditions. *P < 0.05. Error bars denote SEMs.
We also investigated the correlations between refractions vs retinal DA levels, retinal DOPAC levels, and vitreous DOPAC levels; however, no significant correlations were found across the nine conditions (Figs. 5C–E).
Discussion
The present study created artificial environments composed of different wavelengths and temporal frequencies of light to explore the effects of each stimulus on retinal DA, as well as the relationship between DA-related levels and refraction. We found that, among the DA-related levels, the vitreous DOPAC level and retinal DOPAC/DA ratio played a critical role in ocular growth. The refractions in green and white light conditions were correlated positively with retinal DOPAC/DA ratios. Our findings supported the hypothesis that myopic shifts induced by light at a low temporal frequency and long wavelength were associated with lower retinal DOPAC/DA ratios. High temporal frequencies inhibited these myopic shifts with higher retinal DOPAC/DA ratios. However, hyperopic shifts induced by short wavelength light were associated with lower (rather than higher) retinal DOPAC/DA ratios.
Biomarkers of DA Metabolism in Refractive Development
A previous study has indicated that myopia is specifically associated with DA released from amacrine cells rather than the total DA content in the retina.65 Retinal DA levels only represent general stored content. DOPAC, the main metabolite of DA, is generally produced after DA has been released from dopaminergic neurons.59 Therefore, vitreous DOPAC levels can be used as a robust index of retinal DA release.59 The retinal DOPAC/DA ratio, an indicator of retinal DA turnover, can accurately represent the metabolic efficiency of DA.58 A decreased retinal DOPAC/DA ratio has been reported to be associated with an increased susceptibility to form-deprivation myopia in mice, with no changes in other DA-related levels.66–68
In the present study, retinal DA and retinal DOPAC levels were not correlated with refractions. In addition to interindividual variability, measurements of static retinal DA and retinal DOPAC levels may not be representative measures of the gain of dopaminergic signaling because of the balance between DA production and metabolism.69 After light exposure in our present study, we found that only retinal DOPAC/DA ratios (retinal DA turnover) and vitreous DOPAC levels (retinal DA release) were dependent on wavelength, frequency, and their interaction, in which the retinal DOPAC/DA ratio was found to be a more sensitive measure of retinal DA activity for reflecting refractive changes.
Narrowband Light
In the present study, we found that at 0 Hz, even though the refractive changes in green and blue light were in opposite directions, the retinal DOPAC/DA ratios and vitreous DOPAC levels were similar, being lower than those in white light. This trend persisted at 0.5 and 20 Hz in terms of retinal DOPAC/DA ratios. The lack of chromatic contrast between blue and green in monochromatic light, which is present in white light, may account for the lower retinal DOPAC/DA ratios and vitreous DOPAC levels.70–72 Retinal contrast is more blurred in blue light than in green light as a result of longitudinal chromatic aberration, which may explain why retinal DOPAC/DA ratios in blue light tended to be even lower than those in green light in our present study. As early as 1997, Ohngemach et al.65 proposed that retinal DA release may serve as a “blur detector.” If contrast decreases because of defocus or diffuser wear, the release of DA is reduced. They found a decrease in vitreous DOPAC levels after treatment with +7 diopter lenses in chicks. In 2019, Sarfare et al.73 found that whether diffusers or lenses of either sign were unilaterally worn in chicks, vitreous DOPAC levels were decreased compared with those in the contralateral eye.
If the blur detector theory is not the entire story, how does retinal DA underlie refractive errors from light exposure with different wavelengths? The myopic shifts induced in green light were associated with lower retinal DOPAC/DA ratios and vitreous DOPAC levels in the present study, which supports the hypothesis that myopic shifts induced by long wavelength light would be associated with lower retinal DA turnover and release. However, the hyperopic shifts induced in blue light were associated with higher retinal DA levels but lower retinal DOPAC/DA ratios and vitreous DOPAC levels. Consistent with our present results, Wang et al.42 found that blue light yielded less deprivation-induced myopia compared with white light in chicks. The retinal DA level in blue light was higher than that in white light, but the vitreous DOPAC level in blue light was lower than that in white light. Recently, Strickland et al.43 found that violet light induced hyperopia and inhibited lens-induced myopia compared with the effects in mice exposed to white light. However, there were no changes in the levels of DA or its metabolite.
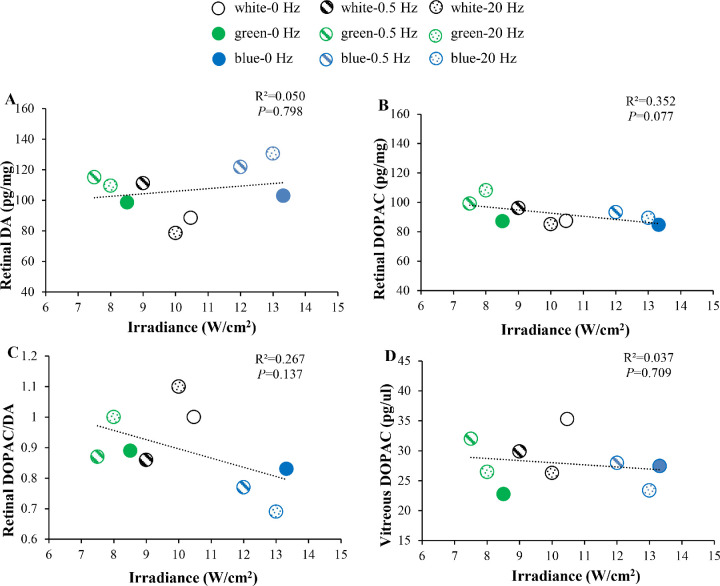
As mentioned in a previous review,74 it is possible that differences in DA-related levels between blue and green/white light are due to an increased irradiance in blue light. However, in our present study, we found that irradiance levels were not significantly correlated with retinal DA levels, retinal DOPAC levels, retinal DOPAC/DA ratios, or vitreous DOPAC levels (Fig. 6). Therefore, we suspect that the difference in DA-related levels between blue and green/white light in our present study may not have been due to an increase in irradiance. The higher retinal DA levels in blue light (Fig. 2A) may be due to the stimulation of intrinsically photosensitive retinal ganglion cells, which are spectrally tuned to short wavelength light.75 Further studies measuring levels of DA transporters and receptors may be useful to better explain retinal DA metabolism in blue light.
Figure 6.
Relationship between irradiance and DA-related levels. (A) Relationship between irradiance and retinal DA levels across the nine conditions. (B) Relationship between irradiance and retinal DOPAC levels across the nine conditions. (C) Relationship between irradiance and retinal DOPAC/DA ratios across the nine conditions. (D) Relationship between irradiance and vitreous DOPAC levels across the nine conditions. *P < 0.05. Error bars denote SEMs.
Temporal Frequency
The refractive results in the present study were consistent with previous studies showing that lighting at a low temporal frequency produces myopic shifts with longer axial lengths,53–57 whereas lighting at a high temporal frequency produces hyperopic shifts with shorter axial lengths57 or shorter anterior chambers.55
The role of retinal DA in these temporal growth responses need to be clarified. In the present study, we investigated the influence of temporal frequency on retinal DA in white light. Low temporal frequencies induced myopic shifts with lower retinal DOPAC/DA ratios and vitreous DOPAC levels compared with those at 0 Hz. High temporal frequencies induced the same refraction and retinal DOPAC/DA ratio as those at 0 Hz. Consistently, in green light, low temporal frequencies induced myopic shifts with low retinal DOPAC/DA ratios, whereas high temporal frequencies inhibited myopic shifts by recovering the retinal DOPAC/DA ratio. In blue light, the effects of different temporal frequencies on eye growth and DA-related levels were fairly weak. Only high temporal frequencies tended to inhibit hyperopic shifts with low retinal DOPAC/DA ratios and vitreous DOPAC levels.
It is interesting to note that the vitreous DOPAC level at a high temporal frequency was lower than that at other frequencies for all wavelengths (Fig. 4B). Luft et al.48 measured vitreous DOPAC levels in different temporal frequencies in chicks, but found no difference among 2, 5, 10, 15, or 20 Hz, except for a significant decrease at 20 Hz compared with that at 15 Hz. These results indicated that high temporal frequency stimulation could decrease the vitreous DOPAC level. However, it remains to be explored how high temporal frequencies induce lower vitreous DOPAC levels.
Possibility of the Retinal DOPAC/DA Ratio as a Final Common Pathway in Myopia
An important outcome in our present study was in whether retinal DA perfectly correlated with refractive changes across all lighting conditions. If it did, that would suggest that retinal DA is a part of a final common pathway. If it did not, that would indicate that different biochemical pathways may be involved in emmetropization. In our present study, retinal DOPAC/DA ratios and vitreous DOPAC levels were two sensitive measures indicating DA activity, in which retinal DOPAC/DA ratios were found to be positively correlated with refractions in white and green light conditions. The myopic shifts induced by light at a low temporal frequency and long wavelength were accompanied by lower retinal DOPAC/DA ratios. The inhibition of myopic shifts at high temporal frequencies were associated with higher retinal DOPAC/DA ratios. Therefore, we suggest that the retinal DOPAC/DA ratio may underlie myopic shifts in light with a long wavelength and low temporal frequency.
However, the hyperopic shift induced by short wavelength light decreased the retinal DOPAC/DA ratio and vitreous DOPAC level. High temporal frequency tended to further reduce these levels in blue light. These results suggested that retinal DA turnover and release cannot be used to predict the refractive development in blue light. Some studies have reported that agents like pirenzepine-a cholinergic antagonist,76 and neurotoxins against catecholaminergic neurons (6-OHDA, Reserpine)77 block only the development of myopia, not hyperopia. These observations are in line with the assumption that hyperopia involves different biochemical pathways.
Conclusions
Our findings demonstrate that the retinal DOPAC/DA ratio, indicating the metabolic efficiency of retinal DA, was correlated with ocular growth, which likely underlies myopic shifts from light exposure with a long wavelength and low temporal frequency. However, different biochemical pathways may be involved in the hyperopic shifts from short wavelength light exposure.
Acknowledgments
The authors thank LetPub (www.letpub.com) for its linguistic assistance and scientific consultation during the preparation of this article.
Supported by the Non-Profit Central Research Institute Fund of the Chinese Academy of Medical Sciences (2018PT32019), National Nature Science Foundation of China (82070997, 81770957, 81900901), and Shanghai Municipal Health Commission Program (201940485).
Disclosure: T. Tian, None; L. Zou, None; S. Wang, None; R. Liu, None; H. Liu, None
References
- 1.Dong F, Zhi Z, Pan M, et al.. Inhibition of experimental myopia by a dopamine agonist: different effectiveness between form deprivation and hyperopic defocus in guinea pigs. Mol Vis. 2011; 17: 2824–2834. [PMC free article] [PubMed] [Google Scholar]
- 2.Mao J, Liu S, Qin W, et al.. Levodopa inhibits the development of form-deprivation myopia in guinea pigs. Optom Vis Sci. 2010; 87(1): 53–60. [DOI] [PubMed] [Google Scholar]
- 3.Gao Q, Liu Q, Ma P, et al.. Effects of direct intravitreal dopamine injections on the development of lid-suture induced myopia in rabbits. Graefes Arch Clin Exp Ophthalmol. 2006; 244(10): 1329–1335. [DOI] [PubMed] [Google Scholar]
- 4.Rohrer B, Spira A-W, Stell W-K.. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigmented epithelium. Vis Neurosci. 1993; 10(3): 447–453. [DOI] [PubMed] [Google Scholar]
- 5.Schmid K-L, Wildsoet C-F.. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optom Vis Sci. 2004; 81(2): 137–147. [DOI] [PubMed] [Google Scholar]
- 6.Stone R-A, Lin T, Laties A-M, et al.. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci USA. 1989; 86(2): 704–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boatright J-H, Hoel M-J, Iuvone P-M.. Stimulation of endogenous dopamine release and metabolism in amphibian retina by light- and K+-evoked depolarization. Brain Res. 1989; 482(1): 164–168. [DOI] [PubMed] [Google Scholar]
- 8.Megaw P-L, Boelen M-G, Morgan I-G, et al.. Diurnal patterns of dopamine release in chicken retina. Neurochem Int. 2006; 48(1): 17–23. [DOI] [PubMed] [Google Scholar]
- 9.Nir I, Haque R, Iuvone P-M.. Diurnal metabolism of dopamine in the mouse retina. Brain Res. 2000; 870(1–2): 118–125. [DOI] [PubMed] [Google Scholar]
- 10.Zawilska J-B, Bednarek A, Berezinska M, et al.. Rhythmic changes in metabolism of dopamine in the chick retina: the importance of light versus biological clock. J Neurochem. 2003; 84(4): 717–724. [DOI] [PubMed] [Google Scholar]
- 11.Godley B-F, Wurtman R-J.. Release of endogenous dopamine from the superfused rabbit retina in vitro: effect of light stimulation. Brain Res. 1988; 452(1–2): 393–395. [DOI] [PubMed] [Google Scholar]
- 12.Contini M, Lin B, Kobayashi K, et al.. Synaptic input of ON-bipolar cells onto the dopaminergic neurons of the mouse retina. J Comp Neurol. 2010; 518(11): 2035–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumitrescu O-N, Pucci F-G, Wong K-Y, et al.. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J Comp Neurol. 2009; 517(2): 226–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prigge C-L, Yeh P-T, Liou N-F, et al.. M1 ipRGCs influence visual function through retrograde signaling in the retina. J Neurosci. 2016; 36(27): 7184–7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rose K-A, Morgan I-G, Ip J, et al.. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008; 115(8): 1279–1285. [DOI] [PubMed] [Google Scholar]
- 16.Dirani M, Tong L, Gazzard G, et al.. Outdoor activity and myopia in Singapore teenage children. Br J Ophthalmol. 2009; 93(8): 997–1000. [DOI] [PubMed] [Google Scholar]
- 17.Rose K-A, Morgan I-G, Smith W, et al.. Myopia, lifestyle, and schooling in students of Chinese ethnicity in Singapore and Sydney. Arch Ophthalmol. 2008; 126(4): 527–530. [DOI] [PubMed] [Google Scholar]
- 18.Pei-Chang Wu, Chen Chueh-Tan, Lin Ken-Kuo, et al.. Myopia prevention and outdoor light intensity in a school-based cluster randomized trial. Ophthalmology. 2018; 125(8): 1239–1250. [DOI] [PubMed] [Google Scholar]
- 19.Jones-Jordan L-A, Mitchell G-L, Cotter S-A, et al.. Visual activity before and after the onset of juvenile myopia. Invest Ophthalmol Vis Sci. 2011; 52(3): 1841–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones-Jordan L-A, Sinnott L-T, Cotter S-A, et al.. Time outdoors, visual activity, and myopia progression in juvenile-onset myopes. Invest Ophthalmol Vis Sci. 2012; 53(11): 7169–7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashby R, Ohlendorf A, Schaeffel F.. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2009; 50(11): 5348–5354. [DOI] [PubMed] [Google Scholar]
- 22.Chen S, Zhi Z, Ruan Q, et al.. Bright light suppresses form-deprivation myopia development with activation of dopamine D1 receptor signaling in the ON pathway in retina. Invest Ophthalmol Vis Sci. 2017; 58(4): 2306–2316. [DOI] [PubMed] [Google Scholar]
- 23.Cohen Y, Peleg E, Belkin M, et al.. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp Eye Res. 2012; 103:33–40. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Lan W, Yang S, et al.. The effect of spectral property and intensity of light on natural refractive development and compensation to negative lenses in guinea pigs. Invest Ophthalmol Vis Sci. 2014; 55(10): 6324–6332. [DOI] [PubMed] [Google Scholar]
- 25.EL-Rd Smith, L-F Hung, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012; 53(1): 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Ding H, Stell W-K, et al.. Exposure to sunlight reduces the risk of myopia in rhesus monkeys. PLoS One. 2015; 10(6): e127863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroger R-H, Wagner H-J.. The eye of the blue acara (Aequidens pulcher, Cichlidae) grows to compensate for defocus due to chromatic aberration. J Comp Physiol A. 1996; 179(6): 837–842. [DOI] [PubMed] [Google Scholar]
- 28.Timucin O-B, Arabaci M, Cuce F, et al.. The effects of light sources with different spectral structures on ocular axial length in rainbow trout (Oncorhynchus mykiss). Exp Eye Res. 2016; 151: 212–221. [DOI] [PubMed] [Google Scholar]
- 29.Foulds W-S, Barathi V-A, Luu C-D.. Progressive myopia or hyperopia can be induced in chicks and reversed by manipulation of the chromaticity of ambient light. Invest Ophthalmol Vis Sci. 2013; 54(13): 8004–8012. [DOI] [PubMed] [Google Scholar]
- 30.Rucker F-J, Wallman J.. Chicks use changes in luminance and chromatic contrast as indicators of the sign of defocus. J Vis. 2012; 12(6): 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu R, Qian Y-F, He J-C, et al.. Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Exp Eye Res. 2011; 92(6): 447–453. [DOI] [PubMed] [Google Scholar]
- 32.Qian Y-F, Dai J-H, Liu R, et al.. Effects of the chromatic defocus caused by interchange of two monochromatic lights on refraction and ocular dimension in guinea pigs. PLoS One. 2013; 8(5): e63229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gawne T-J, JT-Jr Siegwart, Ward A-H, et al.. The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Exp Eye Res. 2017; 155: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gawne T-J, Ward A-H, Norton T-T.. Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vision Res. 2017; 140: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith EL-Rd, Hung L-F, Arumugam B, et al.. Effects of long-wavelength lighting on refractive development in infant rhesus monkeys. Invest Ophthalmol Vis Sci. 2015; 56(11): 6490–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu R, Hu M, He J-C, et al.. The effects of monochromatic illumination on early eye development in rhesus monkeys. Invest Ophthalmol Vis Sci. 2014; 55(3): 1901–1909. [DOI] [PubMed] [Google Scholar]
- 37.Tian T, Zou L, Wu S, et al.. Wavelength defocus and temporal sensitivity affect refractive development in guinea pigs. Invest Ophthalmol Vis Sci. 2019; 60(6): 2173–2180. [DOI] [PubMed] [Google Scholar]
- 38.Rucker F-J, Wallman J.. Chick eyes compensate for chromatic simulations of hyperopic and myopic defocus: evidence that the eye uses longitudinal chromatic aberration to guide eye-growth. Vision Res. 2009; 49(14): 1775–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rucker F-J, Wallman J.. Chicks use changes in luminance and chromatic contrast as indicators of the sign of defocus. J Vis. 2012; 12(6): 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rucker F-J.The role of luminance and chromatic cues in emmetropisation. Ophthalmic Physiol Opt. 2013; 33(3): 196–214. [DOI] [PubMed] [Google Scholar]
- 41.Chuang K-K, Rucker F-J.. The role of dopamine in eye growth responses to color and luminance flicker in chicks. Exp Eye Res. 2019; 189:107822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang M, Schaeffel F, Jiang B, et al.. Effects of light of different spectral composition on refractive development and retinal dopamine in chicks. Invest Ophthalmol Vis Sci. 2018; 59(11): 4413–4424. [DOI] [PubMed] [Google Scholar]
- 43.Strickland R, Landis E-G, Pardue M-T.. Short-wavelength (violet) light protects mice from myopia through cone signaling. Invest Ophthalmol Vis Sci. 2020; 61(2): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nowak J-Z, Zurawska E.. Dopamine in the rabbit retina and striatum: diurnal rhythm and effect of light stimulation. J Neural Transm. 1989; 75(3): 201–212. [DOI] [PubMed] [Google Scholar]
- 45.Kirsch M, Wagner H-J.. Release pattern of endogenous dopamine in teleost retinae during light adaptation and pharmacological stimulation. Vision Res. 1989; 29(2): 147–154. [DOI] [PubMed] [Google Scholar]
- 46.Kramer S-G.Dopamine: a retinal neurotransmitter. I. Retinal uptake, storage, and light-stimulated release of H3-dopamine in vivo. Invest Ophthalmol. 1971; 10(6): 438–452. [PubMed] [Google Scholar]
- 47.Gottlieb M-D, Wallman J.. Retinal activity modulates eye growth: evidence from rearing in stroboscopic illumination. Soc Neurosci Abstr. 1987; 13: 1297. [Google Scholar]
- 48.Luft W-A, Iuvone P-M, Stell W-K.. Spatial, temporal, and intensive determinants of dopamine release in the chick retina. Vis Neurosci. 2004; 21(4): 627–635. [DOI] [PubMed] [Google Scholar]
- 49.Iuvone P-M, Galli C-L, Garrison-Gund C-K, et al.. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science. 1978; 202(4370): 901–902. [DOI] [PubMed] [Google Scholar]
- 50.Kee C-S, Marzani D, Wallman J.. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Invest Ophthalmol Vis Sci. 2001; 42(3): 575–583. [PubMed] [Google Scholar]
- 51.Rohrer B, Iuvone P-M, Stell W-K.. Stimulation of dopaminergic amacrine cells by stroboscopic illumination or fibroblast growth factor (bFGF, FGF-2) injections: possible roles in prevention of form-deprivation myopia in the chick. Brain Res. 1995; 686(2): 169–181. [DOI] [PubMed] [Google Scholar]
- 52.Schwahn H-N, Schaeffel F.. Flicker parameters are different for suppression of myopia and hyperopia. Vision Res. 1997; 37(19): 2661–2673. [DOI] [PubMed] [Google Scholar]
- 53.Cremieux J, Orban G-A, Duysens J, et al.. Experimental myopia in cats reared in stroboscopic illumination. Vision Res. 1989; 29: 1033–1036. [DOI] [PubMed] [Google Scholar]
- 54.Yu Y, Chen H, Tuo J, et al.. Effects of flickering light on refraction and changes in eye axial length of C57BL/6 mice. Ophthalmic Res. 2011; 46(2): 80–87. [DOI] [PubMed] [Google Scholar]
- 55.Zhi Z, Pan M, Xie R, et al.. The effect of temporal and spatial stimuli on the refractive status of guinea pigs following natural emmetropization. Invest Ophthalmol Vis Sci. 2013; 54(1): 890–897. [DOI] [PubMed] [Google Scholar]
- 56.Di Y, Liu R, Chu R-Y, et al.. Myopia induced by flickering light in guinea pigs: a detailed assessment on susceptibility of different frequencies. Int J Ophthalmol. 2013; 6(2): 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rucker F, Britton S, Taylor C.. Color and temporal frequency sensitive eye growth in chick. Invest Ophthalmol Vis Sci. 2018; 59(15): 6003–6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Witkovsky P.Dopamine and retinal function. Doc Ophthalmol. 2004; 108(1): 17–40. [DOI] [PubMed] [Google Scholar]
- 59.Megaw P, Morgan I, Boelen M.. Vitreal dihydroxyphenylacetic acid (DOPAC) as an index of retinal dopamine release. J Neurochem. 2001; 76(6): 1636–1644. [DOI] [PubMed] [Google Scholar]
- 60.Glickstein M, Millodot M.. Retinoscopy and eye size. Science. 1970; 168(3931): 605–606. [DOI] [PubMed] [Google Scholar]
- 61.Zhang S, Yang J, Reinach P-S, et al.. Dopamine receptor subtypes mediate opposing effects on form deprivation myopia in pigmented guinea pigs. Invest Ophthalmol Vis Sci. 2018; 59(11): 4441–4448. [DOI] [PubMed] [Google Scholar]
- 62.Jiang L, Zhang S, Chen R, et al.. Effects of the tyrosinase-dependent dopaminergic system on refractive error development in guinea pigs. Invest Ophthalmol Vis Sci. 2018; 59(11): 4631–4638. [DOI] [PubMed] [Google Scholar]
- 63.Wu X-H, Qian K-W, Xu G-Z, et al.. The role of retinal dopamine in C57BL/6 mouse refractive development as revealed by intravitreal administration of 6-hydroxydopamine. Invest Ophthalmol Vis Sci. 2016; 57(13): 5393–5404. [DOI] [PubMed] [Google Scholar]
- 64.Wu X-H, Li Y-Y, Zhang P-P, et al.. Unaltered retinal dopamine levels in a C57BL/6 mouse model of form-deprivation myopia. Invest Ophthalmol Vis Sci. 2015; 56(2): 967–977. [DOI] [PubMed] [Google Scholar]
- 65.Ohngemach S, Hagel G, Schaeffel F.. Concentrations of biogenic amines in fundal layers in chickens with normal visual experience, deprivation, and after reserpine application. Vis Neurosci. 1997; 14(3): 493–505. [DOI] [PubMed] [Google Scholar]
- 66.Chakraborty R, Park Hn, Aung M-H, et al.. Comparison of refractive development and retinal dopamine in OFF pathway mutant and C57BL/6J wild-type mice. Mol Vis. 2014; 20: 1318–1327. [PMC free article] [PubMed] [Google Scholar]
- 67.Park Hn, Jabbar S-B, Tan C-C, et al.. Visually-driven ocular growth in mice requires functional rod photoreceptors. Invest Ophthalmol Vis Sci. 2014; 55(10): 6272–6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chakraborty R, Park H-N, Hanif A-M, et al.. ON pathway mutations increase susceptibility to form-deprivation myopia. Exp Eye Res. 2015; 137: 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feldkaemper M, Schaeffel F.. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013; 114: 106–119. [DOI] [PubMed] [Google Scholar]
- 70.Kruger P-B, Rucker F-J, Hu C, et al.. Accommodation with and without short-wavelength-sensitive cones and chromatic aberration. Vision Res. 2005; 45(10): 1265–1274. [DOI] [PubMed] [Google Scholar]
- 71.Rucker F-J, Kruger P-B.. The role of short-wavelength sensitive cones and chromatic aberration in the response to stationary and step accommodation stimuli. Vision Res. 2004; 44(2): 197–208. [DOI] [PubMed] [Google Scholar]
- 72.Seidemann A, Schaeffel F.. Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Res. 2002; 42(21): 2409–2417. [DOI] [PubMed] [Google Scholar]
- 73.Sarfare Shanta, Stone Richard-A, Iuvone P-Michael, et al.. Dopamine: mechanistic conundrums in eye growth control. Invest Ophthalmol Vis Sci. 2019; 60(9): 5883. [Google Scholar]
- 74.Rucker F.Monochromatic and white light and the regulation of eye growth. Exp Eye Res. 2019; 184: 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dacey D-M, Liao H-W, Peterson B-B, et al.. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005; 433(7027): 749–754. [DOI] [PubMed] [Google Scholar]
- 76.Rickers Markus, Schaeffel Frank. Dose-dependent effects of intravitreal pirenzepine on deprivation myopia and lens-induced refractive errors in chickens. Exp Eye Res. 1995; 61(4): 509–516. [DOI] [PubMed] [Google Scholar]
- 77.Schaeffel Frank, Bartmann Marieluise, Hagel Gabi, et al.. Studies on the role of the retinal dopamine/melatonin system in experimental refractive errors in chickens. Vision Res. 1995; 35(9): 1247–1264. [DOI] [PubMed] [Google Scholar]